Abstract
Background
Wolbachia incompatible insect technique (IIT) programs have been shown in field trials to be highly effective in suppressing populations of mosquitoes that carry diseases such as dengue, chikungunya, and Zika. However, the frequent and repeated release of Wolbachia-infected male mosquitoes makes such programs resource-intensive. While the need for optimization is recognized, potential strategies to optimize releases and reduce resource utilization have not been fully explored.
Results
We developed a process-based model to study the spatio-temporal metapopulation dynamics of mosquitoes in a Wolbachia IIT program, which explicitly incorporates climatic influence in mosquito life-history traits. We then used the model to simulate various scale-down and redistribution strategies to optimize the existing program in Singapore. Specifically, the model was used to study the trade-offs between the intervention efficacy outcomes and resource requirements of various release program strategies, such as the total number of release events and the number of mosquitoes released. We found that scaling down releases in existing sites from twice a week to only once a week yielded small changes in suppression efficacy (from 87 to 80%), while requiring 44% fewer mosquitoes and release events. Additionally, redistributing mosquitoes from already suppressed areas and releasing them in new areas once a week led to a greater total suppressive efficacy (83% compared to 61%) while also yielding a 16% and 14% reduction in the number of mosquitoes and release events required, respectively.
Conclusions
Both scale-down and redistribution strategies can be implemented to significantly reduce program resource requirements without compromising the suppressive efficacy of IIT. These findings will inform planners on ways to optimize existing and future IIT programs, potentially allowing for the wider adoption of this method for mosquito-borne disease control.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-024-02070-1.
Keywords: Wolbachia, Incompatible insect technique, Simulation, Mosquitoes, Aedes aegypti, Compartmental model, Neglected tropical diseases
Background
Mosquitoes are vectors for several tropical diseases, including malaria, dengue, chikungunya, Zika, and lymphatic filariasis. It is estimated that up to 700 million people worldwide are infected and more than a million people die from mosquito-borne diseases every year [1]. At present, vector control methods such as breeding site reduction and insecticide spraying are the most effective tools for combating these mosquito-borne diseases, as pharmaceutical interventions are not widely available [2]. Given growing concerns of emerging insecticide resistance [2], public health organizations and governments worldwide have been exploring novel vector control methods to supplement existing toolkits.
One promising approach for some diseases is the use of mosquitoes infected with Wolbachia, a naturally occurring intracellular alpha-proteobacterium that is commonly found in insect species [3]. Wolbachia has been found to confer the mosquito host with inhibitory effects against certain viral pathogens, thereby reducing the transmission potential for dengue, chikungunya, and Zika [4–7]. Population replacement trials, in which both male and female Wolbachia-infected mosquitoes are released, have also been conducted in Australia, Vietnam, Malaysia, and Indonesia, resulting in significant decreases in dengue incidence [8].
Wolbachia also affects arthropod reproduction by inducing cytoplasmic incompatibility. When male insects infected with Wolbachia mate with females that either lack Wolbachia or have different Wolbachia strains, cytoplasmic incompatibility can cause the offspring to die during the early stages of embryonic development [9]. Wolbachia-mediated cytoplasmic incompatibility has been tested in field trials in Australia, China, Italy, the USA, and Singapore through the incompatible insect technique (IIT), where only male mosquitoes with the Wolbachia infection are released, and has been found to effectively suppress the mosquito population [10–15] and consequently reduce dengue incidence [16, 17]. A cluster-randomized controlled trial is also underway in Singapore to provide gold-standard evidence of the technology’s epidemiological efficacy [18]. IIT is also sometimes supplemented with the sterile insect technique, in which a low-dose of sterilizing radiation is applied to counteract the accidental release of fertile Wolbachia-infected female mosquitoes due to imperfect sex-sorting methods [18].
Although cost-effective in specific settings [19], Wolbachia-based IIT programs are resource-intensive since they require the repeated and frequent release of large quantities of male Wolbachia-infected mosquitoes over the treatment period [20]. This is especially in cases where high-fidelity sex-sorting techniques are employed to ensure a low risk of accidentally releasing female mosquitoes [12]. In Singapore, for example, a total of 11 million male IIT mosquitoes are released every week [21], with each treatment site receiving mosquitoes twice a week and some sites requiring releases at different floors in high-rise apartment blocks [18]. As such, it is crucial to identify optimization strategies to safely scale down the IIT release program once the wild-type mosquito population is effectively suppressed. Scale-down strategies can also reduce the risk of releasing female Wolbachia mosquitoes by accident, especially in IIT programs that do not adopt a sterile insect technique approach, since fewer mosquitoes need to be released [22]. An alternative way to increase the cost-effectiveness of Wolbachia IIT programs is by expanding program coverage while keeping the quantity of mosquitoes constant through redistributing IIT mosquitoes from already-suppressed areas to new ones. While prior work has explored scale-down strategies in which reduced quantities of IIT mosquitoes were released, to our knowledge, no work has been done on either frequency-based scale-down strategies or redistribution strategies to expand program coverage, despite their practical importance.
Mathematical models have been used for studying mosquito population dynamics [23] and the intervention efficacy of traditional vector control measures such as insecticide-based measures [24] and the sterile insect technique [25]. While mathematical models exist for studying Wolbachia IIT release optimization [20, 22, 26, 27], these models cannot model large spatial scales and more importantly, do not consider exogenous factors affecting the intervention efficacies of Wolbachia-based programs such as precipitation, temperature, and mosquito migration. In addition to playing a significant role in the mosquito life cycle [28, 29], climatic factors such as temperature have been found to impact the intervention efficacy of Wolbachia replacement programs [30], and the same could hold for IIT programs. Prior evidence from randomized controlled trials and field trials also suggests that the migration of Wolbachia mosquitoes in treatment boundary areas has non-negligible effects on both IIT as well as replacement programs [15, 31], which makes the characterization of mosquito population dynamics over both spatial and temporal scales important.
To address these issues, we propose a process-based model that explicitly accounts for precipitation, temperature, and spatio-temporal mosquito population dynamics. We employed the model to study the intervention efficacy of various release optimization strategies for an existing Wolbachia IIT program that targets Aedes aegypti mosquitoes. We used Singapore as the study site because it has an established network of mosquito surveillance traps which systematically collects national data on mosquito abundance [32], and extensive Wolbachia IIT field trial information for model validation purposes [14, 15]. Using the model, we sought to understand the consequences of:
-
(i)
Quantity- and frequency-reduction scale-down strategies in existing release areas to reduce the number of mosquitoes released, and
-
(ii)
Redistribution strategies that cover more release areas while keeping the overall mosquito release quantity constant,
with the goal of comparing the trade-offs between intervention efficacy and the program resources required to adopt each Wolbachia IIT release strategy, using Singapore as a case study.
Results
To assess various Wolbachia IIT release strategies for mosquito population control, a 34-compartment process-based model was developed to simulate the life cycle dynamics of both Wolbachia-infected and uninfected Aedes aegypti mosquitoes. This model incorporated climatic factors such as temperature and precipitation and was spatially structured to account for mosquito movement. The model was validated against empirical data from a previous Wolbachia release program described in Bansal et al. [15]. Two main classes of release strategies were evaluated: scale-down strategies aimed at optimizing the existing program by reducing the number of released mosquitoes or release frequency, and redistribution strategies designed to expand program coverage without increasing resource consumption. The primary outcome measures were the percentage reduction in wild-type adult female mosquito abundance and the resource consumption footprint of each strategy.
We ran a total of 270 strategy simulations in a high-performance computing cluster (1 set of 10 strategies for the main experiment, and 26 sets of 10 strategies for sensitivity analyses). Each strategy took approximately 11 h to run on a 12-core compute node and used 9.6 GB of memory.
Model validation
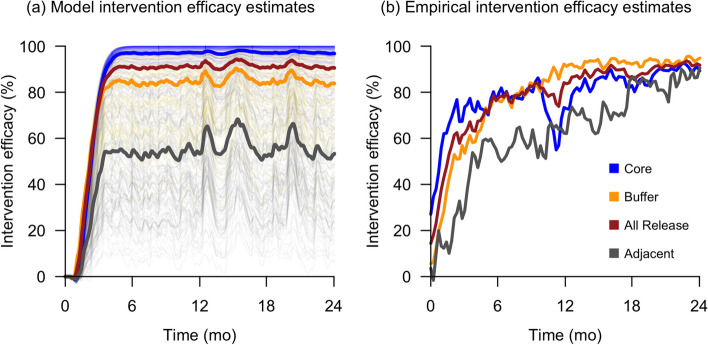
The intervention efficacy estimated by the model outputs was compared against the empirically derived estimates in Bansal et al. [15], as shown in Fig. 1. The model captured the dynamics observed in the early stages of the field trial: a larger suppression effect was observed in core hexagons than in buffer hexagons, and non-negligible suppression effects were observed in adjacent non-release hexagons. Additionally, the model outputs and field trial estimates both reached > 90% mean intervention efficacy at the end of the 2-year intervention period. However, the model outputs differed from the trial estimates as time went on. Specifically, the field trial intervention efficacy in the core release areas fell below that of the buffer release areas in the latter half, and intervention efficacies across all release and adjacent non-release areas converged towards the end of the 2-year period. We discuss the possible reasons for these deviations in detail later in the discussion section.
Fig. 1.
Model-based simulation (a) and the empirically estimated (b) intervention efficacies of the early release program in Singapore. The x-axis represents time since the intervention began. The model-based intervention efficacy was defined as the difference in the adult ovipositing female abundance between the intervention and baseline (no intervention) simulations. Each thin line represents the intervention efficacy for a single hexagon, whereas each thick line represents the mean intervention efficacy for an entire hexagon class. Core, buffer, and adjacent non-release hexagons are colored in blue, orange, and gray, respectively. The mean intervention efficacy line for all release hexagons (core and buffer) is shown in red
Scale-down strategies to optimize the current release program
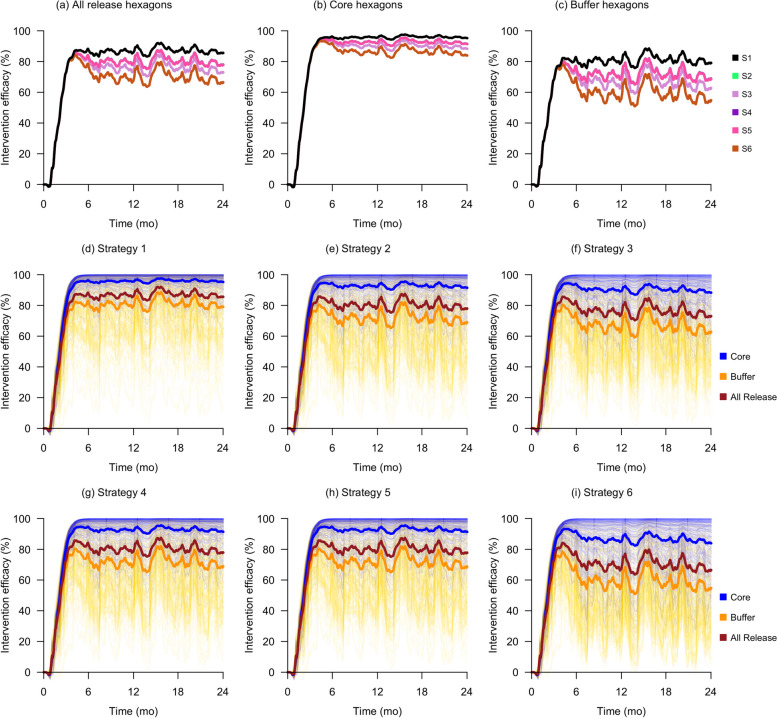
As expected, the constant release strategy (Strategy 1) had the best suppression with IEfinal values of 87% across all release hexagons. The half-quantity (Strategy 2) and quarter-quantity (Strategy 3) release strategies had IEfinal values of 80% and 75% in all release hexagons but required approximately 44% and 63% fewer IIT mosquitoes respectively. The weekly (Strategy 4) and alternate weeks (Strategy 5) strategies both had IEfinal values of 80% in all release hexagons and required 44% fewer IIT mosquitoes and release events. The fortnightly strategy (Strategy 6), on the other hand, produced a markedly lower IEfinal value of 70% across all release hexagons, but required approximately 66% fewer IIT mosquitoes and release events. These results are shown visually in Fig. 2 as well as in table form in Table 1.
Fig. 2.
Intervention efficacy outcomes of various scale-down strategies. Plots a, b, and c show the mean intervention efficacy in all release, core-only, and buffer-only hexagons respectively for all scale-down strategies. The remaining plots d to i show the detailed intervention efficacies in all hexagons for Strategies 1 to 6, respectively
Table 1.
Comparison of intervention efficacy and resource consumption for various scale-down strategies
| Strategy | Name | Average mean intervention efficacy over the final 24 weeks (%), (95% CI) | Relative resource consumption change (%) | |||
|---|---|---|---|---|---|---|
| Core | Buffer | All release | Number of Wolbachia mosquitoes released | Number of release events | ||
| 1 | Constant | 96 (81–100) | 81 (44–99) | 87 (50–100) | Reference | Reference |
| 2 | Half quantity | 93 (69–100) | 71 (34–100) | 80 (37–100) | -44 | 0 |
| 3 | Quarter quantity | 90 (64–100) | 66 (31–97) | 75 (34–100) | -63 | 0 |
| 4 | Weekly | 93 (68–100) | 71 (34–98) | 80 (37–100) | -44 | -44 |
| 5 | Alternate weeks | 93 (68–100) | 71 (34–98) | 80 (37–100) | -44 | -44 |
| 6 | Fortnightly | 86 (50–100) | 58 (20–96) | 70 (23–100) | -66 | -66 |
CI Confidence interval
Redistribution strategies to increase program coverage
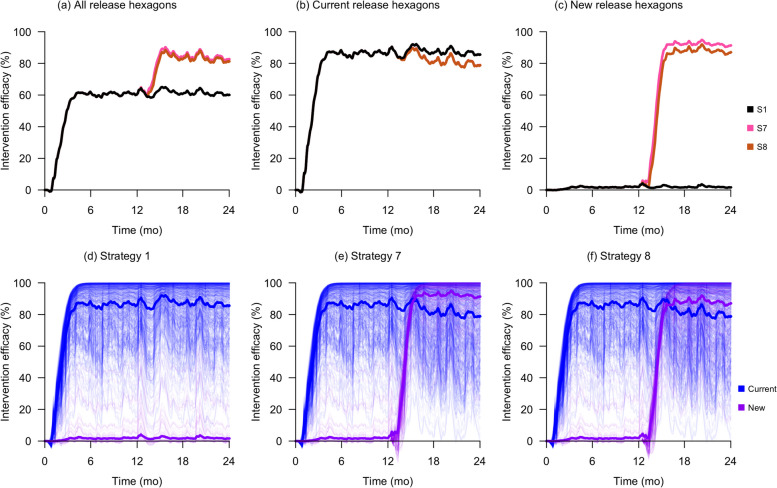
We analyzed the IEfinal values and resource consumption of each redistribution strategy in the same manner as in the scale-down strategies, described in the previous section. Both the redistribution by quantity (Strategy 7) and redistribution by frequency (Strategy 8) strategies had better overall mean intervention efficacies across current and new hexagons than the constant release strategy (Strategy 1) because they led to a minimal decrease in suppression for current release hexagons while creating a strong suppression effect in the new hexagons. Strategies 7 and 8 had overall IEfinal values of 84% and 83% respectively, compared to 61% in Strategy 1. In current hexagons, Strategy 1 produced an IEfinal of 87%, which was only slightly higher than 81% in both Strategies 7 and 8. However, in new hexagons, Strategy 1 produced an IEfinal of only 1%, whereas Strategies 7 and 8 resulted in much higher overall IEfinal values of 92%, and 88% respectively. In terms of resource consumption, although Strategy 7 required 7% fewer mosquitoes than Strategy 1, it required 22% more release events; Strategy 8 on the other hand, required approximately 16% fewer mosquitoes and 14% fewer release events. These results are shown visually in Fig. 3 as well as in table form in Table 2.
Fig. 3.
Intervention efficacy outcomes of various redistribution strategies. The first row of plots shows the mean intervention efficacy in both current and new hexagons (a), current hexagons (b), and new hexagons only (c). The remaining plots d, e, and f show the intervention efficacies in all hexagons for Strategies 1, 7, and 8, respectively
Table 2.
Comparison of intervention efficacy and resource consumption for various redistribution strategies
| Strategy | Name | Average mean intervention efficacy over the final 24 weeks (%), (95% CI) | Relative resource consumption (% change) | |||
|---|---|---|---|---|---|---|
| Current | New | All release | Number of Wolbachia mosquitoes released | Number of release events | ||
| 1 | Constant | 87 (50–100) | 1 (0–28) | 61 (0–100) | Reference | Reference |
| 7 | Redistribution by quantity | 81 (35–100) | 92 (56–100) | 84 (37–100) | -7 | 22 |
| 8 | Redistribution by frequency | 81 (35–100) | 88 (41–100) | 83 (35–100) | -16 | -14 |
CI Confidence interval
Discussion
As the Wolbachia incompatible insect technique becomes a more commonly used tool to combat mosquito-borne diseases across various regions in the world, there is a growing need to optimize release programs to maximize cost-effectiveness. However, there are ethical challenges with trialing novel release strategies in the field, because they could result in a rebound in wild-type mosquito abundance and hence an increase in mosquito-borne disease transmission. Given the potential risks of releasing too few IIT mosquitoes, it is pertinent to use mathematical models to explore optimal release strategies beforehand to filter out strategies that are unlikely to work in the field. Additionally, in silico simulations can eliminate the inherent variability due to fluctuations in environmental conditions and also provide the advantage of exploring a wider range of release strategies than would be feasible in field trials. While there exist mathematical models to study release strategies, they are unable to explore IIT programs over large spatial scales. Models by Pagendam et al. [22], Soh et al. [27], and Matsufuji and Seirin-Lee [20] do not take into account important climatic factors affecting the life cycle of mosquitoes such as precipitation and temperature. Additionally, the models by Soh et al. [27] and Matsufuji and Seirin-Lee [20] assume a well-mixed ecological environment, which does not account for heterogeneous geographical release coverage in real Wolbachia IIT programs. While an agent-based model proposed by Magori et al. [26] accounts for climatic factors, it requires extremely fine-grained descriptions of individual houses and breeding containers as well as tracks individual mosquitoes, making it extremely challenging for studying IIT programs over a large city. Therefore, it was important for us to develop a general model that could explore program resource requirements over large spatial scales while accounting for precipitation, temperature and mosquito migration.
Resource consumption, as measured by the number of IIT males released, as well as the number of discrete release events, is correlated with overall IIT program cost [19]. Therefore, we explored scale-down strategies which involved reducing resource consumption, as well as redistribution strategies which involved expanding program coverage without requiring the production of more IIT males using a process-based model to optimize an existing Wolbachia-Aedes aegypti IIT program in Singapore. While previous studies, such as Pagendam et al. [22], have explored quantity-based scale-down strategies, our study is unique in its exploration of redistribution strategies and frequency-based scale-down strategies. One of the quantity-based scale-down strategies that Pagendam et al. [22] explored was the crude adaptive strategy. The strategy begins with a constant release of IIT mosquitoes;once the wild-type population is reduced to 50%, the number of IIT mosquitoes released is halved. Finally, when the wild-type population falls to 10% of its initial size, the number of IIT mosquitoes is reduced to 10% of the initial release quantity. Pagendam et al. [22] found that the crude adaptive strategy was able to completely eliminate wild-type mosquitoes at a very high probability, almost identical to that of the constant release strategy.
We replicated a quantity-based scale-down strategy similar to the crude adaptive strategy in our model simulations which we term the quarter quantity release strategy (Strategy 3). We observed that while the quarter quantity release strategy achieved effective suppression in release areas, in contrast to findings from Pagendam et al. [22], the mean intervention efficacy across all release hexagons fell noticeably by 12% (from 87 to 75%) compared to the constant release strategy (Strategy 1), as shown in Table 1. We posit that the appreciable decrease observed in the simulation outputs was because our model factored in the migratory effects of mosquitoes at the buffer hexagons. As shown in Table 1, the IEfinal for core hexagons only fell by 6% (from 96 to 90%), whereas the decrease was 15% (from 81 to 66%) for buffer hexagons. These results highlight the dilutive effects of mosquito migration on intervention efficacy at release boundary areas, which is corroborated by field trial results in both Wolbachia IIT and replacement programs [15, 31].
In general, our simulations showed that implementing certain quantity- or frequency-based scale-down strategies could lead to significantly less resource consumption while having minimal impact on the overall mean intervention efficacy of Wolbachia IIT programs. For example, as shown in Table 1, the half-quantity (Strategy 2) and weekly (Strategy 4) release strategies resulted in a small decrease in overall intervention efficacy of 7% (from 87 to 80%) compared to the constant release strategy (Strategy 1). This is despite them requiring significantly fewer resources compared to Strategy 1; Strategies 2 and 4 both required 44% fewer mosquitoes, and Strategy 4 additionally reduced the number of release events by 44%. Given that they have similar overall intervention outcomes, reducing the release frequency from twice to only once a week would lead to the most cost-effective program outcomes especially if release events contribute significantly to program costs.
Simulations of redistribution strategies also showed that both strategies led to significantly higher overall intervention efficacy compared to the constant release strategy (Strategy 1), because they caused strong suppression in new release areas while having minimal reduction in intervention efficacy in existing release areas. The redistribute-by-quantity (Strategy 7) strategy, however, required 22% more release events compared to the constant release strategy (Strategy 1), whilst the redistribute-by-frequency (Strategy 8) strategy required 16% fewer mosquitoes and 14% fewer release events, as shown in Table 2. Since both redistribution strategies have similar intervention outcomes, the redistribute-by-frequency strategy is more cost-effective, since it would require fewer mosquitoes and release events whilst having greater intervention efficacy than the existing program.
While the simulation results suggest that strategies to reduce the release frequency in both scale-down and redistribution strategies will significantly reduce program resources while maintaining strong suppression, it is also important to consider them in the context of other existing mosquito control measures that could affect the released Wolbachia mosquitoes. For example, the actual intervention efficacy of the strategy could be affected by the frequency and timing of existing insecticide-spraying activities in release areas.
Therefore, the recommended strategies should be implemented in a phased approach. First, a few release areas with strong suppression should be selected to adopt a scale-down strategy and monitored over a period of time. Based on field observations [15], we propose a minimum monitoring period of 6 months to allow for the stabilization of intervention efficacy. Our simulation results in Figs. 2 and 3 concur with these findings, showing that a new release regime generally requires between 3 and 6 months to stabilize. If suppression remains effective after 6 months, the excess mosquitoes that were produced for these areas can then be released in new areas using one of the redistribution strategies. Then, both existing and new release areas that have adopted the new release strategy should be observed for another 6 months. If there remains effective suppression in both existing and new areas, these steps can subsequently be repeated for the other existing release areas.
Limitations
There were deviations between the model outputs and the empirical intervention efficacy estimates, possibly due to complexities that arise in the conduct of an actual Wolbachia release program. The model simulations were run with the assumption that the number of mosquitoes released remained constant throughout the 2-year period and the core and buffer hexagons remained the same due to the lack of data availability. However, in reality, the release program began with a smaller group of sites and expanded over time [15]. This meant that release sites switched identities from adjacent non-release to buffer, and buffer to core sites over time in the empirical study. Therefore, the aggregation of intervention efficacy by event time was also confounded by anthropogenic and environmental characteristics across different locations and time points due to the staggered introduction of Wolbachia releases in real sites. Consequently, we observed some suppression in the empirical intervention efficacy estimates even at the start of the field trial period across release and adjacent non-release areas. It should be noted that the suppression effect observed at the start of the trial period could also be due to the fact that although the field trial analyzed data from 2020 to 2022, Wolbachia releases had already begun in Singapore in 2016 [15]. Moreover, the empirical results were compared against those from counterfactual locations in the same time period using the synthetic control method, whereas our study used outputs from the simulation where no releases had occurred as the baseline for comparison. Furthermore, while our model incorporates the fitness cost associated with Wolbachia infection in IIT male mosquitoes, it does not account for other potential fitness costs associated with an IIT program, for example, the use of irradiation [14] or in-breeding [33]. Additionally, the carrying capacity functions assumed a direct proportionality between the human-dependent component of carrying capacity and the hexagon-specific human population size. However, this might have been too simple to accurately represent the availability of oviposition sites, which could be influenced by other climatic factors such as temperature and humidity, as well as complex urban environmental and human behavioral factors. For example, Fernandez et al. [34] found that the presence of certain architectural features (gully traps), housing age, housing price, and corridor cleanliness were highly predictive of Aedes aegypti abundance in high-rise apartments in Singapore. These factors could have also contributed to the discrepancies between the model outputs and empirical estimates.
Further applications of the spatio-temporal mosquito metapopulation model
As the model was designed to be flexible and configurable with various climate and geospatial inputs, we foresee its application in further Wolbachia IIT-based studies. For example, the model can be used in the planning and optimization of new and existing Wolbachia IIT release programs in a variety of regions around the world, including those with subtropical and temperate climates with seasonal Aedes aegypti population fluctuations [35, 36]. Additionally, the existing model can also be configured to study and optimize Wolbachia replacement programs where both adult male and female Wolbachia mosquitoes are released [37, 38], or modified to model replacement programs where mosquitoes are released at different lifecycle points such as the egg stage [39].
Modeling studies have been conducted on the effects of climate change on Wolbachia replacement programs [30]. Similarly, our model can be used to predict the effects of climate change on existing Wolbachia IIT release programs and study various strategies to make such programs more resilient to future climatic variations. The model can also easily be configured to model the population dynamics and Wolbachia IIT-based control of other mosquito species such as Aedes albopictus, which is becoming an increasingly important vector in Europe because of their potentially expanding range due to climate change [40].
Finally, given that the keen interest in mosquito population dynamics stems from its implications for mosquito-borne disease burden, we expect that our model can also be used to study the intervention efficacy and cost-effectiveness of various Wolbachia IIT program strategies on disease dynamics and burden. Studies have been done in the past where outputs from the mosquito dynamics model were used as inputs to mosquito-borne disease transmission models [20]. Our model therefore provides the ability to tie Wolbachia IIT program inputs to mosquito population dynamics and eventually human disease burden.
Conclusions
We developed a new modeling tool to study Wolbachia IIT programs over large spatial scales that explicitly account for climatic factors such as precipitation and rainfall as well as the movement of mosquitoes. Several scale-down and redistribution strategies were simulated to understand the trade-offs between intervention efficacy and program resource consumption. Results from our study suggest that both scale-down and redistribution strategies can be adopted to significantly reduce the resources required by Wolbachia IIT programs without compromising on intervention efficacy.
Methods
Modeling mosquito metapopulation dynamics with a process-based framework
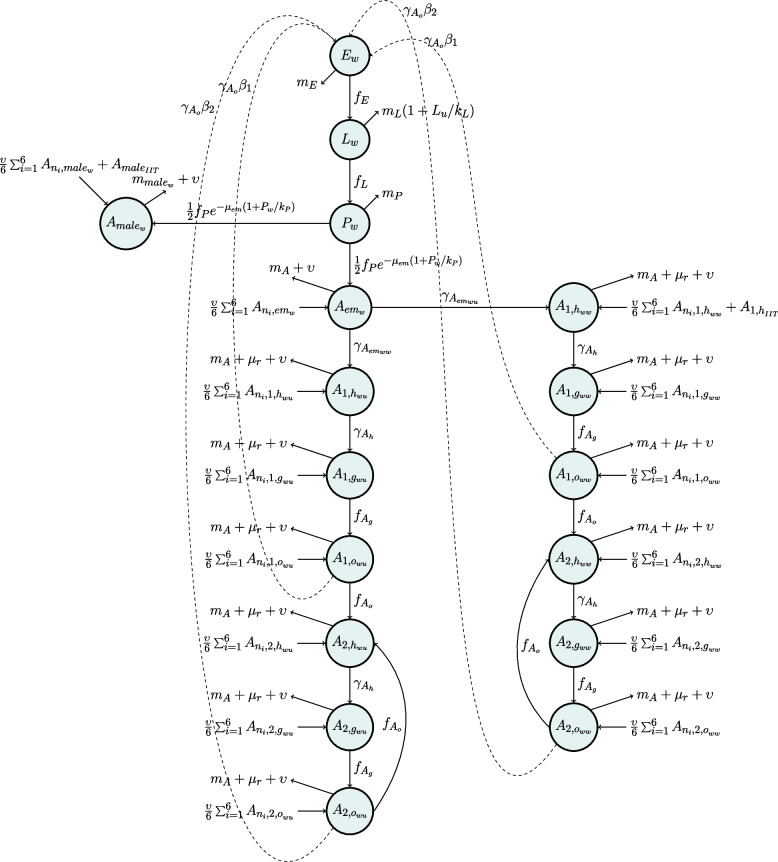
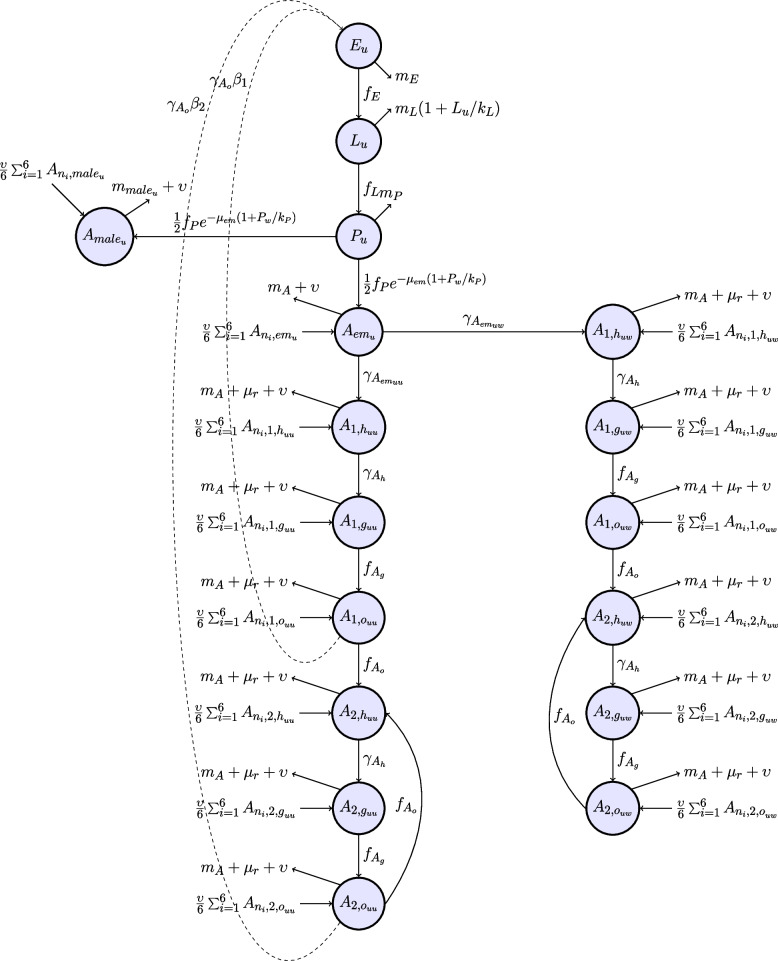
We developed a process-based framework, described as a system of ordinary differential equations that represents various life cycle stages of both Wolbachia-positive and Wolbachia-negative Aedes aegypti mosquitoes. This model was adapted from Cailly et al. (2012) [41] and modified by adding new compartments to explicitly represent male mosquitoes, Wolbachia-positive mosquitoes, and mating-cross outcomes in adult female mosquitoes. Fluxes were also added to account for the migration of adult mosquitoes and the introduction of Wolbachia-positive mosquitoes through the IIT program.
The compartment models shown in Figs. 4 and 5 represent Wolbachia-positive (w) and Wolbachia-negative (u) mosquitoes respectively in the aquatic and adult life cycle compartments. The aquatic stages comprise the egg (E), larvae (L), pupae (P) compartments; for example, Pu represents the compartment for Wolbachia-negative pupae.The adult stages are divided into emergent (Aem), nulliparous (A1), and parous (A2) female adults, and male adults (Amale). Nulliparous and parous female adults are further divided into host-seeking (h), gravid (g), and ovipositing (o). Additionally, adult nulliparous and parous females are divided into their mating crosses (for example, A2,huw represents nulliparous host-seeking Wolbachia-negative females that mated with a Wolbachia-positive male). The full set of equations describing the process-based model is in Additional File 1: Fig. S1.
Fig. 4.
Model diagram for Wolbachia-positive mosquitoes across various life cycle stages
Fig. 5.
Model diagram for Wolbachia-negative mosquitoes across various life cycle stages
The parameter values and functions used in this model were either directly obtained from Bonnin et al. [42] and Tran et al. [43] or derived from data in the literature. Details on the parameter values and functions, as well as their derivations are available in Additional File 1: Tables S1 and S2, and Additional File 2 [26, 42–46] respectively. For a given compartment X, βX is the egg-laying rate, μX is the mortality rate, μr is the additional mortality rate associated with host-seeking behavior, and υ is the mosquito migration rate. γX is the transition rate to the subsequent compartment. For example, γh is the rate at which host-seeking mosquitoes become gravid, which is the next mosquito life cycle stage. The functions represent the time-varying temperature- and precipitation-dependent transition rates (fX), mortality rates (mX), and total carrying capacity indices (kX). As described by Tran et al. [43], the total carrying capacity is a sum of two components: a human-dependent (kXfix) and a precipitation-dependent one (kXvar). The human-dependent component represents the availability of man-made oviposition sites inside of homes such as stagnant water in flower pots and vases, whereas the precipitation-dependent component represents the availability of oviposition sites that occur due to rain. We decided to derive parameters individually from the existing literature, rather than the empirical results from Bansal et al. [15] because we were unable to obtain abundance data for most life-cycle compartments (except for ovipositing adult females), thus making it challenging to make good inferences due to the large number of hidden observations.
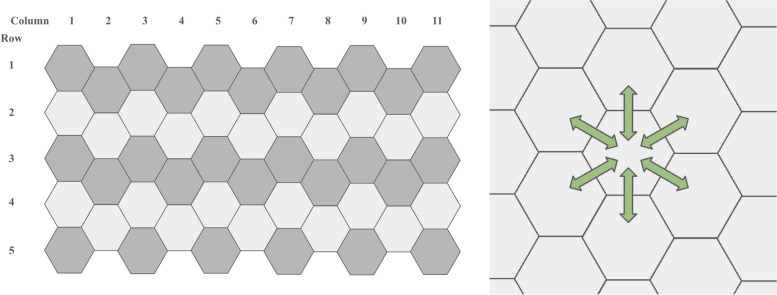
Given the effects of adult mosquito migration on mosquito abundance and treatment program efficacy, the framework was designed to factor in migration by explicitly defining the study area as a grid of vertically aligned hexagons, as illustrated in Fig. 6. A single hexagon approximates a planning sector, which is the primary spatial resolution used for planning the surveillance and control of environment-related infectious diseases in Singapore [15]. Mosquitoes are modeled to mix homogeneously within a hexagon, and some adult mosquitoes migrate out of a hexagon into its six neighbors at a total daily rate of υ; conversely adult mosquitoes migrate into a hexagon from each of its six neighbors at a daily rate of υ/6. The number of mosquitoes in each of the neighboring hexagons is denoted by An. Details on the derivation of the daily mosquito migration rate can be found in Additional File 2.
Fig. 6.
The spatial distribution of mosquitoes was represented by a grid of vertically aligned hexagons. Mosquitoes mix freely within each hexagon (left). Some mosquitoes move between neighboring hexagons at each timestep (right)
Climate, geospatial, and human population data
In this study, we ran all simulations over a 2-year period using the historical climate data from the Meteorological Service Singapore website [47]. Specifically, daily mean temperature and precipitation data were obtained from 15 and 45 weather stations respectively for the 2-year period starting 1 January 2020 and ending 31 December 2021. Each hexagon was assigned the set of climate data belonging to its nearest weather station. The list of weather stations can be found in Additional File 3. Additionally, a 300-day burn-in period was added prior to the start of each simulation using the mean values of the 2-year climate dataset to allow mosquito abundances to reach equilibrium before interventions began.
The grid of vertically aligned hexagons was designed to span the main island of Singapore, Pulau Ujong. Each hexagon was designed to have a side length of 184 m to match the average planning sector area of 0.088 km2 [15]. A hexagon was classified as a land or water one based on the geospatial subzone in which its center lies. The subzone boundary latitude and longitude coordinates were obtained from the Singapore government data portal [48]. Land hexagons were assigned the maximum human- and precipitation-dependent carrying capacity indices of 100 and 50, respectively. Water hexagons were given 0 for both indices since mosquito breeding is taken not to occur there.
We assumed that the effective human-dependent mosquito-carrying capacity was proportional to the number of residents in each hexagon. Subzone-level resident data from 2023 was obtained from the Singapore Department of Statistics [49] and each hexagon was then assigned a normalized human-density value (Hnorm) by taking the number of residents in the subzone in which its center lies, and normalizing that against the number of residents in the most populous subzone.
Model validation
Validation was performed by comparing model-based estimates of intervention efficacy against empirically derived estimates in Bansal et al. [15]. Specifically, we ran a 2-year simulation modeled after the early Wolbachia IIT release program conducted in four townships across Singapore from 2020 to 2022. We identified the approximate subzones belonging to the release townships based on publicly available release maps [50] and used them to select release hexagons whose centers fell in the abovementioned subzones. The list of subzones used for the model validation can be found in Additional File 4: Table S3.
We studied the intervention efficacy in release hexagons as well as adjacent non-release hexagons, as shown in Fig. 7. Release hexagons were classified into core or buffer, where buffer hexagons have at least one non-release neighboring hexagon, while core hexagons do not have any non-release neighboring hexagons. Adjacent non-release hexagons were defined to have at least one release neighboring hexagon. The intervention efficacy was defined as the percentage reduction of adult ovipositing female mosquito abundance between the intervention and the baseline where no intervention occurred. Wild-type adult ovipositing female mosquito abundance was used as the empirical study used data from Gravitraps, which trap gravid females in search of oviposition sites [32].
Fig. 7.
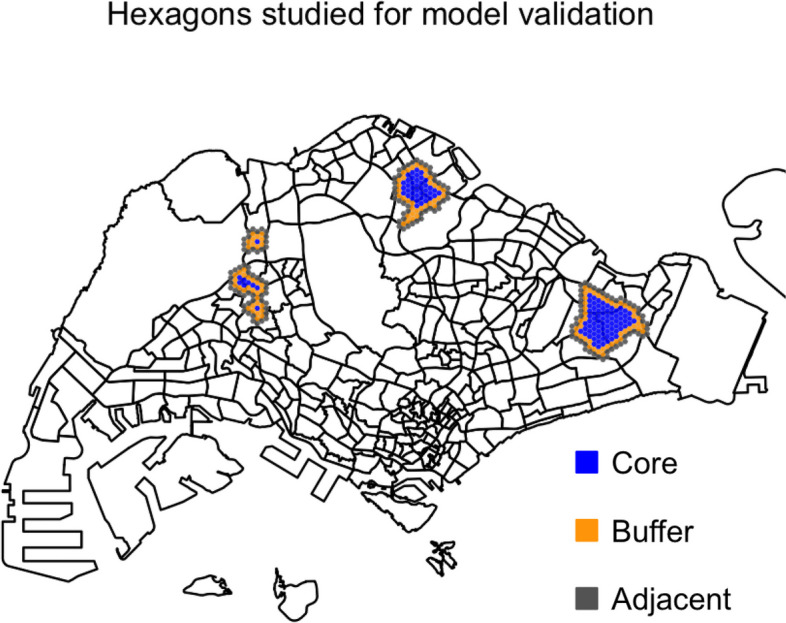
Release hexagons modeled after the early release program in Singapore. Core, buffer, and adjacent non-release hexagons are colored in blue, orange, and gray, respectively
Two simulations were run: a baseline simulation where no releases occurred, while the intervention simulation had releases twice a week at an initial overflooding ratio of 10:1. The initial overflooding ratio is defined as the ratio of Wolbachia IIT male mosquitoes released at each release event relative to the adult wild-type males at the start of the intervention. An initial overflooding ratio of 10:1 was chosen to mirror the mean of the two overflooding ratios (5:1 and 15:1) studied by Pagendam et al. [22].
Scale-down and redistribution strategies
We studied two classes of release strategies: scale-down strategies that optimize the current release program, and redistribution strategies that expand geographical program coverage without requiring more Wolbachia IIT mosquitoes. The release hexagons for scale-down and redistribution strategies are highlighted in Fig. 8, while each strategy is detailed in Table 3. The list of subzones used in the scale-down and redistribution strategies can be found in Additional File 4: Tables S4 and S5, respectively.
Fig. 8.
Release hexagons for the current release program in Singapore, core and buffer release hexagons colored in blue and orange, respectively (a). Release hexagons for the proposed expanded release program in Singapore, current and new release hexagons colored in blue and purple, respectively (b)
Table 3.
Definition of scale-down and redistribution strategies
| Strategy | Type | Name | Initial intervention condition | Threshold(s) to switch to new intervention condition | New intervention condition(s) |
|---|---|---|---|---|---|
| 0 | - | Baseline | No intervention | - | - |
| 1 | - | Constant | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week (e.g. every Tuesday and Friday) | - | - |
| 2 | Scale-down | Half quantity | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week | When wild-type adult male abundance reaches 50% of the initial value | Release in current treatment areas at an initial overflooding ratio of 5:1 twice a week |
| 3 | Scale-down | Quarter quantity | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week | When wild-type adult male abundance reaches 50% and 25% of the initial value | Release in current treatment areas at initial overflooding ratios of 5:1 and 2.5:1 twice a week respectively |
| 4 | Scale-down | Weekly | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week | When wild-type adult male abundance reaches 50% of the initial value | Release in current treatment areas at the initial overflooding ratio of 10:1 once a week (e.g. every Tuesday) |
| 5 | Scale-down | Alternate weeks | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week | When wild-type adult male abundance reaches 50% of the initial value | Release in current treatment areas at the initial overflooding ratio of 10:1 twice every alternate week (e.g. every other week on Tuesday and Friday) |
| 6 | Scale-down | Fortnightly | Release in current treatment areas at the initial overflooding ratio of 10:1 twice a week | When wild-type adult male abundance reaches 50% of the initial value | Release in current treatment areas at the initial overflooding ratio of 10:1 once every two weeks (e.g. every other Tuesday) |
| 7 | Redistribution | Redistribution by quantity | Release in current treatment areas at an initial overflooding ratio of 10:1 twice a week for the first year | After 1 year |
Release in: - Current treatment areas at an initial overflooding ratio of 5:1 twice a week for the second year - New treatment areas at an initial overflooding ratio of 10:1 twice a week for the second year |
| 8 | Redistribution | Redistribution by frequency | Release in current treatment areas at an initial overflooding ratio of 10:1 twice a week for the first year | After 1 year |
Release in: - Current treatment areas at an initial overflooding ratio of 10:1 once a week for the second year - New treatment areas at an initial overflooding ratio of 10:1 once a week for the second year |
The Wolbachia IIT release program in Singapore was expanded to cover fifteen townships in 2024. We simulated five scale-down release strategies to optimize the current release program, which all start with an initial overflooding ratio of 10:1. To compare intervention efficacies for each release optimization strategy, release maps were used to identify release hexagons of these townships using the same method as per model validation. Strategy 1 is a constant release strategy modeled after the current program where IIT male mosquitoes are released twice a week. Strategies 2 and 3 are quantity reduction strategies where the number of released IIT male mosquitoes is reduced when the wild-type male abundance falls below specific thresholds. Strategies 4 to 6 are frequency reduction strategies where the number of release events is reduced when the wild-type male abundance falls below specific thresholds.
Given the possibility of reducing the number of mosquitoes released in already-suppressed hexagons without significantly affecting intervention efficacy, we also explored two redistribution strategies to increase program coverage without changing the overall number of mosquitoes produced. This was done by taking some mosquitoes from current release hexagons and releasing them in new hexagons belonging to four large townships. New hexagons were selected based on subzones with substantial population sizes, ensuring that the combined population of the newly released subzones was approximately half of that already covered by the current release program. Both Strategies 7 and 8 adopted the same constant release strategy for the first year in current release hexagons, and new hexagons did not receive any mosquitoes in the first year.
Strategy 7 is a redistribution-by-quantity strategy where in the second year, the quantity of mosquitoes released in the current release hexagons is reduced by half, but the release frequency is kept constant at twice a week; new hexagons receive redistributed mosquitoes twice a week at the initial overflooding ratio of 10:1. Strategy 8 is a redistribution-by-frequency strategy where in the second year, the frequency of mosquito releases in the current release hexagons is reduced to once a week, while the release quantity is kept constant at the initial value; new hexagons receive redistributed mosquitoes once a week at the initial overflooding ratio of 10:1.
The intervention efficacy was computed as the percentage reduction in wild-type adult female mosquito abundance for each strategy compared to the baseline simulation where no releases were conducted. We analyzed the average mean intervention efficacies over the final 24 weeks of the simulation for all release hexagons (IEfinal) and the resource consumption footprint for each strategy, specifically the total number of mosquitoes released and the total number of release events required for the entire two-year program.
Sensitivity analyses
To assess the robustness of our findings, we conducted sensitivity analyses by varying key model parameters. The results can be found in the sensitivity analysis results sections in Additional File 5.
Supplementary Information
Additional file 1: Figure S1, Tables S1 and S2. Figure S1 – Equations for the process-based model. Table S1 – Process-based model parameters. Table S2 – Process-based model functions.
Additional file 2: Parameter derivations and Figures S2 and S3. Figure S2 – Derived probability distribution of mosquitoes leaving a hexagon. Figure S3 – Carrying capacities for hexagons.
Additional file 3: Weather station list.
Additional file 4: Tables S3-5. Table S3 – List of subzones in the model validation study. Table S4 – List of subzones in the scale-down strategies study. Table S5 – List of new subzones in the redistribution strategies study.
Additional file 5: Sensitivity analysis results.
Acknowledgments
Not applicable.
Abbreviations
- IIT
Incompatible insect technique
- IEfinal
Final intervention efficacy
Authors’ contributions
Made substantial contributions to the conception: P.LJ.L., A.R.C., JT.L. Made substantial contributions to the analysis or interpretation of the data: P.LJ.L., A.R.C., JT.L, S.B. Made substantial contributions to the creation of software: P.L., JY.C. Made substantial contributions to or substantively revised the drafts: P.LJ.L., A.R.C., JT.L.,S.B.,JY.C. All authors read and approved the final manuscript.
Funding
This research is hosted by CNRS@CREATE and supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program (Alex R Cook, Jue Tao Lim), and is funded by the Lee Kong Chian School of Medicine—Ministry of Education Start-Up Grant (Jue Tao Lim).
Data availability
The model was written in R (version 4.21), and the code and data required to run the simulations can be accessed at Zenodo [51].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We would like to declare that the primary author, Preston Lim, is an employee of Verily Life Sciences, which produces Wolbachia mosquitoes for mosquito control programs. However, he is pursuing this research in the capacity of his PhD studentship with the National University of Singapore. The opinions expressed in this article are those of the individual authors, and do not reflect those of the primary author’s employer (Verily Life Sciences LLC).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vector-borne diseases. World Health Organization. https://www.who.int/News-Room/Fact-Sheets/Detail/Vector-Borne-Diseases. Accessed 2 May 2024.
- 2.A global brief on vector-borne diseases. World Health Organization. https://iris.who.int/handle/10665/111008. Accessed 2 May 2024.
- 3.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito–Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:S150–63. [DOI] [PubMed] [Google Scholar]
- 4.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–78. [DOI] [PubMed] [Google Scholar]
- 5.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep. 2016;6:28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gesto JSM, Ribeiro GS, Rocha MN, Dias FBS, Peixoto J, Carvalho FD, et al. Reduced competence to arboviruses following the sustainable invasion of Wolbachia into native Aedes aegypti from Southeastern Brazil. Sci Rep. 2021;11(1):10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pocquet N, O’Connor O, Flores HA, Tutagata J, Pol M, Hooker DJ, et al. Assessment of fitness and vector competence of a New Caledonia wMel Aedes aegypti strain before field-release. PLoS Negl Trop Dis. 2021;15(9): e0009752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ant TH, Mancini MV, McNamara CJ, Rainey SM, Sinkins SP. Wolbachia-Virus interactions and arbovirus control through population replacement in mosquitoes. Pathog Glob Health. 2023;117(3):245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelstädter J, Telschow A. Cytoplasmic incompatibility and host population structure. Heredity (Edinb). 2009;103(3):196–207. [DOI] [PubMed] [Google Scholar]
- 10.Beebe NW, Pagendam D, Trewin BJ, Boomer A, Bradford M, Ford A, et al. Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia. Proc Natl Acad Sci U S A. 2021;118(41). 10.1073/pnas.2106828118. [DOI] [PMC free article] [PubMed]
- 11.Zeng Q, She L, Yuan H, Luo Y, Wang R, Mao W, et al. A standalone incompatible insect technique enables mosquito suppression in the urban subtropics. Commun Biol. 2022;5(1):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford JE, Clarke DW, Criswell V, Desnoyer M, Cornel D, Deegan B, et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol. 2020;38(4):482–92. [DOI] [PubMed] [Google Scholar]
- 13.Caputo B, Moretti R, Virgillito C, Manica M, Lampazzi E, Lombardi G, et al. A bacterium against the tiger: further evidence of the potential of noninundative releases of males with manipulated Wolbachia infection in reducing fertility of Aedes albopictus field populations in Italy. Pest Manag Sci. 2023;79(9):3167–76. [DOI] [PubMed] [Google Scholar]
- 14.Ng LC. Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv. 2021:2021.06.16.21257922.
- 15.Bansal S, Lim JT, Chong C-S, Dickens B, Ng Y, Deng L, et al. Effectiveness of Wolbachia-mediated sterility coupled with sterile insect technique to suppress adult <em>Aedes aegypti</em> populations in Singapore: a synthetic control study. Lancet Planet Health. 2024;8(9):e617–28. [DOI] [PubMed] [Google Scholar]
- 16.Lim JT, Bansal S, Chong CS, Dickens B, Ng Y, Deng L, et al. Efficacy of Wolbachia-mediated sterility to reduce the incidence of dengue: a synthetic control study in Singapore. Lancet Microbe. 2024;5(5):e422–32. [DOI] [PubMed] [Google Scholar]
- 17.Lim JT, Mailepessov D, Chong C-S, Dickens B, Lai YL, Ng Y, et al. Assessing Wolbachia-mediated sterility for dengue control: emulation of a cluster-randomized target trial in Singapore. J Travel Med. 2024;31(7):taae103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong J, Ho SH, Soh SXH, Wong Y, Ng Y, Vasquez K, et al. Assessing the efficacy of male Wolbachia-infected mosquito deployments to reduce dengue incidence in Singapore: study protocol for a cluster-randomized controlled trial. Trials. 2022;23(1):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soh S, Ho SH, Seah A, Ong J, Dickens BS, Tan KW, et al. Economic impact of dengue in Singapore from 2010 to 2020 and the cost-effectiveness of Wolbachia interventions. PLOS Glob Public Health. 2021;1(10): e0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsufuji T, Seirin-Lee S. The optimal strategy of incompatible insect technique (IIT) using Wolbachia and the application to malaria control. J Theor Biol. 2023;569: 111519. [DOI] [PubMed] [Google Scholar]
- 21.Ganesan N. Wolbachia mosquitoes released at Clementi site as part of project expansion to fight dengue. CNA. 2024. https://www.channelnewsasia.com/singapore/wolbachiaaedes-mosquitoes-clementi-dengue-nea-4135106.
- 22.Pagendam DE, Trewin BJ, Snoad N, Ritchie SA, Hoffmann AA, Staunton KM, et al. Modelling the Wolbachia incompatible insect technique: strategies for effective mosquito population elimination. BMC Biol. 2020;18(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roques L, Bonnefon O. Modelling Population Dynamics in Realistic Landscapes with Linear Elements: A Mechanistic-Statistical Reaction-Diffusion Approach. PLoS ONE. 2016;11(3): e0151217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbosa S, Kay K, Chitnis N, Hastings IM. Modelling the impact of insecticide-based control interventions on the evolution of insecticide resistance and disease transmission. Parasit Vectors. 2018;11(1):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufourd C, Dumont Y. Impact of environmental factors on mosquito dispersal in the prospect of sterile insect technique control. Comput Math Appl. 2013;66(9):1695–715. [Google Scholar]
- 26.Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, et al. Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis. 2009;3(9): e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soh S, Ho SH, Ong J, Seah A, Dickens BS, Tan KW, et al. Strategies to Mitigate Establishment under the Wolbachia Incompatible Insect Technique. Viruses. 2022;14(6):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhold JM, Lazzari CR, Lahondère C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects. 2018;9(4). 10.3390/insects9040158. [DOI] [PMC free article] [PubMed]
- 29.Alto BW, Juliano SA. Precipitation and Temperature Effects on Populations of Aedes albopictus (Diptera: Culicidae): Implications for Range Expansion. J Med Entomol. 2001;38(5):646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vásquez VN, Kueppers LM, Rašić G, Marshall JM. wMel replacement of dengue-competent mosquitoes is robust to near-term climate change. Nat Clim Chang. 2023;13(8):848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N Engl J Med. 2021;384(23):2177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong J, Chong CS, Yap G, Lee C, Abdul Razak MA, Chiang S, et al. Gravitrap deployment for adult Aedes aegypti surveillance and its impact on dengue cases. PLoS Negl Trop Dis. 2020;14(8): e0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross PA, Endersby-Harshman NM, Hoffmann AA. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol Appl. 2019;12(3):572–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez SA, Sun H, Dickens BL, Ng LC, Cook AR, Lim JT. Features of the urban environment associated with Aedes aegypti abundance in high-rise public apartments in Singapore: An environmental case-control study. PLoS Negl Trop Dis. 2023;17(2): e0011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mores GB, Schuler-Faccini L, Hasenack H, Fetzer LO, Souza GD, Ferraz G. Site Occupancy by Aedes aegypti in a Subtropical City is Most Sensitive to Control during Autumn and Winter Months. Am J Trop Med Hyg. 2020;103(1):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benitez EM, Estallo EL, Grech MG, Frías-Céspedes M, Almirón WR, Robert MA, et al. Understanding the role of temporal variation of environmental variables in predicting Aedes aegypti oviposition activity in a temperate region of Argentina. Acta Trop. 2021;216: 105744. [DOI] [PubMed] [Google Scholar]
- 37.Velez ID, Uribe A, Barajas J, Uribe S, Ángel S, Suaza-Vasco JD, et al. Large-scale releases and establishment of wMel Wolbachia in Aedes aegypti mosquitoes throughout the Cities of Bello, Medellín and Itagüí, Colombia. PLoS Negl Trop Dis. 2023;17(11): e0011642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann AA, Ahmad NW, Keong WM, Ling CY, Ahmad NA, Golding N, et al. Introduction of Aedes aegypti mosquitoes carrying wAlbB Wolbachia sharply decreases dengue incidence in disease hotspots. iScience. 2024;27(2). 10.1016/j.isci.2024.108942. [DOI] [PMC free article] [PubMed]
- 39.Allman MJ, Lin Y-H, Joubert DA, Addley-Cook J, Mejía-Torres MC, Simmons CP, et al. Enhancing the scalability of Wolbachia-based vector-borne disease management: time and temperature limits for storage and transport of Wolbachia-infected Aedes aegypti eggs for field releases. Parasit Vectors. 2023;16(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira S, Rocha J, Sousa CA, Capinha C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci Rep. 2021;11(1):9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cailly P, Tran A, Balenghien T, L’Ambert G, Toty C, Ezanno P. A climate-driven abundance model to assess mosquito control strategies. Ecol Modell. 2012;227:7–17. [Google Scholar]
- 42.Bonnin L, Tran A, Herbreteau V, Marcombe S, Boyer S, Mangeas M, et al. Predicting the Effects of Climate Change on Dengue Vector Densities in Southeast Asia through Process-Based Modeling. Environ Health Perspect. 2022;130(12):127002. 10.1289/EHP11068. [DOI] [PMC free article] [PubMed]
- 43.Tran A, Mangeas M, Demarchi M, Roux E, Degenne P, Haramboure M, et al. Complementarity of empirical and process-based approaches to modelling mosquito population dynamics with Aedes albopictus as an example-Application to the development of an operational mapping tool of vector populations. PLoS ONE. 2020;15(1): e0227407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosquito Breeding Habitats Found In Homes Doubled In January Compared To Last Year. National Environment Agency. https://www.nea.gov.sg/media/news/news/index/mosquito-breeding-habitats-found-in-homes-doubled-in-january-compared-to-last-year#:~:text=2%20The%20number%20of%20Aedes,ornamental%20containers%20(e.g.%20vases). Accessed 2 May 2024.
- 45.Phase 1 Small-Scale Field Study. National Environment Agency. https://www.nea.gov.sg/corporate-functions/resources/research/environmental_health_institute/wolbachia-aedes-mosquito-suppression-strategy/phase-1-small-scale-field-study. Accessed 2 May 2024.
- 46.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14(1): e1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Historical Daily Records. Meterological Service Singapore. https://www.weather.gov.sg/climate-historical-daily/. Accessed 2 May 2024.
- 48.Master Plan 2014 Subzone Boundary (No Sea). data.gov.sg. https://data.gov.sg/collections/1707/view. Accessed 2 May 2024.
- 49.Singapore Residents by Planning Area / Subzone, Age Group, Sex and Type of Dwelling, June 2023. Department of Statistics Singapore. https://www.singstat.gov.sg/find-data/search-by-theme/population/geographic-distribution/latest-data. Accessed 2 May 2024.
- 50.Wolbachia-Aedes Release Schedule. National Environment Agency. https://www.nea.gov.sg/corporate-functions/resources/research/environmental_health_institute/wolbachia-aedes-mosquito-suppression-strategy/wolbachia-aedes-release-schedule. Accessed 2 May 2024.
- 51.Lim PL, Cook AR, Bansal S, Chow JY, Lim JT. Wolbachia IIT program modeling software (1.0.0). 2024. Zenodo 10.5281/zenodo.14007713.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1, Tables S1 and S2. Figure S1 – Equations for the process-based model. Table S1 – Process-based model parameters. Table S2 – Process-based model functions.
Additional file 2: Parameter derivations and Figures S2 and S3. Figure S2 – Derived probability distribution of mosquitoes leaving a hexagon. Figure S3 – Carrying capacities for hexagons.
Additional file 3: Weather station list.
Additional file 4: Tables S3-5. Table S3 – List of subzones in the model validation study. Table S4 – List of subzones in the scale-down strategies study. Table S5 – List of new subzones in the redistribution strategies study.
Additional file 5: Sensitivity analysis results.
Data Availability Statement
The model was written in R (version 4.21), and the code and data required to run the simulations can be accessed at Zenodo [51].