Abstract
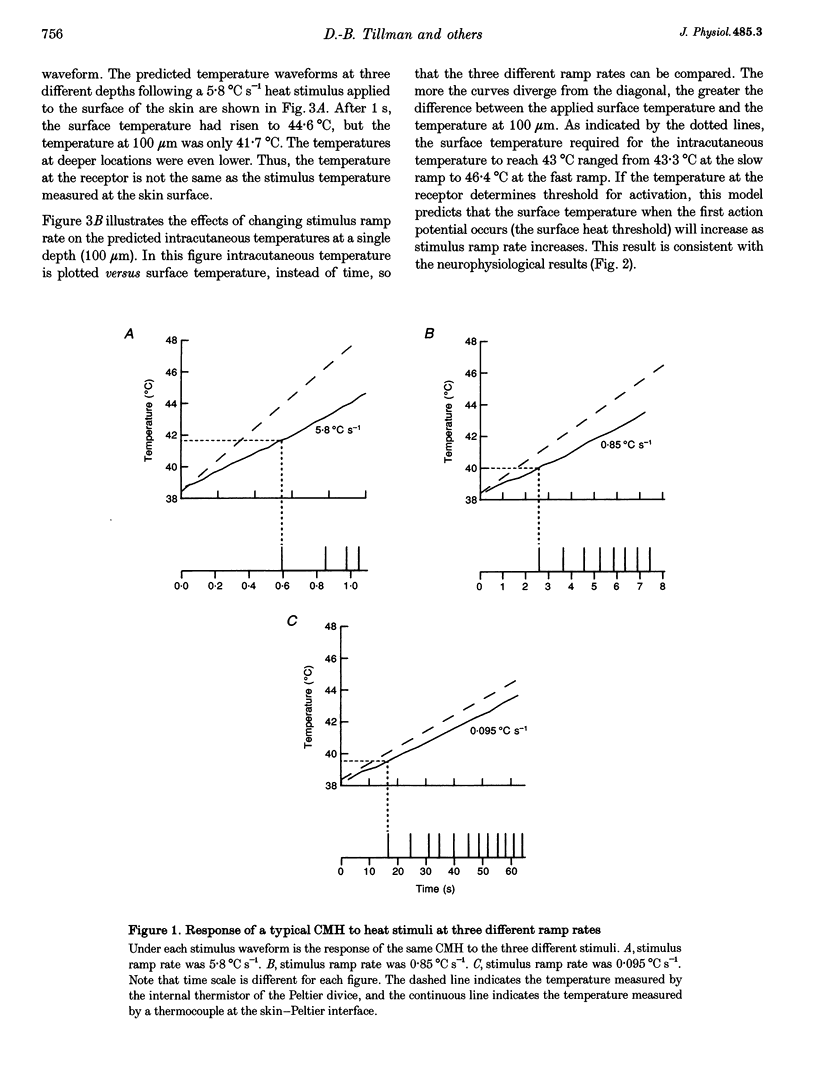
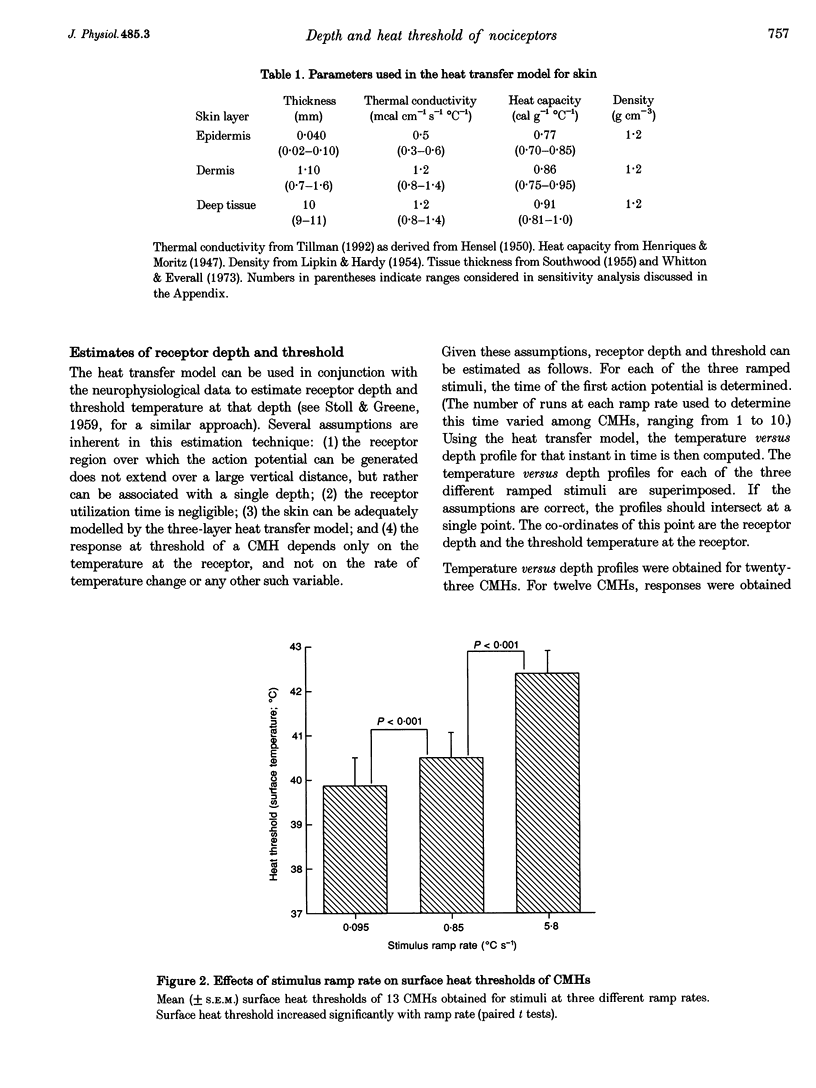
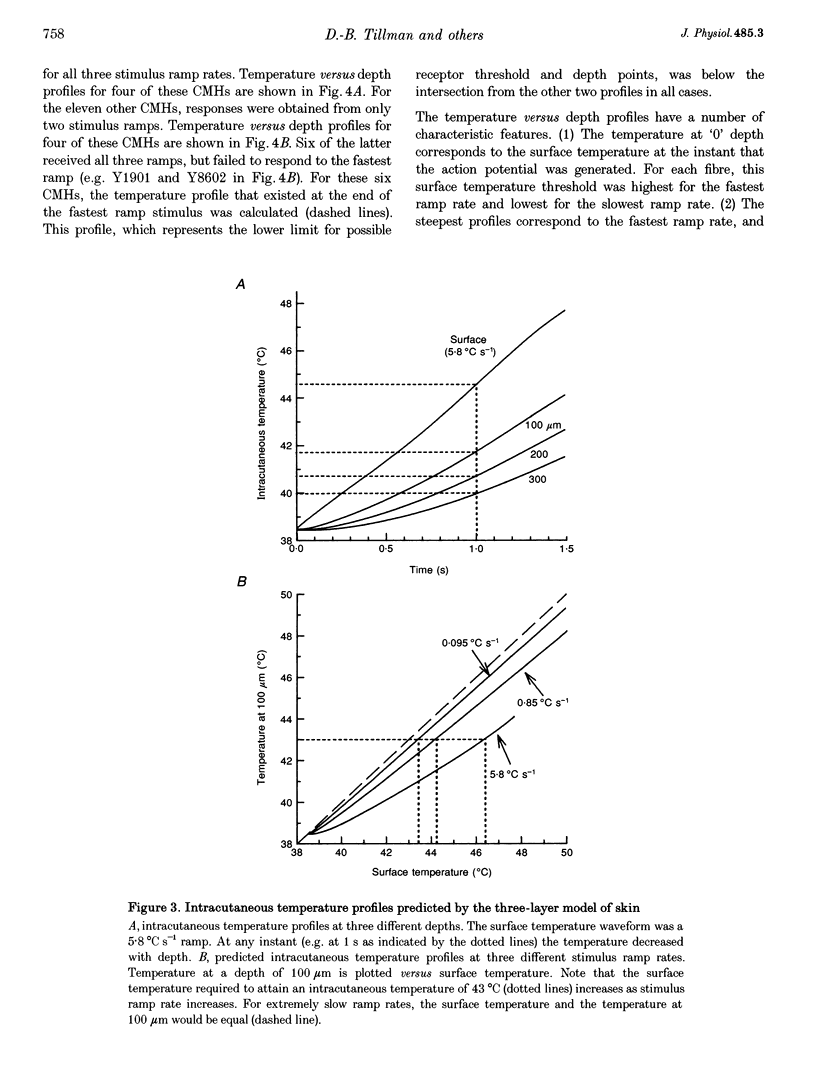
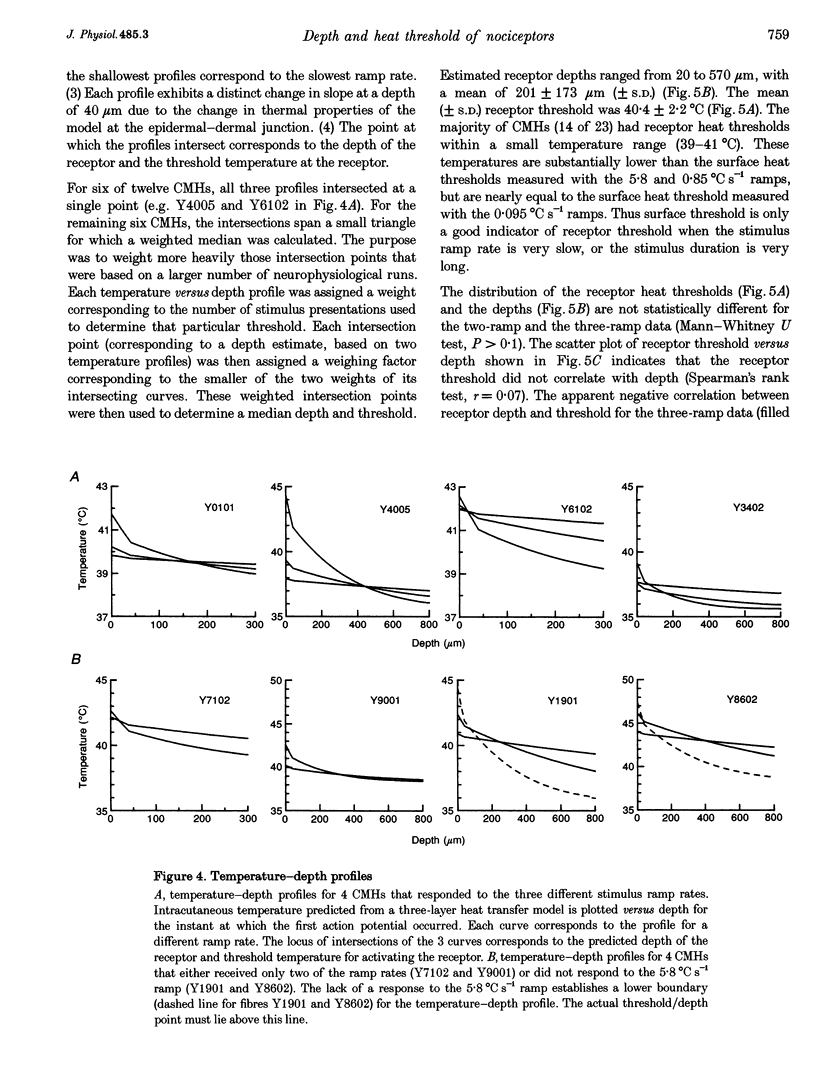
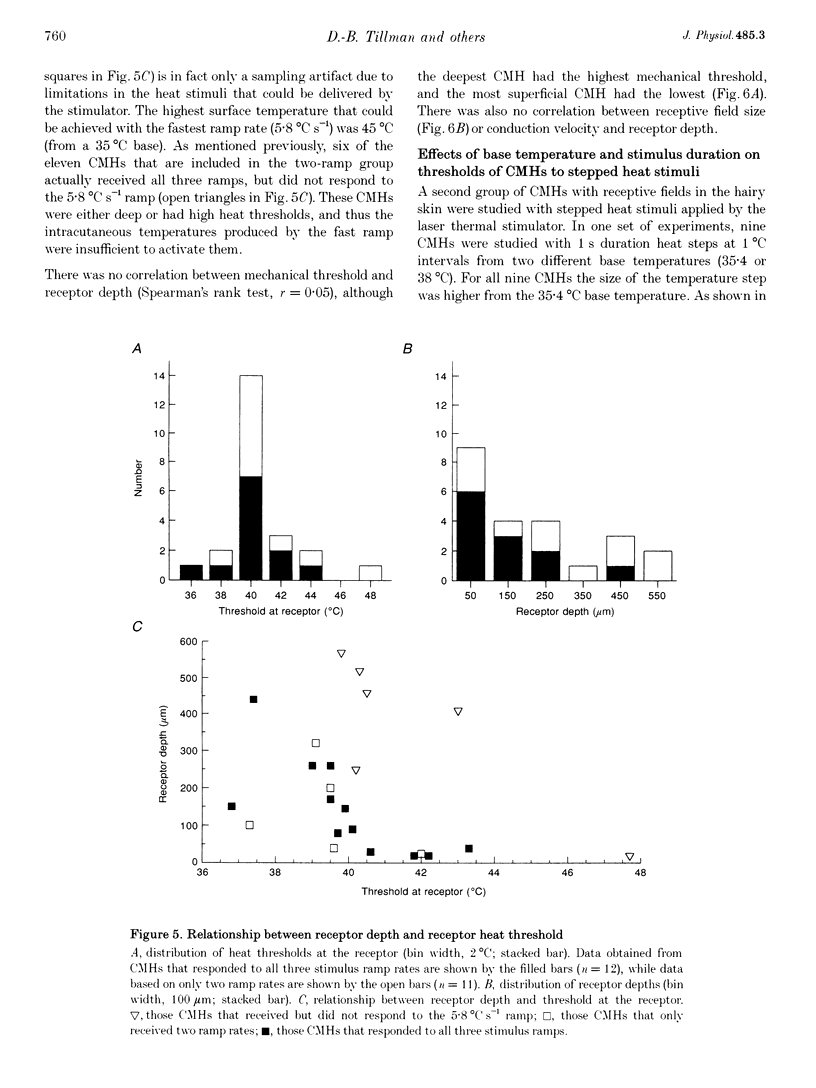
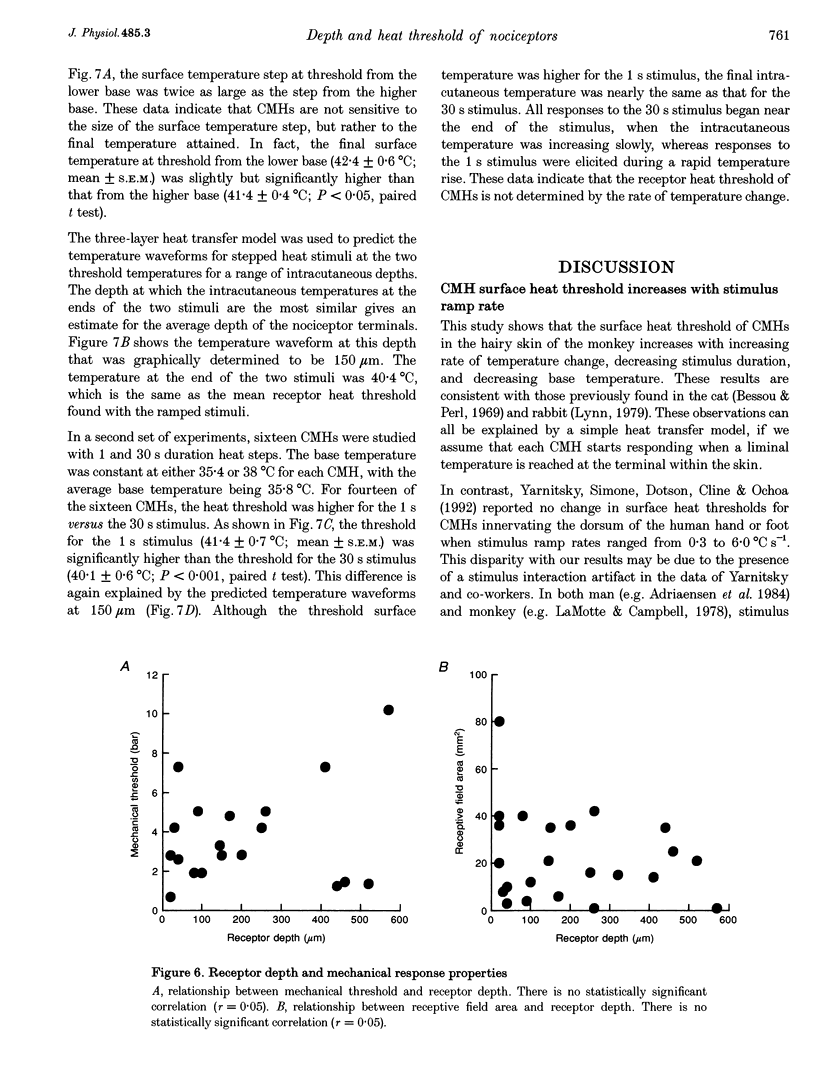
1. Responses to ramped or stepped temperature stimuli were obtained from fifty-three cutaneous C fibre mechano-heat nociceptors (CMHs) in the hairy skin of the pentobarbitone-morphine anaesthetized monkey. A three-layer heat transfer model was developed to describe the temperature distribution within the skin and to estimate receptor depth and heat threshold. 2. Surface heat threshold, defined as the surface temperature when the first action potential occurs, increased as: (a) the rate of temperature rise for the ramped stimuli increased from 0.095 to 5.8 degrees C s-1; (b) the duration of stepped heat stimuli decreased from 30 to 1 s; and (c) the base temperature of stepped heat stimuli decreased from 38 to 35 degrees C. These results suggest that the heat threshold for CMHs is determined by the temperature at the depth of the receptor. 3. Receptor depth estimates from responses to ramped stimuli ranged from 20 to 570 microns with a mean of 201 microns. The estimated mean receptor heat threshold was 40.4 +/- 2.2 degrees C (+/- S.D.). No correlation was observed between depth and thermal or mechanical threshold. The average receptor depth and threshold, estimated from the responses to stepped heat stimuli, were 150 microns and 40.2 degrees C, respectively. 4. We conclude that: (a) the receptor endings of CMHs occur in the epidermis and dermis; (b) temperature at the level of the receptor determines threshold; (c) temperature at the receptor ending is much lower than skin surface temperature at threshold; and (d) the tight distribution of receptor heat thresholds suggests a uniform transducer mechanism for heat in CMHs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARTHUR R. P., SHELLEY W. B. The innervation of human epidermis. J Invest Dermatol. 1959 Mar;32(3):397–411. doi: 10.1038/jid.1959.69. [DOI] [PubMed] [Google Scholar]
- Adriaensen H., Gybels J., Handwerker H. O., Van Hees J. Suppression of C-fibre discharges upon repeated heat stimulation may explain characteristics of concomitant pain sensations. Brain Res. 1984 Jun 8;302(2):203–211. doi: 10.1016/0006-8993(84)90232-4. [DOI] [PubMed] [Google Scholar]
- BUETTNER K. Effects of extreme heat and cold on human skin. II. Surface temperature, pain and heat conductivity in experiments with radiant heat. J Appl Physiol. 1951 Jun;3(12):703–713. doi: 10.1152/jappl.1951.3.12.703. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Breathnach A. S. Electron microscopy of cutaneous nerves and receptors. J Invest Dermatol. 1977 Jul;69(1):8–26. doi: 10.1111/1523-1747.ep12497857. [DOI] [PubMed] [Google Scholar]
- Bromm B., Jahnke M. T., Treede R. D. Responses of human cutaneous afferents to CO2 laser stimuli causing pain. Exp Brain Res. 1984;55(1):158–166. doi: 10.1007/BF00240510. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Meyer R. A. Sensitization of unmyelinated nociceptive afferents in monkey varies with skin type. J Neurophysiol. 1983 Jan;49(1):98–110. doi: 10.1152/jn.1983.49.1.98. [DOI] [PubMed] [Google Scholar]
- Cauna N. Fine morphological characteristics and microtopography of the free nerve endings of the human digital skin. Anat Rec. 1980 Dec;198(4):643–656. doi: 10.1002/ar.1091980409. [DOI] [PubMed] [Google Scholar]
- Cauna N. The free penicillate nerve endings of the human hairy skin. J Anat. 1973 Jul;115(Pt 2):277–288. [PMC free article] [PubMed] [Google Scholar]
- Croze S., Duclaux R., Kenshalo D. R. The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol. 1976 Dec;263(3):539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSEL H., ZOTTERMAN Y. Action potentials of cold fibres and intracutaneous temperature gradient. J Neurophysiol. 1951 Sep;14(5):377–385. doi: 10.1152/jn.1951.14.5.377. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Neher K. D. Characteristics of C-fibre receptors in the cat's foot responding to stepwise increase of skin temperature ot noxious levels. Pflugers Arch. 1976 Sep 30;365(2-3):221–229. doi: 10.1007/BF01067022. [DOI] [PubMed] [Google Scholar]
- Hardy J. D., Stolwijk J. A., Hammel H. T., Murgatroyd D. Skin temperature and cutaneous pain during warm water immersion. J Appl Physiol. 1965 Sep;20(5):1014–1021. doi: 10.1152/jappl.1965.20.5.1014. [DOI] [PubMed] [Google Scholar]
- Henriques F. C., Moritz A. R. Studies of Thermal Injury: I. The Conduction of Heat to and through Skin and the Temperatures Attained Therein. A Theoretical and an Experimental Investigation. Am J Pathol. 1947 Jul;23(4):530–549. [PMC free article] [PubMed] [Google Scholar]
- Kruger L., Perl E. R., Sedivec M. J. Fine structure of myelinated mechanical nociceptor endings in cat hairy skin. J Comp Neurol. 1981 May 1;198(1):137–154. doi: 10.1002/cne.901980112. [DOI] [PubMed] [Google Scholar]
- LIPKIN M., HARDY J. D. Measurement of some thermal properties of human tissues. J Appl Physiol. 1954 Sep;7(2):212–217. doi: 10.1152/jappl.1954.7.2.212. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Campbell J. N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978 Mar;41(2):509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Lynn B. The heat sensitization of polymodal nociceptors in the rabbit and its independence of the local blood flow. J Physiol. 1979 Feb;287:493–507. doi: 10.1113/jphysiol.1979.sp012672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER M. R., RALSTON H. J., 3rd, KASAHARA M. The pattern of cutaneous innervation of the human hand. Am J Anat. 1958 Mar;102(2):183–217. doi: 10.1002/aja.1001020203. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Walker R. E., Mountcastle V. B., Jr A laser stimulator for the study of cutaneous thermal and pain sensations. IEEE Trans Biomed Eng. 1976 Jan;23(1):54–60. doi: 10.1109/tbme.1976.324616. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Halata Z. The sensory innervation of primate facial skin. I. Hairy skin. Brain Res. 1983 Jan;286(1):45–80. doi: 10.1016/0165-0173(83)90021-8. [DOI] [PubMed] [Google Scholar]
- Novotny G. E., Gommert-Novotny E. Intraepidermal nerves in human digital skin. Cell Tissue Res. 1988 Oct;254(1):111–117. doi: 10.1007/BF00220023. [DOI] [PubMed] [Google Scholar]
- SOUTHWOOD W. F. The thickness of the skin. Plast Reconstr Surg (1946) 1955 May;15(5):423–429. doi: 10.1097/00006534-195505000-00006. [DOI] [PubMed] [Google Scholar]
- STOLL A. M., GREENE L. C. Relationship between pain and tissue damage due to thermal radiation. J Appl Physiol. 1959 May;14(3):373–382. doi: 10.1152/jappl.1959.14.3.373. [DOI] [PubMed] [Google Scholar]
- Stolwijk J. A., Hardy J. D. Skin and subcutaneous temperature changes during exposure to intense thermal radiation. J Appl Physiol. 1965 Sep;20(5):1006–1013. doi: 10.1152/jappl.1965.20.5.1006. [DOI] [PubMed] [Google Scholar]
- Tillman D. B., Treede R. D., Meyer R. A., Campbell J. N. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: correlation with pain threshold in humans. J Physiol. 1995 Jun 15;485(Pt 3):767–774. doi: 10.1113/jphysiol.1995.sp020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede R. D., Meyer R. A., Campbell J. N. Comparison of heat and mechanical receptive fields of cutaneous C-fiber nociceptors in monkey. J Neurophysiol. 1990 Nov;64(5):1502–1513. doi: 10.1152/jn.1990.64.5.1502. [DOI] [PubMed] [Google Scholar]
- Whitton J. T., Everall J. D. The thickness of the epidermis. Br J Dermatol. 1973 Nov;89(5):467–476. doi: 10.1111/j.1365-2133.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]
- Wilcox G. L., Giesler G. J., Jr An instrument using a multiple layer Peltier device to change skin temperature rapidly. Brain Res Bull. 1984 Jan;12(1):143–146. doi: 10.1016/0361-9230(84)90227-2. [DOI] [PubMed] [Google Scholar]
- Woollard H. H. Intra-Epidermal Nerve Endings. J Anat. 1936 Oct;71(Pt 1):54–60.5. doi: 10.1097/00005053-193707000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnitsky D., Ochoa J. L. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain. 1990 Jan;40(1):85–91. doi: 10.1016/0304-3959(90)91055-N. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D., Simone D. A., Dotson R. M., Cline M. A., Ochoa J. L. Single C nociceptor responses and psychophysical parameters of evoked pain: effect of rate of rise of heat stimuli in humans. J Physiol. 1992 May;450:581–592. doi: 10.1113/jphysiol.1992.sp019144. [DOI] [PMC free article] [PubMed] [Google Scholar]



