Abstract
Background
Porcine circovirus 3 (PCV3) is a recently discovered swine pathogen associated with reproductive disease. To date, clinical problems linked to PCV3 have been described in intensive rearing pig farms. The present case describes an Iberian semi-outdoors sow farm affected by PCV3 reproductive disease.
Case presentation
The affected farm was composed of 420 self-replaced Iberian sows, working in 3-week batches (60 sows per batch). The farm was free from porcine reproductive and respiratory syndrome virus (PRRSV) and had been previously affected by porcine circovirus 2 (PCV2) reproductive disease, which was successfully managed through sow vaccination. In spring 2022, reproductive disease was noticed with a high increase in the number of mummified foetuses and stillborn piglets from gilts as the most remarkable finding; multiparous sows were not affected. A first analysis with pooled stillborn tissues ruled out most swine reproductive pathogens and revealed detection of PCV3. To further elucidate PCV3 implication in the reproductive disease, a complete post-mortem examination of stillborn and mummified foetuses from two affected litters was conducted. Pooled tissue samples yielded high PCV3 loads by quantitative PCR. Grossly, one (out of 5) stillborn had an enlarged, flaccid heart. Histopathological evaluation revealed PCV3 lesions consisting of lymphohistiocytic and systemic periarteritis (3/5). The grossly affected heart had lymphohistiocytic myocarditis with fibrosis and lymphohistiocytic endocarditis. By in situ hybridization, high amounts of PCV3 genome were observed within histological lesions. Moreover, immunohistochemistry against PRRSV and PCV2 resulted negative in the same tissues.
Conclusions
This is the first report of PCV3 reproductive disease in a semi-extensive production Iberian pig farm, affecting exclusively gilts. Moreover, this is the first description of grossly apparent myocarditis associated to PCV3 infection. Therefore, PCV3 should be considered within the differential diagnostic list of swine reproductive problems in non-intensive pig rearing production.
Keywords: Semi-outdoor, Extensive, Porcine circovirus 3 (PCV3), Reproductive disease, PCV3 reproductive disease (PCV3-RD), In situ hybridization (ISH), Periarteritis, Myocarditis
Background
Porcine circovirus 3 (PCV3) was discovered in 2016 in sows affected by reproductive disease and porcine dermatitis and nephropathy syndrome (PDNS)-like lesions [1]. Although initial doubts were raised regarding the PCV3 pathogenic role, its involvement in reproductive disorders was subsequently confirmed. Main associated clinical signs are late abortions, mummified and stillborn foetuses, and weak-born piglets that either die soon after birth or experience a marked decrease in growth rate in later stages [2, 3]. Furthermore, histopathological lesions in cases of PCV3-reproductive disease (PCV3-RD) are also consistent, which include systemic lymphohistiocytic periarteritis and non-suppurative myocarditis [2, 4–6]. The potential of PCV3 to cause transuterine infection and histopathological lesions in piglets has been recently further characterized by means of an experimental infection, which successfully established a link between the etiological agent and the lesions, partially fulfilling Koch’s postulates [7].
Similar to PCV3, porcine circovirus 2 (PCV2) has been known for long to cause reproductive disease, which is characterized by similar clinical signs to those indicated above (abortion, stillbirths, mummifications, preweaning mortalities…) [8]. Interestingly, the pathogenesis of PCV2 reproductive disease (PCV2-RD) depends on the age of foetal infection of tissues, most remarkably cardiomyocytes, which in turn cause a fatal myocarditis [9]. These cardiac lesions can result in a cardiorespiratory insufficiency, grossly apparent as hepatomegaly and anasarca [10–13]. Histologically, the myocardium is infiltrated by lymphocytes and plasma cells, and in most severe cases, necrosis of myocardiocytes and replacement by fibrous tissue appears, which is then referred as necrotizing and/or fibrosing myocarditis [14]. Similarly, foetuses aborted by PCV3-RD have evidence of cardiomyocyte infection and myocarditis [4], and it has been proven experimentally a gestational age-dependent infection of the heart [7].
PCV3-RD has been so far exclusively reported in intensive production herds [4, 5]. Whether PCV3 can produce reproductive disease in extensively reared herds was unknown. Therefore, the purpose of this report is to describe a case of PCV3-RD in an Iberian semi-outdoors herd.
Case presentation
Farm description
The case occurred in a semi-outdoors farrow-to-nursery farm, consisting of 420 Iberian sows using a self-replacement policy. The farm was organized in a 3-week batch farrowing system, with 60 sows per batch, and weaning at 4 weeks of age (average 29.1 days). Replacement gilts were introduced during 4 consecutive batches in spring and then 4 more consecutive batches during autumn (overall, 8 out of 17 batches in a year included gilts). Each batch containing replacement stock consisted of 40% gilts and 60% multiparous sows. Gilt management was as follows: the future gilts were housed with their litter mattes and were raised together in the nursery and early fattening stages with the rest of the pigs. At 3 months of age, animals were moved outdoors in fenced areas, and future gilts were allocated in individual fenced areas separated from the rest of fattening pigs. In this area, animals were clinically monitored by visual inspection every day. One month prior to artificial insemination (AI), gilts were relocated indoors within the same building where multiparous sows were also present. At around 40 days after AI, gestation was confirmed using ultrasonography and then multiparous sows and gilts were moved outdoors in separate fields with no contact (in the so-called soleras, which are outdoors fenced areas where gilts were allowed to move freely and have access to commercial compound feed and roofed areas). Each group of approximately 20 sows was allocated in a 90 m2 space, where they were kept during most of the gestation. One week prior to farrowing, they were reallocated to a maternity room where they farrowed naturally.
The farm had prior history of outbreaks of reproductive disease associated to PCV2 and Leptospira spp. (around spring 2021), which were successfully managed with sow vaccination and antibiotic treatment, respectively. At the moment of the present case, the farm’s gilt vaccination program included immunization against Erysipelothrix rhusiopathiae, porcine parvovirus (PPV), PCV2, Leptospira spp., swine herpesvirus-1, Brachyspira hyodysenteriae, Escherichia coli, and Clostridium perfringens type C. Sows were revaccinated every cycle with the same program. Routinary monitoring of PRRSV was made by means of quantitative PCR (qPCR) in foetuses, stillborn piglets and growing piglets, and ELISA in growing piglets and sows, with continuous negative results. Monitoring of PCV2 was made through qPCR in foetuses, stillborn piglets and growing pigs until 10 weeks of age.
Clinical description
A reproductive clinical problem was detected during spring 2022, coinciding with the farrowing of a new batch of gilts. Around October 2022 when the next gilt batch was introduced, reproductive problems were observed again, and they were further investigated. All gilts within the four consecutive batches were affected. A minority of the gilts either returned to oestrus or aborted before farrowing date. The remaining ones reached farrowing date, delivering low numbers of alive piglets and an increased number of mummified foetuses and stillborn piglets. The live-born piglets were often weak and failed to grow correctly during the first weeks of life.
During this outbreak of reproductive disease, pooled samples of nine stillborn foetuses (including liver, lung and gastric content) were used to screen reproductive pathogens in an external laboratory by qPCR, including PRRSV, PCV2, PCV3, PPV, Leptospira spp., Chlamydia spp., and Brucella spp. All analyses yielded negative results, except for high PCV3 loads in tissue pools. In consequence, five piglets from two gilt litters were submitted to the Servei de Diagnòstic en Patologia Veterinaria (UAB, Barcelona, Spain) to further determine the cause of the reproductive problems and the potential role of PCV3 in them.
Post-mortem examination
Animals submitted for post-mortem examination consisted of a weak born piglet (animal 1) and a stillborn piglet (animal 2) from one litter, and a weak born piglet (animal 3) and two mummified foetuses (animals 4 and 5) from a second litter. Weight and crown-to-rump length (CRL) of each piglet are detailed in Table 1. Gross lesions were only observed in the stillborn piglet 2, which included generalized oedema (Fig. 1a) affecting the subcutaneous tissue and cavitary effusions (anasarca), as well as cardiomegaly with multifocal dark areas of melanosis (Fig. 1b). Fresh heart, lung, spleen, mesenteric lymph node and brain were collected from all the five piglets and pooled (by animal) for viral load determination through qPCR as described previously [4]. All animals yielded low to high viral loads in pooled tissues (Table 1).
Table 1.
Summary of histopathological and virological results from necropsied piglets
| Heart | Mesenteric arteries | |||||||
|---|---|---|---|---|---|---|---|---|
| Litter | Animal No. | CRL (cm) |
Status | Viral load* | Myocarditis | ISH | Periarteritis | ISH |
| 1 | 1 | 27 | W | 1.6 × 109 | - | - | +++ | +++ |
| 2 | 25 | S | 2.9 × 109 | ++ | +++ | +++ | +++ | |
| 2 | 3 | 20 | W | 1.6 × 104 | - | - | ++ | + |
| 4 | 19 | M | 7.4 × 104 | - | - | - | - | |
| 5 | 20 | M | 1.3 × 104 | - | - | - | - | |
*Viral load in a pool of tissues is expressed as PCV3 copies/mL of supernatant of macerated tissue samples. CRL: crown-to-rump length; ISH: in situ hybridization; W: weak born piglet; S: stillborn piglet; M: mummified foetus
Fig. 1.
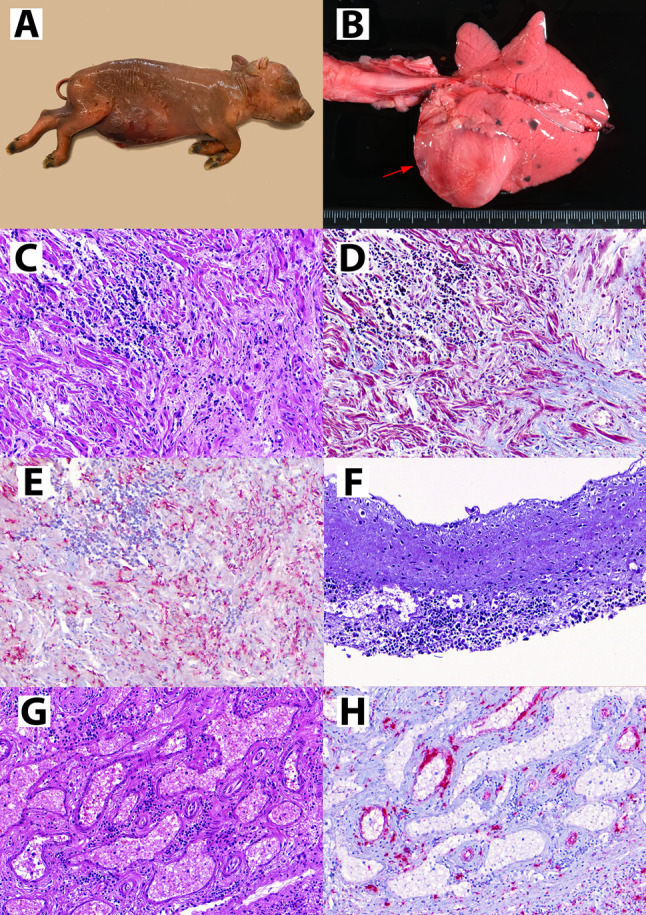
Postmortem examination of stillborn foetus (animal No. 2). (A) Severe generalized oedema (anasarca) of the autolytic foetus. (B) Grossly evident cardiomyopathy featuring a severe dilation of both ventriculus and depressed reddish areas (red arrow). The lung showed melanosis (black spots), which is a usual finding of Iberian pigs with no disease implications. (C) Histopathological evaluation of the heart reveals foci of mononuclear inflammation (top left) and areas devoid of cardiomyocytes and replaced by fibrous tissue (bottom right). (D) Masson trichrome stain confirms the presence of collagen fibres (blue colour) in the fibrosed areas. (E) ISH against PCV3 reveals moderate amount of PCV3 genome (red staining) within the cardiac tissue. (F) Mitral valve, showing subendocardial infiltration of mononuclear cells. (G) Mesenteric arteries displaying lymphohistiocytic periarteritis. (H) In the same mesenteric arteries, ISH against PCV3 reveals abundant viral genome (red staining) in the arterial walls and -to a lesser extent- within the inflammatory infiltrate
For histopathological studies, the following samples were collected from all studied piglets and fixed in 10% neutral-buffered formalin: heart, lung, spleen, mesenteric, tracheobronchial and superficial inguinal lymph nodes, brain, tonsil, liver, kidney, duodenum, jejunum, ileum, colon, placenta and umbilical cord. The tissues were evaluated histopathologically through haematoxilyn-eosin staining, and PCV3-like associated lesions were graded as previously described [15]. Moreover, an in situ hybridization (ISH) targeting the PCV3 rep gene was also performed as previously described [4]. Additional slides were cut and stained using Masson’s trichrome staining to evaluate fibrosis within cardiac tissue. Further slides were obtained to perform immunohistochemistry (IHC) against PCV2 and PRRSV, as previously described [16, 17].
Histopathological evaluation of the heart of animal 2 revealed a moderate lymphohistiocytic myocarditis. Multifocally, loss of cardiomyocytes was observed (Fig. 1c), which was replaced by fibrous tissue as evidenced through Masson’s trichrome stain (Fig. 1d). Abundant PCV3 genome was evidenced through ISH in the myocardium (Fig. 1e). Some mononuclear aggregates could be observed subjacent to the endothelial lining of the endocardium, especially in the heart valves (Fig. 1f).
Lymphohistiocytic arteritis and periarteritis were observed in animals 1, 2 and 3, mainly in the mesenteric arterial plexus and – to a lesser extent – in the renal arteries (Fig. 1g). Score for each lesion is available in Table 1. The same lesions were observed in animal 2 in the placenta and umbilical cord, affecting small and medium caliber arteries but sparing the umbilical artery. All vascular lesions had intense labelling by PCV3 ISH (Fig. 1h). A small degree of interstitial pneumonia was observed in animals 1 and 2, and PCV3 genome was found in alveolar septa in both animals. No lesions were observed in the brain and lymph nodes, although labelling was frequently observed in lymphoid germinal centres and in neuroparenchyma. The other studied tissues were histologically unremarkable. All piglets had negative PCV2 and PRRSV IHC results.
Discussion
The present case in an Iberian pig farm fulfilled the proposed PCV3-RD diagnostic criteria in three out of five animals, i.e., compatible clinical reproductive signs, vascular histologic lesions, and moderate to high amount of PCV3 genome within the lesions [2]. Importantly, other common swine pathogens (such as PCV2 and PRRSV) potentially linked to reproductive disease [18] were ruled out. Other pathogens such as enteroviruses, teschoviruses and encephalomyocarditis virus were not specifically ruled out; however, to date they have not been reported to cause systemic non-suppurative periarteritis.
In addition, this case provides a previously non-described post-mortem finding in PCV3-RD. One of the stillborn piglets (animal 2) had a grossly identifiable cardiomyopathy, characterized by severe myocarditis with loss of cardiomyocytes and scarring of cardiac tissue with fibrosis. The anasarca observed in the affected animal suggests that cardiovascular failure may play a role in foetal and neonatal death in PCV3-RD. Importantly, the observed myocarditis was similar to that described in PCV2-RD [10, 19–21]. However, subendocardial mononuclear infiltrates had not been reported in previous cases of PCV3-RD although it has been described in a case of PCV2-RD in Norway [22]. Despite these similarities in cardiac lesions between reproductive cases associated to PCV2 and PCV3, a particular difference in the vascular lesions can be noted. Although lymphohistiocytic periarteritis had been occasionally described in PCV2 associated diseases [23], it is a major feature in PCV3-RD cases [4, 5, 7]. Therefore, the thorough histological examination of vascular structures is considered fundamental to support the diagnosis of PCV3-RD together with the detection of the virus in damaged tissues.
In the present case, PCV3-RD affected gilts exclusively. The increased susceptibility of gilts to viral diseases has also been described for PCV2-RD [22], which is probably related to naïve animals being introduced to a herd with endemic circulation of the virus and subsequent infection. In the present case, gilts were initially housed separately from the rest of the herd. As PCV3 likely circulated endemically within the herd, gilts were probably non-exposed during the first months of age and lost maternally derived immunity thereafter, becoming probably naïve unprotected animals. One month before AI, they were transferred to the AI facility in which they were kept in individual cages, but within the same barn than multiparous sows. One month after AI, animals were allocated in groups outdoors (gilts and multiparous sows separately). It is therefore likely that gilts were infected between one month prior and one month after insemination, since it was in this time period where they were kept gilts and multiparous sows in the same environment. It is worth mentioning that PCV3 can be found in semen [24, 25]; however, the role of semen as a potential infectious vehicle for PCV3 is speculative, as for PCV2 the loads in semen of naturally infected boars are not capable of generating PCV2-RD in sows [26]. The moment of infection is presumably a major factor determining the outcome of circoviral infections. Multiple studies already assessed the age-related factors in PCV2 intrauterine infection [9, 27]. Similarly, an experimental PCV3 infection of pregnant gilts revealed that the earlier the infection, the higher viral loads in piglets at farrowing and the more severe PCV3 associated lesions [7]. Although PCV3-RD has been reported in multiple cases affecting intensive farms, this is the first report of PCV3-RD in a semi-outdoor swine herd, which implies that PCV3 may also be a pathogen of concern in facilities with a presumably lower infection pressure. Although speculative, in outdoor housing practices, pigs might have been exposed to wild boar which are well-known natural PCV-3 reservoirs [28] or to infected ticks, which have been suggested to act as vectors [29]. However, this aspect could not be clarified.
The economic burden of PCV3-RD is currently unknown. The most evident impact in PCV3-RD is attributed to direct loss of animals in abortions, mummified and stillborn foetuses. However, affected weak born piglets in this case showed overall poor growth performance in the first weeks of life. This effect has also been reported in previous cases PCV3-RD in the field [5, 6] as well as in piglets from experimentally infected pregnant gilts [7].
PCV3 may produce reproductive losses including mummified and stillborn foetuses. In the present case, mummies had lower viral loads compared to stillborn or weak born piglets, which is similar to previous studies [7]. Moreover, histologic lesions are rarely reported in mummified foetuses due to advanced autolysis. Therefore, for diagnostic considerations, stillborn and/or weak born foetuses are deemed as the best options for diagnostic workup.
Despite PCV3-RD has been known to affect pig herds worldwide for almost a decade, a commercially available vaccine has not been developed. The overall economic impact of PCV3 has not been determined yet. However, it may potentially be prevented through vaccination, as the example of PCV2, which largely prevents PCV2-RD in herds nowadays [26, 30–32]. Some PCV3 vaccine developments are already in place [33].
Conclusions
This is the first report of PCV3-RD in a semi-outdoor (extensive) swine herd. This case displayed typical PCV3-RD clinical and pathological features, fulfilling PCV3-RD proposed criteria. Moreover, this case offers new undescribed features, such as a grossly visible cardiomyopathy characterized by myocarditis, loss of cardiomyocytes and fibrosis, and subendocardial inflammation.
Acknowledgements
The authors would like to thank the personnel of the Servei de Diagnòstic en Patologia Veterinària for their contribution to this work.
Abbreviations
- PCV2
Porcine circovirus 2
- PCV3
Porcine circovirus 3
- PCV2-RD
Porcine circovirus 2 reproductive disease
- PCV3-RD
Porcine circovirus 3 reproductive disease
- PRRSV
Porcine respiratory and reproductive syndrome virus
- PDNS
Porcine dermatitis and nephropathy syndrome
- qPCR
Quantitative PCR
- ISH
In situ hybridization
- AI
Artificial insemination
Author contributions
AC performed the necropsies, histopathological assessment, in situ hybridization and manuscript writing. EH performed the qPCRs. MP performed the in situ hybridization. MM, RM, MJ and LG visited the farm. MS and JS analysed and interpreted the data and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
No applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Palinski R, Piñeyro P, Shang P, Yuan F, Guo R, Fang Y, et al. A Novel Porcine Circovirus distantly related to known circoviruses is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive failure. J Virol. 2017;91(1):e01879–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saporiti V, Franzo G, Sibila M, Segalés J. Porcine circovirus 3 (PCV-3) as a causal agent of disease in swine and a proposal of PCV-3 associated disease case definition. Transbound Emerg Dis. 2021;68(6):2936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molossi FA, de Almeida BA, de Cecco BS, da Silva MS, Mósena ACS, Brandalise L, et al. A putative PCV3-associated disease in piglets from Southern Brazil. Braz J Microbiol. 2022;53(1):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saporiti V, Valls L, Maldonado J, Perez M, Correa-Fiz F, Segalés J, et al. Porcine Circovirus 3 detection in aborted fetuses and Stillborn piglets from Swine Reproductive failure cases. Viruses. 2021;13(2):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molossi FA, de Cecco BS, de Almeida BA, Henker LC, da Silva MS, Mósena ACS, et al. PCV3-associated reproductive failure in pig herds in Brazil. Trop Anim Health Prod. 2022;54(5):293. [DOI] [PubMed] [Google Scholar]
- 6.Arruda B, Piñeyro P, Derscheid R, Hause B, Byers E, Dion K, et al. PCV3-associated disease in the United States swine herd. Emerg Microbes Infect. 2019;8(1):684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobos À, Ruiz A, Pérez M, Llorens A, Huerta E, Correa-Fiz F, et al. Experimental inoculation of Porcine Circovirus 3 (PCV-3) in pregnant gilts causes PCV-3-Associated lesions in Newborn piglets that persist until Weaning. Transbound Emerg Dis. 2023;1:5270254. [Google Scholar]
- 8.Gillespie J, Opriessnig T, Meng XJ, Pelzer K, Buechner-Maxwell V. Porcine circovirus type 2 and Porcine Circovirus-Associated Disease. J Vet Intern Med. 2009;23(6):1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez RE, Meerts P, Nauwynck HJ, Pensaert MB. Change of porcine circovirus 2 target cells in pigs during development from fetal to early postnatal life. Vet Microbiol. 2003;95(1–2):15–25. [DOI] [PubMed] [Google Scholar]
- 10.West KH, Bystrom JM, Wojnarowicz C, Shantz N, Jacobson M, Allan GM, et al. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J Vet Diagn Invest. 1999;11(6):530–2. [DOI] [PubMed] [Google Scholar]
- 11.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng XJ, Opriessnig T. Reproductive failure experimentally induced in sows via artificial insemination with semen spiked with porcine circovirus type 2. Vet Pathol. 2009;46(4):707–16. [DOI] [PubMed] [Google Scholar]
- 12.Mateusen B, Maes DGD, Van Soom A, Lefebvre D, Nauwynck HJ. Effect of a porcine circovirus type 2 infection on embryos during early pregnancy. Theriogenology. 2007;68(6):896–901. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor B, Gauvreau H, West K, Bogdan J, Ayroud M, Clark EG, et al. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can Vet J. 2001;42(7):551–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6(2):119–42. [DOI] [PubMed] [Google Scholar]
- 15.Cobos À, Sibila M, Alomar J, Pérez M, Huerta E, Segalés J. Retrospective assessment of porcine circovirus 3 (PCV-3) in formalin-fixed, paraffin-embedded tissues from pigs affected by different clinical-pathological conditions. Porcine Health Manag. 2022;8(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, et al. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999;120(1):59–78. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Lobo FJ, de Lome LC, Díez-Fuertes F, Segalés J, García-Artiga C, Simarro I, et al. Safety of Porcine Reproductive and respiratory syndrome modified live virus (MLV) vaccine strains in a young pig infection model. Vet Res. 2013;44(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes D, Peltoniemi O, Malik M. Abortion and fetal death in sows. Reprod Domest Anim. 2023;58:125–36. [DOI] [PubMed] [Google Scholar]
- 19.Mikami O, Nakajima H, Kawashima K, Yoshii M, Nakajima Y. Nonsuppurative myocarditis caused by porcine circovirus type 2 in a weak-born piglet. J Vet Med Sci. 2005;67(7):735–8. [DOI] [PubMed] [Google Scholar]
- 20.Brunborg IM, Jonassen CM, Moldal T, Bratberg B, Lium B, Koenen F, et al. Association of myocarditis with high viral load of porcine circovirus type 2 in several tissues in cases of fetal death and high mortality in piglets. A case study. J Vet Diagn Invest. 2007;19(4):368–75. [DOI] [PubMed] [Google Scholar]
- 21.Harding JCS. The clinical expression and emergence of porcine circovirus 2. Vet Microbiol. 2004;98(2):131–5. [DOI] [PubMed] [Google Scholar]
- 22.Oropeza-Moe M, Delgado AJO, Framstad T. Porcine circovirus type 2 associated reproductive failure in a specific pathogen free (SPF) piglet producing herd in Norway: a case report. Porcine Health Manag. 2017;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opriessnig T, Langohr I. Current state of knowledge on Porcine Circovirus type 2-Associated lesions. Vet Pathol. 2013;50(1):23–38. [DOI] [PubMed] [Google Scholar]
- 24.Qi S, He Q, Zhang Z, Chen H, Giménez-Lirola L, Yuan F, et al. Detection of Porcine Circovirus Type 3 in serum, semen, oral fluid, and Preputial Fluid Samples of Boars. Vet Sci. 2023;10(12):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddicks M, Müller M, Fux R, Ritzmann M, Stadler J. Detection of porcine circovirus type 3 DNA in serum and semen samples of boars from a German boar stud. Vet J. 2022;279:105784. [DOI] [PubMed] [Google Scholar]
- 26.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng XJ, Opriessnig T. Effect of porcine circovirus type 2 (PCV2) vaccination of the dam on PCV2 replication in utero. Clin Vaccine Immunol. 2009;16(6):830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pensaert MB, Sanchez RE, Ladekjær-Mikkelsen AS, Allan GM, Nauwynck HJ. Viremia and effect of fetal infection with porcine viruses with special reference to porcine circovirus 2 infection. Vet Microbiol. 2004;98(2):175–83. [DOI] [PubMed] [Google Scholar]
- 28.Zhai SL, Lu SS, Wei WK, Lv DH, Wen XH, Zhai Q, et al. Reservoirs of Porcine circoviruses: a Mini Review. Front Vet Sci. 2019;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzo G, Grassi L, Tucciarone CM, Drigo M, Martini M, Pasotto D, et al. A wild circulation: high presence of porcine circovirus 3 in different mammalian wild hosts and ticks. Transbound Emerg Dis. 2019;66(4):1548–57. [DOI] [PubMed] [Google Scholar]
- 30.Pleguezuelos P, Sibila M, Cuadrado R, López-Jiménez R, Pérez D, Huerta E, et al. Exploratory field study on the effects of porcine circovirus 2 (PCV-2) sow vaccination at different physiological stages mimicking blanket vaccination. Porcine Health Manag. 2021;7(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Rodriguez A, Dewulf J, Meyns T, Del-Pozo-sacrist R, Andreoni C, Goubier A, et al. Effect of sow vaccination against porcine circovirus type 2 (PCV2) on virological profiles in herds with or without PCV2 systemic disease. Can Vet J. 2016;57(6):619–28. [PMC free article] [PubMed] [Google Scholar]
- 32.Fraile L, Sibila M, Nofrarías M, López-Jimenez R, Huerta E, Llorens A, et al. Effect of sow and piglet porcine circovirus type 2 (PCV2) vaccination on piglet mortality, viraemia, antibody titre and production parameters. Vet Microbiol. 2012;161(1–2):229–34. [DOI] [PubMed] [Google Scholar]
- 33.Pranoto S, Wu H, Chu C. Porcine circovirus type 3: Diagnostics, genotyping, and challenges in Vaccine Development. Transbound Emerg Dis. 2023;1:8858447. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


