Abstract
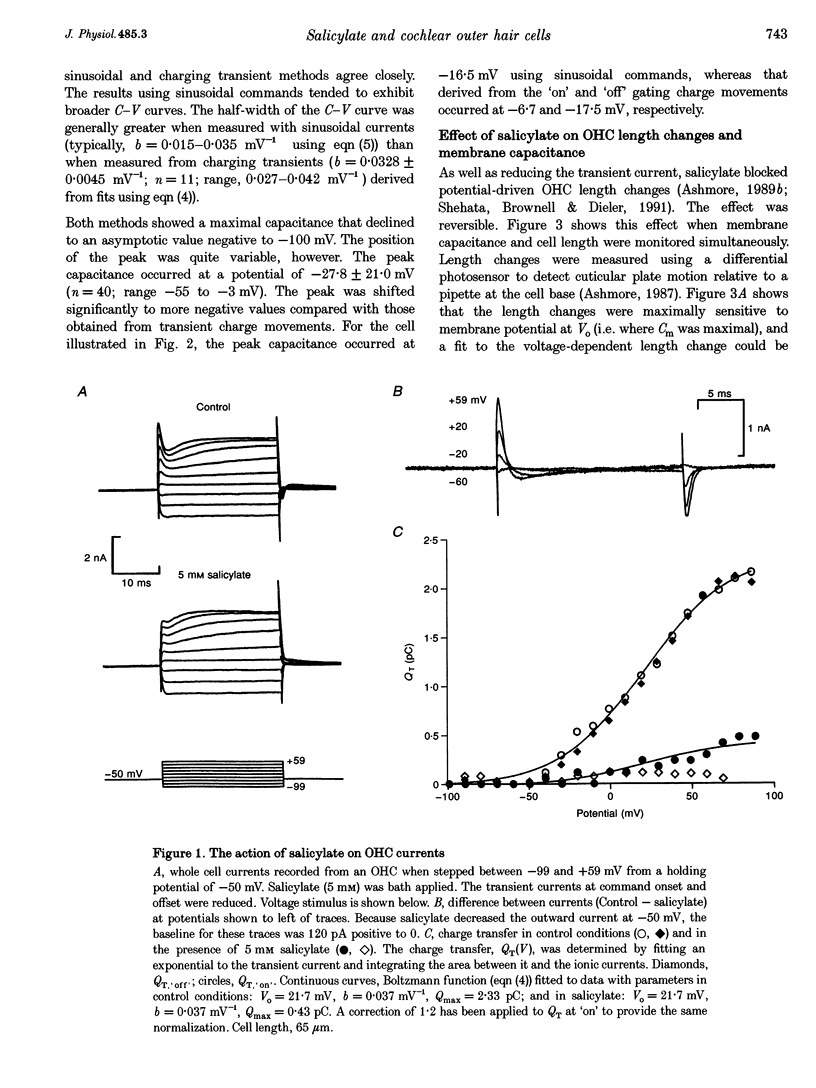
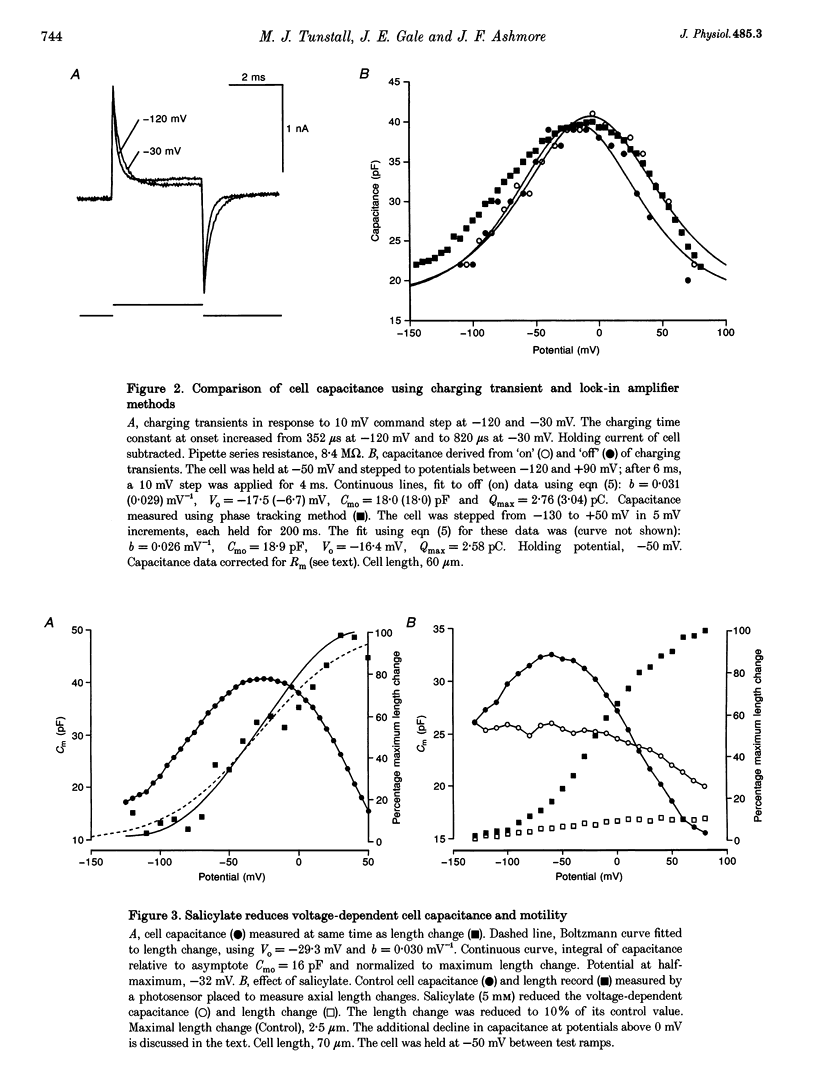
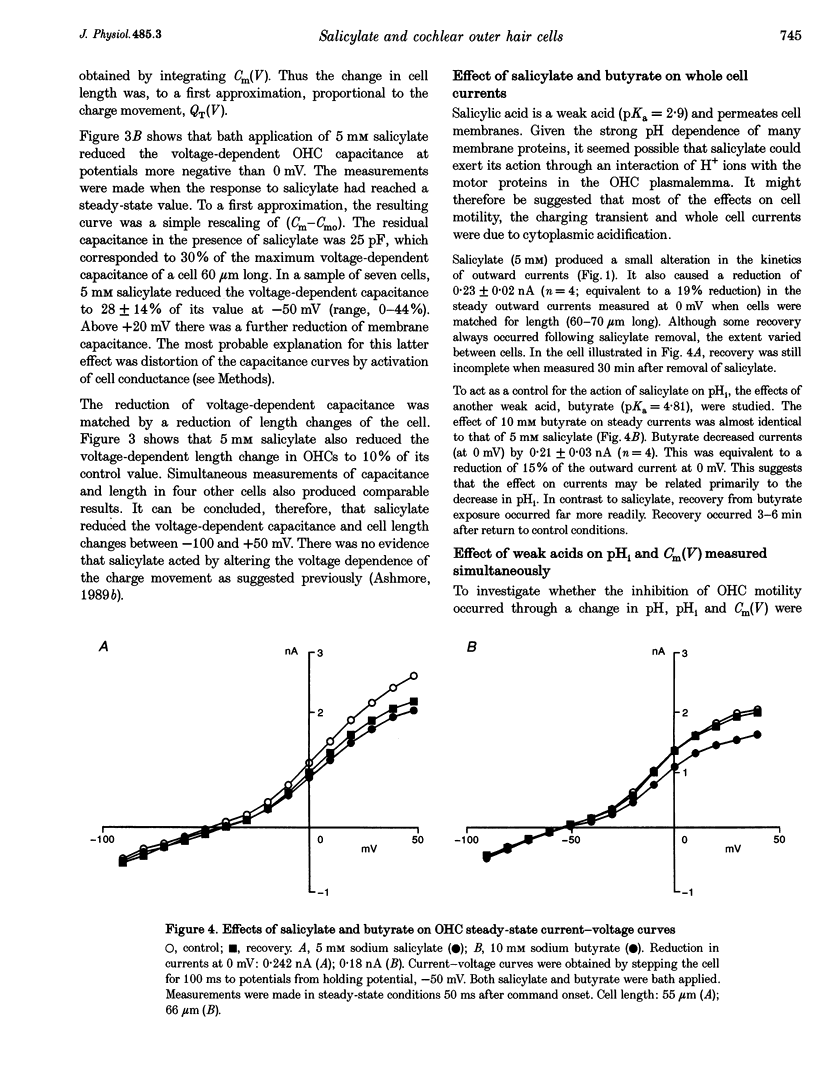
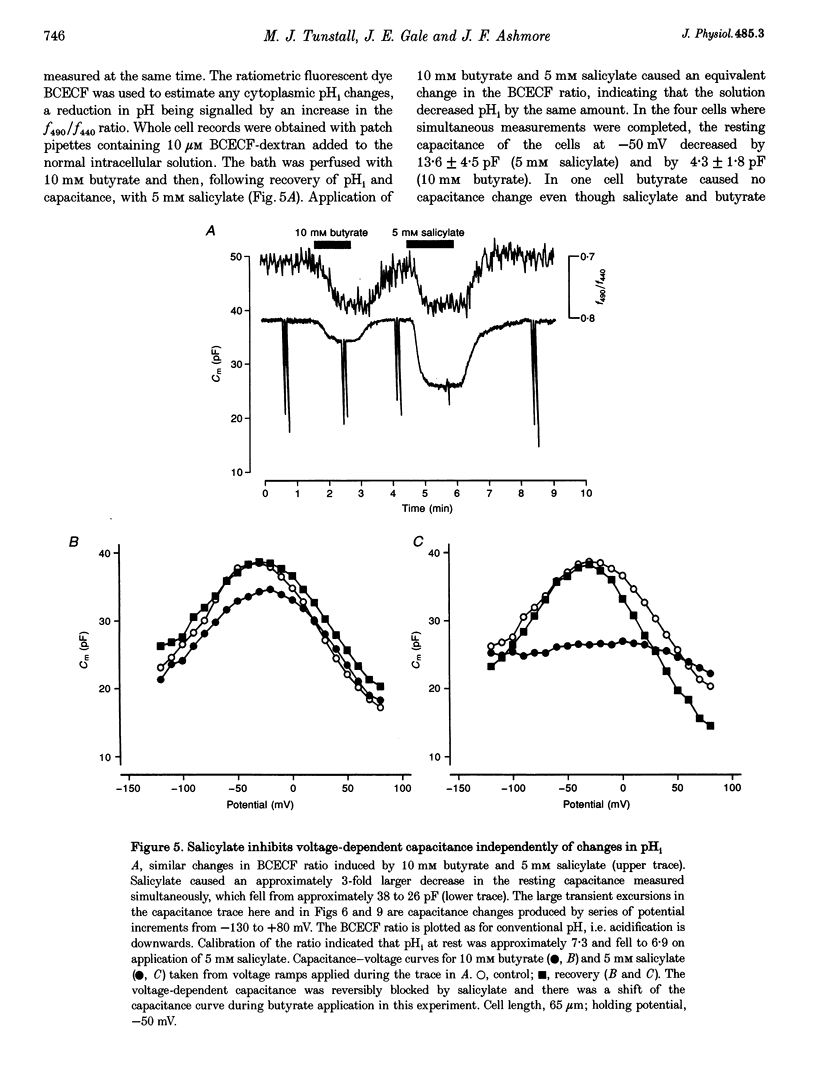
1. The effect of salicylate on membrane capacitance and intracellular pH has been measured in isolated outer hair cells (OHCs) during whole cell recording. Cell membrane capacitance was measured using a lock-in amplifier technique. 2. Salicylate applied in the bath reduced the fast charge movement, equivalent to a voltage-dependent membrane capacitance, present in OHCs. Simultaneous measurement of membrane capacitance and voltage-driven cell length changes showed that salicylate reduced both together. 3. A small effect of salicylate on outward currents at 0 mV was observed. Sodium salicylate (5 mM) reduced the currents by 19% and another weak acid, sodium butyrate (10 mM), reduced outward currents in OHCs by 15%. 4. The ratiometric dye 2,7-bis(2-carboxymethyl)-5,6-carboxyfluorescein (BCECF) was used to measure pHi changes in OHCs during weak acid exposure. Membrane capacitance and pHi were measured simultaneously in OHCs exposed first to 10 mM sodium butyrate and then to 5 mM sodium salicylate. Although both compounds produced a similar reduction in pHi, butyrate decreased the resting capacitance from a mean resting capacitance of 35 pF (at -30 mV) by 5.4 +/- 2.1 pF, whereas salicylate decreased it by 15.7 +/- 2.3 pF (n = 4). 5. Exposure of OHCs to 10 mM sodium benzoate, an amphiphilic anion, reduced resting membrane capacitance at -30 mV by 9.2 +/- 3.2 pF (n = 3). Outward currents, measured at 0 mV, were reduced by 0.25 +/- 0.05 nA during benzoate application, comparable with the effect of salicylate. 6. Capacitance was measured during slow bath application of salicylate. The resulting dose-capacitance curve had a Hill coefficient of 3.40 +/- 0.85 (n = 4) and a half-maximal dose of 3.95 +/- 0.34 mM. The dose-capacitance curve was not significantly voltage dependent. 7. Salicylate had no detectable effect on the resting capacitance of Deiters' cells, a non-sensory cell type of the organ of Corti. 8. It is concluded that many of the described effects of salicylate on hearing may arise from the partitioning of the salicylate molecule into the membrane of the OHC and consequent inhibition of OHC motility.
Full text
PDF



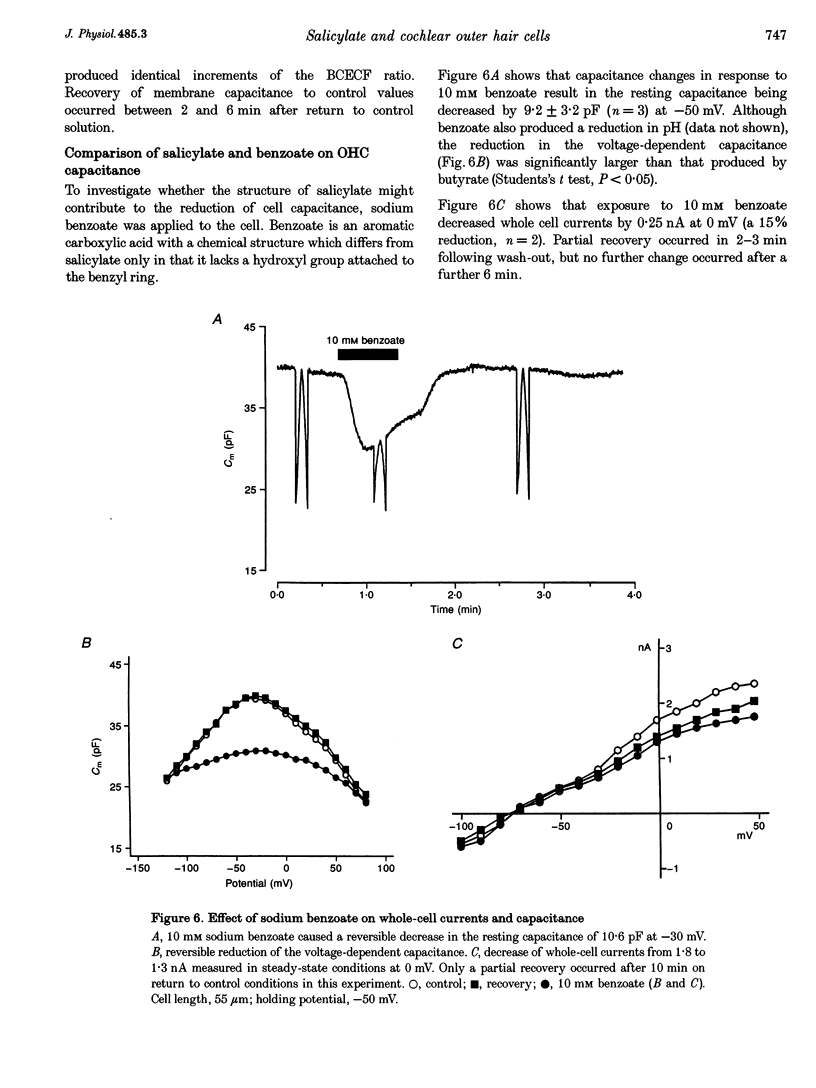
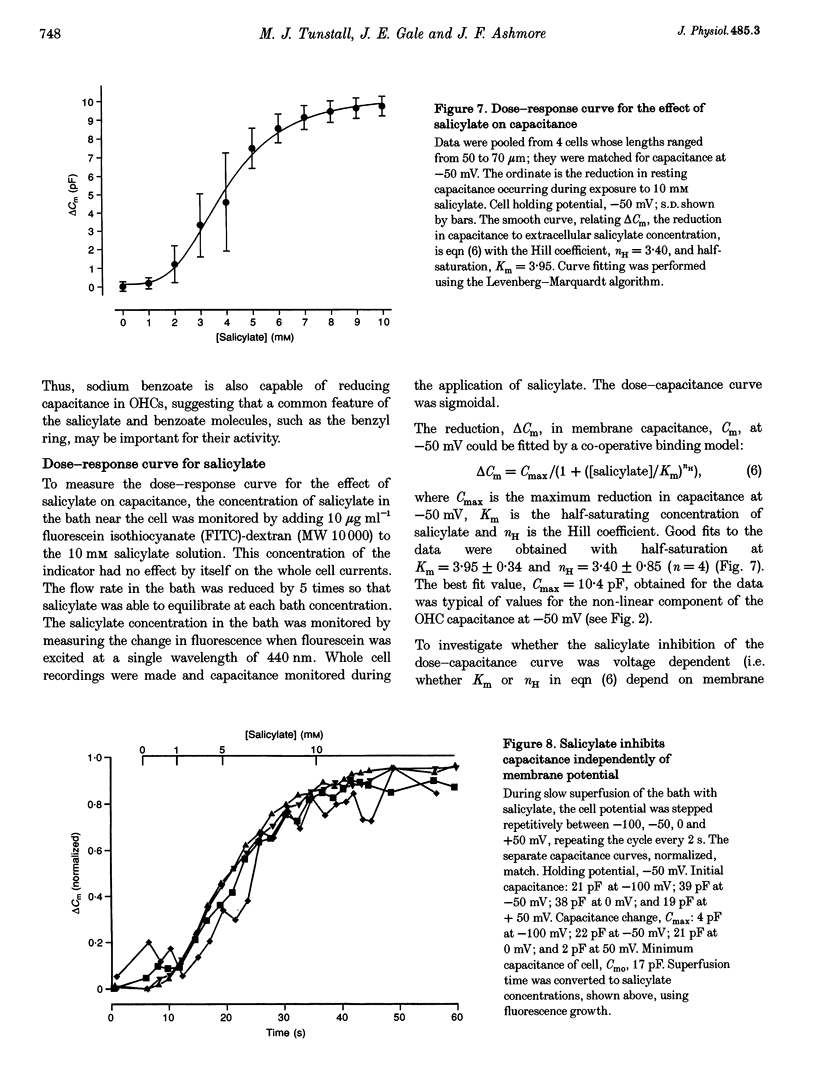
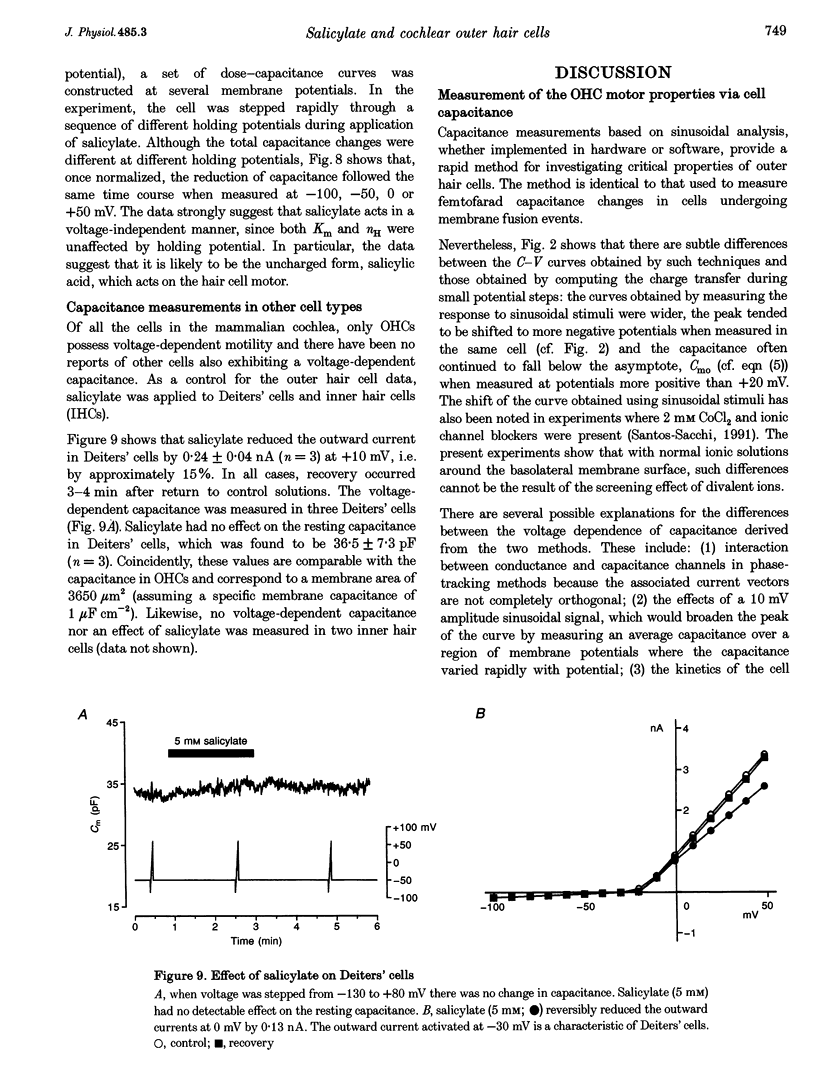



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. Mammalian hearing and the cellular mechanisms of the cochlear amplifier. Soc Gen Physiol Ser. 1992;47:395–412. [PubMed] [Google Scholar]
- Brown A. M., Williams D. M., Gaskill S. A. The effect of aspirin on cochlear mechanical tuning. J Acoust Soc Am. 1993 Jun;93(6):3298–3307. doi: 10.1121/1.405714. [DOI] [PubMed] [Google Scholar]
- Carlyon R. P., Butt M. Effects of aspirin on human auditory filters. Hear Res. 1993 Apr;66(2):233–244. doi: 10.1016/0378-5955(93)90143-o. [DOI] [PubMed] [Google Scholar]
- Copello J., Segal Y., Reuss L. Cytosolic pH regulates maxi K+ channels in Necturus gall-bladder epithelial cells. J Physiol. 1991 Mar;434:577–590. doi: 10.1113/jphysiol.1991.sp018487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier A., Miller J. M., Nuttall A. L. The vascular component of sodium salicylate ototoxicity in the guinea pig. Hear Res. 1993 Sep;69(1-2):199–206. doi: 10.1016/0378-5955(93)90108-d. [DOI] [PubMed] [Google Scholar]
- Douek E. E., Dodson H. C., Bannister L. H. The effects of sodium salicylate on the cochlea of guinea pigs. J Laryngol Otol. 1983 Sep;97(9):793–799. doi: 10.1017/s0022215100095025. [DOI] [PubMed] [Google Scholar]
- Evans E. F., Borerwe T. A. Ototoxic effects of salicylates on the responses of single cochlear nerve fibres and on cochlear potentials. Br J Audiol. 1982 May;16(2):101–108. doi: 10.3109/03005368209081454. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:529–551. doi: 10.1113/jphysiol.1990.sp018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale J. E., Ashmore J. F. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc Biol Sci. 1994 Mar 22;255(1344):243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Ashmore J. F. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol. 1992 Mar;448:73–98. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G., Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J. 1993 Nov;65(5):2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Saito Y., Nishiyama A., Takasaka T. Intracellular pH regulation in isolated cochlear outer hair cells of the guinea-pig. J Physiol. 1992 Feb;447:627–648. doi: 10.1113/jphysiol.1992.sp019021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993 Jul;65(1):492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff P. J., Hansen R., Sasaki P. G., Sasaki C. T. Differential uptake of salicylate in serum, cerebrospinal fluid, and perilymph. Arch Otolaryngol Head Neck Surg. 1986 Oct;112(10):1050–1053. doi: 10.1001/archotol.1986.03780100038004. [DOI] [PubMed] [Google Scholar]
- Kalinec F., Holley M. C., Iwasa K. H., Lim D. J., Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G. R., Tubis A. Modification of spontaneous and evoked otoacoustic emissions and associated psychoacoustic microstructure by aspirin consumption. J Acoust Soc Am. 1988 Oct;84(4):1343–1353. doi: 10.1121/1.396633. [DOI] [PubMed] [Google Scholar]
- Mammano F., Ashmore J. F. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature. 1993 Oct 28;365(6449):838–841. doi: 10.1038/365838a0. [DOI] [PubMed] [Google Scholar]
- McFadden D., Plattsmier H. S. Aspirin abolishes spontaneous oto-acoustic emissions. J Acoust Soc Am. 1984 Aug;76(2):443–448. doi: 10.1121/1.391585. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. Salicylates and phospholipid bilayer membranes. Nature. 1973 May 25;243(5404):234–236. doi: 10.1038/243234a0. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Akarasereenont P., Thiemermann C., Flower R. J., Vane J. R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan E., Kelly P., Nies K., Porter W. W., Paulus H. E. Tinnitus as an indication of therapeutic serum salicylate levels. JAMA. 1973 Oct 8;226(2):142–145. [PubMed] [Google Scholar]
- Myers E. N., Bernstein J. M. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol. 1965 Nov;82(5):483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel J. L., Bledsoe S. C., Jr, Bobbin R. P., Ceasar G., Fallon M. Comparative actions of salicylate on the amphibian lateral line and guinea pig cochlea. Comp Biochem Physiol C. 1989;93(1):73–80. doi: 10.1016/0742-8413(89)90013-3. [DOI] [PubMed] [Google Scholar]
- Reinhart W. H., Chien S. Red cell rheology in stomatocyte-echinocyte transformation: roles of cell geometry and cell shape. Blood. 1986 Apr;67(4):1110–1118. [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991 Oct;11(10):3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata W. E., Brownell W. E., Dieler R. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngol. 1991;111(4):707–718. doi: 10.3109/00016489109138403. [DOI] [PubMed] [Google Scholar]
- Spedding M. Changing surface charge with salicylate differentiates between subgroups of calcium-antagonists. Br J Pharmacol. 1984 Sep;83(1):211–220. doi: 10.1111/j.1476-5381.1984.tb10137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M. S., Thomas R. C. New method for calculating pHi from accurately measured changes in pHi induced by a weak acid and base. Pflugers Arch. 1986 Jul;407(1):59–63. doi: 10.1007/BF00580721. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Wu M. L. pH dependence of intrinsic H+ buffering power in the sheep cardiac Purkinje fibre. J Physiol. 1990 Jun;425:429–448. doi: 10.1113/jphysiol.1990.sp018112. [DOI] [PMC free article] [PubMed] [Google Scholar]


