Abstract
Heart failure remains a significant clinical challenge due to the difficulty in managing refractory fluid overload. Combination diuretic strategies, involving various combinations, such as metolazone and furosemide, have been proposed to improve diuresis and patient outcomes. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of combination therapies. A comprehensive search of PubMed, Cochrane Library, Embase, and Web of Science identified 61 articles, of which seven randomised controlled trials met the inclusion criteria. Data on mortality, hospital readmission rates, symptom improvement, electrolyte imbalances, renal function, and adverse events were extracted and analysed. The risk of bias was assessed using the established guidelines. The analysis revealed that combination diuretic therapies significantly reduced body weight (P=0.001) but did not significantly impact mortality (RR: 0.99, 95% CI: 0.90-1.09) or hospital readmission rates (RR: 1.05, 95% CI: 0.98-1.12) compared to placebo. Adverse effects, particularly electrolyte imbalances, such as hypo and hypernatraemia and hypokalaemia, and renal function deterioration were noted in the combined diuretic group. In contrast, serious adverse events were observed more in the placebo group. The mean difference of the Kansas City Cardiomyopathy Questionnaire (KCCQ) score was 2.43 (95% CI: 0.95-3.92). The risk ratio for hospital readmission was 1.05 (95% CI: 0.98-1.12), which was statistically non-significant. We used fixed-effect models for most variables due to less heterogeneity between the studies, and the corresponding I2 values were <50% for most variables. Funnel plots indicated minimal publication bias, although some heterogeneity was observed. Comparisons with other studies in the literature, such as the DAPA-HF and EVEREST trials, supported these findings but also highlighted the need for individualised treatment approaches. Combination diuretic therapies effectively manage fluid overload and reduce body weight in patients with heart failure but do not significantly affect mortality or hospital readmission rates. The potential for adverse events, particularly electrolyte imbalances and renal function deterioration, underscores the need for careful monitoring and personalised treatment plans. Future research should focus on optimising diuretic combinations and dosing strategies to enhance their safety and efficacy. These findings align with the current guidelines emphasising individualised treatment in heart failure management and highlight the importance of integrating combination diuretics into a comprehensive care plan to improve patient outcomes.
Keywords: combination diuretics in heart failure, decompensated heart failure, heart failure advancements, heart failure and medical education, heart failure hospitalization, heart failure management programmes, heart failure prognosis, heart failure with reduced ejection fraction, mortality in acute heart failure, valvular heart disease
Introduction and background
Pulmonary congestion is a common cause of presentation among individuals requiring hospital admission for acute heart failure (AHF) [1]. Therefore, one of the primary goals of in-hospital care for these patients is to alleviate congestion signs and symptoms (also known as decongestion) [2-4]. Unfortunately, after hospital admission, adequate decongestion is frequently not obtained, and this has been associated with increased mortality and hospitalisation rates for heart failure (HF) [5-6]. The persistence of congestion post-admission necessitates the exploration of targeted strategies to enhance decongestion and improve patient outcomes. Currently, loop diuretics are the cornerstone of treatment for patients with HF who present with congestion [7-8]. However, the effectiveness of loop diuretics can diminish over time due to progressive disease and the development of diuretic resistance, necessitating higher doses, which can lead to adverse effects, such as electrolyte imbalances, renal dysfunction, and worsening cardiovascular outcomes [9]. Consequently, there is growing interest in the use of combination diuretic therapies, which involve the concurrent use of different classes of diuretics to optimise fluid management while minimising the adverse effects associated with high-dose monotherapy [8,10]. Combination diuretic therapies employ various classes of diuretics, including loop diuretics, thiazide diuretics, and mineralocorticoid receptor antagonists (MRAs). By targeting multiple sites of renal sodium reabsorption, these combinations aimed to enhance diuresis and achieve better decongestion outcomes. The rationale behind this approach is to overcome the limitations of single-agent therapy, which often fails to provide adequate decongestion in advanced HF cases.
Rationale for evaluating combination diuretic therapies
Combination diuretic strategies involving the concurrent use of different classes of diuretics, such as loop diuretics, thiazide diuretics, and MRAs, to target multiple sites of renal sodium reabsorption and enhance diuresis while minimizing adverse effects associated with high-dose monotherapy are being evaluated as potential methods of achieving adequate decongestion among HF patients. There are several challenges to evaluating the effectiveness of combination diuretic therapies for HF. First, the lack of standardised protocols or guidelines for these therapies results in significant variability in dosing regimens and drug combinations across studies. This variability makes it difficult to compare results and draw definitive conclusions. Additionally, individual patient characteristics such as renal function, electrolyte status, and comorbidities can significantly influence the response to diuretic therapy, complicating the interpretation of the study results. For instance, patients with compromised renal function may experience different outcomes than those with normal renal function, making it challenging to establish a one-size-fits-all approach. Moreover, there are concerns regarding the potential synergistic effects of combination diuretic therapy on electrolyte imbalance, renal function decline, and neurohormonal activation. These potential adverse effects underscore the importance of rigorous evaluations to assess the effectiveness and safety of these therapies. The complexity of managing HF with combination diuretics necessitates a thorough understanding of how these therapies interact with various physiological parameters [11-12]. Given the current scarcity of high-quality evidence and the clinical uncertainty surrounding combination diuretic therapies for HF, there is a pressing need for a comprehensive systematic review. The goal of this systematic review was to inform evidence-based clinical decision-making and help clinicians optimise fluid management strategies in patients with HF. By identifying the most effective and safest diuretic combinations, healthcare providers can tailor treatments according to individual patient needs, ultimately improving the overall management of HF. Additionally, this review aimed to highlight areas where further research is needed to guide future studies to address unresolved questions and improve clinical practice.
Review
Methodology
This was a systematic review and therefore adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. A comprehensive literature search was conducted using the electronic databases PubMed and Cochrane Database of Systematic Reviews from May 1, 2023, to May 31, 2023. In addition, the systematic review register PROSPERO was searched. The search strategy involved a combination of Medical Subject Headings (MeSH) terms and keywords related to AHF, diuretic therapies, and study design. The search terms included “acute heart failure" OR "acute decompensated heart failure" AND "diuretics" OR "combination therapy" AND "randomized controlled trial" OR "quasi-experimental. “To ensure thoroughness, a manual search of the reference lists of studies meeting the PICO criteria and inclusion/exclusion criteria was performed. Additional database searches were conducted to include existing guidelines and recommendations on diuretic therapies in HF. The study selection process included articles evaluating the effects of combination diuretic therapies in patients hospitalized with AHF.
Two reviewers independently assessed the titles and abstracts of articles for relevance, duplication, and inclusion. The full texts of potentially relevant studies were retrieved and assessed for inclusion based on the predefined Population, Intervention, Comparison, and Outcome (PICO) model and inclusion/exclusion criteria. Any disagreements were resolved by consensus or by a third-party adjudicator, and eligibility criteria were established before the selection process. Articles were eligible if they were systematic reviews, meta-analyses, or randomised controlled studies and included a predefined PICO model. Articles on animals or non-human subjects and studies involving hospitalized patients for reasons other than decongestion were excluded. Specifically, the criteria were as follows. The target population in this study were patients hospitalized with AHF requiring intravenous diuretics. Patients hospitalized for other management that did not involve decongestion were also excluded. Studies of combination diuretic therapy were included. Studies without combination diuretic therapy were excluded, and studies comparing combination diuretic therapies to placebo or monotherapy with diuretics were included. Studies not reporting the effectiveness and safety outcomes, including mortality, hospital readmission rates, symptom improvement, electrolyte abnormalities, and renal function, were excluded. Studies with outcomes unrelated to effectiveness and safety were excluded. We included studies that were randomised controlled trials (RCTs) and quasi-experimental studies, whereas case studies, letters, case reports, editorials, and comments were excluded from the analysis.
Publication type
Only peer-reviewed articles published in English were included, as shown in Table 1.
Table 1. Inclusion and exclusion criteria based on the PICO framework .
PICO, Population, Intervention, Comparison, and Outcome
| Criteria | Inclusion | Exclusion |
| Population | Patients hospitalized with acute heart failure for decongestion | Patients with acute heart failure hospitalized for other management that does not include decongestion |
| Intervention | Combination diuretic therapies | No combination diuretic therapy |
| Comparison | Placebo or monotherapy with diuretics | No comparison group |
| Outcome | Effectiveness and safety outcomes, including mortality, hospital readmission rates, symptom improvement, electrolyte abnormalities, and renal function | Outcomes not related to effectiveness and safety |
| Study design | Randomised controlled trials and quasi-experimental studies | Articles, case studies, letters, case reports, editorials, comments |
| Publication type | Peer-reviewed articles published in English | Studies not published in the English language were excluded due to potential costs associated with professional translational services |
Full-text articles of potentially relevant studies were retrieved and assessed for inclusion based on eligibility criteria. Data extraction was performed using the systematic review software COVIDENCE.
Risk-of-bias assessment
Two reviewers independently assessed the risk of bias using the appropriate tools for different types of studies. For RCTs, the Cochrane Risk of Bias tool was used to critically appraise methodological quality. For quasi-experimental studies, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used. The results of these assessments were compared to determine inter-rater reliability. Each tool consists of various domains used to evaluate the risk of bias and the overall quality of the studies. The studies were rated accordingly based on the presence of flaws and accuracy. This rigorous process helps identify high-quality studies and ensures the reliability of the review. The quality assessments of all studies that met the PICO and the inclusion criteria are reported in Figure 1. The summary of the risk of bias assessment for all seven selected studies is illustrated in Figure 2.
Figure 1. Traffic light chart for the risk-of-bias assessment.
Figure 2. Risk-of-bias assessment for included studies.
Results
Study Selection
Four databases (PubMed, Cochrane Library, Embase, and Web of Science) provided 61 articles. In total, 15 duplicate records were removed, and 10 records were removed because they were irrelevant to the study. Following the full-text screening of the remaining articles, seven RCTs made up the final selection were included in the systematic review and meta-analysis. Figure 3 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram representing the search strategy [12].
Figure 3. PRISMA flow diagram.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Characteristics of incorporated RCTs
Table 2 provides a summary of the study characteristics of the RCTs that were included in this review. The sample size in the obtained articles ranges from 51 to 4,133 participants, and the average age of the participants was between 58 and 83 years [13-19]. Most studies reported that most of the population among the participants were male. Here, all the articles mainly focused on acute decompensated HF, but one study investigated HF with reduced ejection fraction [17]. Most of the studies focused on loop diuretics, particularly furosemide (FSM). Two studies investigated thiazide diuretics (hydrochlorothiazide [HCTZ]) [14,16]. One study compared empagliflozin (a sodium-glucose cotransporter-2 [SGLT2] inhibitor) to a placebo, alongside standard diuretic therapy [18]. Another study compared tolvaptan (a vasopressin receptor antagonist) to a placebo [19].
Table 2. A summary of study characteristics of the RCTs.
HCTZ, hydrochlorothiazide; RCT, randomised controlled trial
| Author, Year | Study Design | Sample Size | Age | Gender | Type of Heart Failure | Types of Diuretics | Dosages | Duration of Treatment | Primary Outcome | Secondary Outcome |
| Shah et al., 2014 [13] | Prospective, randomised controlled trial | N=90. Group 1: 30; group 2: 30; group 3: 30 | Mean age: 58.22 ± 15.45 years | Male: 66 (73.3%) | Acute decompensated heart failure | Loop diuretics (furosemide), intravenous | Group 1: Furosemide 100 mg/24h + Dopamine 2.5 mg/kg/min; Group 2: Furosemide 100 mg/24h; Group 3: Furosemide 100 mg/24h | 48 hrs | Negative fluid balance at 24 hrs | Negative fluid balance at 48, 72, 96 hrs; trend of serum sodium, potassium, blood urea, creatinine; hospital stay duration; clinical outcomes at 30 days |
| Piardi et al., 2021 [14] | Randomised, single-centre, double-blind, placebo-controlled trial | N=51. HCTZ group: 26; placebo group: 25 | Mean age: 64 years | HCTZ group: 69% male; placebo group: 48% | Acute decompensated heart failure | Furosemide + HCTZ; placebo | HCTZ: 50 mg or placebo (oral) | 3 days | Daily weight change over 3 days | Change in creatinine, need for vasoactive drugs, change in natriuretic peptides, congestion score, dyspnoea scale, thirst scale, length of stay, in-hospital mortality, hypernatremia, hypokalaemia, renal function, need for haemodialysis, ventricular arrhythmias |
| Mullens et al., 2022 [15] | Multicentre, parallel-group, double-blind, placebo-controlled trial | N=519. Acetazolamide group: 259; placebo group: 260 | Placebo: 78.5±8.8; acetazolamide: 77.9 ±9.0 | Placebo: 59.6% male; acetazolamide: 65.6% male | Acute decompensated heart failure | Acetazolamide with loop diuretic | Acetazolamide: 500 mg once daily | 3 days | Successful decongestion | Death from any cause or rehospitalisation for heart failure; safety and adverse events |
| Trullàs et al., 2023 [16] | Multicentre, prospective, randomised, double-blind, placebo-controlled trial | N=230. Placebo: 116; HCTZ: 114 | Mean age: 83 years | 48% women | Acute decompensated heart failure | HCTZ with intravenous furosemide | HCTZ: 25-100 mg daily based on glomerular filtration rate; Furosemide: 80 mg/day | 5 days | Changes in body weight and patient-reported dyspnoea at 72 hrs | Diuretic response metrics, mortality/rehospitalisations at 30 and 90 days, safety outcomes (renal function/electrolytes) |
| Jackson et al., 2020 [17] | Randomised, double-blind, placebo-controlled trial | N=736 (no diuretic). Total with diuretic: 3,880 | Average age: 66.1 years across groups | Female: 23.6% overall | Heart failure with reduced ejection fraction | Loop diuretics (azosemide, bumetanide, furosemide, torsemide); combination of loop and thiazide-like diuretics | Mean: 57.0 ± 94.4 mg Furosemide-Equivalent; Median: 40 mg (range 20-80 mg) | Nil | Dapagliflozin reduced the risk of the primary endpoint across diuretic subgroups | Improvement in symptoms and treatment toleration, consistency across diuretic subgroups, unchanged diuretic dose during follow-up, no difference in mean diuretic dose post-randomisation |
| Biegus et al., 2023 [18] | Multinational, multicentre, randomised, double-blind trial | N=530. Empagliflozin: 265; placebo: 265 | Empagliflozin: 71 (62–78); placebo: 70 (59–78) | Empagliflozin: 67.5% male; placebo: 64.9% | Acute heart failure | Furosemide (intravenous and oral), torasemide, bumetanide | Empagliflozin: 10 mg once daily; furosemide: 40 mg intravenous; torasemide: 20 mg; bumetanide: 1 mg | 90 days | Weight loss, WL adjusted for mean daily loop diuretic dose | Area under the curve of change in N-terminal pro-B-type natriuretic peptide levels, haemoconcentration, clinical congestion score |
| Konstam et al., 2007 [19] | Randomised, double-blind, placebo-controlled study | N=4,133. Tolvaptan: 2,072; placebo: 2,061 | Tolvaptan: 65.9 years (SD = 11.7); placebo: 65.6 years (SD = 12.0) | Tolvaptan: 73.4% male; placebo: 75.4% | Acute decompensated heart failure | Furosemide (loop diuretic) | Low-dose: equal to outpatient dose; high-dose: 2.5 times outpatient dose; continuous infusion; intermittent bolus | 60 days | Death from cardiovascular causes or first hospitalisation for heart failure | Composite of cardiovascular death or hospitalisation, incidence of cardiovascular mortality, clinical worsening of heart failure |
Study findings on clinical outcomes and adverse events
The seven RCTs included in this analysis provided a comprehensive overview of the clinical outcomes and adverse events associated with various diuretics in HF management. These results indicate that while some diuretics improve symptoms and patient-reported outcomes, they may also pose risks related to electrolyte imbalances and renal function deterioration. Table 3 summarises the clinical outcomes and adverse events.
Table 3. Comparison of clinical outcomes and adverse events in diuretic therapy trials.
HCTZ, hydrochlorothiazide; KCCQ, Kansas City Cardiomyopathy Questionnaire
| Author, Year | Mortality | Hospital Readmission Rates | Symptom Improvement | Electrolyte Abnormalities | Renal Function | Other Adverse Events |
| Shah et al., 2014 [13] | 0 in dopamine infusion group; 2 in bolus group; 1 in infusion group | 9 in infusion + dopamine group; 8 in bolus group; 7 in infusion group | None reported | No statistically significant difference in serum sodium and potassium levels (p > 0.05) | No statistically significant difference in blood urea and serum creatinine levels (p > 0.05) | None reported |
| Piardi et al., 2021 [14] | In-hospital mortality: HCTZ 3.8%, Placebo 0% (p = 1.00) | None reported | Slight improvement in dyspnoea and congestion scores with HCTZ, but not statistically significant | Hypernatremia: HCTZ 0%, placebo 4.8%. Hypokalaemia: HCTZ 3.8%, placebo 4.5%. Increase in creatinine: HCTZ 58%, placebo 41% (p = 0.05) | Significant increase in creatinine levels in the HCTZ group compared to placebo | Ventricular arrhythmias: HCTZ 3.8%, placebo 4%. Haemodialysis: HCTZ 3.8%. placebo 0% (p = 1.00) |
| Mullens et al., 2022 [15] | Death: acetazolamide 29.7%, placebo 27.8% (HR 1.07; 95% CI, 0.78-1.48) | Acetazolamide: 18.4%; placebo: 17.4% | None reported | Hypokalaemia: acetazolamide 5.5%, placebo 3.9% | Renal safety endpoint: acetazolamide 2.7%, placebo 0.8%. Doubling of serum creatinine: acetazolamide 0.8%, placebo 0%. ≥50% decrease in GFR: acetazolamide 1.6%, placebo 0.4% | Serious adverse events: acetazolamide 48.0%, placebo 47.9%. Cardiovascular adverse events: acetazolamide 44.1%, placebo 47.1% |
| Trullàs et al., 2023 [16] | 30-day mortality: HCTZ 9.6%, placebo 6.0%. 90-day mortality: HCTZ 20.2%, placebo 16.4% | HCTZ: 37.7%; placebo: 34.5% | Slight improvement in dyspnoea in the HCTZ group at 72 and 96 hours | Sodium ≤ 130 mmol/L: HCTZ 8.8%, placebo 5.2%. Sodium ≤ 125 mmol/L: HCTZ 2.6% placebo 1.7%. Potassium ≤ 3.5 mmol/L: HCTZ 44.7%, placebo 19.0%. Potassium ≤ 3.0 mmol/L: HCTZ 40.6%, placebo 16.1% | Impaired renal function: HCTZ 46.5%, placebo 17.2%. Increase in serum creatinine > 26.5 μmol/L: HCTZ 46.5%, placebo 17.2% (p < 0.001) | None reported |
| Jackson et al., 2020 [17] | Placebo: 289 deaths. Dapagliflozin: 245 deaths (across all diuretic doses) | None reported | Significant improvement in KCCQ total symptom score with dapagliflozin across all diuretic doses | None reported | Slight increase in creatinine levels with dapagliflozin, but fewer renal adverse events | None reported |
| Biegus et al., 2023 [18] | None reported | None reported | Significant weight loss and slight improvement in congestion score with empagliflozin | None reported | None reported | None reported |
| Konstam et al., 2007 [19] | Composite outcomes of death and unscheduled urgent clinic visit: orthodema score 0: 50% event rate; orthodema score 3-4: 68% event rate | Rehospitalisation: Orthodema score 1-2: 52% event rate | Weight loss from baseline to discharge: 12.2 lbs (low-grade orthodema); 14.2 lbs (high-grade orthodema at baseline); 14.6 lbs (high-grade orthodema at discharge) | None reported | None reported | None reported |
Statistical analysis
We used RevMan statistical software version 5.4.0 (The Cochrane Collaboration, Copenhagen, Denmark) to perform meta-analysis on data extracted from these studies. Fixed or random effect models was used according to the Mantel-Haenszel model to assess study heterogeneity for most variables, and funnel plots were used for detecting publication bias. We calculated the risk ratio or mean difference for variables with corresponding confidence intervals, as shown in the forest plots. The risk ratio with 95% confidence interval was used as a measure of the treatment effect for the various variables such as electrolyte imbalance, mortality, adverse events, and serious adverse events. The mean difference was measured for continuous variables, and a p-value of less than or equal to 0.05 was considered statistical significance for each outcome of interest. I2 statistics was used to determine statistical heterogeneity for each outcome. I2 values of 50% or less showed mild or moderate heterogeneity, whereas I2 values of 75% showed high level of heterogeneity. A fixed effect model was used for outcome of interest with I2 values less than 50%, and random effect model was used for outcome of interest with I2 value greater than 50%.
Main outcomes
Mortality
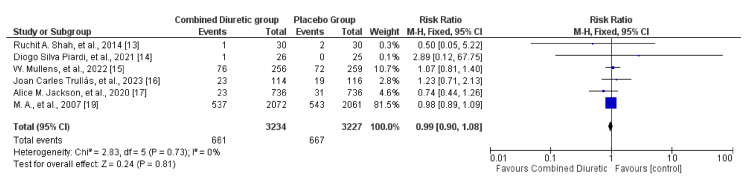
Combination diuretic regimens did not show a statistically significant difference in mortality risk compared to the placebo group, as indicated by the overall risk ratio of 0.99 and the 95% confidence interval of 0.90 to 1.09 (Figure 4). The included studies were consistent with each other (I² = 0%), and the overall effect was not statistically significant (P = 0.85). A fixed effect model was used due to the lack of heterogeneity in the included studies. This suggests that, based on the available evidence, combined diuretic therapy does not significantly affect the mortality risk outcome compared with placebo. Concerning the individual agents based on the studies included in the review, FSM and HCTZ did not significantly impact mortality rates when compared with placebo. In contrast, acetazolamide was associated with increased mortality rates. Dapagliflozin, on the other hand, showed a reduction in deaths across all dosage groups compared with placebo.
Figure 4. Forest plot showing mortality with 95% confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
There did not seem to be a significant publication bias, as the funnel plot (Figure 5) was relatively symmetrical. Some level of heterogeneity is indicated by the spread of studies, which is typical of meta-analyses. Overall, the funnel plot suggests that the meta-analysis results on mortality are robust, with a minimal indication of publication bias, although some heterogeneity among the studies was present.
Figure 5. Funnel plots showing mortality for the combined diuretic and placebo groups.
Hospital Readmission Rates
Most studies reported no significant differences between diuretic treatment and placebo in terms of hospital readmission rates. The forest plot of hospital readmission rates (Figure 6) demonstrates that the combined diuretic group does not have a statistically significant difference in hospital readmission rates compared to the placebo group, as indicated by the overall risk ratio of 1.05 (95% CI, 0.98-1.12). Additionally, the funnel plot (Figure 7) did not show significant asymmetry, suggesting minimal or no publication bias.
Figure 6. Forest plots of hospital readmission showing the comparison between the combined diuretic and placebo groups.
Figure 7. Funnel plots of hospital readmission showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Symptom Improvement
The effect of combination diuretics on symptom improvement compared to placebo is shown in Figures 8, 9. Combination diuretics significantly reduced body weight (p=0.001). Additionally, there were trends towards improvement in dyspnoea (p=0.09) and thirst scale scores (p=0.06), but these did not reach statistical significance. These results suggest that while combination diuretics are effective in reducing body weight, their impact on other symptoms such as dyspnoea, KCCQ score, congestion, and thirst was not statistically significant, although some trends towards improvement were observed.
Figure 8. Forest plot showing comparison between the combined diuretics and placebo groups for dyspnoea, KCCQ, and congestion scores.
KCCQ, Kansas City Cardiomyopathy Questionnaire
Figure 9. Forest plot showing body weight and thirst level comparison between the diuretics and placebo groups.
The funnel plot (Figure 10) does not show significant asymmetry, suggesting minimal to no publication bias. The studies appear to be relatively homogeneous, with a consistent pattern around the central line.
Figure 10. Funnel plots of symptom improvement showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Electrolyte Abnormalities
Figure 11 shows the overall electrolyte imbalances compared with placebo in combination with diuretics. The meta-analysis suggests that the use of combined diuretics does not significantly impact serum potassium or sodium levels compared with placebo. The evidence for serum sodium was limited to one study, and the heterogeneity in the results for serum potassium indicates some variability among the included studies.
Figure 11. Forest plot showing electrolyte abnormalities for the combined diuretic and placebo groups.
The funnel plot illustrated in Figure 12 shows that the studies on serum potassium and serum sodium are symmetrically distributed around the mean difference. This symmetry suggests that there is no significant publication bias or small-study effects in the included studies regarding electrolyte abnormalities in the comparison of the combined diuretic group with the placebo group.
Figure 12. Funnel plots of electrolyte abnormalities showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
The analysis illustrated in Figure 13 did not show a statistically significant difference in the risk of hypokalaemia or hypernatraemia between the combined diuretic and placebo groups.
Figure 13. Forest plot for hypokalaemia and hypernatremia showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
In Figure 14, the open circles (studies) are spread around the combined-effect estimate line for hypokalaemia, showing some symmetry. However, there are fewer red diamonds (studies) for hypernatraemia, and they appear more dispersed, which might suggest variability or potential bias (Figure 15).
Figure 14. Funnel plot for hypokalaemia showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Figure 15. Funnel plot for hyponatraemia showing hypernatremia showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Renal Function
Figure 16 suggests that the use of combined diuretics does not significantly impact serum creatinine or blood urea levels compared to placebo. Heterogeneity was moderate for both outcomes, suggesting some variability among the included studies. However, higher rates of worsening renal function were observed with HCTZ and high-dose FSM than with placebo. Dapagliflozin showed a slight increase in creatinine levels compared with placebo. Figure 17 provides a visual assessment indicating that the studies on renal function (serum creatinine and blood urea) do not exhibit significant publication bias.
Figure 16. Forest plot for renal function showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Figure 17. Funnel plot for renal function showing confidence interval and Mantel–Haenszel ratio for the combined diuretic and placebo groups.
Adverse and Serious Adverse Events
Adverse events varied among the studies. Some studies reported higher incidences of specific events such as ventricular arrhythmias, haemodialysis, and cardiovascular adverse events. Figure 18 illustrates the risk of adverse and serious events between the combined diuretic and placebo groups. The pooled data indicates that combined diuretic therapy does not significantly affect the risk of adverse or serious adverse events compared to placebo. The overall effect estimate is close to 1.00, with no statistically significant differences observed. The I2 value for the groups was 65%, and the chi-square value was 11.37.
Figure 18. Forest plot for any adverse events and any serious adverse events for the combined diuretic and placebo groups.
Figure 19 shows that the studies on "any adverse event" and "any serious adverse event" are symmetrically distributed around the mean difference. The presence of one outlier for "any adverse event" could suggest some publication bias or variability in study results, but the general symmetry suggests that significant bias is unlikely.
Figure 19. Funnel plot showing results for adverse events and serious adverse events for the combined diuretic and placebo groups.
Subgroup analysis or senstivity analysis of the studies
Subgroup analyses were inadequate in the reviewed studies, but few reports provided insights. Shah et al. assessed serum sodium, potassium, and creatinine blood urea levels across different infusion methods (infusion + dopamine, bolus, infusion alone) [13]. There were no statistically significant differences between the treatment groups. Jackson et al. performed subgroup analyses of patients taking different dosages of loop diuretics and found that dapagliflozin improved symptoms across all dosage groups [17]. Additionally, Piardi et al. found no significant differences in the HCTZ and placebo groups while comparing creatinine levels, dyspnoea scale, congestion score, hypernatraemia, and hypokalaemia. Recently, Mullens et al. compared renal safety parameters between acetazolamide and placebo groups. The results revealed a slightly adverse renal outcome in the former group. These findings highlight the need for customised treatments based on individual patient characteristics and responses.
This systematic review highlights the effectiveness of combination diuretic strategies in managing fluid overload in patients with HF. Studies have consistently demonstrated that various combinations, such as metolazone (MTZ) with FSM, FSM with quinethazone, and bendroflumethiazidewith bumetanide, significantly improved diuresis and weight loss. Refractory fluid overload in HF, characterized by decreased diuretic responsiveness, inadequate sodium and water excretion, and fluid accumulation in the body, remains a significant challenge in managing HF, despite considerable advancements in therapeutics. The combination of MTZ with FSM or empagliflozin showed promising potential for improving symptom management.
Discussion
In this systematic review and meta-analysis, numerous studies explored the effectiveness of combining diuretics to alleviate HF symptoms. The findings from Konstam et al. on tolvaptan and recent trials by Biegus et al. on empagliflozin highlight the rapid advancement of combination therapies [18-19]. SGLT2 inhibitors are increasingly used in HF and have been shown to have diuretic effects. Biegus et al. found that the use of empagliflozin in HF patients resulted in more decongestion, in addition to a reduction in HF hospitalisations and mortality [18]. This aligns with the findings of the DAPA-HF trial, which also demonstrated significant reductions in cardiovascular deaths and worsening HF events with the use of dapagliflozin, another SGLT2 inhibitor [17]. The consistent efficacy of SGLT2 inhibitors across different studies underscores their potential as standard components of combination diuretic therapy for patients with HF.
Similarly, Felker et al. indicated that tolvaptan can be effective for decongestion and alleviation of symptoms in patients with AHF [20]. Tolvaptan is the only clinically approved medication for the treatment of dilutional hyponatraemia and has been shown to improve dyspnoea and oedema while mitigating adverse effects. Tolvaptan can be useful as a diuretic in decongestion, although it may cause side effects, such as activation of the renin-angiotensin-aldosterone system, electrolyte disturbances, and worsening renal function. This study is comparable to one of the RCTs included in this review, the EVEREST trial, which showed that tolvaptan improved congestion symptoms without significantly affecting long-term mortality and morbidity [19].
Comparisons between different diuretic combinations revealed varying levels of effectiveness. For example, the combination of FSM with MTZ was shown to be highly effective in resolving oedema [16,21], whereas agents such as empagliflozin demonstrated superior weight loss and congestion relief compared to placebo [18,22-24]. These findings are similar to those of earlier studies that demonstrated more effective diuresis with combination regimens [21-22,25].
Rasoul et al. performed a meta-analysis on several RCTs involving patients with acute decompensated HF treated with intravenous loop diuretic infusion and boluses. This study demonstrated that there was little to no difference in mortality between the two groups and that the net weight loss was slightly higher in the continuous intravenous infusion group [26]. Additionally, this also study also suggested that there was not much difference for the hospital stay duration and hospital readmission rate between the two groups. Similarly, there was no statistically significant difference between the groups regarding electrolyte imbalance and complications such as ototoxicity, hypotension, and acute renal impairment. Another study by Karedath et al. showed that all-cause mortality was higher in patients group receiving continuous infusion of diuretics compared to bolus injection group; however, this difference was statistically not significant [27]. There was no significant between the two groups regarding patients’ length of hospital stay. The incidence of hypokalaemia was lower in the bolus injection group than the infusion group.
Safety concerns in combination with diuretic therapy remain a significant issue. The analysis of adverse events in this study indicated that while combination diuretic therapy did not significantly increase the overall risk of adverse events compared with placebo, specific adverse events were noted. The risks of hyponatraemia and hypokalaemia have been evident in several studies. However, individual studies have shown variability in outcomes. Piardi et al, reported an increased risk of hypokalaemia in patients receiving HCTZ than in those receiving placebo [14]. Similarly, Trullàs et al. found a higher incidence of hypokalaemia in the HCTZ group [16]. Additionally, Mullens et al. reported higher rates of renal impairment in patients treated with acetazolamide compared to placebo [15]. These findings are critical, as they underscore the need for careful monitoring of electrolytes and renal function during combination diuretic therapy. Therefore, while combination diuretic therapies offer significant benefits in managing fluid overload in patients with HF, their use must be carefully balanced with potential risks.
Tailored protocols considering patient-specific factors such as baseline renal function, comorbidities, and previous diuretic response are therefore key to optimizing therapy and reducing adverse outcomes. Mullens et al. highlighted the need for careful monitoring of renal function and electrolytes when using high-dose diuretics [8]. Additionally, since a significant proportion of hospitalized patients with HF are discharged with residual congestion, there is still a need for future research to explore optimal diuretic combinations and dosing strategies to enhance the efficacy and safety of HF management. By integrating combination diuretics into a comprehensive care plan and addressing the individual needs of patients, clinicians can improve outcomes and enhance the quality of life of those living with HF.
Clinical implications
The findings of this systematic review and meta-analysis suggest that combination diuretics have important clinical implications. Healthcare providers managing patients with HF should weigh the risks and benefits of combination diuretic therapies. These findings also highlight the need for individualized treatment approaches based on patient characteristics and responses. This aligns with the recommendations of the American Heart Association and the European Society of Cardiology, which emphasise personalized treatment plans in HF management [4]. By considering individual patient factors such as age, comorbidities, and baseline renal function, clinicians can optimize therapeutic outcomes and minimise adverse effects.
Study strengths and limitations
The strengths of this systematic review include its comprehensive search strategy, inclusion of RCTs, and rigorous quality assessment of the studies. Methodological rigor ensures that the findings are robust and reliable. Additionally, by reviewing the years of research, this systematic review not only offers valuable insights into effective treatment strategies but also indicates the need for tailored methods in clinical practice. Nevertheless, there were limitations, such as the heterogeneity of the included studies, variations in diuretic combinations and dosages, and potential biases in some studies. In addition, the lack of long-term outcome data in some studies limits the ability to draw definitive conclusions regarding the sustained efficacy and safety of combination diuretic therapy. Future research should address these gaps by conducting large-scale long-term studies to better understand the chronic effects of these therapies.
Conclusions
While combination diuretic therapies offer significant benefits in managing fluid overload in patients with HF, their use must be carefully balanced with potential risks. This systematic review underscores the importance of personalized medicine, rigorous monitoring, and ongoing research to optimize HF treatment. By integrating combination diuretics into a comprehensive care plan and addressing the individual needs of patients, clinicians can improve outcomes and enhance the quality of life for those living with HF.
Acknowledgments
Rebecca Ayoti and Zahid Khan contributed equally to the work and should be considered co-first authors
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Zahid Khan, Rebecca Ayoti, Animesh Gupta, Gideon Mlawa
Acquisition, analysis, or interpretation of data: Zahid Khan, Rebecca Ayoti
Drafting of the manuscript: Zahid Khan, Rebecca Ayoti, Animesh Gupta, Gideon Mlawa
Critical review of the manuscript for important intellectual content: Zahid Khan, Rebecca Ayoti, Animesh Gupta, Gideon Mlawa
Supervision: Zahid Khan
References
- 1.Quality of care and outcomes in acute decompensated heart failure: The ADHERE Registry. Yancy CW, Fonarow GC. Curr Heart Fail Rep. 2004;1:121–128. doi: 10.1007/s11897-004-0021-8. [DOI] [PubMed] [Google Scholar]
- 2.Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Am J Med. 2006;119:0. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Lucas C, Johnson W, Hamilton MA, et al. Am Heart J. 2000;140:840–847. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 4.Erratum. Eur Heart J. 2018;39:860. [Google Scholar]
- 5.Relief and recurrence of congestion during and after hospitalization for acute heart failure: Insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF) Lala A, McNulty SE, Mentz RJ, et al. Circ Heart Fail. 2015;8:741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Ambrosy AP, Pang PS, Khan S, et al. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 7.2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. McDonagh TA, Metra M, Adamo M, et al. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 8.The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Mullens W, Damman K, Harjola VP, et al. Eur J Heart Fail. 2019;21:137–155. doi: 10.1002/ejhf.1369. [DOI] [PubMed] [Google Scholar]
- 9.Long-term adaptation of renal ion transporters to chronic diuretic treatment. Kim GH. Am J Nephrol. 2004;24:595–605. doi: 10.1159/000082314. [DOI] [PubMed] [Google Scholar]
- 10.Heart failure. Braunwald E. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Combination diuretic treatment in severe heart failure: a randomised controlled trial. Channer KS, McLean KA, Lawson-Matthew P, Richardson M. Br Heart J. 1994;71:146–150. doi: 10.1136/hrt.71.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A prospective, randomized study to evaluate the efficacy of various diuretic strategies in acute decompensated heart failure. Shah RA, Subban V, Lakshmanan A, et al. Indian Heart J. 2014;66:309–316. doi: 10.1016/j.ihj.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Author Correction: Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial. Piardi DS, Butzke M, Mazzuca AC, et al. Sci Rep. 2021;11:17370. doi: 10.1038/s41598-021-96943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acetazolamide in Decompensated Heart Failure with Volume Overload trial (ADVOR): baseline characteristics. Mullens W, Dauw J, Martens P, et al. Eur J Heart Fail. 2022;24:1601–1610. doi: 10.1002/ejhf.2587. [DOI] [PubMed] [Google Scholar]
- 16.Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Trullàs JC, Morales-Rull JL, Casado J, et al. Eur Heart J. 2023;44:411–421. doi: 10.1093/eurheartj/ehac689. [DOI] [PubMed] [Google Scholar]
- 17.Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Jackson AM, Dewan P, Anand IS, et al. Circulation. 2020;142:1040–1054. doi: 10.1161/CIRCULATIONAHA.120.047077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Biegus J, Voors AA, Collins SP, et al. Eur Heart J. 2023;44:41–50. doi: 10.1093/eurheartj/ehac530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 20.Tolvaptan in Patients Hospitalized With Acute Heart Failure: Rationale and Design of the TACTICS and the SECRET of CHF Trials. Felker GM, Mentz RJ, Adams KF, et al. Circ Heart Fail. 2015;8:997–1005. doi: 10.1161/CIRCHEARTFAILURE.115.002259. [DOI] [PubMed] [Google Scholar]
- 21.Synergistic action of metolazone with "loop" diuretics. Ghose RR, Gupta SK. Br Med J (Clin Res Ed) 1981;282:1432–1433. doi: 10.1136/bmj.282.6274.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diuretic strategies in patients with acute decompensated heart failure. Felker GM, Lee KL, Bull DA, et al. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF Jr. Eur J Heart Fail. 2007;9:1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sodium glucose cotransporter-2 inhibitors and heart disease: current perspectives. Mondal S, Pramanik S, Khare VR, Fernandez CJ, Pappachan JM. World J Cardiol. 2024;16:240–259. doi: 10.4330/wjc.v16.i5.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The supra-additive natriuretic effect addition of bendroflumethiazide and bumetanide in congestive heart failure. Permutation trial tests in patients in long-term treatment with bumetanide. Sigurd B, Olesen KH, Wennevold A. Am Heart J. 1975;89:163–170. doi: 10.1016/0002-8703(75)90041-1. [DOI] [PubMed] [Google Scholar]
- 26.Continuous infusion versus bolus injection of loop diuretics for acute heart failure. Rasoul D, Zhang J, Farnell E, et al. Cochrane Database Syst Rev. 2024;5:0. doi: 10.1002/14651858.CD014811.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Continuous infusion versus bolus injection of loop diuretics for patients with congestive heart failure: a meta-analysis. Karedath J, Asif A, Tentu N, et al. Cureus. 2023;15:0. doi: 10.7759/cureus.34758. [DOI] [PMC free article] [PubMed] [Google Scholar]