Abstract
Microfluidic-engineered hydrogel microspheres have emerged as a promising avenue for advancements in tissue engineering and regenerative medicine, particularly through the precise manipulation of fluids to achieve personalized composite biomaterials. In this study, we employed microfluidic technology to fabricate hydrogel microspheres (HMs) using Chinese herbal Bletilla striata polysaccharide (BSP) as the primary material. Concurrently, the natural active ingredient 20(S)-protopanaxadiol (PPD) was encapsulated within the HMs in the form of liposomes (PPD-Lipo), resulting in the formation of nanocomposite hydrogel microspheres (PPD-Lipo@HMs) intended for diabetic wound tissue repair. PPD-Lipo@HMs are characterized by the expansive specific surface area, adjustable mechanical properties, and exceptional biocompatibility. PPD-Lipo@HMs can stimulate the production of vascular endothelial factors, which in turn enhances the migration of endothelial cells, the creation of tubes, angiogenesis, and tissue repair. Moreover, the PPD-Lipo@HMs accumulation produces a microsphere scaffold that effectively covers damaged tissues, promoting the attachment, spread, and multiplication of fibroblast and endothelial cells. The polysaccharide material BSP within PPD-Lipo@HMs can modulate the immune microenvironment of the damaged tissue, reducing inflammation, encouraging re-epithelialization and granulation tissue formation, accelerating angiogenesis and collagen deposition, ultimately leading to tissue repair. The findings highlight the superior therapeutic efficacy of the microfluidic-engineered PPD-Lipo@HMs in addressing the complex challenges of diabetic wound tissue repair, thereby affirming the significant potential of microfluidic engineering technology in tissue repair applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02998-0.
Keywords: Microfluidic, Nanocomposite hydrogel microspheres, Bletilla Striata Polysaccharide, 20(S)-Protopanaxadiol, Diabetic wound
Highlights
Microfluidic-engineered nano-composite microspheres are extensively explored for tissue engineering.
Microfluidic and photocrosslinking techniques were used to construct Bletilla striata polysaccharide nano-composite microspheres for the first time.
Tissue-engineered nano-composite microspheres efficiently fill irregular wounds to promote cell migration and proliferation.
20(S)-protopanaxadiol combined with Bletilla striata polysaccharide synergistic angiogenesis and immune regulation to promote diabetic wound tissue regeneration.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02998-0.
Introduction
Diabetes is a multifaceted metabolic illness marked by elevated levels of glucose in the blood, resistance to insulin, and excessive production of insulin. It is a significant worldwide public health issue [1]. Diabetic ulcers are a significant outcome of diabetes. Presently, diabetic foot ulcers affect around 25% of individuals with diabetes, and out of this group, 20% experience the development of moderate to severe ulcers that require amputation [2, 3]. This poses a substantial challenge for the worldwide healthcare system. The process of repairing skin wounds is an intricate and organized pathophysiological process that involves various stages including wound stress, inflammatory response, formation of granulation tissue, and regeneration of epidermal and other tissues [4]. Diabetic wounds occur predominantly as a result of wound ischemia, hypoxia, hyperglycemia, and chronic inflammatory reactions [5, 6]. These pathways cause inflammatory cell infiltration in the diabetic wound milieu, neovascularization problems, diminished collagen deposition, poor granulation tissue development, ultimately, sluggish healing of diabetes wounds [7–9]. Therefore, addressing these critical concerns through combination treatment has emerged as a potential strategy for diabetic wound management. Impaired immune response and decreased angiogenesis in the diabetic wound microenvironment are major contributors to delayed diabetic wound tissue repair [10–13].
Engineering polymer hydrogels have garnered significant interest as tissue repair dressings due to their outstanding biocompatibility with the extracellular matrix structure [14–16]. However, traditional hydrogels have a nano-scale hole structure that allows small molecules to diffuse but prevents the infiltration of cells and blood vessels, thereby hindering tissue regeneration [17, 18]. Researchers are currently focusing on producing microstructured hydrogels as an alternative to regular hydrogels, also known as hydrogel microspheres (HMs) or microgels [19]. HMs have qualities properties such as a high specific surface area, precision control, and injectability [18, 20]. HMs can be fabricated using various techniques including mechanical emulsification [21], electrospray [22], lithography [23], and microfluidic technology [24]. Microfluidic engineering is the use of microfluidic chips to precisely manipulate and regulate fluids within microchannels [25]. By designing and regulating microfluidic systems, researchers can manipulate and process different substances, cells, and particles within microfluidic environments [26, 27]. This approach has several advantages, including high accuracy, reproducibility, and automation, making it an important tool in tissue engineering and regenerative medicine [28]. Microfluidic-engineered HMs can load various drugs, cells, or cytokines, and effectively fill irregularly shaped injured tissues [29]. The spatial network of HMs accumulates scaffolds that promote cell adhesion, proliferation, and migration, allowing for the fast repair of injured tissues [30–33]. Currently, HMs typically use biological materials such as polyethylene glycol [34], hyaluronic acid [35], chitosan [36], and gelatin [37]. The exploration of novel natural materials has become increasingly prominent in the field of tissue regeneration.
Natural Chinese herbal ingredients exhibit favorable biocompatibility and biosafety, making them widely utilized in wound treatment [38, 39]. Bletilla striata (Thunb.) Reichb.F. is a traditional Chinese herbal known for its detumescence, hemostasis, astringent properties, and granulation promotion. Its primary active component, Bletilla striata polysaccharide (BSP), is a glucomannan consisting of α-mannose, β-glucose, and β-mannose [40]. BSP has been extensively researched for its wound-healing properties, facilitating cell proliferation, migration, and collagen deposition [41]. Moreover, the D-mannose in BSP exhibits a robust affinity for macrophages via the mannose receptor, promoting the transition of macrophages to the M2-like phenotype [42]. This targeted approach can modulate the immune microenvironment of diabetic wounds. Currently, an oxidized BSP-based spray hydrogel dressing has been developed, demonstrating excellent wound adaptability and biocompatibility. It effectively influences the macrophage phenotype transformation to the M2-like subtype, reducing inflammatory responses in wound tissues. This dressing has shown promising wound healing capabilities in both rat-infected wound models and miniature pig wound models [43]. 20(S)-Protopanaxdiol (PPD) is a compound derived from various ginseng plants within the Araliaceae family, such as Panax ginseng, and Panax notoginseng. It serves as a fundamental aglycone of dammarane saponins found in Panax notoginseng [44]. PPD has been shown to enhance the secretion of vascular endothelial factors, leading to the stimulation of proliferation and lumen formation in human umbilical vein endothelial cells (HUVECs). Through the activation of the PI3 K/Akt/mTOR and Raf/MEK/ERK signaling pathways, along with their downstream p70S6 kinase (p70S6K), ultimately facilitating angiogenesis. Despite its promising effects, challenges such as low solubility hinder its clinical utility, underscoring the need for innovative approaches to enhance its bioavailability [45].
In this study, PPD-Lipo was prepared using the thin-film hydration method, as shown in Fig. 1a. BSP, a natural Chinese herbal component, was utilized as the material to develop PPD-Lipo-loaded composite BSP hydrogel microspheres (PPD-Lipo@HMs) through microfluidic and photocrosslinking techniques (Fig. 1b). The PPD-Lipo@HMs demonstrated the ability to modulate the diabetic wound microenvironment and promote angiogenesis, which would hasten the healing process. Evaluations were conducted on PPD-Lipo and PPD-Lipo@HMs’ morphology and release kinetics. Examining the hemostatic qualities, cell compatibility, migration, angiogenic potential, and macrophage polarization brought on by PPD-Lipo@HMs were among the in vitro assessments conducted. Liposomal delivery of PPD facilitated angiogenesis. The incorporation of BSP in the preparation of PPD-Lipo@HMs conferred the PPD-Lipo@HMs with the capacity to regulate macrophage phenotypic changes. Leveraging these attributes, PPD-Lipo@HMs could be administered at the wound site to expedite tissue repair. Application of PPD-Lipo@HMs in a full-thickness skin injury model in diabetic rats demonstrated their ability to modulate the wound microenvironment, reduce inflammation, enhance re-epithelialization, promote granulation tissue formation, stimulate angiogenesis, and increase collagen deposition, leading to accelerated wound closure (Fig. 1c). These findings strongly support the composite multifunctional properties of PPD-Lipo@HMs and their promising potential for diabetic wound tissue repair.
Fig. 1.
Schematic diagram of nanocomposite hydrogel microspheres that accelerate diabetic wound healing by promoting angiogenesis and reducing inflammatory response. a The synthesis process of liposomes loaded with natural active ingredient PPD. b Preparation of PPD-Lipo@HMs. c The application of PPD-Lipo@HMs in the skin injury model of diabetic rats shows that PPD-Lipo@HMs can accurately deliver PPD, regulate the immune microenvironment of the wound to reduce the inflammatory response and promote angiogenesis to accelerate diabetic wound tissue repair
Experimental and methods
Materials
PPD (purity > 98%) was purchased from Chengdu Dexter Biotechnology Co., Ltd. (Chengdu China). Bletilla striata polysaccharides were extracted by the methods available in the laboratory. Lecithin was purchased from A.V.T Pharmaceutical Technology Co., Ltd. (Shanghai, China). Methacrylic anhydride, 5-isothiocyanate fluorescein, and streptozotocin were purchased from Zhengzhou Feiman Biotechnology Co., Ltd. (Zhengzhou, China, mall.shiyanjia.com). Cell counting kit (CCK-8) and Live/Dead cell staining kit were provided by Wuhan Boster Bioengineering Co., Ltd. (Wuhan, China). Dulbecco’s modified Eagle medium (DMEM), trypsin-EDTA, and penicillin-streptomycin were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Matrigel Matrix was purchased from Corning Incorporated (New York, USA). APC anti-mouse CD 86 antibody and PE anti-mouse CD 206 (MMR) antibody were purchased from BioLegend (San Diego, USA). All other reagents were analytical grade.
Fabrication and characterization of PPD-Lipo
PPD-Lipo was prepared using a film dispersion method [33]. Initially, 30 mg of egg yolk lecithin and 6 mg of PPD were precisely weighed and dissolved in 20 ml of absolute ethanol. The resulting lipid film adhered to the bottom of a round-bottom flask after complete evaporation of the organic solvent at 50 °C. Subsequently, 6 mL of deionized water was added to the flask, and the mixture was hydrated at 37 °C for 30 min until the film dissolved completely in the water. PPD-Lipo was then obtained through sonication for 5 min (3 s probing, 2 s intermittent). The liposome solution was diluted to 1 mg/mL, and the measurements of particle size distribution and potential of the drug-loaded liposome were performed using dynamic light scattering (LITESIZER500, Anton Paar, Austria), at a test temperature of 25 °C. The morphology of PPD-Lipo was examined and imaged using transmission electron microscopy (FEI Tecnai G2 12, USA). The liposome suspension was appropriately diluted, dropped onto a copper mesh, left to stand for 5–10 min, excess liquid removed with a sharp-angle filter paper, stained with a 3% phosphotungstic acid water negative staining solution for 1–2 min, excess staining solution removed, and finally observed and photographed after drying.
The measurement of encapsulation efficiency: a certain amount of PPD-Lipo solution was taken and filtered through a 0.22 μm microporous membrane, and 1 mL of the solution was accurately taken and dissolved with 9 mL of methanol. The ultrasonic demulsification was used to determine the loading amount of PPD, which was recorded as an M package. In addition, 1 mL of PPD-Lipo solution was taken without membrane, and 9 mL of methanol was directly added to dissolve and ultrasonic demulsification. The total content of PPD was determined and recorded as M total.
The formula for encapsulation efficiency is as follows:
Encapsulation efficiency (%) = Mpackage/Mtotal×100%.
The in vitro release of PPD-Lipo was investigated by dialysis method [46]. PBS buffer solution (pH = 7.2–7.4) was used as the release medium, and 3 mL of PPD-Lipo suspension was placed in a dialysis bag (Mw8500) and placed in 20 mL of ethanol normal saline solution (mass fraction of ethanol was 50%). All samples were kept in a constant temperature shaker at 37 °C, 100 r/min. At 0.5,1,2,6,4,8,12,24,48,96 h, 1mL of drug release medium was taken, and the same amount of isothermal ethanol normal saline was added. Each group was parallel to three batches of samples. After ultrasonic demulsification with methanol, the release solution was filtered through a 0.22 μm filter membrane, and the drug content was determined by HPLC. The average cumulative drug release rate at each time point was calculated, and the drug release curve was drawn. The cumulative release was calculated by the following formula:
Er
Er: cumulative drug release; Ve: the replacement volume of the medium; V0: volume of release medium; Ci: the concentration of the drug in the release solution at the time of I th displacement sampling; Mdrug: the mass of the drug in the drug-loaded nanoparticles for release; n: The number of times the medium is replaced.
Synthesis of BSPMA
BSP was dissolved in deionized water, followed by the addition of methacrylic anhydride. The solution was then adjusted with 4% NaOH (4 g/100 ml) to maintain a pH between 8.0 ~ 8.5. After 24 h of reaction, the supernatant was centrifuged in a dialysis bag and subsequently dialyzed with deionized water for 3 days. The resulting dialyzed solution was freeze-dried to yield BSPMA. The degree of substitution of BSPMA was determined using 1H-NMR [42].
Preparation of BSPMA pregel
The experimental mold was designed to accommodate the volume needed for the experimental instrument. A 2 ml BSPMA solution was injected into the vial and exposed to ultraviolet light (405 nm, 3.5 mWcm−2) for 1 min. Photos were taken before and after the ultraviolet light exposure to confirm that the BSPMA solution could solidify into a gel.
Preparation of HMs
HMs were synthesized using a 0.5% LAP and BSPMA aqueous solution mixed with 95wt% paraffin oil and 5wt% Span80 as the oil phase [35]. The two liquid phases were injected into a microfluidic chip using a precision push pump, where the external phase sheared the solution into droplets that flowed into a collection device. Subsequently, HMs were formed through crosslinking under ultraviolet light. The HMs were then washed with 75% ethanol and deionized water to remove surfactants and paraffin oil. To investigate the impact of external and internal phase flow rates on HMs morphology, experiments were conducted with varying external phase flow rates (2–7 mL/h) while keeping the internal phase flow rate constant. Similarly, experiments were carried out by adjusting the internal phase flow rate (0.2–0.7 mL/h) while maintaining a fixed external phase flow rate. Additionally, the effect of the internal phase concentration on HMs characteristics was studied by altering the BSPMA solution concentration in the internal phase (ranging from 0.5 to 2%). The morphology and diameter distribution of HMs were analyzed using an optical microscope and Image J software.
Preparation of PPD-Lipo@HMs
The internal phase consisted of 0.5% LAP and a 1% BSPMA/PPD-Lipo solution (50 mg BSPMA dissolved in 5 mL PPD-Lipo solution). PPD-Lipo@HMs were created using 95 wt% paraffin oil and 5 wt% Span80 as the external phase. The internal and external phases were introduced into the microfluidic chip at flow rates of 0.4 ml/h and 5 ml/h, respectively. The internal solution was fragmented into droplets by the external phase due to shear stress from both liquid phases and then directed into a collection device. Subsequently, the droplets were cross-linked under ultraviolet light to form solid PPD-Lipo@HMs. The resulting PPD-Lipo@HMs underwent multiple washes with 75% ethanol and deionized water to eliminate surfactants and paraffin oil, before being stored in deionized water. The morphology and diameter of the PPD-Lipo@HMs were examined using a bright field microscope, and the diameter distribution was analyzed with Image J software. To assess the uniform loading distribution of PPD-Lipo within PPD-Lipo@HMs, FITC, and rhodamine-B were utilized to label PPD-Lipo and HMs, respectively. Laser confocal microscopy was employed to observe the loading of PPD-Lipo in HMs from various perspectives. The morphology of the PPD-Lipo@HMs was further analyzed using scanning electron microscopy (SEM, Axio Imagerm2 EVO10, Germany). Samples were freeze-dried, fixed on the SEM stage, and observed after vacuum sputtering and gold plating for 60 s to reveal morphological characteristics under different visual fields. Fourier transform infrared spectroscopy (FTIR) was conducted to identify chemical bonds within the sample, with each spectrum accumulating 32 scans in the wavenumber range of 4000–1000 cm−1 at a resolution of 4 cm-1 using the KBr pelleting technique.
Swelling performance
The 10 mg freeze-dried HMs were fully immersed in 4 mL of deionized water and shaken at 37 °C and 80 rpm. The supernatant was removed via centrifugation at predetermined time intervals (0, 30, 60, 90, 120, 150, 180 min), and the sample weight was compared to its initial weight. The sample was placed in an EP tube for the experiment, and the mass of the sample + EP tube was weighed at 0 min (W0). The microspheres were submerged in PBS and placed in a shaker at 37 °C and 80 rpm/min. At specific time points, the supernatant was removed by centrifugation, dried, and weighed (recorded as Wt). Each group underwent a minimum of three parallel experiments. The swelling ratio (SR) of the microspheres was calculated using the formula:
SR (%) = (Wt - W0) / W0 * 100%.
Here, W0 represents the initial weight of the microspheres, and Wt is the weight after swelling.
Additionally, the HMs and PPD-Lipo@HMs were labeled with rhodamine, and their swelling behavior was observed under a fluorescence microscope post-freeze-drying.
Thermogravimetric analysis (TGA)
To assess the thermal stability and thermal degradation of HMs and PPD-Lipo@HMs, a thorough thermal analysis was conducted utilizing a thermogravimetric analyzer (Mettler TGA2 Switzerland) with a temperature range from 30 to 800 °C and a heating rate of 10 °C/min.
Degradation tests
The biodegradability of the microsphere system was assessed through in vitro enzymatic degradation experiments. Freeze-dried microspheres were placed in PBS with 1% lysozyme, while a control group was also prepared. The system was incubated at 37 °C and 80 rpm for 5 weeks with regular PBS changes every two days. HMS and PPD-Lipo@HMs were retrieved at the 1st, 3rd, and 5th weeks, cleaned, freeze-dried, and observed for morphological changes using SEM. The weight of the microspheres was recorded to calculate the degradation rate using the formula:
Degradation rate (%) = (Wt) / (W0) × 100%.
Here, W0 represents the initial weight of the microspheres, and Wt is the weight after degradation.
Drug encapsulation and release
The in vitro release properties of PPD-Lipo@HMs were studied using the dialysis method. A dialysis bag (Mw8500) containing 3 mL of PPD-Lipo@HMs was immersed in a normal saline solution as the release medium. Samples of the release medium were collected at time points of 0.5, 1,2, 4, 6,8, 12, 24, 48, and 96 h, with 1mL of fresh buffer added each time. The collected samples were then subjected to ultrasonic demulsification with methanol, filtered through a 0.22 μm filter membrane, and analyzed for drug content using HPLC. The cumulative drug release rate at each time point was calculated to generate a drug release profile.
Hemolysis assay
The blood compatibility of HMS and PPD-Lipo@HMs was assessed through an in vitro hemolysis test [47]. A 0.5 g sample was combined with 5 mL of normal saline and incubated at 37 °C for 48 h in a biochemical incubator. Red blood cells were isolated from 3 to 4 mL of ear marginal venous blood from New Zealand white rabbits by centrifugation at 2000 rpm for 10 min, followed by three washes with normal saline. The purified red blood cells were diluted with normal saline to create a red blood cell suspension (5%, v/v). Various samples (PPD-Lipo, HMs, and PPD-Lipo@HMs) were added to 1 mL of the RBC suspension at final concentrations of 100 and 300 mg/mL. The mixture was then centrifuged at 1500 rpm for 5 min. Positive and negative controls were 0.1% Triton X-100 (T8200, Solarbio) and saline, respectively. The absorbance of the supernatant at 540 nm was measured using a microplate reader. Each experiment was conducted in triplicate, and the hemolysis rate was calculated using the formula:
Hemolysis rate (%) = (As-An) / (Ap-An) × 100%.
where As, Ap and An represent the absorbance of the sample, positive control, and negative control, respectively.
In vitro hemostasis assay
The hemostatic efficacy of PPD-Lipo@HMs in vivo was evaluated using a mouse liver bleeding model. Kunming mice, aged 5–6 weeks, weighing 26–33 g, and male, were anesthetized for the experiment. The mouse liver was exposed in the abdominal cavity, and a pre-weighed filter paper was positioned beneath the liver. Subsequently, a 20 mm liver slice was excised from the root of the liver lobe to induce bleeding. Following 0.5 s of uncontrolled bleeding, a specific quantity of either HMs or PPD-Lipo@HMs was administered to the bleeding site. The amount of blood loss was determined by comparing the weight of the filter paper before and after blood absorption.
Cell culture
L929 fibroblasts and HUVEC were cultured in high glucose medium (DMEM, GIBCO) supplemented with 10% (v/v) fetal bovine serum at 37 °C and 5% CO2. The medium was refreshed every two days, and the cells were subcultured when they reached 80% confluence.
Biocompatibility of PPD-Lipo@HMs
The cytotoxicity of PPD-Lipo@HMs on L929 fibroblasts and HUVECs in vitro was assessed using the CCK-8 method. Samples were prepared, sterilized, and placed in a 5 ml EP tube, then immersed in DEME culture medium with 10% fetal bovine serum at 37 °C for 24 h to create sample extracts at different concentrations (0.2, 0.4, 0.6, 0.8 mg/mL). The cells were seeded in a 96-well plate and cultured for 24 h before being exposed to varying concentrations of PPD-Lipo, HMs, and PPD-Lipo@HMs co-culture solutions. Control and blank groups were also included in the experiment and cultured in a cell incubator under specific conditions. On the 1st and 2nd day, CCK-8 reagent was added to each well, followed by a 2 h incubation in the dark. The OD value at 450 nm wavelength was measured using a microplate reader to calculate the cell survival rate using the formula:
Cell viability (%) = (ODs-ODb)/(ODc-ODb) x 100%.
Here, ODs represent the OD value of the experimental group, while ODb represents the OD value of the blank group.
The L929 fibroblasts and HUVEC cell suspension, containing 10^5 cells per milliliter in the logarithmic growth phase, were cultured directly with the sterilized sample on a confocal dish for 24, 48, and 72 h. Following staining with the Live/Dead staining reagent, the cells were observed using laser confocal microscopy. Cell proliferation was assessed through cytoskeleton staining, where L929 cells and HUVECs were stained with iFluor 488 phalloidin and 4’,6-diamidino-2-phenylindole (DAPI) according to the provided instructions. Post-culture, the samples were gently washed three times with PBS, and the stained cells were visualized using an inverted fluorescence microscope.
The migration and angiogenesis in vitro
A wound healing assay was conducted to assess the migratory ability of cells co-cultured with PPD-Lipo or PPD-Lipo@HMs. L929 cells and HUVECs (5 × 10^5 cells/well) were plated in 6-well plates for 24 h. A 200 µL pipette tip was used to create a straight scratch on the lower chamber surface, followed by removal of detached cells with PBS. The adherent cells were then co-cultured with different sample groups for 0, 12, 24, and 48 h. Subsequently, the width of the scratches was observed under a microscope and analyzed using Image J software. The change in scratch width (ΔScratch) was calculated using the formula:
Wound area ratio (%) = (WA0 - WAt) / WA0 × 100%.
where WA0 and WAt represent the initial wound area and the wound area at time t, respectively.
HUVECs were utilized to investigate angiogenesis. Before the experiment, the 96-well plate was pre-cooled at −20℃. Matrix adhesive was then added to each well and cured at 37 °C for 2 h. Subsequently, HUVECs (2 × 10^4 cells per well) were seeded in the 96-well plates and incubated at 37 °C for 4 h. Cell staining was carried out using calcein, and angiogenesis was observed through a laser confocal microscope. The tubular structure was quantitatively analyzed using Angiotool software.
Macrophage polarization in vitro
To assess the impact of PPD-Lipo@HMs on macrophage polarization, RAW264.7 cells were seeded in a 12-well plate at a concentration of 2 × 10^5 cells per well. They were then treated with 200 mg of sterile (PPD-Lipo, HMs, PPD-Lipo@HMs) in DMEM medium dissolved in fetal bovine serum (100 µg/ml) and incubated at 37 °C with 5% CO2 for 24 h. Subsequently, 100 ng/ml LPS was added and the cells were cultured for an additional 24 h. The group treated with 100 ng/ml LPS was designated as the positive control (M1-like). Flow cytometry was employed to analyze the impact of the various treatments on macrophage polarization. Macrophages were collected before and after treatment, as well as from the control group. These cells were incubated with 100 µl of PBS containing CD86/APC antibody and CD206/PE antibody at 4 °C for 30 min followed by room temperature for 1 h. The expression levels of CD86 and CD206 were then assessed using flow cytometry.
Wound healing in diabetic rats
All rats used in the study were obtained from SPF Biotechnology Co., Ltd. (Beijing, China). The experimental environment maintained a temperature of 24 ± 2 °C and a relative humidity of 55 ± 5%. The bedding was changed daily during the experiment. The research protocol was approved by the Experimental Animal Management Association of Chengdu University of Traditional Chinese Medicine, adhering to the guidelines for the treatment and use of experimental animals set forth by the National Institutes of Health. SD rats weighing 200–250 g were intraperitoneally injected with a 60 mg/kg dose of Streptozotocin (STZ) solution. Blood glucose levels were monitored in the SD rats after 3 days of continuous injections. Rats with blood glucose levels exceeding 16.67 mmol/L were selected for the type I diabetic model. Following the establishment of diabetes, the rats were randomly assigned to a control group and an experimental group. The rats’ dorsal hair was removed, and an 8 mm full-thickness skin wound was created. Various treatments including normal saline (control), PPD-Lipo, HMs, and PPD-Lipo@HMs were applied to the wounds. Wound images were captured on the 3rd, 7th, 10th, and 14th days post-treatment, and the wound area was quantified using ImageJ software. Subsequently, the rats were euthanized, and wound tissues were collected for histological and immunofluorescence analyses. Tissue Sect. (5 μm) were prepared for histological examination. H&E staining was utilized to assess wound closure, Masson trichrome staining for collagen deposition, CD31, and α-SMA immunofluorescence staining for angiogenesis evaluation, CD86 and CD206 immunofluorescence staining for macrophage polarization and inflammation analysis, and MPO immunohistochemistry for assessing the inflammatory response at the wound site.
Statistical analysis
The experimental data were presented as the mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test to assess the significance levels of the software Origin 2022. Statistical significance was defined as *p < 0.05, **p < 0.01, ***p < 0.001.
Results and discussion
Preparation and characterization of PPD-Lipo
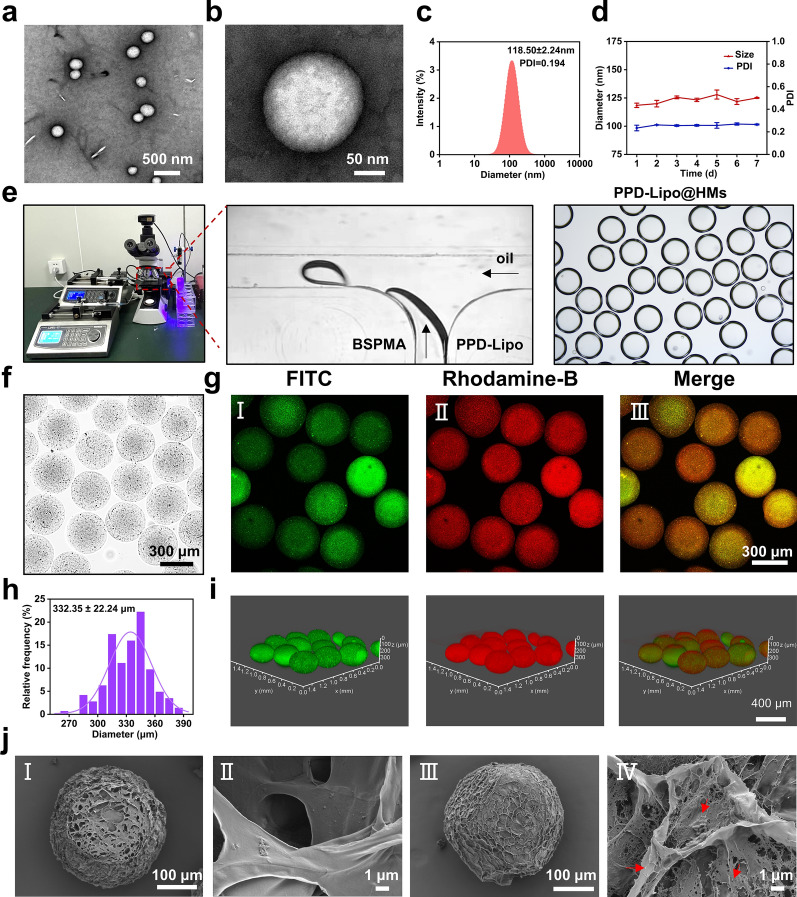
HSPC is known for its excellent stability and is commonly utilized as a lipid material to enhance the stability of liposomes. PPD, a key aglycone of notoginsenosides, shares a similar structure to cholesterol and can efficiently act as a substitute liposome material [48]. The transmission electron microscope micrographs of PPD-Lipo prepared using the thin film hydration method, revealed a spherical vesicle morphology across various magnifications (Fig. 2a and b). The mean particle diameter of PPD-Lipo was determined to be 118.50 ± 2.24 nm, with a polydispersity index of 0.194 (Fig. 2c), suggesting excellent dispersion properties. In addition, the stability of PPD-Lipo was verified by conducting successive measurements of particle size and PDI for 7 days (Fig. 2d). The results consistently showed the same values, highlighting the strong stability of PPD-Lipo. Consistent stability is essential for the ongoing release of PPD and the resulting therapeutic effectiveness.
Fig. 2.
Characterization of PPD-Lipo and PPD-Lipo@HMs. a, b Transmission electron microscopy images of PPD-Lipo in different fields of view: a 500 nm, b 50 nm. c The particle size distribution of PPD-Lipo. d Stability of PPD-Lipo. e Schematic diagram of droplet microfluidic device, and microsphere encapsulating PPD-Lipo. f The bright field microscope image of PPD-Lipo@HMs. Scale bar:300 μm. g CLSM images of PPD-Lipo@HMs: I FITC-labeled PPD-Lipo image, (II) Rhodamine-labeled HMs image, (III) PPD-Lipo@HMs merge image, Scale bar:300 μm. h The particle size distribution of PPD-Lipo@HMs. i CLSM 3D scanning pictures of PPD-Lipo@HMs. Scale bar:400 μm. j SEM images of HMs and PPD-Lipo@HMs. (I, II) SEM images of HMs in different fields of view, Scale bar:100 μm, 1 μm. (III, IV) SEM images of PPD-lipo@HMs in different fields of view. Scale bar: 100 μm,1 μm. The data are expressed as mean ± SD, n ≥ 3
Synthesis and characterization of BSPMA
BSP, a natural polysaccharide derived from the Chinese herbal Bletilla striata, exhibits favorable biocompatibility and biodegradability [43]. Studies reveal that BSP has multiple pharmacological properties and can function as a carrier substance for HMs by imitating the extracellular matrix. BSP is a linear polymer glucomannan consisting of mannose and glucose connected by β−1,4-glycosidic linkages [41]. Through activation with NaOH and esterification with methacrylic anhydride (MA) (Figure S1a), the hydroxyl groups on the molecular chain can be modified to introduce methacrylic acid groups, resulting in the derivative methacrylate BSP (BSPMA) with light-curing capabilities [42]. The presence of characteristic proton peaks at 5.67ppm and 6.11ppm in the 1H-NMR spectrum (Figure S1b) confirms the successful grafting of the MA group onto BSP. The degree of substitution for BSPMA was determined to be 43% through peak area calculations. The confirmation of BSPMA synthesis was achieved through Fourier transform infrared spectroscopy (FT-IR). The spectrum in (Figure S1d) exhibited a prominent absorption peak at 3399 cm−1, corresponding to the hydroxyl O-H stretching vibration peak of polysaccharides. Additionally, a weaker absorption peak at 2927 cm−1 indicated the stretching vibration peak of C-H, while peaks at 1151 cm−1, 1062 cm−1, and 1031 cm−1 confirmed the presence of pyranose. BSPMA displayed an intensified carbonyl (C = O) stretching vibration absorption peak at 1735 cm−1, suggesting the conversion of some hydroxyl groups into carbonyl groups post-reaction with MA. Furthermore, the successful solidification of BSPMA into a hydrogel was validated through a small bottle inversion experiment (Figure S1c). In this study, HMs and PPD-Lipo@HMs were prepared by microfluidic equipment. The precursor solution was a BSPMA solution and a mixed solution of BSPMA and PPD-Lipo. The adjustable mechanical properties in this article can control the mechanical properties of HMs and PPD-Lipo@HMs hydrogel microspheres by controlling the mechanical properties of the precursor solution. Indirect modulation of the mechanical properties of microspheres by determining the mechanical properties of prerequisite gels with different concentrations of BSPMA. The experimental data showed that the maximum compression force increased with the increase of BSPMA solution concentration (Figure S1f, Figure S1g). Therefore the mechanical properties of microspheres can be adjusted by the concentration of BSP solution.
Preparation and process screening of HMs
Microfluidic technology enables precise control of two or more immiscible micro-volume liquids in micron-scale channels, allowing for the continuous and controlled preparation of emulsions or droplets with excellent monodispersity, controllable particle size, and morphology [49]. This technology offers the advantages of monodisperse particle size, a straightforward preparation process, and easy control, making it widely utilized in the production of HMs. In this research, we utilized microfluidic technology to fabricate HMs. The internal phase consisted of a BSPMA aqueous solution (dispersed phase), while the external phase comprised paraffin oil with 5% Span 80 (continuous phase). The hydrogel pre-mixed solution was dispersed within the oil phase to generate uniformly sized pre-gel droplets, which were then crosslinked using ultraviolet light to form HMs. To investigate the impact of internal and external phase flow rates on the morphology of HMs, various flow rates were employed. The internal phase flow rates ranged from 0.2 to 0.7 mL/h, while the external phase flow rates spanned from 2 to 7 mL/h. The formation of HMs under different flow rate ratios was examined using an orthogonal method. Analysis of the results (Figure S2, Figure S3) revealed that HMs exhibited a spherical shape across varying internal and external phase flow rates. ImageJ software was utilized to randomly measure the diameter of 100 HMs, demonstrating an unimodal distribution in the diameter of all HMs groups. Under fixed external phase flow rate conditions, altering the internal phase flow rate results in varying sizes of corresponding HMs, as illustrated in Figure S3b. Larger internal phase flow rates lead to larger HMs sizes; however, excessively high internal phase flow rates prevent the formation of droplets and instead generate fibers. Conversely, when maintaining a constant internal phase flow rate and adjusting the external phase flow rate, depicted in Figure S3c, increasing the external phase flow rate results in smaller particle sizes of the corresponding HMs. Excessive external phase flow rates also inhibit HMs formation. To investigate the impact of internal phase concentration on HMs morphology and size, four different concentrations of BSPMA solution (0.5%, 1%, 1.5%, 2%) were utilized as the internal phase. The flow rates of both internal and external phases were fixed, and droplets were formed using microfluidics and solidified into HMs. As shown in Figure S4a and Figure S4b, the size of HMs generated from various internal phase concentrations at the same flow rate differs. HMs size increases with higher internal phase concentrations, while the rate of HMs formation decreases with concentration.
Preparation and characterization of PPD-Lipo@HMs
HMs have garnered significant attention in the biomedicine and tissue engineering sectors as effective carriers for drug and cytokine delivery [19]. In contrast to conventional hydrogels, HMs offer distinct advantages such as injectability, modularity, and porosity [17]. The preparation of PPD-Lipo@HMs using the 3.3 process is illustrated in Fig. 2e. HMs generated through microfluidic technology appeared uniform and intact under an optical microscope (Fig. 2f). To confirm the successful loading of PPD-Lipo into HMs, we utilized different fluorescent dyes to label PPD-Lipo (FITC) and HMs (Rhodamine-B), observing the presence of liposomes within the HMs via confocal laser scanning microscope (CLSM). The presence of evenly dispersed green fluorescence within the HMs, as depicted in Fig. 2g, serves as evidence of the successful loading and distribution of PPD-Lipo within the HMs. Given the 3D spherical nature of HMs, we further conducted 3D fluorescence scanning using CLSM on the fluorescently labeled HMs. The results, as shown in (Fig. 2i), revealed a uniformly dispersed sphere of green fluorescence from the PPD-Lipo, corroborating its successful dispersion within the HMs. Particle size analysis indicated a single peak distribution for the diameter of PPD-Lipo@HMs, with an average diameter of (332.35 ± 22.24) µm (Fig. 2h). Examination of the surface morphology of PPD-Lipo@HMs via scanning electron microscopy (SEM) highlighted the presence of a porous structure, facilitating the release of PPD (Fig. 2j). Furthermore, a comparative analysis between HMs and PPD-Lipo@HMs demonstrated the surface distribution of PPD-Lipo within a 1 μm field of view, further confirming the successful loading of PPD-Lipo onto PPD-Lipo@HMs.
In vitro swelling, thermogravimetric, and degradation analysis of PPD-Lipo@HMs
The highly porous 3D spherical network structure of HMs allows them to effectively absorb wound exudate and create a moist environment conducive to healing [18]. Our research focused on investigating the swelling behavior of HMs and PPD-Lipo@HMs in deionized water at room temperature. The results depicted in Fig. 3a and b demonstrate that both HMs and PPD-Lipo@HMs exhibit significant swelling rates, reaching 1843% and 1658% within 30 min, respectively. Moreover, they maintain this swollen state over time, indicating their ability to rapidly absorb liquid and sustain a moist environment, which is crucial for promoting diabetic wound healing. Additionally, the thermal properties of HMs and PPD-Lipo@HMs were analyzed using thermogravimetric analysis (TGA) as shown in Fig. 3c. The weight loss profiles revealed that the initial weight loss between 30 ~ 100 °C was attributed to water evaporation, while the major weight loss between 200 ~ 400 °C was due to the degradation of the molecular chain of BSP. Importantly, the thermal stability of HMs was not compromised by the addition of PPD-Lipo, as both HMs and PPD-Lipo@HMs exhibited good thermal stability. The biodegradability of 3D network structures of wound dressing, such as HMs, plays a crucial role in maintaining the deposition of ECM in skin tissue and controlling the release of drug components [50]. Results from in vitro degradation experiments (Fig. 3d) indicate that both HMs and PPD-Lipo@HMs exhibit a slow degradation pattern, promoting a sustained release of PPD-Lipo for long-term treatment. Furthermore, SEM analysis of freeze-dried HMs and PPD-Lipo@HMs at various time points reveals changes in the 3D structure as a degradation process (Fig. 3e). The PPD-Lipo@HMs initially collapse on the surface after the first week, followed by the exposure of their internal framework by the third week, aligning with the degradation data presented in Fig. 3d. These findings demonstrate the potential of continuous degradation of microspheres for achieving sustained release of PPD.
Fig. 3.
Characterization of swelling, thermogravimetric, and degradation properties of HMs and PPD-Lipo@HMs. a Swelling fluorescence microscope images of HMs and PPD-Lipo@HMs. Scale bar:200 μm. b The swelling curves of HMs and PPD-lipo@HMs. c TGA of HMs and PPD-Lipo@HMs. d Degradation curves of HMs and PPD-Lipo@HMs. e SEM images of HMs and PPD-Lipo@HMs after degradation at different times. Scale bar: 100 μm and 30 μm. The data are expressed as mean ± SD, n ≥ 3
PPD encapsulation and release properties of PPD-Lipo@HMs
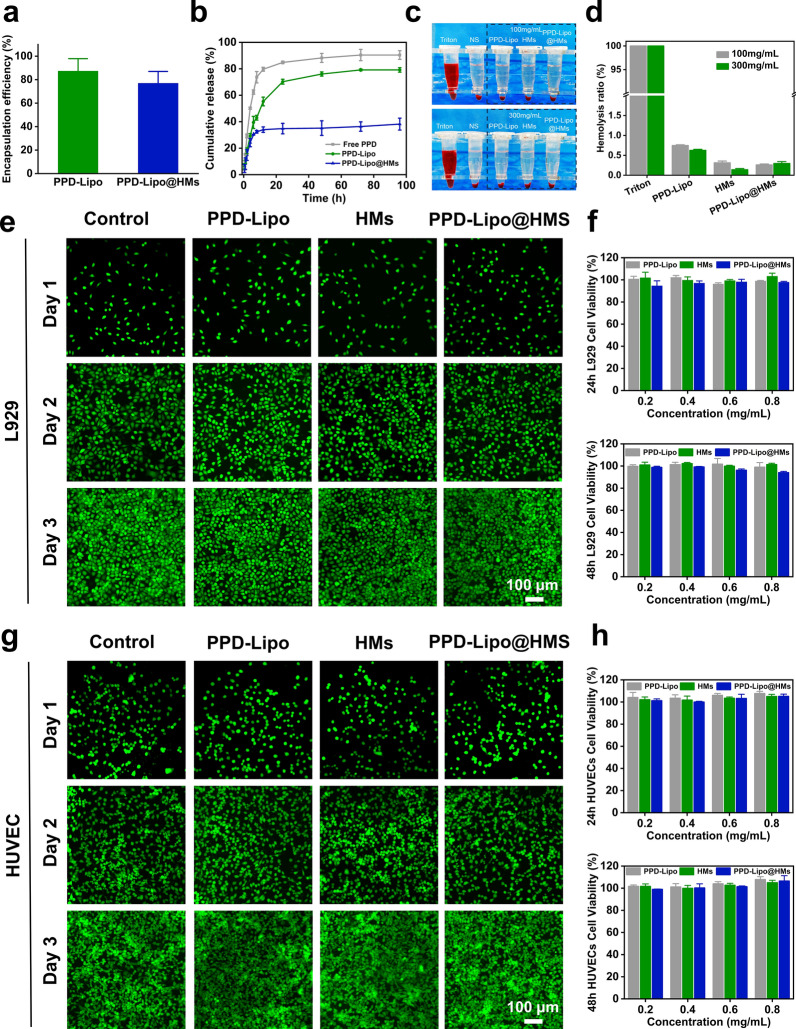
PPD exhibits poor bioavailability, a narrow therapeutic index, and is easily cleared as a water-insoluble drug in the body [51]. The encapsulation of PPD in liposomes can improve its water solubility and enable a slow and sustained release. Furthermore, incorporating these liposomes into HMs can enhance the stability of the drug delivery system, allowing PPD to effectively promote angiogenesis and wound healing. The encapsulation efficiencies of PPD in PPD-Lipo and PPD-Lipo@HMs were measured at 87.38 ± 10.51% and 76.99 ± 10.01%, respectively (Fig. 4a). The slight decrease in encapsulation efficiency observed in PPD-Lipo@HMs may be attributed to drug leakage and the exposure of liposomes during the oil-removing process [33]. Subsequent drug release experiments revealed a biphasic release pattern for PPD loaded in both PPD-Lipo and PPD-Lipo@HMs, characterized by an initial rapid release followed by a sustained slow release. Notably, PPD-Lipo@HMs demonstrated a more sustained drug release compared to free PPD and PPD-Lipo (Fig. 4b), indicating their potential as an effective drug delivery system for controlled PPD release. In the context of diabetic wound treatment, PPD-Lipo@HMs could offer prolonged drug therapy benefits and prevent the rapid clearance of PPD-Lipo in wound settings.
Fig. 4.
In vitro release and biocompatibility of PPD-Lipo@HMs. a Drug encapsulation of PPD-Lipo and PPD-Lipo@HMs. b In vitro drug release of PPD-Lipo and PPD-Lipo@HMs. c Hemolysis images were treated with different concentrations of samples. d Hemolysis rate. e, g Live/Dead staining of L929 and HUVECs co-cultured with different samples (Control, PPD-Lipo, HMs, PPD-Lipo@HMs). Scale bar:100 μm. f, h The cytotoxicity of co-cultured with different samples for 24 h and 48 h (L929 and HUVECs), f L929, h HUVECs. The data are expressed as mean ± SD, n ≥ 3
Hemostasis and hemolysis experiments in vitro
The biocompatibility of tissue engineering materials is crucial for their potential clinical use [47]. The in vitro hemolysis activity of different groups was assessed, with TritonX-100 serving as the positive control and saline as the negative control. The in vitro blood compatibility of PPD-Lipo, HMs, and PPD-Lipo@HMs was tested. The supernatant color of the sample group resembled that of the saline group, while the TritonX-100 positive group exhibited a distinct bright red hemolysis phenomenon (Fig. 4c). Quantitatively, PPD-Lipo, HMs, and PPD-Lipo@HMs all demonstrated lower hemolysis rates (Fig. 4d). Importantly, the hemolysis rates of each group at various concentrations were below the accepted limit of 5%. This suggests that PPD-Lipo and PPD-Lipo@HMs exhibit excellent blood compatibility as materials for wound tissue engineering. In diabetic wounds, managing post-debridement bleeding is a critical challenge, and effective hemostasis is essential for wound healing. Hemostatic powder offers clear advantages for incompressible wounds [47, 52]. In this study, HMs and PPD-Lipo@HMs were freeze-dried to evaluate their hemostatic properties. The hemostatic efficacy of PPD-Lipo@HMs was assessed using a mouse liver bleeding model (Figure S5a), and compared to the HMs group (202.53 ± 50.37 mg) and blank group (97.37 ± 41.11 mg). The blood loss in the PPD-Lipo@HMs group (59.37 ± 15.36 mg) was significantly reduced (Figure S5b, Figure S5c). These findings suggest that PPD-Lipo@HMs can serve as effective hemostatic materials in diabetic wound tissues.
Biocompatibility of PPD-Lipo@HMs
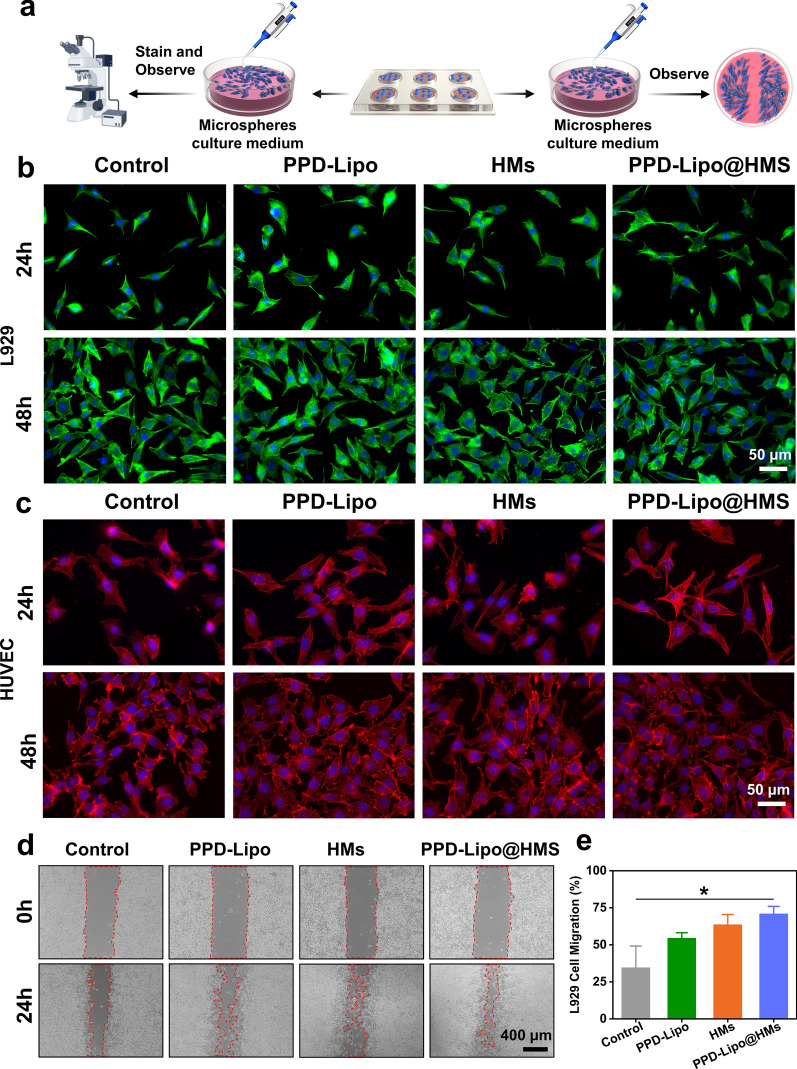
As a wound tissue implant material, tissue-engineered HMs must exhibit good biocompatibility to ensure successful integration [53]. The migration and functional changes of fibroblasts and endothelial cells play a crucial role in the healing process of diabetic skin defects. To assess the biocompatibility of HMs, various tests such as Live/Dead staining, cell counting kit-8 (CCK-8), and skeleton staining were conducted on L929 fibroblasts and HUVEs cells. The experimental groups included a control group, a PPD-Lipo group, an HMs group, and a PPD-Lipo@HMs group, all evaluated using the direct contact method. When L929 fibroblasts and HUVECs cells were co-cultured for 1d, 2d, and 3d, Live/Dead staining revealed good cell morphology and viability across all groups (Fig. 4e and g). Additionally, CCK8 analysis at 24 h and 48 h showed high survival rates of L929 and HUVEC cells, exceeding 90% in all cases (Fig. 4f and h). These results indicated that the PPD-Lipo@HMs met the standard for low-toxicity biomaterials, with no significant differences observed between the experimental groups. Cytoskeleton staining was conducted on co-cultured L929 and HUVEC cells on the 1st and 2nd days to assess proliferation rate and morphology (Fig. 5a), essential for skin wound tissue repair. Cell morphology appeared normal on the 2nd day compared to the 1st day, with no significant changes observed. Filamentous protrusions were present, showing no notable differences between groups (Fig. 5b and c). The results indicate that both L929 and HUVECs cells maintained normal proliferation activity in PPD-Lipo@HMs, laying the groundwork for diabetic wound tissue repair.
Fig. 5.
Cytoskeleton staining and cell scratches of PPD-Lipo@HMs. a A schematic diagram of cytoskeleton staining and L929 cell migration assay. b L929 cytoskeleton staining. Scale bar:50 μm. c HUVECs cytoskeleton staining. Scale bar:50 μm. d L929 cell migration results. Scale bar:400 μm. e Quantitative analysis of L929 migration. The data are expressed as mean ± SD, n ≥ 3. *p < 0.05
Migration and angiogenesis in vitro
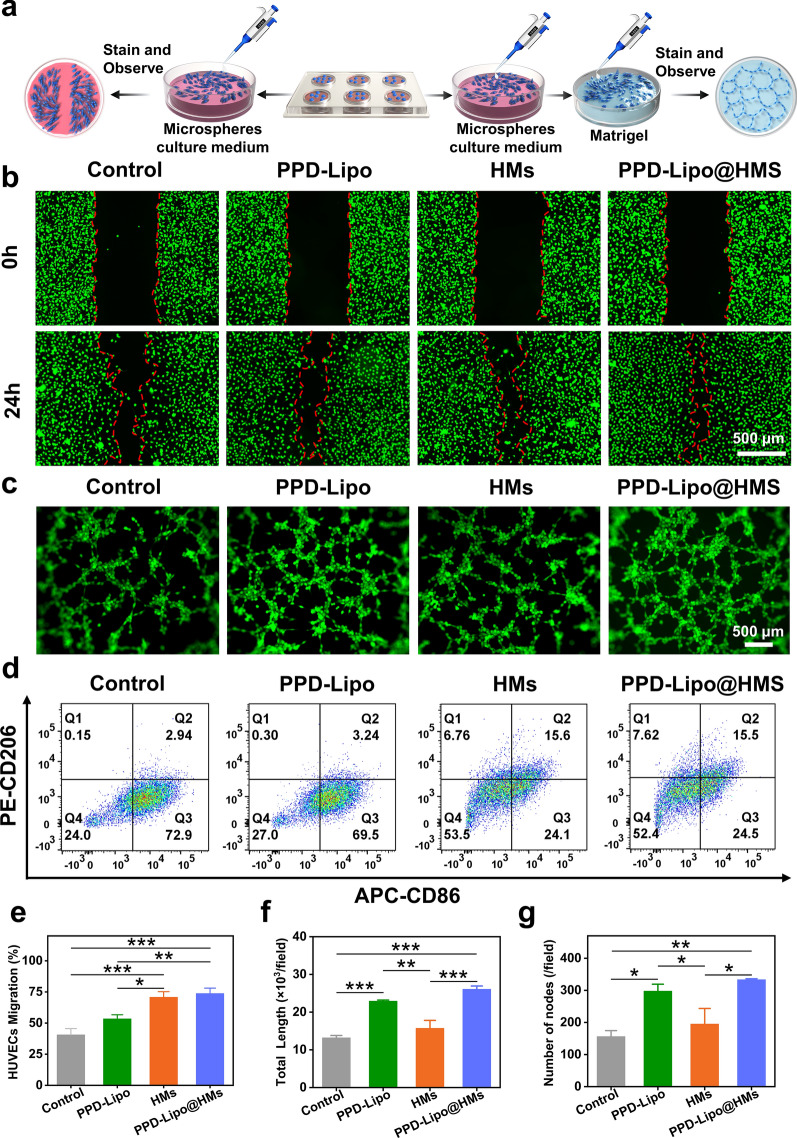
The efficient tissue repair process relies on the development of numerous new blood vessels in the granulation tissue, providing essential nutrients and structural support for extracellular matrix deposition, thereby enhancing wound repair. Angiogenesis dysfunction is a key factor contributing to delayed wound healing in chronic diabetic wound tissues [54]. The migration and proliferation of L929 and HUVEC cells play a crucial role in angiogenesis [4]. To assess the pro-angiogenic effects of different samples, a scratch test and angiogenesis test (Fig. 6a) were conducted on various sample groups: control, PPD-Lipo, HMs, and PPD-Lipo@HMs. Initially, the cell scratch test was performed, revealing enhanced cell migration in the PPD-Lipo, HMs, and PPD-Lipo@HMs groups compared to the control group. Notably, the PPD-Lipo@HMs group exhibited the most significant promotion of cell migration, attributed to the effective release of PPD and the stimulation of BSP (Figs. 5d and 6b). Figures 5e and 6e These dual effects set the stage for PPD-Lipo@HMs to facilitate cell proliferation in angiogenesis. In our study, angiogenesis experiments were conducted in four groups: control group, PPD-Lipo group, HMs group, and PPD-Lipo@HMs group, as illustrated in Fig. 6c. Vascular formation was observed at 4 h, with the control group showing a small number of effective tubes and mostly scattered nodules. In contrast, the PPD-Lipo group, HMs group, and PPD-Lipo@HMs group exhibited lumens formed by HUVECs cells. Vascular nodes and total capillary length were used as metrics to quantify blood vessel formation. The angiogenic effect of the PPD-Lipo group was significantly superior to that of the control and HMs groups. Notably, the PPD-Lipo@HMs group demonstrated the most favorable outcome, possibly due to the accelerated lumen formation induced by PPD. Our results indicate that the PPD-Lipo@HMs group exhibited more prominent blood vessel formation and higher vascularization levels compared to other groups (Fig. 6f and g).
Fig. 6.
Cell scratch, tube formation, and macrophage polarization. a Cell scratch and tube formation experiment schematic diagram. b HUVECs wound scratch healing measurement images of different groups. Scale bar: 500 μm. c The tube formation of HUVECs on matrigel after incubation with different groups for 4 h. Scale bar: 500 μm. d The expression of CD206 and CD86 was analyzed by flow cytometry. RAW264.7 cells were treated with PBS, PPD-Lipo, HMs, and PPD-Lipo@HMs, respectively. e Quantitative analysis of HUVEC migration. f The total length of capillaries in randomly selected regions. g The number of nodes. The data are expressed as mean ± SD, n ≥ 3. *p < 0.05, **p < 0.01, ***p < 0.001
Macrophage polarization
In the context of diabetic wound tissue repair, macrophages play a crucial role in immune regulation. Initially, M1-like macrophages drive the inflammatory response by releasing pro-inflammatory factors like tumor necrosis factor (TNF-a) and interleukin-1 (IL-1) to eliminate pathogens and necrotic tissue in the wound. Subsequently, as the inflammatory phase resolves, macrophages transition to the M2-like phenotype, dampening inflammation and promoting tissue repair by releasing anti-inflammatory factors such as IL-10 [55]. However, in diabetic wound healing, the shift from M1-like to M2-like phenotype is impaired, leading to prolonged inflammation and hindered healing [7]. To investigate the anti-inflammatory properties of PPD-Lipo@HMs and their impact on macrophage polarization, RAW264.7 cells were used as a macrophage model, with lipopolysaccharide (LPS) inducing M1-like polarization. CD86 and CD206 were employed as M1-like and M2-like markers to assess inflammation in diabetic wounds, and flow cytometry was utilized to analyze the effect of PPD-Lipo@HMs on macrophage polarization. Following 24-hour incubation with LPS, the majority of macrophages adopted the M1-like subtype, mimicking the immune environment of diabetic wounds. The proportions of M2-like macrophages treated with PBS, PPD-Lipo, HMs, and PPD-Lipo@HMs were 0.15, 0.30, 6.76, and 7.62(Fig. 6d), respectively. Notably, PPD-Lipo@HMs exhibited the highest proportion of M2-like macrophages indicating that this construct (Figure S6a and b), derived from the natural polysaccharide BSP, could modulate macrophage phenotype transformation to the M2-like subtype and reduce inflammation duration.
In vivo skin tissue regeneration of diabetic rats
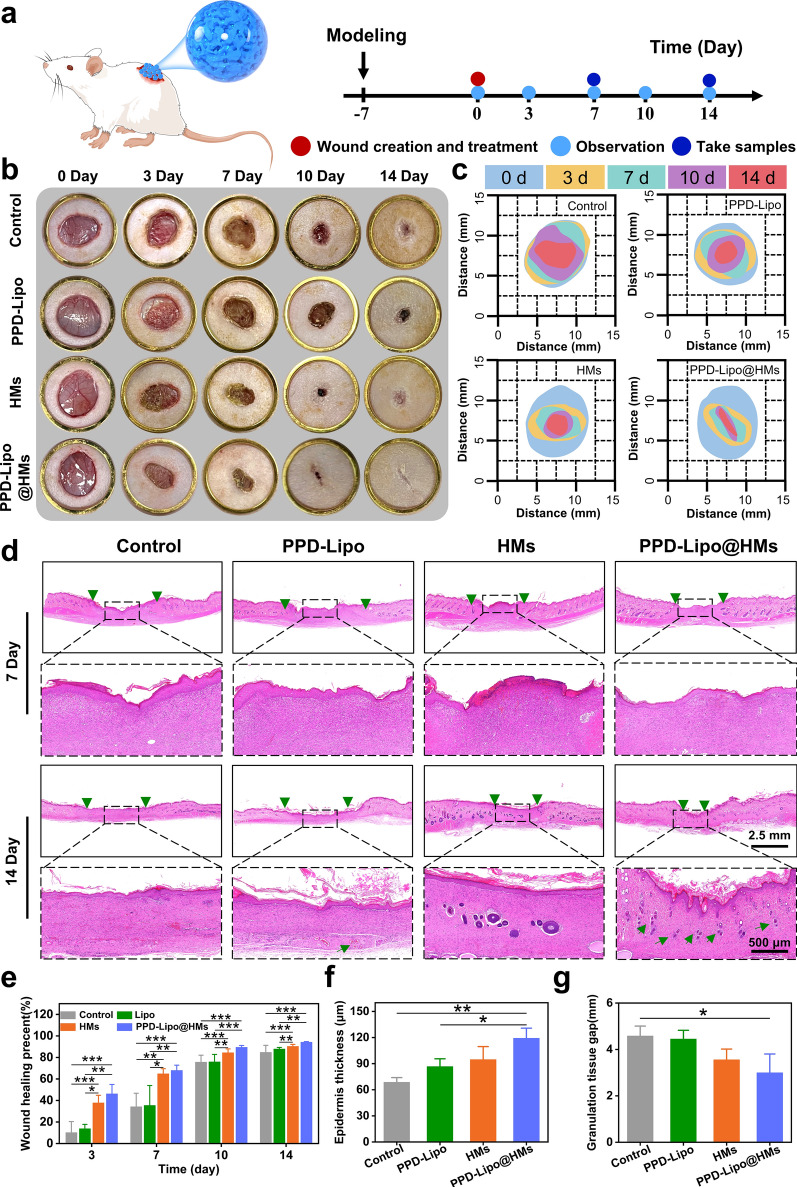
Early studies have demonstrated that HMs and PPD-Lipo@HMs exhibit good shape adjustability and biocompatibility. These materials have been shown to promote the proliferation and migration of fibroblasts and endothelial cells, stimulate angiogenesis, and regulate macrophage phenotype transformation. In this study, a rat model of type I diabetes was successfully established through intraperitoneal injection of STZ. After one week, blood glucose levels were measured via tail vein blood collection, all of which were found to be greater than 16.67 mmol/L (Figure S7). Subsequently, an (8 mm) full-thickness skin injury model was created on the rats’ backs, and they were treated with gauze (Control), PPD-Lipo, HMs, and PPD-Lipo@HMs respectively to investigate how hydrogel microspheres promote wound tissues repair in diabetic rats.
Photographs of the treated wounds were taken on days 0, 3, 7, 10, and 14 to assess healing progress (Fig. 7a). Analysis in Fig. 7b revealed that by day 7, wounds in the HMs group and PPD-Lipo@HMs group exhibited significantly faster healing compared to the PPD-Lipo group and control group. By day 14, wounds in the PPD-Lipo@HMs group showed nearly complete healing with minimal scarring, while the control group still displayed persistent inflammation and dark scabs. Furthermore, a wound closure simulation generated from digital images (Fig. 7C) demonstrated that the HMs group and PPD-Lipo@HMs group had a notably accelerated healing rate compared to the control group. Specifically, the average wound healing areas on the 7th day were 64.84% and 67.99% for the HMs group and PPD-Lipo@HMs group, respectively, higher than the control group (34.27%) and PPD-Lipo group (35.42%). This enhanced healing can be attributed to the PPD-Lipo@HMs derived from BSP, which facilitate the transformation of macrophages to M2-like phenotype, thereby reducing early inflammatory responses and promoting wound closure. By day 14, wounds treated with PPD-Lipo@HMs displayed the highest healing percentage at 94.22%, surpassing the HMs (90.33%), PPD-Lipo (87.84%), and control (84.76%) groups (Fig. 7E).
Fig. 7.
PPD-Lipo@HMs promotes wound healing in a full-thickness skin injury model of diabetic patients. a A schematic diagram of PPD-Lipo@HMs for accelerating wound healing. b Representative pictures of wound healing in different treatment groups on days 0,3,7,10 and 14. c The schematic diagram of the wound healing process. d Representative images of skin tissue sections stained with H&E on days 7 and 14. Scales bar:2.5 mm and 500 μm. Two green triangles represent granulation tissue spaces, and green arrows represent newly formed blood vessels. e Quantitative analysis of wound healing area at different time points. f Quantitative analysis of granulation tissue gap on day 14. g Quantitative analysis of the epidermal thickness of wound healing on day 14. The data are expressed as mean ± SD, n ≥ 3. *p < 0.05, **p < 0.01, ***p < 0.001
On days 7 and 14, we assessed wound bed re-filling, granulation tissue formation, and epithelial re-formation through H&E staining. By the 14th day, we observed a gap in the granulation tissue, marked by two green triangles, with notable differences among the four groups (Fig. 7d). Granulation tissue, comprising fibroblasts, macrophages, matrix proteins, and neovascularization, plays a crucial role in early wound healing stages. Quantitatively, the PPD-Lipo@HMs group exhibited the smallest tissue gap at 3.00 ± 0.80 mm, outperforming the other groups (Fig. 7g). By day 7, small gaps existed between granulation tissue and surrounding tissue in all groups, but by day 14, these gaps had closed in the HMs and PPD-Lipo@HMs groups, indicating strong tissue adhesion. Angiogenesis is vital for wound healing as it enhances oxygen and cytokine delivery, accelerating regeneration. Skin re-epithelialization acts as a protective barrier against infections and water loss. Notably, skin thickness in the PPD-Lipo@HMs group was greater than in other groups (Fig. 7f). Overall, the PPD-Lipo@HMs group demonstrated superior wound healing performance with minimal tissue gaps, strong tissue adhesion, and significant angiogenesis, leading to rapid and effective healing.
Histopathologic evaluations of collagen deposition, neovascularization, and inflammation microenvironment
Collagen, as the primary extracellular matrix in the skin, plays a crucial role in wound healing [56]. Masson trichrome staining was utilized to assess collagen deposition and organization, particularly type I collagen, which enhances wound tensile strength in the initial stages to offer structural support. On day 7, the Control group exhibited a lower fraction of collagen deposition compared to the PPD-Lipo@HMs group, which displayed superior performance in stimulating collagen deposition (Fig. 8c). By the 14th day, the PPD-Lipo@HMs group demonstrated a histological structure resembling healthy rat skin, complete with discernible hair follicles and blood vessels, while the control group exhibited fewer and less organized collagen fibers (Fig. 8a). The collagen deposition fractions for the Control, PPD-Lipo, HMs, and PPD-Lipo@HMs groups were 37.73 ± 3.47%, 39.23 ± 1.07%, 47.70 ± 10.69%, and 56.30 ± 3.64%, respectively (Fig. 8d). These findings suggest that PPD-Lipo@HMs can notably expedite collagen deposition at the wound site.
Fig. 8.
In vivo study of PPD-Lipo@HMs promoting collagen deposition and angiogenesis in diabetic wounds. a Representative images of Masson trichrome staining of wounds treated in different groups on days 7 and 14. Scales bar:2.5 mm and 500 μm. b Representative images of a-SMA and CD31 immunofluorescence on day 7. Scale bar:200 μm. c Collagen deposition density on day 7 in different groups. d 14 collagen deposition density in different groups. e Immunofluorescence quantitative analysis of a-SMA. f Immunofluorescence quantitative analysis of CD31. The data are expressed as mean ± SD, n ≥ 3.* p < 0.05, ** p < 0.01, *** p < 0.001.
In addition to collagen deposition, angiogenesis plays a crucial role in the healing of diabetic wounds by providing essential nutrition through the formation of new blood vessels in the granulation tissue [5]. To confirm the ability of PPD-Lipo@HMs to promote angiogenesis in wound tissue, the expression of smooth muscle actin protein (a-SMA), a marker for angiogenesis in diabetic wounds, was assessed using immunofluorescence. The results depicted in Fig. 8b revealed low positive staining in the control group, indicating minimal blood vessel formation, whereas both the PPD-Lipo group and PPD-Lipo@HMs group exhibited significant positive staining. Notably, the expression of a-SMA was significantly higher in the PPD-Lipo group compared to the HMs group, demonstrating the ability of PPD to enhance a-SMA expression and promote angiogenesis. Moreover, the vascular density in the PPD-Lipo@HMs group was notably higher than in the other groups, suggesting that continuous release of PPD from PPD-Lipo@HMs stimulated the production of vascular growth factors leading to increased angiogenesis (Fig. 8e). CD31, an intercellular adhesion molecule found on endothelial cells, plays a crucial role in vascular formation and stability. Immunofluorescence analysis on the 7th day post-wound repair showed significantly higher expression of CD31 in the healing skin of the PPD-Lipo group and PPD-Lipo@HMs group compared to the Ctrl group and HMs group (Fig. 8f). This observation aligns with the a-SMA results, indicating that PPD effectively enhances early vascularization in diabetic wounds when released by PPD-Lipo@HMs, facilitating the transport of oxygen and nutrients to the wound site and accelerating the healing process.
In the process of diabetic wound tissue repair, chronic inflammation of the wound hinders the healing process. Modulating the immune microenvironment of the wound is crucial for enhancing wound tissue repair [57]. Studies have demonstrated that BSP can target macrophage mannose receptors, prompting M1-like macrophages to transition into M2-like macrophages [7, 41, 42]. This transition leads to a significant decrease in wound inflammation levels and ultimately accelerates wound tissue repair. To assess the extent of wound inflammation post-treatment in each group, the expression of CD86 and CD206 markers associated with M1-like and M2-like macrophages was examined through immunofluorescence. The control group exhibited elevated CD86-positive cell expression (red fluorescence) on the 7th day of wound healing (Fig. 9c), whereas the HMs group and PPD-Lipo@HMs group displayed more pronounced CD206-positive cell expression (green fluorescence) in the subcutaneous layer of the wound area (Fig. 9a). Moreover, compared to the HMs group, the PPD-Lipo@HMs group exhibited a more noticeable expression of CD206-positive cells, indicating a greater induction of macrophages towards the M2-like phenotype (Fig. 9d). MPO serves as both a functional marker of neutrophils and an indicator of neutrophil activation, making it a useful marker for assessing wound inflammation. Following treatment, MPO expression was analyzed through immunohistochemistry to evaluate the inflammatory response across different groups. The control group demonstrated significantly higher MPO expression compared to the other treatment groups. The PPD-lipo group showed slightly elevated levels, while the HMs group and PPD-Lipo@HMs group exhibited lower expression (Fig. 9b and e), suggesting that the control group remained in an inflammatory stage, whereas the PPD-Lipo@HMs group effectively mitigated the inflammatory response at the wound site, thereby expediting wound tissues repair.
Fig. 9.
Effect of PPD-Lipo@HMs on the inflammatory microenvironment of defective skin. a Representative images of CD86 and CD206 immunofluorescence on day 7. Scale bar: 200 μm. b Representative images of MPO immunohistochemistry in 10× and 40× fields on the 7th day. Scale bar: 300 μm and 75 μm. c The quantification for CD86 density from different groups. d Quantitative analysis of CD206 immunofluorescence density in different groups. e Quantitative analysis of MPO immunohistochemistry in different groups. The data are expressed as mean ± SD. n ≥ 3. * p < 0.05, ** p < 0.01, *** p < 0.001
The PPD-Lipo@HMs, consisting of the natural active ingredient PPD and natural polysaccharides, are fabricated using microfluidic technology and a photocrosslinking method. These PPD-Lipo@HMs exhibit remarkable swelling properties that can effectively enhance blood vessel formation and macrophage polarization, ultimately facilitating the healing of diabetic wounds.
Conclusion
In this study, a novel type of natural nanocomposite hydrogel microspheres utilizing BSP as the matrix material was developed. The precise manipulation of microchannel fluid via microfluidic engineering and photocrosslinking technologies, along with adjustments in fluid velocity and matrix material concentration, enabled the accurate control of size, specific surface area, and physical properties of the PPD-Lipo@HMs. Compared to conventional hydrogels, these PPD-Lipo@HMs exhibit a microporous structure that facilitates cell and blood vessel infiltration and proliferation within the interstices of the PPD-Lipo@HMs, laying the groundwork for subsequent tissue regeneration. By loading natural active ingredient PPD with lipid nanostructures, the PPD-Lipo@HMs stimulate angiogenesis through continuous PPD release, significantly expediting the tissue repair process in diabetic conditions. By utilizing Chinese herbal medicine BSP as the matrix material, the PPD-Lipo@HMs were evaluated for their ability to modulate the immune microenvironment both in vitro and in vivo. Results demonstrate that the PPD-Lipo@HMs efficiently fill damaged tissues, promoting cell infiltration, diffusion, and proliferation, while also coordinating angiogenesis and immune macrophage polarization, ultimately enhancing the regeneration and repair of diabetic-damaged tissues. This innovative research amalgamates microfluidic engineering technology with traditional Chinese medicine materials, presenting a sustainable, cost-effective, and scalable platform technology for the production of new natural nano-micro composite materials, and offering a novel approach for the personalized customization of composite biomaterials.
Supplementary Information
Acknowledgements
Thanks to Jiayi Sun at Innovative Institute of Chinese Medicine and Pharmacy, Chengdu University of Traditional Chinese Medicine, for the assistance with laser confocal scanning microscopy.
Author contributions
Peng Guo: Writing–review & editing, Methodology, Investigation, Formal analysis, Data curation. Pengkun Lei: Methodology, Investigation, Formal analysis, Data curation. Lin Luo: Investigation, Conceptualization. Qin Yang: Investigation, Data curation. Qiaolin Yang: Data curation, Conceptualization. Ya Tian: Investigation, Conceptualization. Wen Shi: Investigation, Conceptualization. Yuchun Liu: Investigation, Conceptualization. Rui Zeng: Investigation, Conceptualization. Yunxia Li: Data curation, Conceptualization. Yan Qu: Writing–review &editing, Supervision, Methodology, Funding acquisition, Conceptualization. Chen Zhang: Writing–review & editing, Supervision, Funding acquisition, Conceptualization.
Funding
This work was supported by the National Natural Science Foundation of China (82374303), Natural Science Foundation of Sichuan Province (2023YFS0348), China Postdoctoral Science Foundation (2021M690488, 2023T160071).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine (SYXK2024-092).
Consent for publication
All authors read and agreed to submit the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Qu, Email: quyan028@126.com.
Chen Zhang, Email: chenzhang_1990@126.com.
References
- 1.Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest 2021. 10.1172/JCI142243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and their recurrence. N Engl J Med. 2017;376:2367–75. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Zhang X, Zhang J, Xu Q, Yu X, Xu A, Yi C, Bian X, Shao S. Recent advances in the adjunctive management of diabetic foot ulcer: focus on noninvasive technologies. Med Res Rev. 2024;44:1501. [DOI] [PubMed] [Google Scholar]
- 4.Pena OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:599. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Lin Z, Bu P, Yu T, Endo Y, Zhou W, Sun Y, Cao F, Dai G, Hu Y, et al. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes Diabetic Wound Healing. Adv Mater. 2023;35:e2212300. [DOI] [PubMed] [Google Scholar]
- 6.Han X, Chen S, Cai Z, Zhu Y, Yi W, Guan M, Liao B, Zhang Y, Shen J, Cui W, Bai D. A Diagnostic and Therapeutic Hydrogel to Promote Vascularization via Blood Sugar reduction for Wound Healing. Adv Funct Mater 2023;33:2213008. [Google Scholar]
- 7.Xu N, Gao Y, Li Z, Chen Y, Liu M, Jia J, Zeng R, Luo G, Li J, Yu Y. Immunoregulatory hydrogel decorated with tannic acid/ferric ion accelerates diabetic wound healing via regulating Macrophage polarization. Chem Eng J. 2023;466:143173. [Google Scholar]
- 8.Wen X, Xi K, Tang Y, Bian J, Qin Y, Xiao W, Pan T, Cheng X, Ge Z, Cui W. Immunized microspheres Engineered Hydrogel membrane for reprogramming macrophage and mucosal repair. Small. 2023;19:e2207030. [DOI] [PubMed] [Google Scholar]
- 9.Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Trans Med. 2021;13(585):eabe4839. [DOI] [PubMed] [Google Scholar]
- 10.Zhi Y, Che J, Zhu H, Liu R, Zhao Y. Glycyrrhetinic acid liposomes encapsulated microcapsules from microfluidic electrospray for inflammatory wound healing. Adv Funct Mater. 2023;33:2304353. [Google Scholar]
- 11.Zhou L, Liu F, You J, Zhou B, Guo W, Qu W, Ren X, Gao G. A novel self-pumping janus dressing for promoting wound immunomodulation and diabetic wound healing. Adv Healthc Mater. 2024;13:e2303460. [DOI] [PubMed] [Google Scholar]
- 12.Shao Z, Yin T, Jiang J, He Y, Xiang T, Zhou S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioactive Mater. 2023;20:561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Li F, Shao W, Gao J, Ling D. Promoting angiogenesis in oxidative diabetic wound microenvironment using a nanozyme-reinforced self-protecting hydrogel. ACS Cent Sci. 2019;5:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15:12687–722. [DOI] [PubMed] [Google Scholar]
- 15.Correa S, Grosskopf AK, Lopez Hernandez H, Chan D, Yu AC, Stapleton LM, Appel EA. Translational applications of hydrogels. Chem Rev. 2021;121:11385–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Liu S, Zhang J, Wang Y, Lu H, Zhang Y, Song G, Niu F, Shen Y, Midgley AC, et al. Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration. Nat Commun. 2024;15:1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi F, Farbehi N, Ziaee F, Lau K, Monfared M, Kordanovski M, Joukhdar H, Molly TG, Nordon R, Kilian KA, et al. Photocrosslinked silk fibroin microgel scaffolds for biomedical applications. Adv Funct Mater. 2024. 10.1002/adfm.202313354. [Google Scholar]
- 18.Daly AC, Riley L, Segura T, Burdick JA. Hydrogel microparticles for biomedical applications. Nat Rev Mater. 2020;5:20–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caprio ND, Davidson MD, Daly AC, Burdick JA. Injectable MSC Spheroid and Microgel Granular composites for Engineering tissue. Adv Mater. 2024;36:e2312226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X, Zhang L, Luo Y, Cai Z, Zeng H, Wang T, Liu Z, Chen Y, Sheng X, Mandlate AEG, et al. Charge-driven self‐assembled microspheres hydrogel scaffolds for combined drug delivery and photothermal therapy of diabetic wounds. Adv Funct Mater. 2023;33:2214036. [Google Scholar]
- 21.Li Q, Ma C, Jing Y, Liu X. Multifunctional nanofibrous hollow microspheres for enhanced periodontal bone regeneration. Adv Sci. 2024. 10.1002/advs.202402335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Gu H, Zhang H, Xian J, Li J, Fu C, Zhang C, Zhang J. Oral core–Shell nanoparticles embedded in Hydrogel microspheres for the efficient site-specific delivery of Magnolol and enhanced Antiulcerative Colitis Therapy. ACS Appl Mater Interfaces. 2021;13:33948–61. [DOI] [PubMed] [Google Scholar]
- 23.Helgeson ME, Chapin SC, Doyle PS. Hydrogel microparticles from lithographic processes: novel materials for fundamental and applied colloid science. Curr Opin Colloid Interface Sci. 2011;16:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, Li G, Ruan H, Chen K, Cai Z, Lu G, Li R, Deng L, Cai M, Cui W. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021;15:13041–54. [DOI] [PubMed] [Google Scholar]
- 25.Nunes JK, Stone HA. Introduction: microfluidics. Chem Rev. 2022;122:6919–20. [DOI] [PubMed] [Google Scholar]
- 26.Yeo LY, Chang HC, Chan PPY, Friend JR. Microfluidic devices for Bioapplications. Small. 2010;7:12–48. [DOI] [PubMed] [Google Scholar]
- 27.Marre S, Jensen KF. Synthesis of micro and nanostructures in microfluidic systems. Chem Soc Rev. 2010;39:1183. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell AS, Aguado BA, Anseth KS. Designing microgels for cell culture and controlled assembly of tissue microenvironments. Adv Funct Mater. 2020;30:1907670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin DR, Archang MM, Kuan CH, Weaver WM, Weinstein JS, Feng AC, Ruccia A, Sideris E, Ragkousis V, Koh J, et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat Mater. 2021;20:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q, Chang B, Dong H, Liu X. Functional microspheres for tissue regeneration. Bioact Mater. 2023;25:485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin S, Dai J, Gregory CA, Han A, Alge DL. Creating physicochemical gradients in modular microporous annealed particle hydrogels via a microfluidic method. Adv Funct Mater. 2020;30:1907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Zhu H, Che J, Xu Y, Tan Q, Zhao Y. Stem cell niche-inspired microcarriers with ADSCs encapsulation for diabetic wound treatment. Bioact Mater. 2023;26:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Zhu Y, Wang F, Deng L, Xu X, Cui W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. Chem Eng J. 2020;400:126004. [Google Scholar]
- 34.Li S, Zheng W, Deng W, Li Z, Yang J, Zhang H, Dai Z, Su W, Yan Z, Xue W, et al. Logic-based strategy for spatiotemporal release of dual extracellular vesicles in osteoarthritis treatment. Adv Sci. 2024. 10.1002/advs.202403227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y, Wei G, Deng L, Cui W. Visualizable and lubricating hydrogel microspheres Via NanoPOSS for cartilage regeneration. Adv Sci (Weinh). 2023;10:e2207438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng D, Ding R, Jin X, Lu Y, Bao W, Zhao Y, Chen S, Shen C, Yang Q, Wang Y. Strontium ion-functionalized nano-hydroxyapatite/chitosan composite microspheres promote osteogenesis and angiogenesis for bone regeneration. ACS Appl Mater Interfaces. 2023;15:19951–65. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Han X, Cao Y, Yi W, Zhu Y, Ding X, Li K, Shen J, Cui W, Bai D. Spatiotemporalized hydrogel microspheres promote vascularized osteogenesis via ultrasound oxygen delivery. Adv Funct Mater. 2023;34:2308205. [Google Scholar]
- 38.Wang X, Jia J, Niu M, Li W, Zhao Y. Living Chinese herbal scaffolds from microfluidic bioprinting for wound healing. Res (Wash D C). 2023;6:0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Sun Q, Li Y, Xu R, Li R, Wu D, Huang R, Yang Z, Li Y. Hydrogel encapsulating wormwood essential oil with broad-spectrum antibacterial and immunomodulatory properties for infected diabetic wound healing. Adv Sci (Weinh). 2024;11:e2305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue X, Zhao S, Qiu M, Zhang J, Zhong G, Huang C, Li X, Zhang C, Qu Y. Physical dual-network photothermal antibacterial multifunctional hydrogel adhesive for wound healing of drug-resistant bacterial infections synthesized from natural polysaccharides. Carbohydr Polym. 2023;312:120831. [DOI] [PubMed] [Google Scholar]
- 41.Qiu M, Zhong G, Zhang J, Hou Y, Duan Y, Guo P, Jiang F, Gou K, Zhang C, Qu Y. Biocompatible and biodegradable Bletilla striata polysaccharides hydrogels crosslinked by BDDE for wound healing through the regulating of macrophage polarization. Int J Biol Macromol. 2024;254:128015. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Qu M, Wang C, Xue Y, Huang H, Chen Q, Sun W, Zhou X, Xu G, Jiang X. A dual-cross-linked hydrogel patch for promoting diabetic wound healing. Small. 2022;18:e2106172. [DOI] [PubMed] [Google Scholar]
- 43.Zhong G, Lei P, Guo P, Yang Q, Duan Y, Zhang J, Qiu M, Gou K, Zhang C, Qu Y, Zeng R. A photo-induced cross-linking enhanced A and B combined multi-functional spray hydrogel instantly protects and promotes of irregular dynamic wound healing. Small. 2024;20:e2309568. [DOI] [PubMed] [Google Scholar]
- 44.Hou M, Wang R, Zhao S, Wang Z. Ginsenosides in Panax Genus and their biosynthesis. Acta Pharm Sin B. 2021;11:1813–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang E-Y, Gao B, Shi H-L, Huang L-F, Yang L, Wu X-J, Wang Z-T. 20(S)-Protopanaxadiol enhances angiogenesis via HIF-1α-mediated VEGF secretion by activating p70S6 kinase and benefits wound healing in genetically diabetic mice. Exp Mol Med. 2017;49:e387–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C, Gou K, Yue X, Zhao S, Zeng R, Qu Y, Zhang C. A novel hyaluronic acid-based dissolving microneedle patch loaded with ginsenoside Rg3 liposome for effectively alleviate psoriasis. Mater Design. 2022;224:111363. [Google Scholar]
- 47.Zhang K, Xian Y, Li M, Pan Z, Zhu Z, Yang Y, Wang H, Zhang L, Zhang C, Wu D. Gelable and adhesive powder for lethal non-compressible hemorrhage control. Adv Funct Mater. 2023;33:2305222. [Google Scholar]
- 48.Zhang X, Li S, Dong Y, Rong H, Zhao J, Hu H. A multifunctional cholesterol-free liposomal platform based on protopanaxadiol for alopecia therapy. Nano Res. 2022;15:9498–510. [Google Scholar]
- 49.Moragues T, Arguijo D, Beneyton T, Modavi C, Simutis K, Abate AR, Baret J-C, deMello AJ, Densmore D, Griffiths AD. Droplet-based microfluidics. Nat Reviews Methods Primers. 2023;3:32. [Google Scholar]
- 50.Lei Y, Wang Y, Shen J, Cai Z, Zeng Y, Zhao P, Liao J, Lian C, Hu N, Luo X, et al. Stem cell-recruiting injectable microgels for repairing osteoarthritis. Adv Funct Mater. 2021;31:2105084. [Google Scholar]
- 51.Kim KT, Kim MH, Park JH, Lee JY, Cho HJ, Yoon IS, Kim DD. Microemulsion-based hydrogels for enhancing epidermal/dermal deposition of topically administered 20(S)-protopanaxadiol: in vitro and in vivo evaluation studies. J Ginseng Res. 2018;42:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Z, Tian W, Wen C, Ji X, Diao H, Hou Y, Fan J, Liu Z, Ji T, Sun F, et al. Cellulose-based cryogel microspheres with nanoporous and controllable wrinkled morphologies for rapid hemostasis. Nano Lett. 2022;22:6350–8. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Lin J, Chen L, Deng L, Cui W. Endogenous electric-field-coupled electrospun short fiber via collecting wound exudation. Adv Mater. 2022;34:e2108325. [DOI] [PubMed] [Google Scholar]
- 54.Weng W, Chi J, Yu Y, Zhang C, Shi K, Zhao Y. Multifunctional composite inverse opal film with multiactives for wound healing. ACS Appl Mater Interfaces. 2021;13:4567–73. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Wu Y, Gong H, Xiong Y, Chen Y, Li L, Zhi B, Lv S, Peng T, Zhang H. A multifunctional herb-derived glycopeptide hydrogel for chronic wound healing. Small. 2024. 10.1002/smll.202400516. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S, Rai VK, Narang RK, Markandeywar TS. Collagen-based formulations for wound healing: a literature review. Life Sci. 2022;290:120096. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Z, Deng T, Tao M, Lin L, Sun L, Song X, Gao D, Li J, Wang Z, Wang X, et al. Snail-inspired AFG/GelMA hydrogel accelerates diabetic wound healing via inflammatory cytokines suppression and macrophage polarization. Biomaterials. 2023;299:122141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.