Abstract
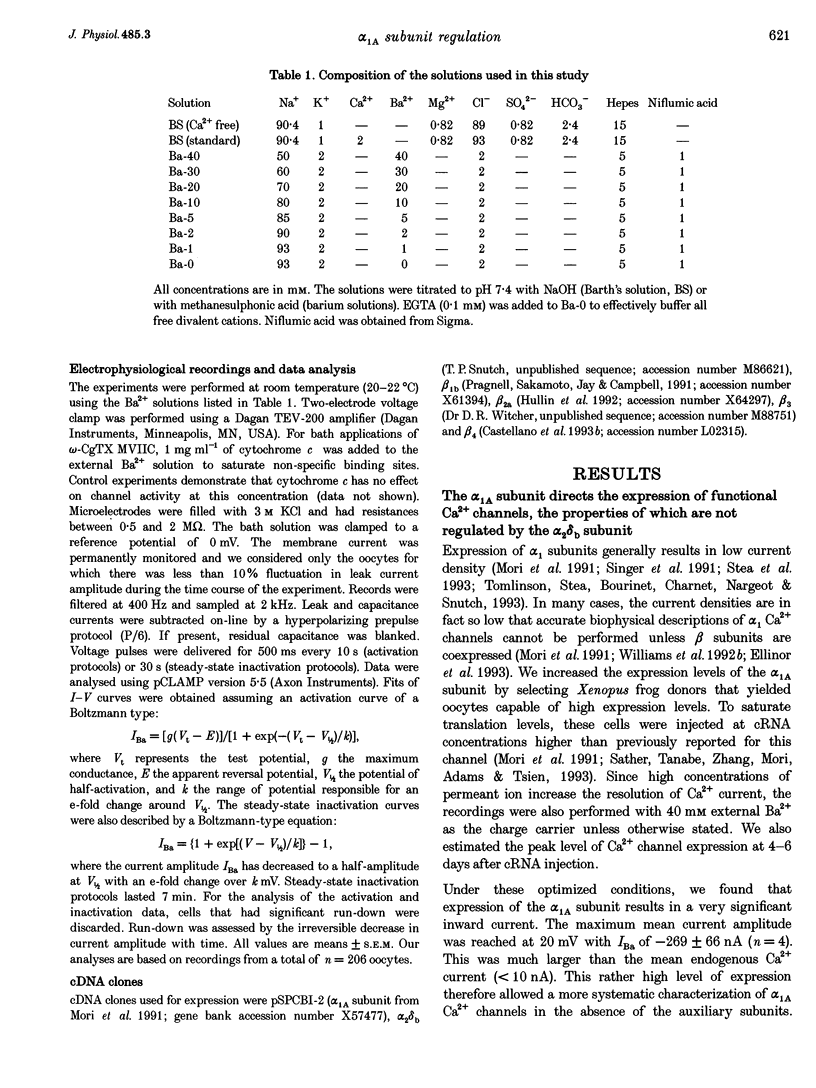
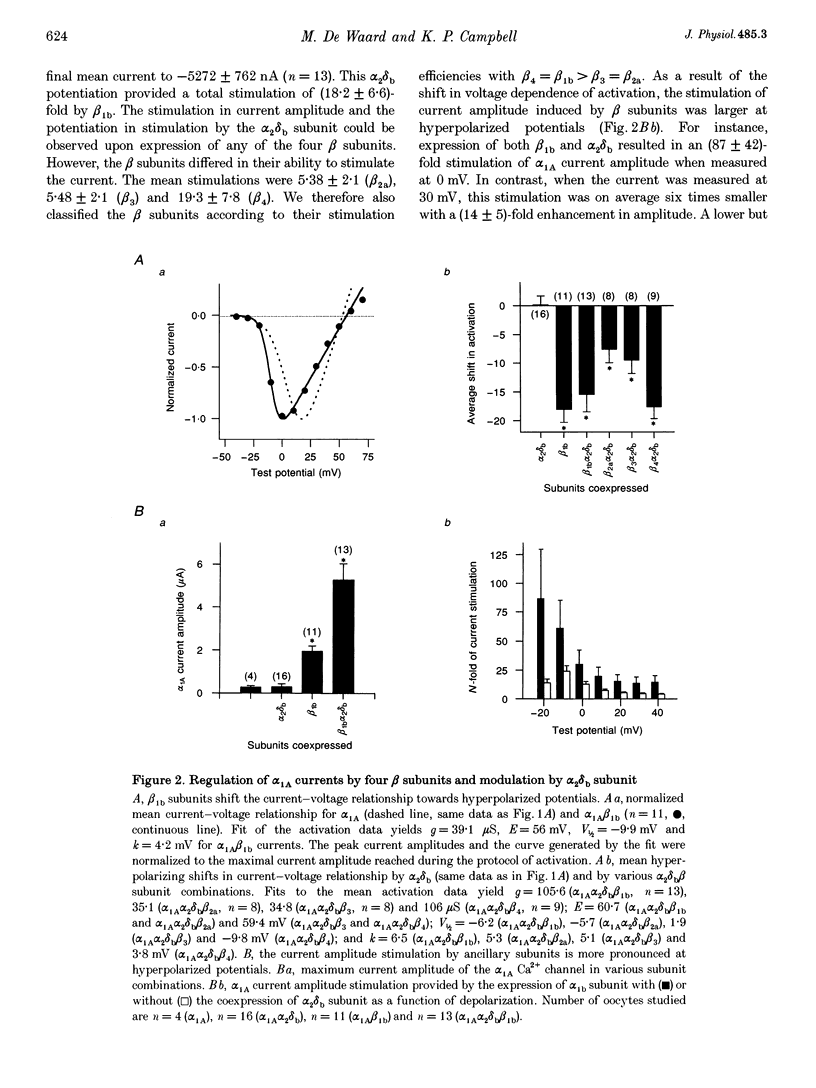
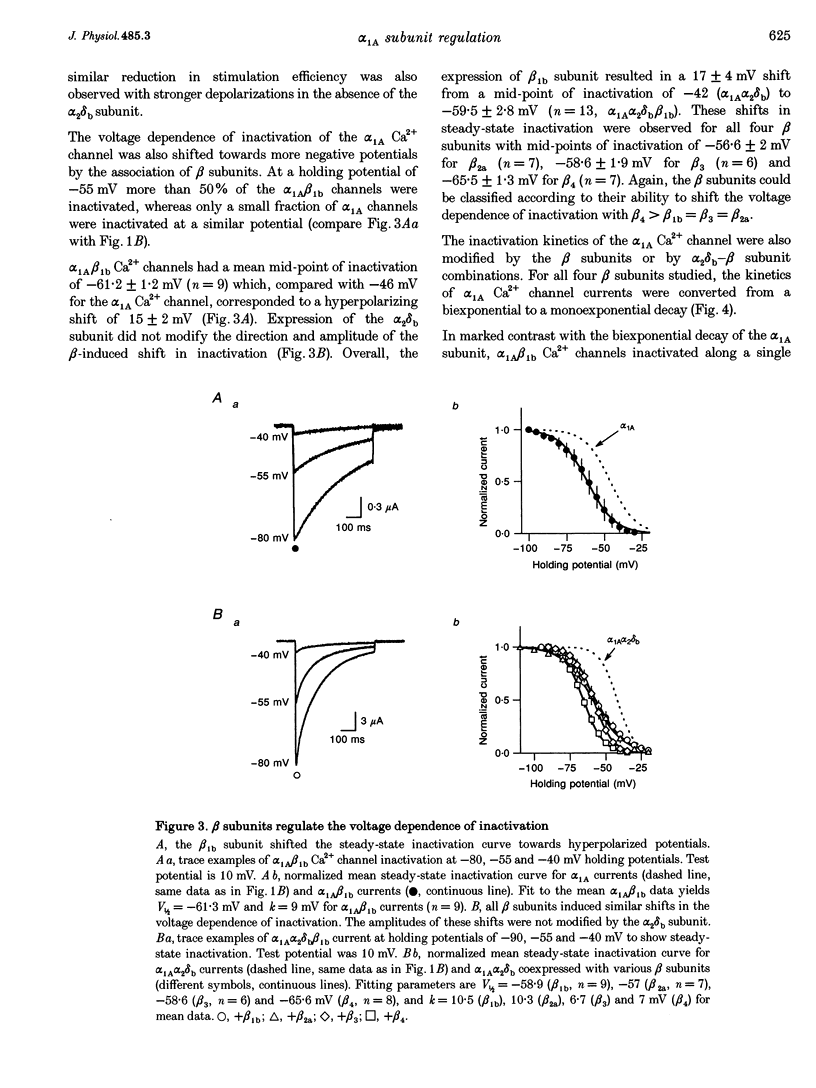
1. Voltage-dependent Ca2+ channels are multi-protein complexes composed of at least three subunits: alpha 1, alpha 2 delta and beta. Ba2+ currents were recorded in Xenopus oocytes expressing the neuronal alpha 1A Ca2+ channel, using the two-electrode voltage-clamp technique. Various subunit combinations were studied: alpha 1A, alpha 1A alpha 2 delta b, alpha 1A beta or alpha 1A alpha 2 delta b beta. 2. The alpha 1A subunit alone directs the expression of functional Ca2+ channels. It carries all the properties of the channel: gating, permeability, voltage dependence of activation and inactivation, and pharmacology. The alpha 1A channel is activated by low voltages when physiological concentrations of the permeant cation are used. Both ancillary subunits alpha 2 delta and beta induced considerable changes in the biophysical properties of the alpha 1A current. The subunit specificity of the changes in current properties was analysed for all four beta gene products by coexpressing beta 1b, beta 2a, beta 3 and beta 4. 3. All beta subunits induce a stimulation in the current amplitude, a change in inactivation kinetics, and two hyperpolarizing shifts--one in the voltage dependence of activation and a second in the voltage dependence of steady-state inactivation. The most significant difference in regulation among beta subunits is the induction of variable rate constants of current inactivation. Rates of inactivation were induced in the following order (fastest to slowest): beta 3 > beta 1b = beta 4 > beta 2a. 4. The alpha 2 delta b subunit does not modify the properties of alpha 1A Ca2+ channels in the absence of beta subunits. However, this subunit increases the beta-induced stimulation in current amplitude and also regulates the beta-induced change in inactivation kinetics. 5. Of all the subunit combinations tested, Ca2+ channels that included a beta subunit were the most prone to decrease in activity. It is concluded that beta subunits are the primary target for the inhibitory mechanisms involved in Ca2+ channel run-down. 6. Both alpha 2 delta b and beta 1 b subunits slightly modified the sensitivity of the alpha 1A subunit to the snail peptide omega-conotoxin MVIIC. 7. The subunit-induced changes in properties of the alpha 1A channel are surprisingly similar to changes reported for other alpha 1 subunits. These modifications in channel activity should therefore represent important functional landmarks in the on-going characterization of subunit-subunit interactions.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Castellano A., Wei X., Birnbaumer L., Perez-Reyes E. Cloning and expression of a neuronal calcium channel beta subunit. J Biol Chem. 1993 Jun 15;268(17):12359–12366. [PubMed] [Google Scholar]
- Castellano A., Wei X., Birnbaumer L., Perez-Reyes E. Cloning and expression of a third calcium channel beta subunit. J Biol Chem. 1993 Feb 15;268(5):3450–3455. [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- De Waard M., Witcher D. R., Campbell K. P. Functional properties of the purified N-type Ca2+ channel from rabbit brain. J Biol Chem. 1994 Mar 4;269(9):6716–6724. [PubMed] [Google Scholar]
- Dubel S. J., Starr T. V., Hell J., Ahlijanian M. K., Enyeart J. J., Catterall W. A., Snutch T. P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Dumont J. N. Defined nutrient medium for the in vitro maintenance of Xenopus laevis oocytes. In Vitro. 1976 Jun;12(6):418–427. doi: 10.1007/BF02806021. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Hui A., Ellinor P. T., Krizanova O., Wang J. J., Diebold R. J., Schwartz A. Molecular cloning of multiple subtypes of a novel rat brain isoform of the alpha 1 subunit of the voltage-dependent calcium channel. Neuron. 1991 Jul;7(1):35–44. doi: 10.1016/0896-6273(91)90072-8. [DOI] [PubMed] [Google Scholar]
- Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992 Mar;11(3):885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S. D., Ellis S. B., McCue A. F., Williams M. E., Vedvick T. S., Harpold M. M., Campbell K. P. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990 Apr 27;248(4954):490–492. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- Jay S. D., Sharp A. H., Kahl S. D., Vedvick T. S., Harpold M. M., Campbell K. P. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991 Feb 15;266(5):3287–3293. [PubMed] [Google Scholar]
- Kim H. L., Kim H., Lee P., King R. G., Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel alpha 2 subunit. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A. E., Kim H. S., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L., Brown A. M. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991 Aug 8;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Voltage-sensitive Ca2+ channels. J Biol Chem. 1992 Jan 25;267(3):1403–1406. [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Morton M. E., Froehner S. C. The alpha 1 and alpha 2 polypeptides of the dihydropyridine-sensitive calcium channel differ in developmental expression and tissue distribution. Neuron. 1989 May;2(5):1499–1506. doi: 10.1016/0896-6273(89)90196-7. [DOI] [PubMed] [Google Scholar]
- Neely A., Wei X., Olcese R., Birnbaumer L., Stefani E. Potentiation by the beta subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993 Oct 22;262(5133):575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- Niidome T., Kim M. S., Friedrich T., Mori Y. Molecular cloning and characterization of a novel calcium channel from rabbit brain. FEBS Lett. 1992 Aug 10;308(1):7–13. doi: 10.1016/0014-5793(92)81038-n. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Takeshima H., Hofmann F., Flockerzi V., Imoto K. Requirement of the calcium channel beta subunit for functional conformation. FEBS Lett. 1993 Jun 21;324(3):283–286. doi: 10.1016/0014-5793(93)80135-h. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Castellano A., Kim H. S., Bertrand P., Baggstrom E., Lacerda A. E., Wei X. Y., Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992 Jan 25;267(3):1792–1797. [PubMed] [Google Scholar]
- Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994 Mar 3;368(6466):67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Pragnell M., Sakamoto J., Jay S. D., Campbell K. P. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 1991 Oct 21;291(2):253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Singer-Lahat D., Lotan I., Itagaki K., Schwartz A., Dascal N. Evidence for the existence of RNA of Ca(2+)-channel alpha 2/delta subunit in Xenopus oocytes. Biochim Biophys Acta. 1992 Oct 6;1137(1):39–44. doi: 10.1016/0167-4889(92)90097-u. [DOI] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991 Sep 27;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Soong T. W., Stea A., Hodson C. D., Dubel S. J., Vincent S. R., Snutch T. P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993 May 21;260(5111):1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Starr T. V., Prystay W., Snutch T. P. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Dubel S. J., Pragnell M., Leonard J. P., Campbell K. P., Snutch T. P. A beta-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel alpha 1-subunit. Neuropharmacology. 1993 Nov;32(11):1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tomlinson W. J., Stea A., Bourinet E., Charnet P., Nargeot J., Snutch T. P. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993 Nov;32(11):1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- Wei X. Y., Perez-Reyes E., Lacerda A. E., Schuster G., Brown A. M., Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. Implications for the structure of cardiac L-type Ca2+ channels. J Biol Chem. 1991 Nov 15;266(32):21943–21947. [PubMed] [Google Scholar]
- Williams M. E., Brust P. F., Feldman D. H., Patthi S., Simerson S., Maroufi A., McCue A. F., Veliçelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992 Jul 17;257(5068):389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., De Waard M., Sakamoto J., Franzini-Armstrong C., Pragnell M., Kahl S. D., Campbell K. P. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993 Jul 23;261(5120):486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]



