Abstract
Objectives
To evaluate the feasibility of using dynamic MRI to measure the features of cranial rhythmic impulse (CRI).
Design and setting
Fifteen healthy participants (9 females and 6 males, aged 25 to 77) underwent dynamic MRI in a sagittal T2 HASTE view at a rate of 0.60 Hz for 30 s. The MRI videos were analyzed using video tracking software. Three points were marked: the glabella, the midpoint of the sella turcica, and a symmetrical point of the glabella on the occiput. The distances between these points were measured across 46 frames. Amplitudes and rates of asymmetrical CRI waves were calculated using Excel formulas.
Results
The mean wave frequencies were 5.65 Hz for the anteroposterior distance, 6.2 Hz from sella turcica to occiput, and 6.76 Hz from sella turcica to glabella. The mean wave amplitudes were 0.39 mm, 0.6 mm, and 0.49 mm for the respective distances. Both intraclass correlation coefficients (ICC) and reliability coefficient (R) indicated excellent reliability (R, ICC > 0.90). The technical error of measurement (TEM) exceeded 1 mm for the anteroposterior and sella-to-occiput distances, while it was 0.32 mm for the sella-to-glabella distance.
Conclusions
Dynamic MRI demonstrates potential in measuring the features of CRI, particularly in assessing CRI wave rate. While the ICC values indicate high reliability, the TEM values suggest that using MRI to measure CRI wave amplitude may only be dependable for the distance from the sella to the glabella.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-08064-y.
Keywords: Sella turcica, Sphenoid, Vomer, MRI, CRI
Contribution of the Paper
What the article adds to the current literature.
Dynamic MRI has proven reliable for validating the measurement of CRI wave frequency based on the four-gear interlinked model interpretation. However, due to a high technical error of measurement, it cannot accurately determine CRI wave amplitude for the glabella-to-occiput and sella-to-occiput distances. In contrast, the distance from the sella turcica to the glabella can serve as an accurate and reliable measurement variable for determining CRI wave amplitude. This article highlights that while the four-gear model interpretation is logical, dynamic MRI can consistently measure CRI wave frequency for all distances but accurately measure wave amplitude only for the sella-to-glabella distance.
Introduction
“Truth is great, certainly, but considering her greatness, it is curious what a long time she is apt to take about prevailing.” TH Huxley, this phrase by T.H. Huxley was mentioned in an article emphasizing the alleged implausibility of cranial osteopathy, arguing that it lacks robust evidence regarding its biological mechanisms, efficacy, and reliability in treatment [1]. While some articles support the theory of cranial osteopathy, others express skepticism. Over time, discussions surrounding cranial osteopathy have evolved from a classical mechanistic approach to embracing alternative interpretations that underpin the cranial model, such as entrainment [2–5]. Nowadays, therapists strive to adhere to evidence-based medicine. In this context, the study aims to evaluate the mechanisms of craniosacral therapy and Cranial Rhythmic Impulse (CRI), which is foundational to the method. CRI assesses the oscillatory patterns in the inspiratory (flexion, external rotation) and expiratory (extension, internal rotation) phases, known as the primary respiratory mechanism in the cranial region [6, 7]. The mechanism operates on the principle of tensegrity, a structural concept where the system functions holistically to respond to external or internal forces. Within the cranium, membranes manage tensile forces while the bony structures handle compressive forces, working synergistically to maintain balance and integrity [8]. With the occiput pushing the temporal bone anteriorly, the temporal bone, in turn, pulls on the tentorium cerebelli. This tension causes the sphenoid to rotate inferiorly while its greater wing rotates anteriorly. Concurrently, the ethmoid and vomer bones rotate in the opposite direction. This model of interpretation for the cranial rhythmic impulse is known as the ‘four gear interlinked’ model. The keystone of this mechanism is the sphenobasilar joint, which connects the sphenoid to the occiput. According to this model, when observing cranial bones laterally, due to primary respiratory mechanism force producers, the anteroposterior diameter of the skull will expand in the inhalation phase and flexion of the sphenobasilar synchondrosis while the sphenoid will rotate inferiorly and anteriorly. In the exhalation phase the anteroposterior diameter of skull will reduce and the sphenoid will return to its former position [9]. This interpretation of the cranial rhythmic impulse suggests that the anteroposterior diameter of the cranium varies in a pattern of increase and decrease with inhalation and exhalation, respectively. Additionally, the distances between the sella turcica, glabella, and the symmetrical opposite point of the glabella also change [10]. Therefore, if we can employ a measurement method that accurately captures these distance changes, it could potentially serve as a useful tool for assessing features of the cranial rhythmic impulse. While CT scans are the most accurate for precisely identifying bony landmarks, they have certain limitations and are not as accessible as MRI. Dynamic MRI, a non-invasive method, offers a viable alternative. It is widely available and capable of capturing video, which enhances the understanding of joint pathomechanics and facilitates the measurement of various distances in medical fields [11, 12]. Considering the minimum frequency detection of dynamic MRI is about 0.6 Hz, and the cranial rhythmic impulse (CRI) rate ranges between 4 and 16 per minute, it raises an important question: can dynamic MRI reliably measure the CRI wave frequency and amplitude? If dynamic MRI can effectively capture these parameters with sufficient accuracy and resolution, it could serve as a valuable, non-invasive tool for understanding CRI’s role in clinical settings.
Participants and methods
Participant selection and study criteria
Fifteen participants, consisting of 9 females and 6 males aged 25 to 77, were selected for the study following a 4-case pilot study using simple random sampling. A rule of thumb was applied to determine an effective sample size. Exclusion criteria included a history of spine or cranial surgery, structural or functional spinal deviations, seizures or epilepsy, spinal tumors, neck pain or lower back pain. Inclusion criteria required no history of shortness of breath or fear of MRI machines or confined spaces. The study was approved by the ethics committee at the University of Social Welfare and Rehabilitation Sciences on March 1 2023 with the ethics committee number IR.USWR.REC.1401.237 and registration code 1,766,135. The health, dignity, and integrity of the participants were ensured and the objectives, procedures, potential risks and benefits of the study were clearly communicated to them. Participation in this study was entirely voluntary, and participants were informed that they could leave the research at any stage if they choose to do so. They were assured that their names and data would be kept confidential and would not be published not even in abbreviations. Informed consent was obtained from all participants, and their rights were protected. Each participant was informed that the MRI measurements would involve evaluating certain distances within the skull.
Distance measurement procedures
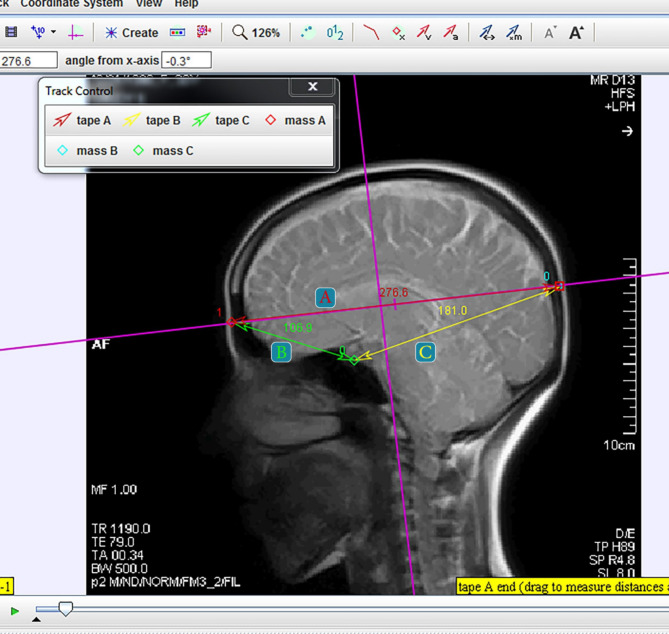
Participants were instructed to lie supine and relax, with the machine’s pointer marker positioned at the level of the eyebrows and nose. Dynamic MRI (1.5 Tesla Siemens Avanto unit) was performed in the sagittal T2 HASTE view of the cranium, a rapid measurement technique that supports very short time intervals. This method involves a single RF-induced proton excitation to acquire half of the K-space with the remainder completed through FFT, enabling scan times of 1 s or less [13]. According to previous studies, the cranial rhythmic impulse wave rate has been reported to range between 4 and 16 cycles per minute [8, 14], In our study, a frequency of 0.6 Hz was used for 30 s to match the cranial rhythmic impulse (CRI) frequency, resulting in 46 frames per participant. The videos were then uploaded to Tracker software, which can distinguish each frame individually. Tracker 4.11.0, a Windows-based application, is capable of measuring distances in 2D motion analysis and has been shown to be valid and reliable for assessing distances and angles related to postural deficits in adults [15]. In each frame, three points were marked: the glabella, the midpoint of the sella turcica, and the symmetrical point of the glabella on the occiput, with the last point remaining consistent along the vertical plumb line passing through C0-C1 and the glabella (Fig. 1).
Fig. 1.
Tracker Software Environment, Marked Points and Measured Distances. A: Glabella to Occiput Distance, B: Glabella to sella turcica Distance, C: Sella turcica to Occiput Distance
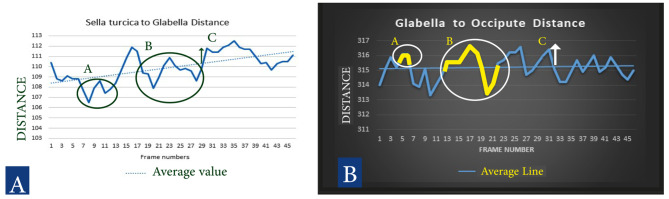
To determine the wave frequency and wave amplitude of the cranial rhythmic impulse, we identified the minimum, maximum, and average values of each tape measurement. Any changes in values that did not cross the average value (baseline) were classified as a change, while those that crossed the baseline and returned were counted as frequency rates within the 30-second interval. Wave amplitude was quantified by identifying peak points and calculating the area under the curve using algebraic formulas (Fig. 2). By using Excel formulas, we were able to determine the number of changes, frequency, and amplitude for each measurement.
Fig. 2.
A (white background): Sella turcica to glabella distance changes, B (Black background): Glabella to Occiput distance changes. Distance Scale: 1 = 0.53 mm (46 frames tracking, CRI asymmetrical wave, Change, Rate and Amplitude description: A: One Change of wave, B: One Rate of Wave, C: Amplitude of Wave)
Examiner experience and measurement scale
The examiner, who had three years of experience using Tracker software for various medical research purposes, measured the distances between the marked points in each frame using three tape measurements. These values were then converted to millimeters based on a pre-determined scale value from the MRI machine, where one unit of tape measurement equaled 0.53 millimeters. The values for all 46 frames across the 15 participants were recorded in an Excel file.
Reliability assessment and repeated measurements
In 10 cases distances were remeasured by the examiner with a 1-month time interval. Intraclass Correlation Coefficient (ICC), Standard Error of Measurement (SEM), Technical Error of Measurement (TEM), Relative Technical Error of Measurement (rTEM), and Coefficient of Reliability (R) were calculated using statistical analysis.
Results
In this section, each parameter- including wave frequency, wave amplitude, the reliability of measuring cranial distances using dynamic MRI and tracker software and the correlation matrix between distances- is elaborated upon and supported by tables and detailed text explanations.
CRI wave frequency(rate)
The mean wave frequency values were 5.65, 6.2, and 6.25 per 30 s for the anteroposterior (AP) measurement, sella to the symmetrical point of the glabella on the occiput, and sella to glabella measurements, respectively. Frequency values varied from 1 to 10.5 for both the AP and sella to occiput measurements, and from 2.5 to 12 for the sella to glabella measurement. The number of changes ranged from 13 to 27 for the AP measurement, 19 to 29 for the sella to glabella measurement, and 19 to 30 for the sella to occiput measurement, with mean values of 20.8, 22.66, and 23.26, respectively (Table 1).
Table 1.
CRI Wave frequency, Wave Amplitude and Number of changes measured for each distances value
| Variables | Wave frequency(Hz) | Lower Bound | Upper bound | Number of changes | Amplitude of changes(mm) | P-Value |
|---|---|---|---|---|---|---|
| Glabella to Occiput value | 5.65 per 30 s | 1 | 10.5 | 20.8 | 0.39 | < 0.001 |
| Sella turcica to glabella value | 6.25 per 30 s | 2.5 | 12 | 23.26 | 0.49 | < 0.001 |
| Sella turcica to Occiput value | 6.2 per 30 s | 1 | 10.5 | 22.66 | 0.60 | < 0.001 |
CRI wave amplitude (Distance changes)
The average wave amplitudes were 0.39 mm, 0.49 mm, and 0.6 mm for the anteroposterior (AP) measurement, sella to the symmetrical point of the glabella on the occiput, and sella to glabella measurements, respectively. The amplitudes ranged from 0.17 mm to 1.17 mm for AP, 0.26 mm to 1.69 mm for sella to occiput, and 0.27 mm to 1.23 mm for sella to glabella measurements (Table 1).
Reliability assessment and correlation matrix
The Intraclass Correlation Coefficient (ICC) was 90.5%, 87.9%, and 95.9% for AP, sella to occiput, and sella to glabella measurements, respectively. The Standard Error of Measurement (SEM) was 7.7 mm for AP, 6.12 mm for sella to occiput, and 1.68 mm for sella to glabella measurements (Table 2). The correlation matrix between the three measured distances reveals that both the sella turcica to glabella and sella turcica to occiput distances are significantly correlated with the glabella to occiput distance (anteroposterior diameter) in all 15 cases. Furthermore, the sella turcica to glabella distance shows a higher Pearson correlation with the anteroposterior distance. However, in some cases, the sella turcica to glabella and sella turcica to occiput distances are also correlated with each other (Table 3).
Table 2.
– output of Intraclass correlation coefficient and other reliability measurements
| Variable | Intraclass Correlation Coefficient |
SEM (mm) | TEM | rTEM (%) | R Reliability Coefficient | P-value |
|---|---|---|---|---|---|---|
| Glabella/Occiput value | 0.905 | 7.77 mm | 1.63 mm | 1.10% | 0.99 | < 0.001 |
| Sella/Glabella value | 0.959 | 1.68 mm | 0.32 mm | 0.54% | 0.99 | < 0.001 |
| Sella/Occiput value | 0.879 | 6.12 mm | 6.23 mm | 1.30% | 0.99 | < 0.001 |
Table 3.
Correlation matrix for three distances (case 1)
| Correlation Matrix (Pearson) | Glabella/Occiput value | Sella/Glabella value | Sella/Occiput value | |||
|---|---|---|---|---|---|---|
| Glabella/Occiput value | 1 | R | P-Value | r | P-Value | |
| 0.891 <0.001 | 0.501 <0.001 | |||||
| Sella/Glabella value | R | P-Value | 1 | r | P-Value | |
| 0.891 <0.001 | 0.194 0.064 | |||||
| Sella/Occiput value | R | P-Value | R | P-Value | 1 | |
| 0.501 <0.001 | 0.194 0.064 | |||||
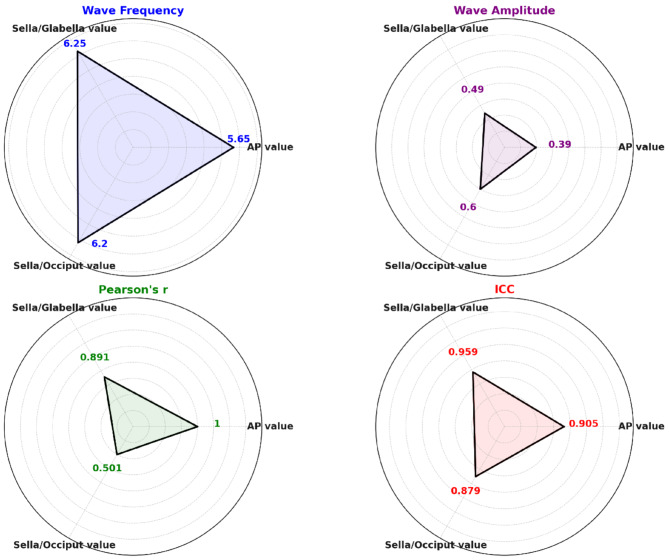
According to the results, there seems to be consistency among each distance value for wave frequency, wave amplitude, ICC and correlations. A summary of the results has been presented in the graph below.
Discussion
In this study, a triangular measurement was conducted using tracker software on each participant’s MRI video to accurately measure the distances between the sella turcica and each side of the skull, as well as the anteroposterior diameter of the skull. These distance changes were assessed in terms of frequency, amplitude, and correlations between each of the measured distances to evaluate the cranial rhythmic impulse (CRI) for frequency, amplitude, and symmetry features. This approach is an innovative method to assess such features of CRI with instruments. The results indicated that, the mean frequency of changes across the three distances was 6.03 per 30 s, and the mean amplitude was 0.49 mm. Furthermore, the distances from the sella turcica to both sides of the skull showed significant correlations with the anteroposterior diameter of the skull (See Graph 1).
Graph 1.
Summary of the wave frequency, wave amplitude, ICC and Correlations for different distances in case 1
Cranial Rhythmic Impulse (CRI) refers to the subtle, rhythmic motion perceived in the cranium, believed to be a result of inherent physiological processes. The key features of CRI include frequency, amplitude, symmetry, and quality which indicate the extent, balance, and smoothness of the motion [16, 17]. This study investigates the frequency, amplitude and symmetry of CRI using dynamic MRI, utilizing a specific procedure and methodology.
CRI Wave frequency(rate) and symmetry
The Cranial Rhythmic Impulse (CRI) wave frequency can be assessed through both instrumentation and palpation. Instruments like the Biodynamic Resonance Recorder and Cranial Oscillation Detectors objectively measure subtle cranial movements, providing data on wave frequency. Manual palpation, used by osteopaths, involves feeling the rhythmic motion of the cranium with the hands. Practitioners estimate CRI frequency to typically be between 6 and 12 cycles per minute. This can range from as low as 2 to 7 cycles per minute with manual palpation, compared to 8 to 14 cycles per minute with instrumental measurements, indicating that instrumental methods tend to show higher CRI frequency values [8, 14]. According to the results of this study, the mean CRI wave frequency ranged between 5 and 6 cycles per 30 s (Table 1), which corresponds to 10 to 12 cycles per minute. This falls within the previously reported range and closely aligns with instrumental measurements of CRI frequency. Comparing the wave frequencies between different cranial measurements, such as glabella to occiput, and sella turcica to glabella and occiput, shows a high level of consistency. This suggests that cranial structures are moving in a coordinated manner, as predicted by the four-gear interlinked model. The correlation matrix between the different distances further emphasizes the symmetric component of the cranial rhythmic impulse. Additionally, the four-gear interlinked model interpretation identifies two sub-components of CRI, the distances between the sella turcica and the glabella, and between the sella turcica and the occiput, which are significantly correlated with a larger component - the distance from the glabella to the occiput (Table 3). This model suggest that the movement of cranial bones is interdependent, much like gears in a machine. When one bone moves, others follow in a predictable pattern, maintaining balance and rhythm. The observed consistency in wave frequencies across different points supports the validity of this model in explaining the dynamics of the cranial rhythmic impulse. The wide spectrum of inter-individual CRI wave frequency in this study may be due to the wide age range of study samples.
CRI wave amplitude (Distance changes)
The amplitude of the Cranial Rhythmic Impulse (CRI) refers to the extent of palpable motion of the cranium during its rhythmic expansion and contraction, reflecting the range of motion of the cranial bones and membranes. Changes in amplitude can be interpreted as indicators of health or dysfunction. Increased amplitude may suggest enhanced physiological activity, while decreased amplitude might indicate restrictions or decreased vitality. The perception of amplitude can vary significantly between practitioners, making it a subjective measure. Unlike frequency, which can be objectively measured, amplitude is more challenging to quantify consistently. Research into the objective measurement of amplitude is still limited. The results of this study regarding CRI wave amplitude appear reasonable in terms of both the mean amplitude and the range of values observed for the displacement of the Sphenobasilar Synchondrosis (SBS) joint, a synchondrosis joint that allows only compression and decompression, However, the technical error of measurement (TEM) suggests that dynamic MRI can be an accurate and reliable method for measuring CRI wave amplitude for the sella-to-glabella distance, but not for the sella-to-occiput and glabella-to-occiput distances. The mean wave amplitude across the three distances is equivalent to the amplitude measured for the sella-to-glabella distance (Tables 1 and 2). The amount of wave amplitude of CRI, reported several tenths of micrometer. This data appears to be consistent with the wave amplitude data of the present study [10, 18, 19].
The technical error of measurement (TEM), preferred over the standard error of measurement (SEM) for assessing measurement errors in biomechanics, anthropometry, or sports sciences, is specifically designed for situations involving direct measurements of physical attributes, such as distance. TEM provides an absolute error value and directly measures frame-to-frame error without adjusting for score variability, offering a clearer indication of the reliability of the measurement process across frames. In contrast, SEM is better suited for situations where the reliability of individual scores needs assessment (e.g., test scores or psychological assessments). A higher SEM value compared to TEM in this study supports this distinction, emphasizing TEM’s advantage for assessing measurement consistency in physical attributes.
Reliability assessment and repeated measurements
A high Intraclass Correlation Coefficient (ICC) and coefficient of reliability (R) indicate that dynamic MRI and the accompanying Tracker software are excellent, reliable tools for measuring cranial distances (Table 2). These tools effectively measure cranial rhythm frequency and, in one specific variable, the amplitude of cranial rhythmic impulse. This capability supports clinicians in developing more precise rehabilitation and treatment plans, particularly when used alongside other reliable methods, such as suboccipital muscle sonography for the neck and cranial regions.
Implications for clinical application
According to the four-gear interlinked model of the cranial rhythmic impulse, cranial structures work rhythmically during inhalation and exhalation to manage tensile and compressive forces. Disruption of this mechanism can lead to symptoms such as headaches, nausea, and neck pain. Measuring CRI wave frequency and amplitude with dynamic MRI, combined with reliable methods like suboccipital muscle sonography and electromyography [20, 21], can provide accurate and valuable insights into craniocervical problems, supporting effective management of these issues.
Limitations
In this study, the authors used dynamic MRI as an innovative approach to evaluate the feasibility of measuring craniometric features of cranial rhythmic impulse. However, several limitations should be considered: (1) Lack of evidence supporting the four-gear interlinked model, (2) Criticism of applying a biomedical and mechanistic paradigm to the cranial field, (3) Limited evidence on the reliability of dynamic MRI in assessing changes at craniometric points, and (4) While the imaging frequency of 1.5 Tesla dynamic MRI is close to the CRI rate reported in previous studies, lower imaging frequencies (below 0.6 Hz) and higher resolution imaging above 1.5 Tesla could provide more precise measurements. Therefore, the authors recommend that future research utilize more advanced instruments to improve measurement accuracy.
Suggestions for future study
Future research should focus on refining methods for objectively measuring CRI wave amplitude, for the other two distances. Additionally, studies should explore the impact of demographic factors such as age, sex, and health status on CRI characteristics to improve understanding of normative ranges and variations. Integrating advanced imaging techniques and biomechanical modeling could also enhance the understanding of physiological and pathological implications of CRI dynamics in both clinical and research settings.
Conclusion
Overall, dynamic MRI, widely used in medicine for diagnosis and research, shows potential in enhancing our understanding of the cranial rhythmic impulse and validating proposed model interpretations. Specifically, it offers practical applications for measuring the wave frequency and amplitude features of CRI, which could be valuable for interventions in various neck and cranial conditions. This article has been written according to TRIPOD Checklist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors should appreciate Dr. Saman Samani and Dr.Leila Rahnama for useful advice in data processing and helping in English proof reading and language editing.
Abbreviations
- MRI
Magnetic Resonance Imaging
- HASTE
Single-shot Turbo Spin Echo Imaging
- CRI
Cranial Rhythmic Impulse
- SEM
Standard Error of Measurement
- ICC
Intraclass Correlation Coefficient
- MDC
Minimal Detectable Change
- TEM
Technical Error of Measurement
- rTEM
Relative Technical Error of Measurement
- R
Coefficient of Reliability
- CI
Confidence Intervals
- AP Distance
AnteroPosterior Distance
- CT Scan, X-Ray
Computed Tomography, X-Ray
- 2D
Two-Dimensional
- MM
Millimeter
Author contributions
P.M. and N.K. and I.A. and S.M. conception and design of the work. P.M. and S.M. and A.A. and E.B. the acquisition, analysis and interpretation of data. P.M. and S.M. and A.A. using software and machine in article. P.M. and N.K. have drafted the work or substantively revised it. P.M. and All authors reviewed all parts of manuscript.
Funding
No funding.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee at the University of Social Welfare and Rehabilitation Sciences, and informed consent was obtained from all subjects, under supervision of a medical practitioner specialist, MRI procedure was done by MR Radiographer with 15 years of experience in MRI and 3 year of experience in dynamic MRI procedure. Health, dignity, integrity of the participants was ensured and objectives, procedures, and potential risks and benefits of the study were clearly communicated to the them. Participation in this study was completely voluntary and participants were informed they are able to leave the research at any stage, they decided to. They were informed their names and data captured would be completely confidential and would not published in even abbreviations. Ethics committee number: (IR.USWR.REC.1401.237).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hartman SE. Cranial osteopathy: its fate seems clear. Australas Chiropr Osteopathy. 2006;14:1–3. 10.1186/1746-1340-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsotti N, Casini A, Chiera M, Lunghi C, Fornari M. editors. 2023 Neurophysiology, Neuro-Immune Interactions, and Mechanobiology in Osteopathy in the cranial field: an evidence-informed perspective for a scientific rationale. Healthcare 11 23 3058 10.3390/healthcare11233058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin RN. Lymphatic drainage of the neuraxis in chronic fatigue syndrome: a hypothetical model for the cranial rhythmic impulse. J Am Osteopath Assoc. 2007;107(6):218–24. 10.7556/jaoa.2007.107.6.218. [PubMed] [Google Scholar]
- 4.Vern BA, Schuette WH, Leheta B, Juel VC, Radulovacki M. Low-frequency oscillations of cortical oxidative metabolism in waking and sleep. J Cereb Blood Flow Metab. 1988;8(2):215–26. 10.1038/jcbfm.1988.52. [DOI] [PubMed] [Google Scholar]
- 5.Farasyn A. New hypothesis for the origin of cranio-sacral motion. J Bodyw Mov Ther. 1999;3(4):229–. 10.1016/S1360-8592(99)80009-6. 37. [Google Scholar]
- 6.Guillaud A, Darbois N, Monvoisin R, Pinsault N. Reliability of diagnosis and clinical efficacy of cranial osteopathy: a systematic review. PLoS ONE. 2016;11(12):e0167823. 10.1371/journal.pone.0167823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth-Pattullo V, Hayes KW. Interrater reliability of craniosacral rate measurements and their relationship with subjects’ and examiners’ heart and respiratory rate measurements. Phys Ther. 1994;74(10):908–16. 10.1093/ptj/74.10.908. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KE, Sergueef N, Glonek T. Recording the rate of the cranial rhythmic impulse. J Am Osteopath Assoc. 2006;106(6):337–41. 10.7556/jaoa.2006.106.6.337. [PubMed] [Google Scholar]
- 9.Chaitow L. Cranial manipulation: theory and practice: osseous and soft tissue approaches. Elsevier Health Sciences; 2005. 10.1016/j.physio.2005.12.005.
- 10.Cook A. The SBS revisited-the mechanics of cranial motion. J Bodyw Mov Ther. 2005;9(177):e88. 10.1016/j.jbmt.2004.12.002. [Google Scholar]
- 11.Subburaj K, Ravi B, Agarwal M. Automated identification of anatomical landmarks on 3D bone models reconstructed from CT scan images. Comput Med Imaging Graph. 2009;33(5):359–68. 10.1016/j.compmedimag.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Nousiainen K, Mäkelä T. Measuring geometric accuracy in magnetic resonance imaging with 3D-printed phantom and nonrigid image registration. MAGMA. 2020;33:401–10. 10.1007/s10334-019-00788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semelka RC, Kelekis NL, Thomasson D, Brown MA, Laub GA. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996;6(4):698–9. 10.1002/jmri.1880060420. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen TR, Meulengracht KC. Direct measurement of the rhythmic motions of the human head identifies a third rhythm. J Bodyw Mov Ther. 2021;26:24–9. 10.1016/j.jbmt.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Paul S, Lester M, Foreman K, Dibble L. Validity and Reliability of Two-Dimensional Motion Analysis for Quantifying Postural Deficits in adults with and without neurological impairment. Anat Rec (Hoboken). 2016;299(9):1165–73. 10.1002/ar.23385. [DOI] [PubMed] [Google Scholar]
- 16.Moskalenko Y, Frymann V, Kravchenko T, Weinstein G. Physiological background of the cranial rhythmic impulse and the primary respiratory mechanism. AAO J. 2003;13(2):2133. Corpus ID: 15090207. [Google Scholar]
- 17.Bordoni B, Escher AR. Rethinking the origin of the primary respiratory mechanism. Cureus. 2023;15(10). 10.7759/cureus.46527. [DOI] [PMC free article] [PubMed]
- 18.Stubbe L, Le Bihan Y, Ozout A-E, Herivan G, Berthelot E. An Eddy current system for the study of the cranial rhythmic impulse. IEEE Trans Magn. 2015;51(1):1–3. 10.13140/RG.2.1.4350.7927.26203196 [Google Scholar]
- 19.Nelson KE, Sergueef N, Lipinski CM, Chapman AR, Glonek T. Cranial rhythmic impulse related to the Traube-Hering-Mayer oscillation: comparing laser-doppler flowmetry and palpation. J Am Osteopath Assoc. 2001;101(3):163–73. 10.7556/jaoa.2001.101.3.163. [PubMed] [Google Scholar]
- 20.Masoudi P, Karimi N, Abdollahi I, Moravej S, Tahamtan A. Reliability of Rehabilitative Musculoskeletal Sonography for measuring the visible cross-sectional area of Suboccipital muscles. Cureus. 2024;16(9). 10.7759/cureus.68772. [DOI] [PMC free article] [PubMed]
- 21.Hallgren RC, Andary MT, Wyman AJ, Rowan JJ. A standardized protocol for needle placement in suboccipital muscles. Clin Anat. 2008;21(6):501–8. 10.1002/ca.20660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.