Abstract
Background
Acute mountain sickness (AMS) is a debilitating condition that individuals may develop on ascent to high altitude. It is characterized by headache, nausea, vomiting, dizziness, and fatigue with the potential to progress to fatal disease. Although the pathophysiology of AMS remains unclear, proposed mechanisms are hypothesized to be similar to migraine. Prochlorperazine, a first-line treatment for acute migraine, has been shown to abort migraine early and thus may be effective in preventing AMS. Its action as a respiratory stimulant additionally makes it a promising novel agent for AMS prevention.
Methods
In this randomized double-blinded trial, participants will be randomized to receive oral prochlorperazine maleate or placebo for 24 h of three times daily dosing on a rapid ascent to 4348 m. Participants will be adults, aged 18, and older who are unacclimatized. Participants will remain at this elevation overnight. The Lake Louise Questionnaire will be utilized to define the primary outcome and presence of AMS and will be assessed the evening of and morning after ascent to peak altitude.
Discussion
Currently, acetazolamide is the preferred option for the chemoprophylaxis of AMS, which has been studied and utilized since the 1970s and involves potential prohibitive side effects. Other more efficacious options with more tolerable side effects are needed. Preventing AMS has the potential to limit both the morbidity and mortality associated with developing AMS and more serious diseases (notably high-altitude cerebral edema). Additionally, there is a substantial economic and environmental impact of AMS that could be prevented.
Trial registration
Clinicaltrial.gov, NCT06450899. Registered on June 2024.
Keywords: Acute mountain sickness, Chemoprophylaxis, Altitude illness, Prevention, Clinical trial
Administrative information
| Title {1} | Prochlorperazine Maleate versus Placebo for the Prevention of Acute Mountain Sickness: Study Protocol for a Randomized Controlled Trial |
| Trial registration {2a and 2b} | Clinicaltrials.gov NCT 06450899 |
| Protocol version {3} | V4; 7/11/24 |
| Funding {4} | 2023 Research Award International Society of Travel Medicine in partnership with the GeoSentinal Foundation |
| Author details {5a} |
1: University of Colorado School of Medicine, Department of Emergency Medicine, Aurora, CO USA 2: Department of Computer Science, University of Colorado School of Medicine, Aurora, CO |
| Name and contact information for the trial sponsor {5b} |
University of Colorado Section of Wilderness Medicine Department of Emergency Medicine Phone: 720–848-6777 E-mail (principal investigator): martin.mus@cuanschutz.edu |
| Role of sponsor {5c} | This trial is funded by the International Society of Travel Medicine however the analysis and trial will be conducted independently by the study team |
Introduction
Background and rationale {6a}
Background
High-altitude travel has become an increasingly popular venture, with roughly 100 million high-altitude visits annually [1]. When unacclimatized individuals travel to altitude, they incur a risk of developing acute mountain sickness (AMS), a potentially debilitating condition characterized by headache and additional symptoms including nausea and vomiting, fatigue, and dizziness with the potential to lead to fatal disease, notably high-altitude cerebral edema (HACE). Acute mountain sickness is altitude-dependent and common, occurring in 50–85% of unacclimatized individuals ascending to 4500–5500 m [2, 3]. Despite decades of research, AMS pathophysiology remains unclear. However, AMS shares clinical and proposed pathophysiological characteristics with migraine [4, 5]. Such similarities suggest that AMS could be prevented using migraine medications [6]. For instance, medications that are effective for migraine such as sumatriptan and metoclopramide have been shown to have potential for AMS prophylaxis [7] and high-altitude headache [8], respectively.
Prochlorperazine maleate is a piperazine phenothiazine with primarily anti-D2 dopaminergic activity [9] and is recommended by the American Headache Society as a first-line migraine agent [10]. The underlying mechanism for prochlorperazine-mediated migraine abatement is unclear, [11] though limited evidence has shown antidopaminergic medications may serve to abort migraines in the early premonitory phase [12]. Given potential similarities between AMS and migraine pathophysiology and demonstrated early migraine abatement with dopamine antagonists, prochlorperazine administration prior to or early in altitude travel may help prevent the cascade leading to AMS development. Additionally, prochlorperazine has been shown to stimulate ventilation and augment hypoxic ventilatory response [13]. Less robust hypoxic ventilatory response has been shown to increase the risk of developing AMS, [14] further supporting prochlorperazine’s utility as a prophylactic agent.
Thus, our objective is to examine the efficacy of prochlorperazine in a randomized controlled trial for the chemoprophylaxis of AMS. Although there is potential for prochlorperazine to reduce the risk of AMS, there are no published randomized controlled trials that demonstrate efficacy and safety when used prior to or early in ascent for unacclimatized individuals.
Risk/benefit assessment
Overall, the risks to potential participants are unlikely, small, and reasonable in relation to the potential benefit, as prochlorperazine has a favorable safety profile and is a commonly used medication in migraines and vomiting. The study drug prochlorperazine has demonstrated excellent tolerability. Serious adverse events are rare, [11] with the most commonly reported side effects being drowsiness and extrapyramidal effects (akathisia and dystonia). Drowsiness, while more common, had been reported as mild [15] and did not show significantly increased pooled odds ratios over other comparison migraine therapeutics [11]. Akathisias did occur in studies utilizing intravenous dosing at rates of around 16%, [16] though in limited data on oral prochlorperazine, there were no reported occurrences of extrapyramidal side effects [15]. Dystonia has also been a reported potential side effect of prochlorperazine maleate, with one large Emergency Department study reporting < 2% when given intravenously [16]. Other less common side effects of prochlorperazine include tardive dyskinesia, which occurs in a dose-dependent fashion and for the limited dosing over 24 h in this study, these effects are unlikely. The scope of outdoor recreation occurring in this study involves minimal risk, including sunburn, dehydration, hiking-related injury, and lightning injury. Sojourning to altitude incurs a risk of altitude illness, most commonly AMS symptoms of which are typically non-serious, temporary, and manageable. More serious altitude illnesses including high altitude pulmonary and cerebral edema are rare and typically take over 24 h to develop, making them exceedingly uncommon for participants in this study.
Given the appeal of prochlorperazine as an AMS prophylactic agent and its potential relevance in AMS pathophysiology, it has significant potential to limit AMS-related morbidity and mortality. Effective prophylaxis of AMS can reduce the sizable negative economic impact related to tourism in high-altitude communities such as in Colorado and reduce the resource expense and environmental toll associated with high-altitude rescue in often remote areas. Additionally, identifying an effective prophylaxis medication with known pharmacological effects may offer insights into the poorly understood pathophysiology of AMS.
Objectives {7}
Objective
The objective of this study is to determine the utility of prochlorperazine maleate for the prophylaxis of AMS.
Hypothesis
We hypothesize that prochlorperazine maleate prophylaxis will prevent the development of AMS when compared to placebo during rapid ascent to high altitude.
Trial design {8}
This study will be a double-blind randomized controlled trial to investigate the efficacy of prochlorperazine maleate versus placebo for the chemoprophylaxis of AMS on rapid ascent to 4348 m in accordance with Strengthening Altitude Research (STAR) core parameters [17]. Participants will be evaluated for AMS utilizing the 2018 LLQ both the evening of and morning after ascent. The primary outcome will be the presence of AMS, defined by a 2018 LLQ score ≥ 3 including the presence of a headache at any measured point during the study. The study will adhere to applicable STAR core reporting parameters [17].
Methods: participants, interventions, and outcomes
Study setting {9}
Study site and ascent profile
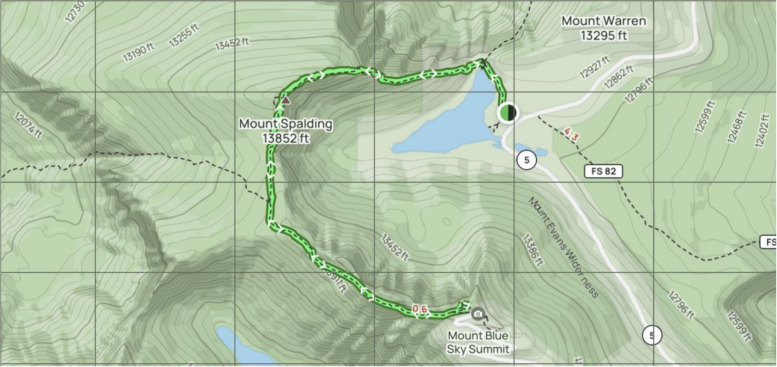
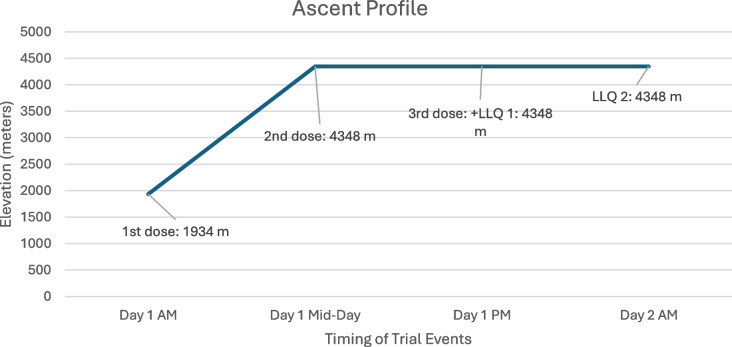
This study will take place on Mount Blue Sky, an iconic peak located in the Rocky Mountain Range with an altitude of 4348 m, with a prominence of 839 m. The study will occur in the month of August. Mount Blue Sky is reachable by both hiking trail and vehicular traffic. Individuals will convene at the meeting point at 1934 m where they will ascend via commercial vehicle to 3911 m. Participants will then hike to the summit of Mount Blue Sky at 4348 m (Fig. 1), where they will spend the night (Fig. 2). Formal approval, including a special research permit, has been obtained from the United States Forest Service.
Fig. 1.
Hiking path from Summit Lake to Mount Blue Sky (formerly Evans) summit
Fig. 2.
Ascent profile with labeled drug administration and outcome assessments
Eligibility criteria {10}
The study population will include adult individuals (≥ 18 years) who meet inclusion criteria and are agreeable to participate under informed consent.
Inclusion criteria
Adults ≥ 18 years old.
Exclusion criteria
Individuals < 18 years old or > 75 years old. Given the inherent risks of outdoor recreation and degree of physical fitness needed to successfully complete the study, the age of 75 was selected as the upper limit in this multifaceted consideration.
Pregnant women
Individuals who reside at or have slept at elevations > 1800 m in the last 2 weeks to eliminate acclimatization.
Individuals who ingested acetazolamide, corticosteroids, ibuprofen, anti-emetics, or additional analgesics (e.g., acetaminophen) within the last 24 h prior to study initiation in order to eliminate their confounding effects
Individuals requiring supplemental baseline oxygen or with chronic disorders known to be significantly impacted by hypoxia (such as chronic obstructive pulmonary disease and interstitial lung disease)
Individuals with known allergies to prochlorperazine or phenothiazines
Individuals taking medications that interact with prochlorperazine: dofetilide, potassium acid phosphate, potassium chloride, potassium citrate, potassium phosphate, Yohimbe.
Individuals living with dementia
Individuals who lack decision-making capacity as this was a stipulation by the ethics review board given the outdoor hiking at altitude component. Decision-making capacity will be assessed by study team members during the consent process to ensure the individuals understand the risks and benefits of the study.
Who will take informed consent? {26a}
Interested individuals will be screened for eligibility. If eligible, they will perform written informed consent using IRB-approved consent forms describing the study interventions, procedures, and risks. The study team will explain the information in the consent verbally in a comprehensible manner. We will obtain consent electronically or face-to-face depending on the availability of the participant. Participants will have the opportunity to review the consent forms and ask questions. The study team will ask questions to ensure comprehension of the consent and study protocol. If agreeable, the participant will sign the consent form with the explicit knowledge that they may leave the study at any time. If non-English speaking, the study team will provide a translator to assist.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable.
Interventions
Explanation for the choice of comparators {6b}
Currently, there are limited options for AMS chemoprophylaxis, with acetazolamide as the most frequently recommended and common option, with one recent meta-analysis demonstrating a pooled risk reduction of 12.8% [18]. Acetazolamide usage comes with the risk of side effects, notably potentially uncomfortable paresthesias that may prove prohibitive for some. Given the mixed utility in the literature and side effect profile, there is no firm usual care for AMS chemoprophylaxis. The Wilderness Medical Society 2024 guidelines specify acetazolamide as the first-line option, though reserved for those individuals at moderate or high risk of AMS and thus not standard usual care [19].
Thus, the usual care arm will receive a non-identical placebo tablet and not an additional chemoprophylactic agent.
Intervention description {11a}
This study will randomize study participants with concealed allocation into either the intervention arm or the placebo arm using a simple random sample methodology generated by the team statistician.
Individuals randomized into the study arm will receive generic prochlorperazine maleate 10 mg orally taken three times daily for one day. Participants will convene in the morning of the trial date at low altitude, where they will receive their first dose roughly around 07:00. They will then be driven to Summit Lake and hike to the summit, where they will receive their second dose approximately 6 h later, around 13:00. They will receive their final dose in the evening, approximately another 6 h later around 19:00.
The placebo arm individuals will receive a non-identical inert generic placebo tablet containing microcrystalline cellulose provided by Belmar Pharma Solutions. Placebo tablets will be taken at the same time and frequency as the study intervention drug.
Criteria for discontinuing or modifying allocated interventions {11b}
Discontinuation of the study intervention will occur if any of the following conditions are met:
Allergic reaction to study interventions
Altitude illness requiring treatment or descent, or any injury/illness requiring treatment with confounding medicines including ibuprofen, acetaminophen, and anti-emetics. Participants will be free and encouraged to seek care and free to utilize these drugs, however, will result in an immediate LLQ assessment at the time of treatment and discontinuation of the study intervention.
Injury/illness requiring descent
Participant wishes to drop out of study
Strategies to improve adherence to interventions {11c}
Study interventions will be directly administered by an unblinded dedicated study team member administering placebo or study drugs and thus will ensure adherence to interventions.
Relevant concomitant care permitted or prohibited during the trial {11d}
Emergency physicians will be present to administer routine care for any injury or illness that may occur while traveling or hiking at altitude. In the event of more serious altitude illness, emergency physicians may administer care including oxygen therapy or acetazolamide. Alcohol (or use of any additional intoxicants) and use of acetazolamide will be prohibited during the trial.
Provisions for post-trial care {30}
As the trial encompasses a 36-h period, no post-trial care will be provided. There will be no compensation for participants who suffer harm from trial participation, but emergency physicians will be able to treat and prevent harm by close monitoring of adverse events.
Outcomes {12}
Primary endpoint
The primary outcome will be the presence of AMS, defined by a 2018 Lake Louise Questionnaire (LLQ) score ≥ 3 including the presence of a headache on any measured LLQ during the study.
Exploratory endpoints
Exploratory endpoints will include moderate AMS defined by a 2018 LLQ score > 5 including the presence of a headache, raw Lake Louise Questionnaire score, demographic variables, Groningen Sleep Quality Scale scores, medication side effects, and vital sign changes.
Participant timeline {13}
Schedule of event matrix
| Time | Location | Visit | Procedures | Application |
|---|---|---|---|---|
| 06:00 | Golden, Colorado Wooley Mammoth Park & Lot (1,934 m) | Medical Screening/Pre-Travel Check in | Basic physical exam, medical history, (informed consent previously obtained) | Re-explain the risks and benefits of participation. Re-assess for inclusion/exclusion criteria. Ensure adequate gear/preparation |
| 07:00 | Study Protocol Dose #1 | Computer randomization assigned individual to study arm or placebo arm | Dispense dose #1 of prochlorperazine maleate 10 mg or non-identical placebo | |
| 7:00 | Golden, CO (1,934 m) to Summit Lake Mount Blue Sky (3,911 m) | Transport participants to Summit Lake via hired vehicles | ||
| 9:00-13:00 | Summit Lake to Mount Blue Sky (3,911 m) | Travel to Study Site | Hike to summit | Travel accompanied by Emergency Physicians in scenic hike to peak altitude and study site destination |
| 13:00-14:00 | Summit of Mount Blue Sky (4,348 m) | Study Protocol Dose #2 | Break for lunch, Dose #2 | Break for lunch, dispense dose #2 of prochlorperazine maleate 10 mg or non-identical placebo. |
| 18:00-20:00 | Summit of Mount Blue Sky (4,348 m) | Study Protocol Dose #3 | Dinner, Biometric Data, AMS assessment, Dose #3 | Eat dinner, obtain biometric data, obtain LLQ for presence of AMS, dispense dose #3 of prochlorperazine maleate 10 mg or non-identical placebo, spend night at summit |
| 07:00-10:00 | Eat breakfast, repeat LLQ for AMS assessment, and biometric data, pack up, transport participants back to Golden, Colorado. | |||
| This will be repeated for each group of 15-30 participants. | ||||
Schedule of enrollment, interventions and assessments
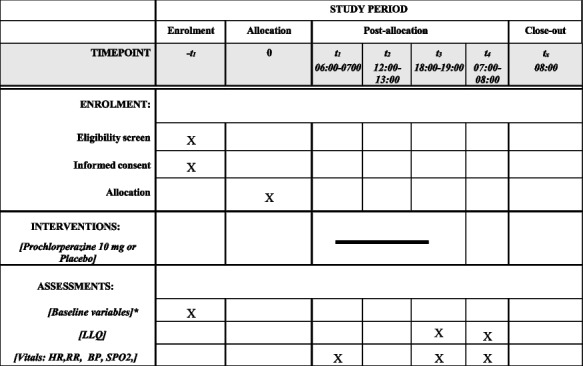
*Baseline variables include sex, age, height, weight, home altitude, prior history of altitude illness, if applicable menopausal status and most recent menstrual cycle, medical history, medications
LLQ Lake Louise Questionnaire, HR heart rate, RR respiratory rate, BP blood pressure, SpO2 oxygen saturation via pulse oximetry
Sample size {14}
Previous literature at similar study sites with similar recruitment catchments demonstrated an AMS incidence range of 45–68% [20, 21]. For example, a prospective double-blinded-placebo controlled trial investigating low-dose acetazolamide versus placebo was conducted on Pikes Peak and recruited subjects residing between 1400 and 1600 m, which includes the elevation of the Denver region [20]. This study reported a total incidence of AMS of 55.5% [20]. A recent meta-analysis reported an absolute risk reduction of 16.7% in preventing AMS with acetazolamide at the currently recommended standard dose [22].
To conduct our power analysis, we utilized STATA. Based on the literature described above, we assumed an AMS incidence rate of 50%. To detect an absolute risk reduction roughly double that of acetazolamide, specifically 33.4%, utilizing an alpha < 0.05 our study would require a total sample size of 62 participants to achieve 80% power. Due to space and budget constraints, the maximum number of individuals enrolled will be 100.
Recruitment {15}
This study will enroll a convenience sample of individuals. We will recruit individuals via word-of-mouth advertising, flyers, e-mails to relevant listservs of organizations, social media as well as constructing a website for the research group and project. The principal investigator or study team member will field responses (usually via e-mail or telephone or in person if possible) from interested individuals. Recruitment will be targeted towards individuals living in the Denver region and areas of lower elevation (e.g., Grand Junction). There will not be pre-screening of participants outside of pre-specified inclusion and exclusion criteria.
Assignment of interventions: allocation
Sequence generation {16a}
As described, this study will randomize study participants with concealed allocation into either the intervention arm or the placebo arm utilizing a simple random sample algorithm generated by the team statistician.
Concealment mechanism {16b}
Randomization to each of the two groups will occur via a simple random sample (generated by the team statistician) without stratification. Allocation will be concealed to all study team members with the exception of the pharmacy, statistician, and the dedicated study team member who will dispense the study drug or placebo.
Implementation {16c}
Study team members will enroll participants. The team statistician will generate the randomized allocation sequence and communicate assignments to the pharmacy.
Assignments of interventions: blinding
Who will be blinded {17a}
This study will be double-blinded and placebo controlled. There will be one study team member dedicated to study drug/placebo administration who will be unblinded. The pharmacist and statistician will additionally unblinded. The remaining members of the study team will be blinded at all times. The participants additionally will be blinded.
Due to budget constraints, constructing an identical placebo is not feasible, thus a non-identical placebo will be utilized. This is an inherent limitation and will be mentioned in the final manuscript as a limitation. However, the placebo and study intervention are overall very similar in appearance as shown below including similarly sized and shaped pills (Fig. 3).
Fig. 3.
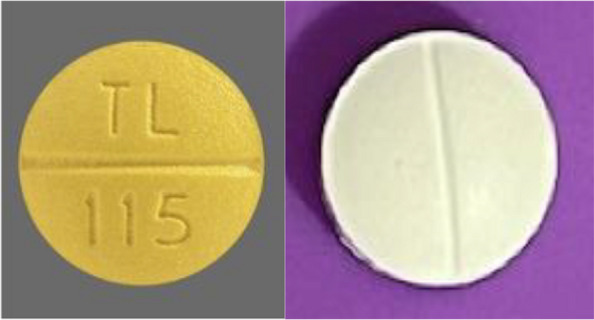
Study intervention pictured on the left, placebo on the right
To provide as much blinding as possible, the drugs will be stored in opaque vials to limit visualization by other individuals. Given the relatively small sample size per trial period, at the indicated time, individuals will proceed one by one to a physically separate location away from other participants or study members. The unblinded study team member will then dispense the study drug or placebo from the opaque vial and then immediately store the vial with the remaining doses back into the secure study container.
The participants will be instructed not to discuss the appearance of their received medication and to not seek to identify it based on any resources available to help avoid any bias.
Procedure for unblinding if needed {17b}
In the event of an emergency requiring unblinding, the dedicated team member assigned to study intervention administration will have the necessary information to unblind. There will be additionally an off-site team member able to unblind.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Acute mountain sickness is a clinical diagnosis, occurring in unacclimatized individuals on ascent to high altitude defined by a constellation of symptoms including headache, nausea and vomiting, dizziness, and headache. For research purposes, there are a number of developed scoring systems to define AMS, the current standard and most commonly utilized being the Lake Louise Questionnaire, first developed in 1993, and updated in 2018 [23]. The LLQ stipulates that a score of ≥ 3, including the presence of a headache, defines AMS. Thus, the primary outcome will be the presence of AMS, defined by 2018 LLQ score ≥ 3 including the presence of a headache, at any measured point during the study.
Plans to promote participant retention and complete follow-up {18b}
As trials will occur over a relatively brief period with continuous oversight by study team members, follow-up and retention will be ensured. This study will be conducted as a modified intention to treat, and any study participants that receive the first dose of study intervention will be included in data analysis in the arm to which they were randomized. We will also perform a per-protocol analysis.
Data management {19}
Data will be collected and stored in the secure data collection software REDCap. We will export data onto secure password-locked computers for statistical analysis by our team statistician.
Confidentiality {27}
All participant information will be entered via REDCap. No personal information or study data will be shared outside the immediate study team.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable; no laboratory of biological specimens will be collected.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
The difference in primary outcome (presence of AMS based on LLQ ≥ 3 including headache measured at any point during the study) between the intervention and placebo group will be investigated using a Chi-squared analysis. We will calculate an absolute risk reduction and number needed to treat for prochlorperazine maleate to prevent AMS. This study will utilize unpaired two-sample t-tests and chi-squared analysis to compare continuous and categorical variables, respectively, in the intervention and placebo arm cohorts (e.g., age, sex, history of altitude illness). The alpha level will be p = 0.05 for all analysis.
Interim analyses {21b}
There will be no formal interim statistical analysis. The study team will continuously monitor for adverse and safety events.
The study may be terminated or suspended for the following reasons:
Insufficient recruitment
Determination of unexpected, significant or unacceptable risk to participants including observed serious adverse intervention effects
Methods for additional analyses (e.g., subgroup analyses) {20b}
While sample size will limit rigorous subgroup analysis, we will additionally conduct a Firth regression to assess the association between intervention and placebo administration and the presence/absence of AMS by adjusting for sex and other relevant factors (e.g., participant historical and demographic factors).
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
This study will analyze participants on a modified intent-to-treat basis, where any individual who has received their first study intervention will be included in the final analysis.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
This study will not grant public access to the participant-level dataset and statistical code. The protocol will be available on clinicaltrials.gov.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee {5d}
As a single-center trial, there is no need for a coordinating center or trial steering committee. The core study team will provide day-to-day trial support and oversee all trial logistics and review. The core study team consists of emergency medicine physicians, research assistants, and a team statistician who will prepare randomization and analyze data. The core study team will generally meet weekly, as well as before and after each trial period.
Composition of the data monitoring committee, its role and reporting structure {21a}
As a single-center trial, there will not be a data monitoring committee. The study team will primarily consist of emergency medicine physicians and a team statistician who will conduct the study and analyze the data.
Adverse event reporting and harms {22}
Adverse events, including serious adverse events, determined by the principal investigator(s) to be related to the study protocol/intervention will be recorded and detailed and reported to the IRB and pertinent authorities according to regulatory guidelines which include:
The principal investigator will report any suspected adverse reaction or adverse event to study treatment that is both serious and unexpected.
Unexpected serious suspected adverse reactions and observations from animal studies suggesting significant risk to human participants will be reported to the FDA as soon as possible but no later than within 15 calendar days following the event.
Unexpected fatal or life-threatening suspected adverse reactions represent especially important safety information and will be reported to the FDA as soon as possible but no later than seven calendar days following the reaction.
Frequency and plans for auditing trial conduct {23}
There will be no planned formal trial auditing. As this was a single-center, low-risk intervention, a data monitoring committee was not considered. The core study team will meet in between each trial date to review trial conduct.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
The trial protocol will be publicly available on clinicaltrials.gov. Any protocol deviations will be documented using standard reporting forms and shared with the ethical institutional review board (COMIRB) and sponsor as well as updated on the clinical trial registry.
Dissemination plans {31a}
The investigators will communicate trial results in form of a peer-reviewed publication.
Participant/public involvement
There was no active public/participant involvement in the design of the protocol.
Discussion
In 1976, Peter Hackett published the landmark first trial investigating the chemoprophylaxis of AMS, using acetazolamide [24]. Despite decades of investigation, there has not been a new agent that has conclusively demonstrated benefit in preventing AMS, making a newer, more efficacious agent long overdue. Prochlorperazine is well tolerated, globally available, and relatively inexpensive, an exciting option that could have significant potential in the field of AMS prevention.
Field studies on various AMS chemoprophylaxis agents are not a novel concept [19, 25]. Field AMS clinical trials involve various logistical challenges, notably safe lodging at high altitude. Overall, availability and access to high-altitude structures able to house participants is severely limited and includes (but not limited to) the White Mountain research station in the Sierra Nevada and at the Capanna Regina Margherita hut located in the Alps. While research has previously been conducted in Colorado at Pikes Peak, recent construction has made this location no longer suitable. The structures present on the summit of Mount Blue Sky, including two A-frame structures and a larger observatory present an exciting opportunity to lodge over 30 participants safely and comfortably. In addition to solid and comfortable summit structures, Mount Blue sky has a paved road allowing easy summit access as well as expeditious evacuation if necessary and is located close to Denver allowing convenience for Denver area residents (thus promoting easier recruitment) highlighting Mount Blue Sky as an exceptional clinical site for high altitude travels. As this will be the first trial conducted at this location, it will serve as a good indicator for the feasibility of future clinical trials at this location.
The study team acknowledges several limitations. Participants overall will spend a relatively short amount of time at altitude (< 48 h), though this does provide adequate time for acute mountain sickness to develop. Additionally, several symptoms of acute mountain sickness include known side effects of the study intervention including dizziness and fatigue, though notably not headache, a requirement for acute mountain sickness diagnosis.
Trial status
This manuscript represents protocol version 4, August 2, 2024. Recruitment began on May 20, 2024 and will end August 26, 2024.
Acknowledgements
We sincerely thank Denver University for their generosity in allowing the study team to use their summit structures. We thank Dr. Peter Hackett for his input and guidance regarding the study. We additionally would like to thank the United States Forest Service for their support.
Abbreviations
- AMS
Acute mountain sickness
- HVR
Hypoxic ventilatory response
- IV
Intravenous
- IRB
Institutional review board
- PO
By mouth, orally
- LLQ
Lake Louise Questionnaire
Authors’ contributions {31b}
ES is the study team lead, including study conception, grant acquisition, and protocol development. MM is the principal investigator and contributed to the study design and protocol development. EG contributed to the protocol development. BS contributed to the study design and protocol development. RP contributed to the study design and protocol development. CP is the team statistician and contributed to the study design and protocol development. LK contributed to the original study conception and grant acquisition and is the senior author, contributed to the study design and protocol development.
Funding {4}
This trial is generously funded by an International Society of Travel Medicine 2023 Research Award in partnership with the GeoSentinal Foundation.
Data availability {29}
The team primary statistician, study team members, and primary investigator will have access to the final trial dataset. There are no contractual agreements that limit access.
Declarations
Ethics approval and consent to participate {24}
This study has been approved by the Colorado Multiple Institutional Review Board (COMIRB) # 23–0958. We will obtain written informed consent to participate from all participants.
Consent for publication {32}
The consent form will be provided if requested.
Competing interests {28}
There are no financial or other competing interests for any study team members.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cornwell WK 3rd, Baggish AL, Bhatta YKD, et al. Clinical implications for exercise at altitude among individuals with cardiovascular disease: a scientific statement from the American Heart Association. J Am Heart Assoc. 2021;10(19):e023225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartsch P, Swenson ER. Clinical practice: acute high-altitude illnesses. N Engl J Med. 2013;368(24):2294–302. [DOI] [PubMed] [Google Scholar]
- 3.Maggiorini M, Buhler B, Walter M, Oelz O. Prevalence of acute mountain sickness in the Swiss Alps. BMJ. 1990;301(6756):853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache: The Journal of Head and Face. Pain. 2018;58(S1):4–16. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MH, Wright A, Imray CH. Intracranial pressure at altitude. High Alt Med Biol. 2014;15(2):123–32. [DOI] [PubMed] [Google Scholar]
- 6.Kim MW, Kim M. Can migraine prophylaxis prevent acute mountain sickness at high altitude? Med Hypotheses. 2011;77(5):818–23. [DOI] [PubMed] [Google Scholar]
- 7.Jafarian S, Gorouhi F, Salimi S, Lotfi J. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol. 2007;62(3):273–7. [DOI] [PubMed] [Google Scholar]
- 8.Irons HR, Salas RN, Bhai SF, Gregorie WD, Harris NS. Prospective double-blinded randomized field-based clinical trial of metoclopramide and ibuprofen for the treatment of high altitude headache and acute mountain sickness. Wilderness Environ Med. 2020;31(1):38–43. [DOI] [PubMed] [Google Scholar]
- 9.Smith HS, Cox LR, Smith BR. Dopamine receptor antagonists. Ann Palliative Med. 2012;1(2):137–42. [DOI] [PubMed] [Google Scholar]
- 10.Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society Evidence Assessment of Parenteral Pharmacotherapies. Headache. 2016;56(6):911–40. [DOI] [PubMed] [Google Scholar]
- 11.Golikhatir I, Cheraghmakani H, Bozorgi F, Jahanian F, Sazgar M, Montazer SH. The efficacy and safety of prochlorperazine in patients with acute migraine: a systematic review and meta-analysis. Headache: 2019;59(5):682–700. [DOI] [PubMed] [Google Scholar]
- 12.Charles A. The evolution of a migraine attack - a review of recent evidence. Headache. 2013;53(2):413–9. [DOI] [PubMed] [Google Scholar]
- 13.Olson LG, Hensley MJ, Saunders NA. Augmentation of ventilatory response to asphyxia by prochlorperazine in humans. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(3):637–43. [DOI] [PubMed] [Google Scholar]
- 14.Richalet JP, Larmignat P, Poitrine E, Letournel M, Canouï-Poitrine F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med. 2012;185(2):192–8. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896–902. [DOI] [PubMed] [Google Scholar]
- 16.Olsen JC, Keng JA, Clark JA. Frequency of adverse reactions to prochlorperazine in the ED. Am J Emerg Med. 2000;18(5):609–11 Accessed date: 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 17.Brodmann Maeder M, Brugger H, Pun M, et al. The STAR data reporting guidelines for clinical high altitude research. High Alt Med Biol. 2018;19(1):7–14 Epub 2018 Feb 9 PMID: 29596018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto Estrada VH, Molano Franco D, Medina RD, Gonzalez Garay AG, Martí‐Carvajal AJ, Arevalo‐Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly‐used classes of drugs. Cochrane Database Syst Rev. 2017;(6):Cd009761. [DOI] [PMC free article] [PubMed]
- 19.Luks AM, Beidleman BA, Freer L, et al. Wilderness Medical Society clinical practice guidelines for the prevention, diagnosis, and treatment of acute altitude illness: 2024 update. Wilderness Environ Med. 2024;35(1_suppl):2S. [DOI] [PubMed] [Google Scholar]
- 20.Leadbetter G, Keyes LE, Maakestad KM, Olson S, van Tissot Patot MC, Hackett PH. Ginkgo biloba does--and does not--prevent acute mountain sickness. Wilderness Environ Med. 2009;20(1):66–71 Spring. [DOI] [PubMed] [Google Scholar]
- 21.van Patot MCT, Leadbetter G, Keyes LE, Maakestad KM, Olson S, Hackett PH. Prophylactic low-dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Altitude Med Biol. 2008;9(4):289–93 2008/12/01. [DOI] [PubMed] [Google Scholar]
- 22.Gao D, Wang Y, Zhang R, Zhang Y. Efficacy of acetazolamide for the prophylaxis of acute mountain sickness: a systematic review, meta-analysis, and trial sequential analysis of randomized clinical trials. Ann Thorac Med. 2021;16(4):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach RC, Hackett PH, Oelz O, et al. The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol. 2018;19(1):4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackett PH, Rennie D, Levine HD. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet. 1976;2(7996):1149–55. [DOI] [PubMed] [Google Scholar]
- 25.Lipman GS, Jurkiewicz C, Winstead-Derlega C, et al. Day of ascent dosing of acetazolamide for prevention of acute mountain sickness. High Alt Med Biol. 2019;20(3):271–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The team primary statistician, study team members, and primary investigator will have access to the final trial dataset. There are no contractual agreements that limit access.