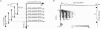Abstract
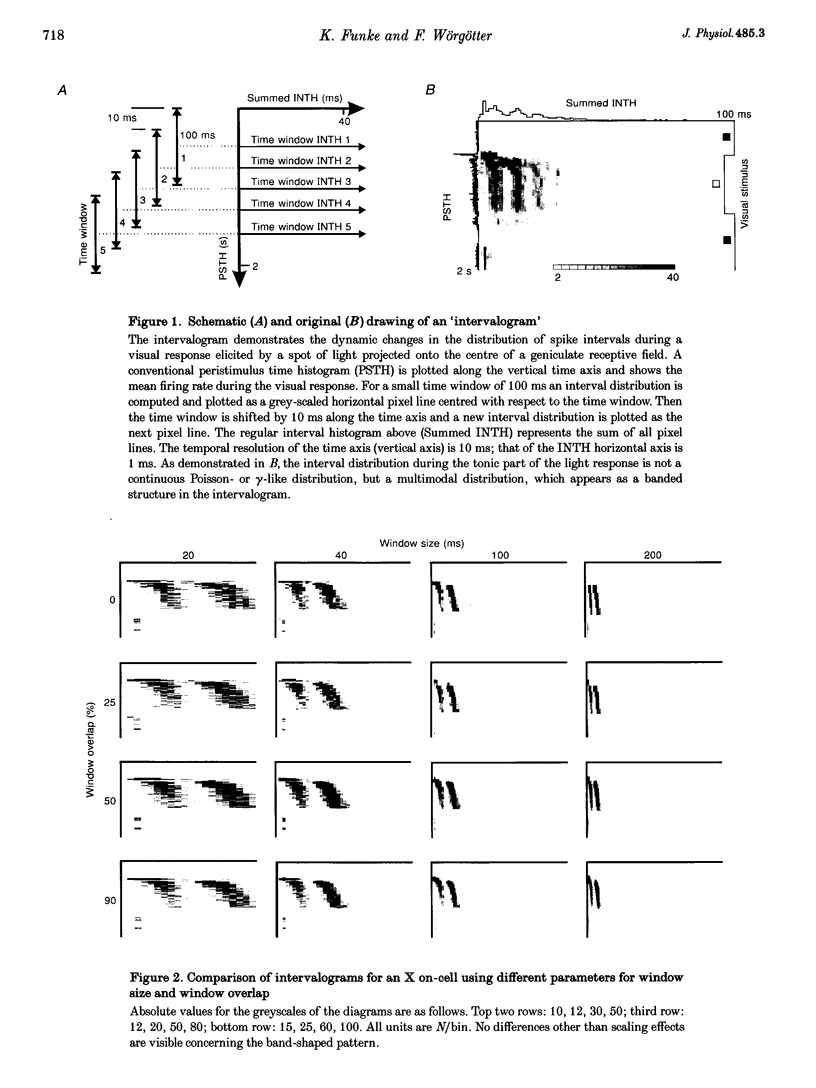
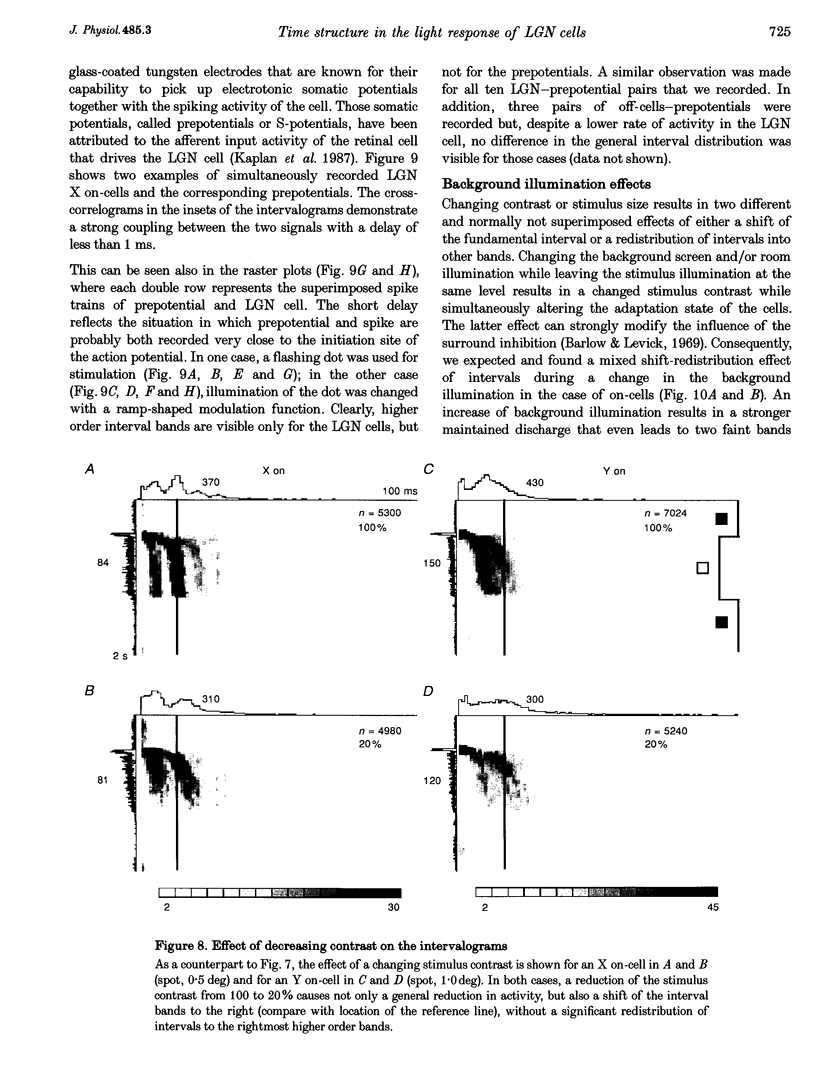
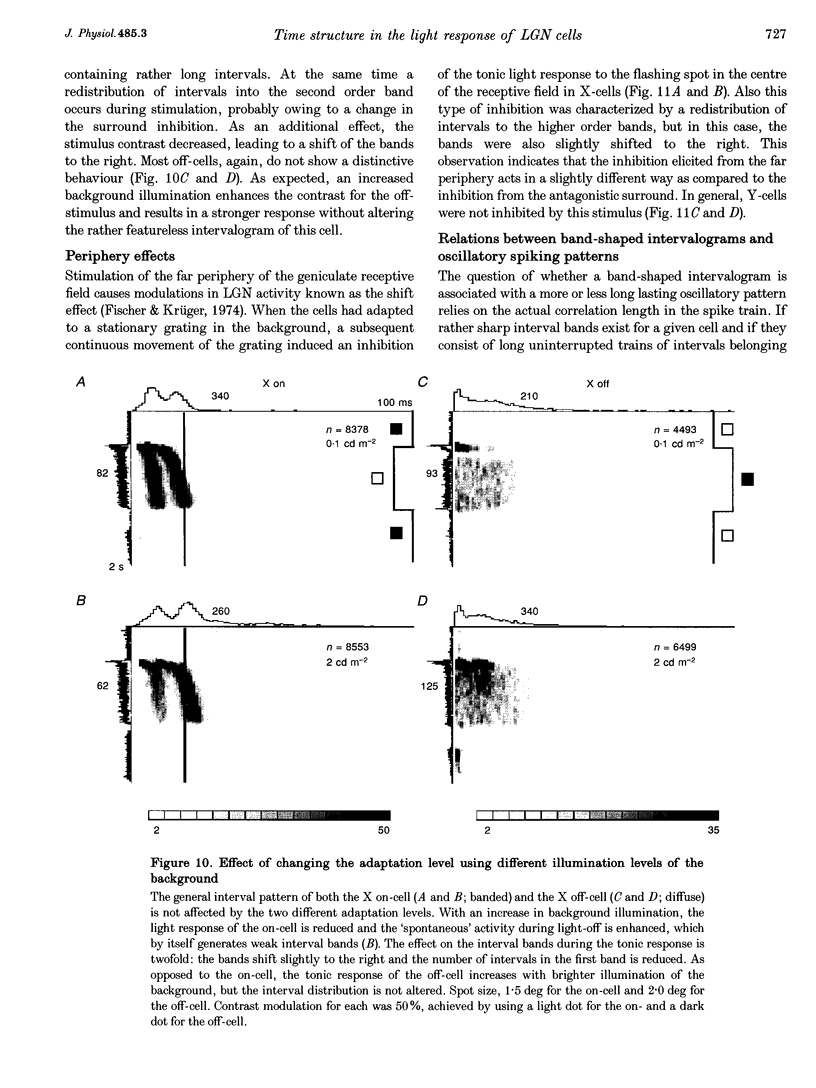
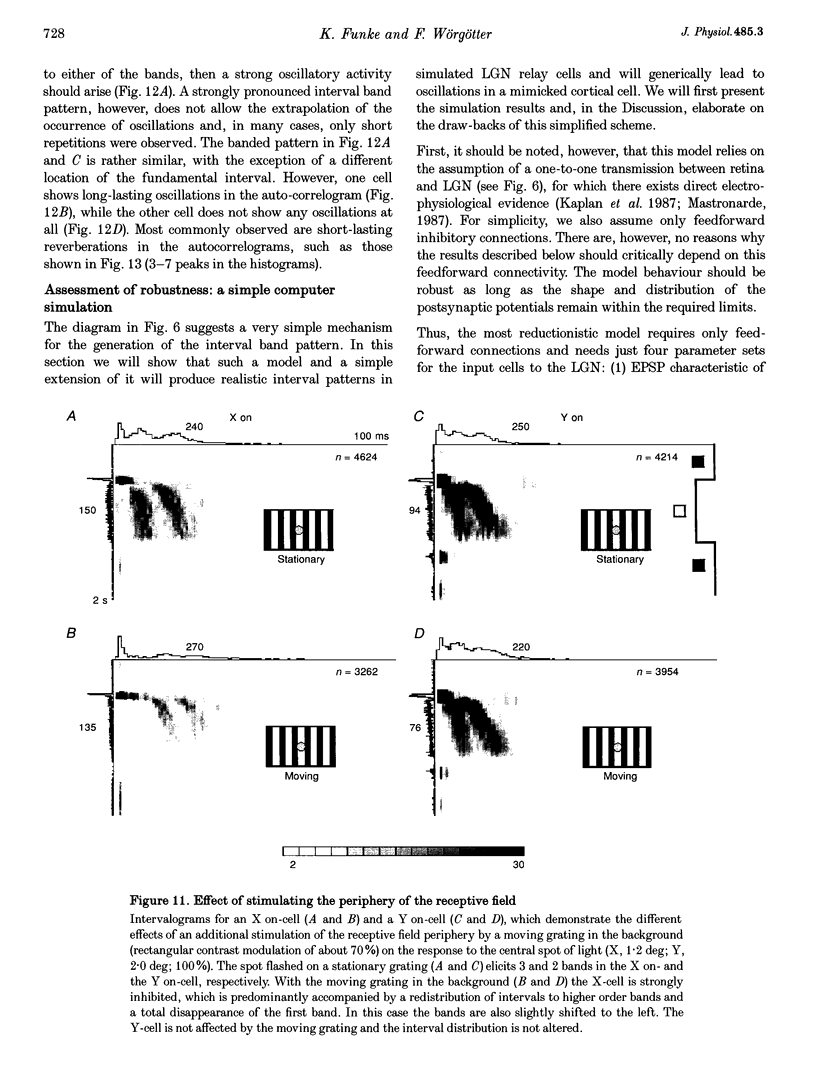
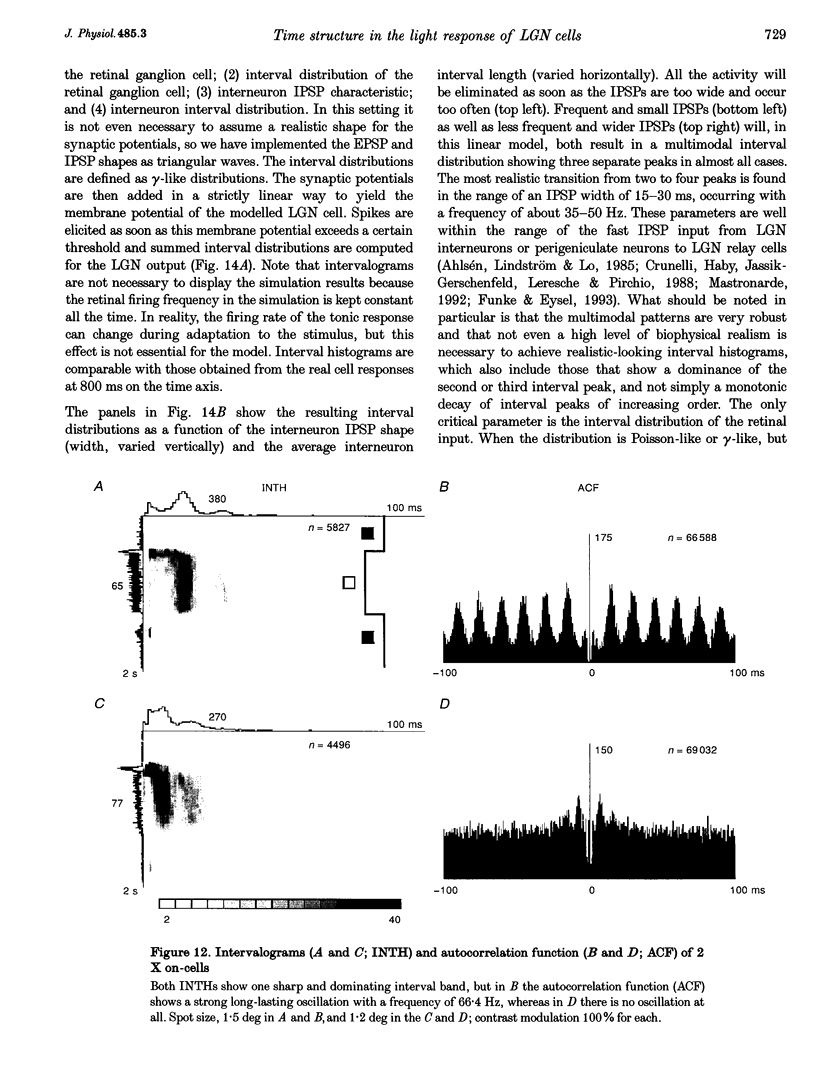
1. The spike interval pattern during the light responses of 155 on- and 81 off-centre cells of the dorsal lateral geniculate nucleus (LGN) was studied in anaesthetized and paralysed cats by the use of a novel analysis. Temporally localized interval distributions were computed from a 100 ms time window, which was shifted along the time axis in 10 ms steps, resulting in a 90% overlap between two adjacent windows. For each step the interval distribution was computed inside the time window with 1 ms resolution, and plotted as a greyscale-coded pixel line orthogonal to the time axis. For visual stimulation, light or dark spots of different size and contrast were presented with different background illumination levels. 2. Two characteristic interval patterns were observed during the sustained response component of the cells. Mainly on-cells (77%) responded with multimodal interval distributions, resulting in elongated 'bands' in the 2-dimensional time window plots. In similar situations, the interval distributions for most (71%) off-cells were rather wide and featureless. In those cases where interval bands (i.e. multimodal interval distributions) were observed for off-cells (14%), they were always much wider than for the on-cells. This difference between the on- and off-cell population was independent of the background illumination and the contrast of the stimulus. Y on-cells also tended to produce wider interval bands than X on-cells. 3. For most stimulation situations the first interval band was centred around 6-9 ms, which has been called the fundamental interval; higher order bands are multiples thereof. The fundamental interval shifted towards larger sizes with decreasing stimulus contrast. Increasing stimulus size, on the other hand, resulted in a redistribution of the intervals into higher order bands, while at the same time the location of the fundamental interval remained largely unaffected. This was interpreted as an effect of the increasing surround inhibition at the geniculate level, by which individual retinal EPSPs were cancelled. A changing level of adaptation can result in a mixed shift/redistribution effect because of the changing stimulus contrast and changing level of tonic inhibition. 4. The occurrence of interval bands is not directly related to the shape of the autocorrelation function, which can be flat, weakly oscillatory or strongly oscillatory, regardless of the interval band pattern. 5. A simple computer model was devised to account for the observed cell behaviour. The model is highly robust against parameter variations.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci. 1982 Jan;18(1):83–92. [PubMed] [Google Scholar]
- Ahlsén G., Lindström S., Lo F. S. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58(1):134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Ariel M., Daw N. W., Rader R. K. Rhythmicity in rabbit retinal ganglion cell responses. Vision Res. 1983;23(12):1485–1493. doi: 10.1016/0042-6989(83)90160-8. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., LEVICK W. R., WILLIAMS W. O. STATISTICAL ANALYSIS OF THE DARK DISCHARGE OF LATERAL GENICULATE NEURONES. J Physiol. 1964 Apr;170:598–612. doi: 10.1113/jphysiol.1964.sp007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier V., Fregnac Y., Debanne D., Shulz D., Baranyi A. Synaptic origin of rhythmic visually evoked activity in kitten area 17 neurones. Neuroreport. 1992 Dec;3(12):1065–1068. doi: 10.1097/00001756-199212000-00008. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Lee B. B. A comparison of visual responses of cat lateral geniculate nucleus neurones with those of ganglion cells afferent to them. J Physiol. 1985 Dec;369:249–268. doi: 10.1113/jphysiol.1985.sp015899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff J. E., Gerstein G. L. Favored patterns in spike trains. II. Application. J Neurophysiol. 1983 Jun;49(6):1349–1363. doi: 10.1152/jn.1983.49.6.1349. [DOI] [PubMed] [Google Scholar]
- Dossi R. C., Nuñez A., Steriade M. Electrophysiology of a slow (0.5-4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J Physiol. 1992 Feb;447:215–234. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Grüsser O. J., Hoffmann K. P. Monocular deprivation and the signal transmission by X- and Y-neurons of the cat lateral geniculate nucleus. Exp Brain Res. 1979 Feb 15;34(3):521–539. doi: 10.1007/BF00239147. [DOI] [PubMed] [Google Scholar]
- Fischer B., Krüger J. The shift-effect in the cat's lateral geniculate neurons. Exp Brain Res. 1974;21(2):225–227. doi: 10.1007/BF00234391. [DOI] [PubMed] [Google Scholar]
- Funke K., Eysel U. T. EEG-dependent modulation of response dynamics of cat dLGN relay cells and the contribution of corticogeniculate feedback. Brain Res. 1992 Feb 28;573(2):217–227. doi: 10.1016/0006-8993(92)90766-3. [DOI] [PubMed] [Google Scholar]
- Funke K., Eysel U. T. Modulatory effects of acetylcholine, serotonin and noradrenaline on the activity of cat perigeniculate neurons. Exp Brain Res. 1993;95(3):409–420. doi: 10.1007/BF00227133. [DOI] [PubMed] [Google Scholar]
- Ghose G. M., Freeman R. D. Oscillatory discharge in the visual system: does it have a functional role? J Neurophysiol. 1992 Nov;68(5):1558–1574. doi: 10.1152/jn.1992.68.5.1558. [DOI] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray Charles M., Engel Andreas K., König Peter, Singer Wolf. Stimulus-Dependent Neuronal Oscillations in Cat Visual Cortex: Receptive Field Properties and Feature Dependence. Eur J Neurosci. 1990;2(7):607–619. doi: 10.1111/j.1460-9568.1990.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Hammond P. Cat retinal ganglion cells: size and shape of receptive field centres. J Physiol. 1974 Oct;242(1):99–118. doi: 10.1113/jphysiol.1974.sp010696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Purpura K., Shapley R. M. Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. J Physiol. 1987 Oct;391:267–288. doi: 10.1113/jphysiol.1987.sp016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc Natl Acad Sci U S A. 1983 May;80(9):2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer M., Verzeano M. Periodic activity in the visual system of the cat. Vision Res. 1967 Mar;7(3):215–229. doi: 10.1016/0042-6989(67)90086-7. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: receptive-field properties and retinal inputs. Vis Neurosci. 1992 May;8(5):407–441. doi: 10.1017/s0952523800004934. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Two classes of single-input X-cells in cat lateral geniculate nucleus. I. Receptive-field properties and classification of cells. J Neurophysiol. 1987 Feb;57(2):357–380. doi: 10.1152/jn.1987.57.2.357. [DOI] [PubMed] [Google Scholar]
- McClurkin J. W., Gawne T. J., Optican L. M., Richmond B. J. Lateral geniculate neurons in behaving primates. II. Encoding of visual information in the temporal shape of the response. J Neurophysiol. 1991 Sep;66(3):794–808. doi: 10.1152/jn.1991.66.3.794. [DOI] [PubMed] [Google Scholar]
- McClurkin J. W., Optican L. M., Richmond B. J., Gawne T. J. Concurrent processing and complexity of temporally encoded neuronal messages in visual perception. Science. 1991 Aug 9;253(5020):675–677. doi: 10.1126/science.1908118. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Perkel D. H., Segundo J. P. Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- Nuñez A., Amzica F., Steriade M. Intrinsic and synaptically generated delta (1-4 Hz) rhythms in dorsal lateral geniculate neurons and their modulation by light-induced fast (30-70 Hz) events. Neuroscience. 1992 Nov;51(2):269–284. doi: 10.1016/0306-4522(92)90314-r. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., VIERNSTEIN L. J. TIME SERIES ANALYSIS OF IMPULSE SEQUENCES OF THALAMIC SOMATIC SENSORY NEURONS. J Neurophysiol. 1964 Jul;27:517–545. doi: 10.1152/jn.1964.27.4.517. [DOI] [PubMed] [Google Scholar]
- Pinault D., Deschênes M. Voltage-dependent 40-Hz oscillations in rat reticular thalamic neurons in vivo. Neuroscience. 1992 Nov;51(2):245–258. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Jones H. E., Gerstein G. L., West D. C. Feature-linked synchronization of thalamic relay cell firing induced by feedback from the visual cortex. Nature. 1994 Jun 9;369(6480):479–482. doi: 10.1038/369479a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H., Golomb D., Kleinfeld D. Global processing of visual stimuli in a neural network of coupled oscillators. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7200–7204. doi: 10.1073/pnas.87.18.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy J. B., Robson J. G. Steady discharges of X and Y retinal ganglion cells of cat under photopic illuminance. Vis Neurosci. 1992 Dec;9(6):535–553. doi: 10.1017/s0952523800001784. [DOI] [PubMed] [Google Scholar]
- Weale R. A. Apparent size and contrast. Vision Res. 1975 Aug-Sep;15:949–955. doi: 10.1016/0042-6989(75)90235-7. [DOI] [PubMed] [Google Scholar]
- Wehrhahn C., Rapf D. ON- and OFF-pathways form separate neural substrates for motion perception: psychophysical evidence. J Neurosci. 1992 Jun;12(6):2247–2250. doi: 10.1523/JNEUROSCI.12-06-02247.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hoopen M. Multimodal interval distributions. Kybernetik. 1966 Jan;3(1):17–24. doi: 10.1007/BF00291086. [DOI] [PubMed] [Google Scholar]
- van de Grind W. A., Koenderink J. J., van der Heyde G. L., Landman H. A., Bouman M. A. Adapting coincidence scalers and neural modelling studies of vision. Kybernetik. 1971 Mar;8(3):85–105. doi: 10.1007/BF00272290. [DOI] [PubMed] [Google Scholar]