Abstract
Background
Knee osteoarthritis (OA) is a leading cause of disability, with current treatment options often falling short of providing satisfactory outcomes. Autologous-cultured adipose-derived mesenchymal stem cells (ADMSCs) and stromal vascular fractions (SVFs) have emerged as potential regenerative therapies.
Methods
A comprehensive search was conducted among multiple databases for studies up to June 2023. The risk of bias was assessed in randomized and non-randomized studies, adhering to PRISMA guidelines. The study has been registered with PROSPERO (CRD 42023433160).
Results
Our analysis encompassed 31 studies involving 1,406 patients, of which, 19 studies with 958 patients were included in a meta-analysis, examining both SVF and autologous-cultured ADMSC methods. Significant pain reduction was observed with autologous-cultured ADMSCs starting at 3 months (MD = −2.43, 95% CI, −3.99, −0.86), whereas significant pain mitigation in response to SVF therapy was found to start at 12 months (MD = −2.13, 95% CI, −3.06, −1.21). Both autologous-cultured ADMSCs and SVF provided significant improvement in knee function starting at 12 months (MD = −9.19, 95% CI, −12.48, −5.90 vs. MD = −9.09, 95% CI, −12.67, −5.51, respectively). We found no evidence of severe adverse events linked directly to ADMSC therapy.
Conclusion
Autologous-cultured ADMSCs offer a promising alternative for more rapid pain relief in knee OA, with both ADMSCs and SVF demonstrating substantial long-term benefits in joint function and cartilage regeneration, in the absence of any severe ADMSC-related adverse events.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-04034-2.
Keywords: Adipose tissue, Knee osteoarthritis, Mesenchymal stem cells, Stromal vascular fraction
Background
Knee osteoarthritis (OA) is a progressive degenerative joint disease that is a leading cause of disability among adults, significantly impacting their quality of life (QoL) [1]. Current treatment options for knee OA, including pharmacological interventions, physiotherapy, and surgery, have notable limitations. Although pharmacological treatments, such as the administration of non-steroidal anti-inflammatory drugs or corticosteroids, can provide temporary pain relief, they tend to be associated with side effects, such as gastrointestinal and cardiovascular risks, and do not address the underlying degeneration of cartilage [2, 3]. By improving joint mobility, strength, and function, physiotherapy plays a vital role in managing OA [4, 5]. However, in more advanced stages of the disease, this therapy may be insufficient to halt disease progression or promote the regeneration of damaged tissues. Comparatively, surgical treatments, including knee arthroscopy or joint replacement, are invasive and typically entail long recovery periods and potential complications [2]. Given these limitations, there is a pressing need for innovative therapies that not only alleviate symptoms but also halt disease progression and contribute to the regeneration of damaged tissues.
Mesenchymal stem cells (MSCs) have gained significant attention in regenerative medicine, owing to their versatility in differentiating into various tissue types, including bone, tendon, fat, cartilage, and ligament. Coupled with their immunomodulatory and angiogenic properties [6, 7], MSCs, particularly adipose-derived mesenchymal stem cells (ADMSCs), have a number of advantages compared with bone marrow-derived cells (BMSCs) in terms of availability and abundance. Adipose tissue serves as a rich source of MSCs, facilitating isolation in substantial quantities compared with BMSCs [2, 6, 8]. Moreover, the less invasive nature of ADMSC isolation, which can be obtained through minimally invasive adipose tissue harvesting, contrasts more invasive processes involving bone marrow aspiration for BMSCs, which can lead to discomfort and complications [2, 9].
Recent studies have highlighted that by promoting cartilage repair and reducing inflammation, ADMSCs can effectively alleviate the symptoms of knee OA [10, 11]. Upon injection into the knee joint, these cells migrate to damaged areas thereby stimulating new cartilage regeneration. Additionally, ADMSCs secrete bioactive molecules that modulate immune responses, further reducing joint inflammation [12]. In the application of ADMSCs for knee OA, their administration via intra-articular injection or arthroscopic implantation is safe, with no significant side effects reported to date [13–15].
The stromal vascular fraction (SVF) is obtained from adipose tissues using mechanical or enzymatic methods and is immediately administered following extraction after the digestion and rinsing of cells, without undergoing any intermediary culturing [16, 17]. Similar to ADMSCs, the SVF has also been recognized for its potential efficacy in managing knee OA. The SVF is a heterogeneous mix of cells, including MSCs, endothelial cells, pericytes, and immune cells [18, 19]. This rich mixture can provide a multifaceted approach to treating OA by aiding tissue regeneration, enhancing angiogenesis, and modulating the immune environment within the joint [18]. The variability in cell types within the SVF and the absence of a culturing process to enrich specific cell populations may contribute to this observed difference in therapeutic outcomes [19, 20].
However, the ongoing discourse in regenerative medicine focuses on selecting non-cultured and cultured MSCs for knee OA treatment. The diverse cellular compositions in MSC cocktails have led to differences in clinical outcomes [21]. Moreover, the number of MSCs introduced into an inflamed joint can adversely influence chondrogenic differentiation and may cause the rapid apoptosis of transplanted cells. This diversity in cellular composition and dosage poses a significant challenge with respect to translating MSC research into practical applications for cartilage regeneration in clinical practice. Hence, understanding the optimal processing type of ADMSCs and elucidating the fine-scale differences in the efficacy of SVFs and cultured ADMSCs can enable researchers to optimize their regenerative potential and thereby enhance their efficacy in cartilage regeneration. Although previous systematic reviews have concluded that ADMSCs are effective and safe for treating knee OA [22, 23], these reviews have generally assessed a limited number of studies and have not differentiated between therapies based on cultured ADMSCs and SVFs. In this systematic review, we seek to fill these gaps by considering a broader range of studies, including the most recently published studies, to provide a more comprehensive evaluation of the efficacy and safety of autologous-cultured ADMSCs and SVFs in treating knee OA, with a particular focus on the relative efficacies of these treatments.
Methods
This review conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) guidelines [24] and is registered at the International Prospective Register of Systematic Reviews (PROSPERO) site as CRD42023433160.
Data sources and search strategy
For the purpose of this review, we searched seven databases, namely, Ovid-MEDLINE, Ovid-Embase, the Cochrane Library, RISS, DBpia, KoreaMed, and KMBASE, for relevant articles up to May 2023. The following keywords were used individually or in all possible combinations: [(“osteoarthritis” OR “osteoarthritides” OR “osteochondritis” OR “osteochondral” OR “lesion” OR “defect” OR “injury” OR “arthritis” OR “arthrosis” OR “arthroses” OR “degenerative joint disease”) AND (“mesenchymal stem cells” OR “mesenchymal” OR “adipose” OR “adipose-derived” OR “adipose tissue-derived” OR “adipocyte-derived” OR “stem cell” OR “stromal cell” OR “progenitor cell” OR “stromal vascular fraction” OR “Lipoaspirate”)].
Selection criteria
Two reviewers selected the appropriate studies based on predefined selection criteria. The inclusion criteria were as follows: (1) randomized controlled trials (RCTs) and non-randomized studies, (2) original articles, (3) patients with knee OA, and (4) intervention as ADMSC therapy, and included appropriate outcomes, such as validated measures of pain relief, functional improvement, QoL, and cartilage regeneration. Studies were excluded if they involved MSCs derived from sources other than adipose tissue or if they had used allogenic-cultured ADMSC. These were excluded due to potential immunogenicity concerns and owing to the fact that our primary focus in this review was autologous therapies, which are more widely accepted in clinical practice for knee OA, given their favorable safety profiles. Additionally, we also excluded non-human studies and non-original articles.
Two reviewers (HL and YL) independently conducted an initial screen of the titles and abstracts, based on which, they subsequently conducted comprehensive full-text reviews. The selection was agreed upon through joint discussion. Any discrepancies and/or disagreements were resolved by discussion with a third reviewer (S-HL).
Data extraction
Two independent reviewers extracted information from the selected studies, including the first author, publication year, country in which the study was conducted, design, knee OA grade, cell source, number of patients enrolled, the age of patients, cell process type, dose of injected cells, intervention methods, outcomes, follow-up time, and results. The efficacy outcomes were as follows: improvement of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Visual Analog Scale (VAS), or Numeric Rating Scale, Lysholm Knee Scale, Short Form 36 (SF-36), cartilage volume, defected cartilage size, cartilage thickness, the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) Score, and the Whole-Organ Magnetic Resonance Imaging Score (WORMS). Adverse effects were documented as indicators of safety.
Risk of bias assessment
The methodological quality of the studies was evaluated by two reviewers, using version 1 of the Cochrane Risk of Bias tool (RoB-1) for RCTs [25]. The evaluation contained six domains of bias, namely, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each item was evaluated in terms of “low,” “high,” and “unclear” risk (Additional File 1).
To assess the risk of bias in the results of non-randomized studies, we used the revised version of the risk of bias in non-randomized studies (RoBANS2) [26]. This tool covers the following eight domains of bias: comparability of participants, selection of participants, confounding variables, measurement of exposure, blinding of outcome assessment, outcome evaluation, incomplete outcome data, and selective reporting. Each item was assessed in terms of “low,” “high,” and “unclear” risk.
Statistical analysis
Data were pooled using Review Manager 5.4, presenting the mead difference (MD) with a 95% CI for continuous outcomes. The median and interquartile range were converted to the mean ± SD. The standardized mean difference (SMD) was used to combine the data when the scale of continuous data differed but measured the same thing [27]. The SD was computed using a validated formula for studies that did not provide the actual mean change [28].
The chi-squared test was used to determine the heterogeneity between studies (p < 0.10) based on I2 quantification. If heterogeneity was less than 60%, we used a fixed-effects model to analyze the data; otherwise, a random-effects model was used [28]. The results were considered statistically significant at p < 0.05.
Results
Search results
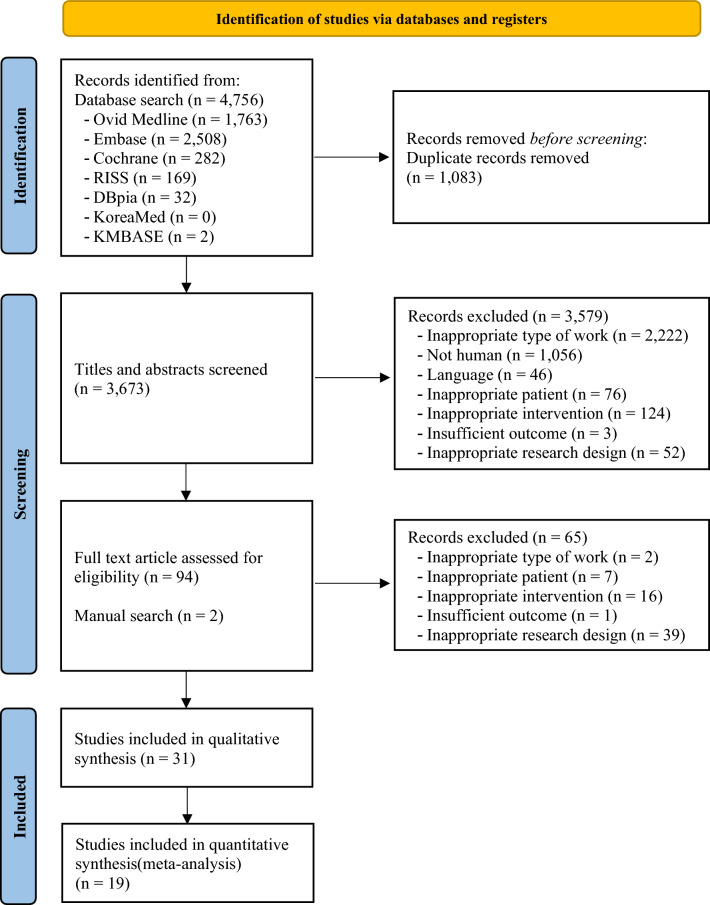
Excluding duplicates, we identified 3673 papers of potential relevance. Following the screening of the titles and abstracts, 3579 papers were retrieved. Subsequently, 65 papers were excluded based on eligibility criteria. Additional papers were included through manual searches of relevant bibliographies, and finally, 31 papers were deemed relevant on the basis of a full-paper review. In total, 31 studies involving 1394 patients were included for systematic review, and 19 studies involving 958 patients were included in our meta-analysis (Fig. 1).
Fig. 1.
PRISMA flow diagram of the included studies
Study characteristics
Table 1 summarizes the included studies published between 2012 and 2022. Twenty-four studies were conducted in Asia–Pacific, four in Europe, and one in Oceania. The study sizes ranged from six to 126 patients, and the follow-up period ranged from 0.25 to 60 months. Sixteen of the studies were RCTs, and the remaining 15 were non-RCTs.
Table 1.
Summary of the included studies
| Author(s), Year Country | Research design | Participant characteristics | Intervention group | Control group | Adverse events (n) | Follow-Up (month) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size N(I/C) | Age (mean ± SD/median [IQR]) | K-L Grade | Source (donor) | Dose (ADMSCs) | Extracted amount (cc/ml) | Delivery method and Frequency | Procedure | Procedure | Intervention group | Control group | |||
| Autologous-culture | |||||||||||||
|
Brazil |
RCT |
26 (6*/6/6/8*) *Patients were divided into 4 groups: 6 in ADMSC and 8 in the control |
I: 58.5 ± 6.17 C: 41 ± 4.67 |
2 to 4 | abdomen | 1 × 107 | 200 |
Injection Once |
Arthroscopic debridement Cultured ADMSCs |
Arthroscopic debridement | Not reported | Not reported | 6 |
|
Korea |
RCT |
26 (13/13) |
I: 58.3 ± 6.4 C: 59.1 ± 5.9 |
2 to 4 | abdomen | 10 × 107 | 20 |
Injection Once |
HTO Cultured ADMSCs |
HTO | No treatment-related adverse events | No adverse events | 24 |
|
Italy |
RCT |
38 (19/19) |
I: 55.8 C: 56.4 |
Outerbridge 4 |
N/A | N/A | N/A |
Injection Once |
Arthroscopic debridement Arthroscopic microfracture PRP Cultured ADMSCs |
Arthroscopic debridement Arthroscopic microfracture PRP |
Pain and swelling (3) | Pain and swelling (2) | 12 |
|
China |
RCT |
30 (10*/10/10*) *Patients were divided into 3 groups: 10 in ADMSC and 10 in the control |
I: 62 ± 8.33 C: 59.7 ± 7.12 *femoro-tibial cartilage defect |
3 | abdomen | 5 × 107 | 50 |
Injection Twice (day1, 22) |
Arthroscopic debridement Arthroscopic microfracture Hyaluronic acid Cultured ADMSCs |
Arthroscopic debridement Arthroscopic microfracture Hyaluronic acid |
Joint effusion/swelling (3) Sciatic pain (2) Low back pain (1) Skin erythema (2) |
Joint effusion/swelling (1) Sciatic pain (2) Low back pain (3) Skin erythema (1) |
24 |
|
Australia |
RCT |
30 (10/10/10) |
Ione: 54.6 ± 6.3 Itwo: 54.7 ± 10.2 C: 51.5 ± 6.1 |
2 to 3 | abdomen | 10 × 107 | 60 |
Injection Once Twice (0, 6 months) |
Ione: One cultured ADMSCs at baseline Itwo: Two cultured ADMSCs at baseline and 6 months |
Conventional management (simple analgesia, weight management, and exercise) |
Minor discomfort and bruising at the fat collection site Discomfort and/or swelling Swelling and pain impacting usual daily activity (2) |
No adverse events | 12 |
|
Korea |
RCT |
24 (12/12) |
I: 62.2 ± 6.5 C: 63.2 ± 4.2 |
2 to 4 | abdomen | 10 × 107 | 20 |
Injection Once |
Cultured ADMSCs | Saline |
Arthralgia (6) Joint effusion (2) |
Joint effusion (1) | 6 |
|
China |
RCT |
52 (26/26) |
I: 55.03 ± 9.19 C: 59.64 ± 5.97 |
1 to 3 | abdomen | 5 × 107 | 50 |
Injection Twice (0, 3 weeks) |
Cultured ADMSCs | Hyaluronic acid | Transient pain and swelling (19) |
Transient pain and swelling (14) Study withdrawal due to infection of the knee joint (1) |
12 |
|
Japan |
Non-RCT (retrospective) |
80 (42/38) |
Iculture: 70.0 ± 9.1 ISVF: 73.0 ± 9.1 |
2 to 4 | abdomen |
Iculture: 1.28 × 107 ISVF: Unknown |
Iculture: 20 ISVF: 200 |
Injection Iculture: Twice** ISVF: Once |
Iculture: Cultured ADMSCs ISVF: SVF |
Iculture Death (1) Gastric cancer (1) Knee pain and swelling (9) Induration at the fat collection site (2) ISVF Knee effusion and swelling (3) Pain at the fat collection site (6) Bleeding at the fat collection site (5) Induration at the fat collection site (12) |
N/A | 24 | |
|
Japan |
Non-RCT (retrospective) |
80 (42/38) |
Iculture: 70.0 ± 9.1 ISVF: 73.0 ± 9.1 |
2 to 4 | abdomen |
Iculture: 1.28 × 107 ISVF: Unknown |
Iculture: 20 ISVF: 200 |
Injection Once |
Iculture: Cultured ADMSCs ISVF: SVF |
Iculture Knee effusion and swelling (1) Induration at the fat collection site (2) ISVF Knee effusion and swelling (3) Pain at the fat collection site (6) Bleeding at the fat collection site (5) Induration at the fat collection site (12) |
N/A | Not reported | |
|
China |
RCT |
18 (6/6/6) |
Ilow: 52.1 ± 11.6 Imed: 59.6 ± 10.2 Ihigh: 52.7 ± 8.7 |
2 to 3 | abdomen |
Ilow: 1 × 107 Imed: 2 × 107 Ihigh: 5 × 107 |
50 |
Injection Twice (3, 6 weeks) |
Cultured ADMSCs |
Ilow Swelling (7) Imiddle Pain (2) Swelling (5) Ihigh Pain (2) Swelling (4) |
N/A | 24 | |
|
Korea |
Non-RCT |
18 (3/3/12) |
Ilow: 63 ± 8.6 Imed: 65 ± 6.6 Ihigh: 61 ± 6.2 |
3 to 4 | abdomen |
Ilow: 1 × 107 Imed: 5 × 107 Ihigh: 10 × 107 |
N/A |
Injection Once |
Cultured ADMSCs | No treatment-related adverse events | N/A | 24 | |
|
France |
Non-RCT |
18 (6/6/6) |
Ilow: 63.2 ± 4.1 Imed: 65.5 ± 8.1 Ihigh: 65.2 ± 2.3 |
3 to 4 | abdomen |
Ilow: 0.2 × 107 Imed: 1 × 107 Ihigh: 5 × 107 |
60 g |
Injection Once |
Cultured ADMSCs |
Ilow Joint effusion and swelling (2) Imiddle No treatment-related adverse events Ihigh Joint effusion and swelling (3) |
N/A | 6 | |
|
Korea |
Non-RCT |
18 (3/3/12) |
Ilow: 63 ± 8.6 Imed: 65 ± 6.6 Ihigh: 61 ± 6.2 |
3 to 4 | abdomen |
Ilow: 1 × 107 Imed: 5 × 107 Ihigh: 10 × 107 |
N/A |
Injection Once |
Cultured ADMSCs | No treatment-related adverse events | N/A | 6 | |
| SVF | |||||||||||||
|
China |
RCT |
6 (6/6) *6 patients /12 knees |
I: 62.17 ± 6.34 C: 62.17 ± 6.34 |
1 to 2 | abdomen | N/A | 200–250 |
Injection Once |
SVF | No treatment | No treatment-related adverse events | N/A | 6 |
|
Russia |
Non-RCT |
20 (10/10) |
I: 52.5 [45.0;57.0] C: 56.5 [52.5;63.5] *median [interquartile range, 25th, 75th percentiles] |
2 to 3 | abdomen | N/A | 150–200 |
Injection Once |
Arthroscopic debridement HTO SVF |
Arthroscopic debridement HTO PRP |
Not reported | Not reported | 18 |
|
Russia |
Non-RCT |
26 (16/10) |
I: 61 [57;64] C: 72 [60;77] *median [interquartile range, 25th, 75th percentiles] |
2 to 3 | abdomen | N/A | 50 |
Injection Once |
SVF | Hyaluronic acid |
Discomfort Painless swelling Minor increase of temperature |
Discomfort Painless swelling Minor increase of temperature |
12 |
|
[32] (A) China |
RCT |
95 (47/48) |
I: 50.83 ± 10.88 C: 52.87 ± 9.35 |
2 to 3 | abdomen | N/A | 100–150 |
Injection Once |
SVF | Hyaluronic acid |
Pain Swelling |
Pain Swelling |
12 |
|
[33] (B) China |
RCT |
126 (56/70) |
I: 53.98 ± 13.69 C: 55.63 ± 12.18 |
2 to 3 | abdomen | N/A | 40 |
Injection Three times (once a month) |
SVF | Hyaluronic acid | No treatment-related adverse events | No adverse events | 60 |
|
China |
RCT |
57 (29/28) |
I: 52.27 ± 1.17 C: 51.77 ± 7.55 |
0 to 3 | Infrapatellar fat pad | 0.391 × 107 | 200 |
Injection Once |
Arthroscopic debridement SVF |
Arthroscopic debridement Saline |
No treatment-related adverse events | No adverse events | 12 |
|
USA |
RCT |
39 (13/13/13) |
Ilow: 60.5 ± 7.9 Ihigh: 59.5 ± 11.7 C: 57.1 ± 9.1 |
2 to 3 | abdomen | N/A | 75 |
Injection Once |
SVF |
Placebo (zero SVF cells) |
Ilow No treatment-related adverse events Ihigh Swelling (1) |
No adverse events | 12 |
|
Korea |
Non-RCT (retrospective) |
60 (30/30) |
I: 63.0 ± 3.2 C: 63.2 ± 3.8 |
1 to 4 | buttock | 0.71 × 107 | 140 |
Injection Once |
Arthrocentesis SVF |
Arthrocentesis Hyaluronic acid |
Swelling (2) Subcutaneous induration at the fat collection site (3) |
Swelling (1) | 12 |
|
Taiwan |
Non-RCT |
33 (18/15) |
I: 59 ± 6.04 C: 58.2 ± 5.7 |
2 to 3 | abdomen | N/A | 50–100 |
Injection Once |
Arthroscopic microfracture SVF |
Arthroscopic microfracture | Not reported | Not reported | 24 |
|
Korea |
Non-RCT |
100 (50/50) |
I: 59.2 ± 4.5 C: 58.3 ± 5.6 |
3 to 4 | buttock | 0.426 × 107 | N/A |
Injection Once |
Arthroscopic debridement HTO SVF |
Arthroscopic debridement HTO |
Not reported | Not reported | 12 |
|
Vietnam |
Non-RCT |
30 (15/15) |
I: 58.6 ± 6.48 C: 58.2 ± 5.71 |
2 to 3 | abdomen | N/A | 100 |
Injection Once |
Arthroscopic microfracture PRP SVF |
Arthroscopic microfracture Saline |
No treatment-related adverse events | No adverse events | 18 |
|
Korea |
RCT |
80 (40/40) |
I: 38.4 ± 6.4 C: 39.1 ± 7.1 |
ICRS 3 to 4 |
buttock | 0.497 × 107 | N/A |
Implantation Once |
Arthroscopic debridement Arthroscopic microfracture SVF with fibrin glue scaffold |
Arthroscopic debridement Arthroscopic microfracture |
Not reported | Not reported | 24 |
|
Korea |
RCT |
44 (21/23) |
I: 54.2 ± 2.9 C: 52.3 ± 4.9 |
1 to 3 | buttock | 0.411 × 107 | 140 |
Injection Once |
Arthroscopic debridement HTO PRP SVF |
Arthroscopic debridement HTO PRP |
Not reported | Not reported | 24 |
|
Korea |
Non-RCT |
50 (25/25) |
I: 54.2 ± 9.3 C: 54.4 ± 11.3 |
1 to 3 | infrapatellar fat pad | 0.189 × 107 | 9.4 g |
Injection Once |
Arthroscopic debridement PRP SVF |
Arthroscopic debridement PRP |
Pain and swelling (1) *The adverse effects are not specified as occurring in the intervention group or the control group |
16.4 (12–18) |
|
|
China |
RCT |
16 (16/16) *16 patients /32 knees |
Ileft: 53 ± 10.97 Iright: 51 ± 5.95 *reported based on the SVF-treated knee's side |
2 to 3 | abdomen | N/A | 100–150 |
Injection Once |
Arthroscopic debridement SVF |
Arthroscopic debridement Hyaluronic acid |
Muscle soreness at the fat collection site (4) Pain and swelling (6) |
N/A | 12 |
|
Japan |
Non-RCT |
60 (30/30) |
Ilow: 69.0 ± 8.3 Ihigh: 70.7 ± 5.3 |
2 to 4 | abdomen | N/A | 290–440 |
Injection Once |
SVF |
Ilow Pain and swelling (3) Ihigh Pain and swelling (2) |
N/A | 12 | |
|
Korea (A) |
Non-RCT |
40 (20/20) |
Iimplantation : 59.1 ± 3.5 Iinjection : 59.4 ± 3.1 |
1 to 2 | buttock |
Iimplantation : 0.396 × 107 Iinjection: : 0.407 × 107 |
140 |
Implantation / Injection Once |
Iimplantation: Arthroscopic debridement SVF with fibrin glue scaffold Iinjection: Arthroscopic debridement PRP SVF |
Not reported | N/A | 28 | |
|
Korea (B) |
Non-RCT (retrospective) |
54 (17/37) |
Iwith : 57.7 ± 5.8 Iwithout : 57.5 ± 5.9 |
1 to 2 | buttock | 0.39 × 107 | 140 |
Implantation Once |
Iwith: Arthroscopic debridement SVF with fibrin glue scaffold Iwithout: Arthroscopic debridement SVF without fibrin glue scaffold |
No treatment-related adverse events | N/A | 29.2 | |
SD standard deviation K-L Grade Kellgren-Lawrence grade RCT randomized controlled trials N/A not available ADMSC Adipose-Derived Mesenchymal Stem Cell HTO Open-wedge High Tibial Osteotomy SVF Stromal Vascular Fracture PRP Platelet-Rich Plasma ICRS International Cartilage Repair Society
**Booster injection for patients whose VAS had decreased by less than 50% from the pre-injection score
Eighteen studies were conducted using the SVF method to isolate ADMSCs [11, 29–45], and 13 studies used autologous-cultured cells from adipose aspirates to obtain the ADMSCs [15, 16, 46–56]. Excluding the three studies that used implantation [11, 44, 45], in the remaining studies, ADMSCs were administered via intra-articular injection [15, 16, 29–43, 46–56]. The number of applied ADMSCs ranged from 0.2 × 107 to 10 × 107, and six studies compared efficacy and safety based on different ADMSC dosages [35, 43, 53–56].
Quality assessment
The quality assessment of RCTs using RoB-1 included 24 studies [11, 15, 29–35, 37, 39–43, 46–51, 53, 55, 56], and seven studies were assessed using the RoBANS2 based on the characteristics of the study [16, 36, 38, 44, 45, 52, 54]. A risk of bias graph and summary are presented in additional Figures S1 and S2.
The quality assessment of RCTs using RoB-1 revealed the following results: 12 studies generated random sequences, although three studies were unclear, three studies conducted unclear allocation concealment, six studies were double-blind, three studies had incompletely reported outcome data, and seven studies reported outcomes according to protocol.
With respect to RoBANS2, quality assessment yielded the following results: seven studies had comparability of participants, five studies were conducted retrospectively, four studies had an unclear measurement of exposure, six studies reported a high risk of bias due to no-blinding, three studies had a high risk of bias due to bias in the missing data, and six studies were unclear regarding selective reporting.
Efficacy analysis
The meta-analysis of outcome variables is shown in additional Tables S1 and S2.
Visual analog scale for pain and numeric pain rating scale
The MD in pain, utilizing autologous-cultured ADMSCs, showed a significant improvement in the intervention group compared with the control group at 3 and 6 months post-treatment (3 months: MD = −2.43, 95% CI [−3.99, −0.86], I2 = 60%; 6 months: MD = −1.62, 95% CI [−2.46, −0.79], I2 = 35%) (Fig. 2). Comparatively, the intervention group treated with SVF showed no significant differences compared with the control group (3 months: MD = −1.47, 95% CI [−3.89, 0.94], I2 = 95%; 6 months: MD = −2.32, 95% CI [−5.15, 0.52], I2 = 96%) [Fig. 2A, B]. However, both autologous-cultured ADMSCs and SVF showed significant pain improvement in the intervention group at 12 months (auto-ADMSCs: MD = −1.97, 95% CI [−3.22, −0.72], I2 = 64%; SVF: MD = −2.13, 95% CI [−3.06, −1.21], I2 = 86%) (Fig. 2).
Fig. 2.
Results for visual analog scale assessments in RCTs at A 3, B 6, C 12, and D 24 months
In the non-RCTs including only SVF intervention, the SMD in pain showed no significant difference between the intervention and control groups at 3, 6, or 12 months (3 months: SMD = −0.36, 95% CI [−1.37, 0.65], I2 = 77%; 6 months: SMD = −0.72, 95% CI [−1.83, 0.39], I2 = 79%; 12 months: SMD = −1.13, 95% CI [−2.53, 0.27], I2 = 85%).
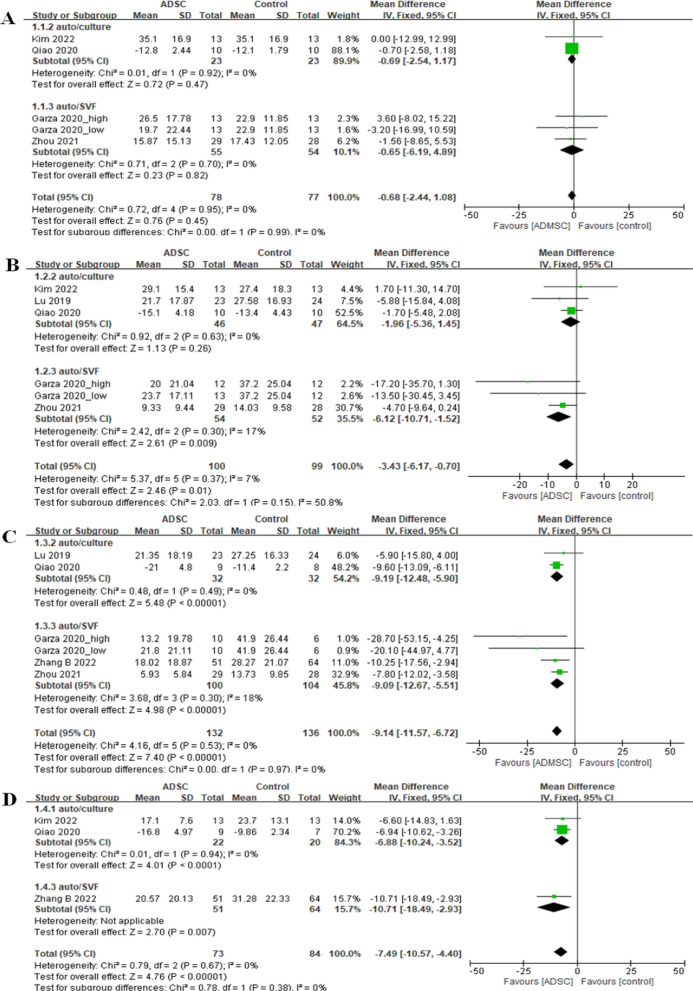
Western ontario and mcmaster universities osteoarthritis index
The MD in WOMAC scores indicated no significant differences between the intervention and control groups three months post-treatment for both autologous-cultured ADMSCs (MD = −0.69, 95% CI [−2.54, 1.17], I2 = 0%) and SVFs (MD = −0.65, 95% CI [−6.19, 4.89], I2 = 0%) (Fig. 3). After six months, there were no significant differences in the WOMAC scores between the autologous-cultured ADMSCs and control groups (MD = −1.96, 95% CI [−5.36, 1.45], I2 = 0%), whereas SVF therapy was associated with a significant increase in WOMAC scores (MD = −6.12, 95% CI [−10.71, −1.52], I2 = 17%) compared with the control group (Fig. 3). Both autologous-cultured ADMSCs and SVFs showed significant differences favoring the intervention after 12 months (auto-ADMSCs: MD = −9.19, 95% CI [−12.48, −5.90], I2 = 0%; SVF: MD = −9.09, 95% CI [−12.67, −5.51], I2 = 18%) (Fig. 3) and 24 months (auto-ADMSCs: MD = −6.88, 95% CI [−10.24, −3.52], I2 = 0%; SVF: MD = −10.71, 95% CI [−18.49, −2.93], I2 = not applicable) (Fig. 3).
Fig. 3.
Results of WOMAC scores in RCTs at A 3, B 6, C 12, and D 24 months
In the non-RCTs, only SVF therapy data were included, which revealed a significant improvement at 12 months (MD = −7.08, 95% CI [−11.63, −2.54], I2 = 35%) and 24 months (MD = −21.68, 95% CI [−26.49, −16.87], I2 = 0%).
Lysholm
In the non-RCTs, there were no significant differences in Lysholm scores between the SVF intervention and control groups at 6, 12, and 24 months post-treatment (6 months: MD = 4.37, 95% CI [−6.95, 15.70], I2 = 0%; 12 months: MD = 5.04, 95% CI [−4.70, 14.78], I2 = 0%; 24 months: MD = 13.60, 95% CI [−0.83, 28.04], I2 = 0%).
SF-36
Six studies using autologous-cultured ADMSCs were reviewed, among which five reported improved patient QoL [46–48, 51, 53]. Carvalho et al. reported significant improvements in SF-36 scores at 6 months [46], whereas Lu et al. also observed significant improvement in SF-36 scores at 6 and 12 months [51], and Qiao et al. reported improvements in QoL only at 24 months [48]. Venosa et al. utilized SF-12 and found that SF-12 scores improved significantly at 12 months, whereas no significant differences were observed compared with the non-ADMSC therapies [47].
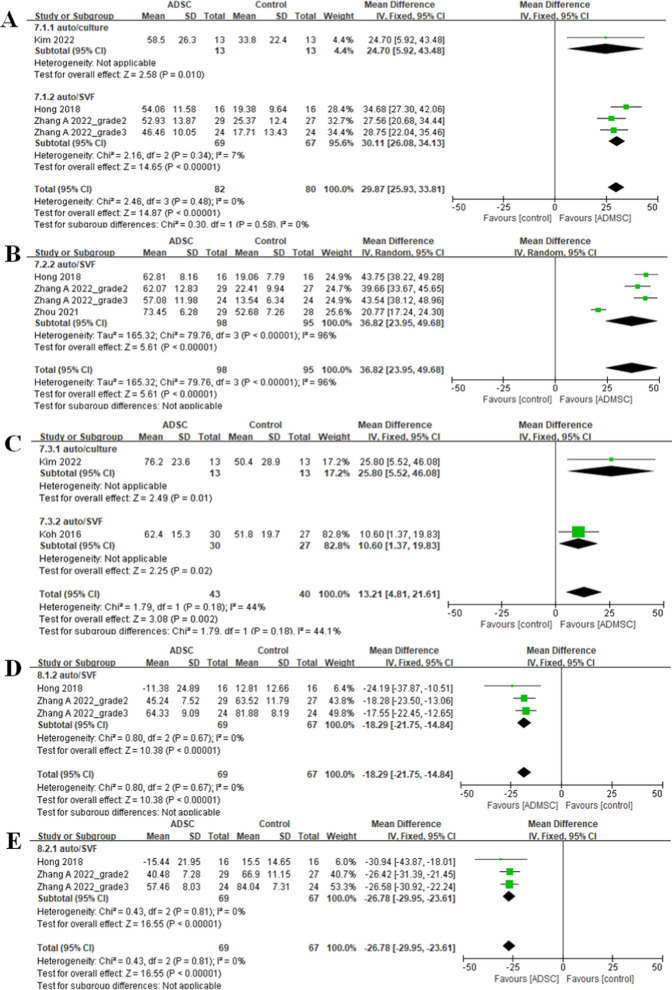
Cartilage repair tissue
The autologous-cultured ADMSCs showed a significant improvement in MOCART at 6 months (MD = 24.70, 95% CI [5.92, 43.48], I2 = not applicable) and 24 months (MD = 25.8, 95% CI [5.52, 46.08], I2 = not applicable) (Fig. 4). Similarly, SVFs showed significant improvements at 6, 12, and 24 months (6 months: MD = 30.11, 95% CI [26.08, 34.13], I2 = 7%; 12 months: MD = 36.82, 95% CI [23.95, 49.68], I2 = 96%; 24 months: MD = 10.60, 95% CI [1.37, 19.83], I2 = not applicable) (Fig. 4).
Fig. 4.
Results of MOCART in RCTs at A 6, B 12, and C 24 months, and results of WORMS in RCTs at D 6 and E 12 months
There were significant improvements in WORMS scores in the SVF intervention group compared with the control group at 6 and 12 months post-treatment (6 months: MD = −18.29, 95% CI [−21.75, −14.84], I2 = 0%; 12 months: MD = −26.78, 95% CI [−29.95, -23.61], I2 = 0%) (Fig. 4).
Effects of ADMSC based on application method
Among the three studies that conducted ADMSC implantation, Koh et al. reported that ADMSC implantation with fibrin glue resulted in a significant improvement in the MOCART and Knee Injury and Osteoarthritis Outcome Score pain and symptom sub-scores compared with those in the group without ADMSC treatment [11]. Kim et al. demonstrated a significant improvement in International Knee Documentation Committee (IKDC) scores in the implantation group compared with the injection group at the final follow-up [44], whereas better International Cartilage Repair Society (ICRS) grades were also obtained in the implantation group. Furthermore, a study comparing implantation with and without a fibrin scaffold reported a significant improvement in the IKDC and Tegner activity scale scores in both groups, with no significant differences being observed between the two groups [45]. ICRS grades were, however, better in the group with fibrin scaffold implantation than in the group without implantation.
Safety analysis
Among 13 studies on autologous-cultured ADMSCs, safety-related results were reported in 12 of these studies [15, 16, 47–56], of which three reported no adverse effects [15, 54, 56], whereas safety was not mentioned in the single remaining study [46]. One study reported serious adverse effects, including one death and one case of gastric cancer [52], whereas a further three studies reported four cases of induration at the fat collection site [16, 49, 52]. Additionally, eight studies reported 93 cases of adverse effects, including pain, swelling, effusion, and arthralgia in response to injection of autologous-cultured ADMSCs [16, 48–53, 55]. Fourteen of 20 studies on SVFs reported safety-related results, among which, adverse effects occurred only in nine studies [16, 31, 33, 35, 36, 41–43, 52]. The remaining six studies failed to mention safety-related results [11, 30, 37, 38, 40, 44]. One case of hospitalization was reported in one study [52], whereas 53 adverse effects occurred at the fat collection site, such as pain, bleeding, induration, and muscle soreness [16, 36, 42, 52], and pain and swelling mainly occurred following SVF injection [16, 31, 33, 35, 36, 41–43, 52]
Discussion
In this systematic review and meta-analysis, we evaluated the safety and efficacy of autologous-cultured ADMSCs and SVFs in the treatment of knee OA, with a particular focus on the more rapid pain relief observed in patients receiving autologous-cultured ADMSCs. To the best of our knowledge, this study is the first to compare the impacts of these two treatments on knee OA. The meta-analysis revealed that whereas both therapies significantly improved knee function, QoL, and cartilage repair, autologous-cultured ADMSCs provided significantly more rapid pain relief. Additionally, our analysis confirmed that ADMSC therapy is not associated with any serious adverse events, regardless of the cell processing method.
A more rapid onset of pain relief with autologous-cultured ADMSCs
The administration of autologous-cultured ADMSCs was found to promote significant improvements in pain relief as early as 3 months post-treatment, with continued efficacy observed at 12 months. This rapid onset contrasts with that achieved using SVFs, in which significant pain reduction was initially observed at 12 months post-treatment in RCTs. This indicates that administering ADMSCs may be conducive to a more rapid alleviation of symptoms, thereby providing more immediate therapeutic benefits for knee OA patients. Yokota et al. have similarly noted that ADMSCs outperform SVFs with respect to early symptom and pain alleviation, further supporting our findings [16].
The rapid pain relief observed in response to treatment with autologous-cultured ADMSCs can be attributed to their high proliferative capacity and ability to differentiate into chondrocytes, which are essential for repairing damaged cartilage. Furthermore, the significant paracrine effects of ADMSCs, characterized by the secretion of bioactive materials, play a vital role in modulating the inflammatory environment within the OA joint, thereby leading to more rapid pain relief. This is consistent with the findings of previous research that have highlighted the chondrogenic differentiation potential of MSCs and their capacity for paracrine-mediated modulation of the OA milieu, thereby facilitating tissue healing and symptom alleviation [56] In contrast, the diverse cellular composition of SVFs, which can include a range of different stem and immune cell types, may require a longer time interval to exert similar therapeutic effects, thereby leading to a delayed onset of pain relief.
The findings of a relevant comparative study using a sheep OA model, in which the authors evaluated the efficacy of autologous SVFs and ADMSCs, both combined with hyaluronic acid (HA), has revealed that whereas both treatments promoted cartilage regeneration, ADMSCs combined with HA produced superior outcomes, particularly with respect to cartilage thickness and macroscopic scores [57], which is consistent with our clinical observations of more rapid pain relief and cartilage repair when using ADMSCs. Although the cellular heterogeneity of SVFs was found to contribute to anti-inflammatory and trophic effects, notably increasing the production of growth factors, including stromal cell-derived factor 1, ADMSCs exhibited a stronger capacity to reduce inflammatory cytokines (IL-1β and IL-6), consistent with their targeted modulation of the OA environment [57]. However, whereas both therapies show promise, ADMSCs appear more effective for rapid cartilage regeneration and symptom relief in knee OA. To optimize therapeutic outcomes, future research should further examine these biological mechanisms.
Sustained functional improvement and cartilage repair
Although the primary advantage of autologous-cultured ADMSCs lies in the rapidity of their relief of pain, both ADMSCs and SVFs can significantly enhance knee function and cartilage repair over time. WOMAC scores indicated that both treatments provide substantial functional improvement at the latter stages post-treatment, particularly after 12 months, with continued benefits thereafter. In contrast, the Lysholm Knee Scale, which focuses on specific knee functions, revealed no significant differences with SVF treatment in non-RCTs, thus indicating a disparity in assessments based on WOMAC and Lysholm scoring [58, 59].
Additionally, MOCART and WORMS assessments validated the positive effects of these therapies on cartilage repair, with MOCART indicating the notable benefits obtained using autologous-cultured ADMSCs at 6 and 24 months, and with SVFs at 6 and 12 months. Although both intervention types show histological efficacy in knee cartilage improvement, there remain challenges in quantification due to subjective interpretations and the absence of standardized criteria among different studies. The inherent limitations in MOCART and WORMS, including their susceptibility to inter-observer variability, highlight the need for more advanced objective evaluation techniques in future research to accurately assess and better understand cartilage regeneration.
The discrepancies in the timing of pain relief and functional improvement can be explained in terms of the different underlying biological and mechanical processes. Pain relief, which occurs relatively rapidly following treatment with autologous-cultured ADMSCs, is primarily mediated by a reduction in inflammation and the alleviation of joint stress [60]. However, functional improvement is a more complex and gradual process, involving the recovery of joint mobility, strength, and stability, which are dependent on the repair and regeneration of cartilage and other joint structures [61, 62]. This process may take several months to fully translate into noticeable functional improvements, thereby explaining why functional outcomes appear at approximately the same time when using both ADMSCs and SVFs, despite the earlier pain relief provided by autologous-cultured ADMSCs.
Dosage consideration and application methods
Although autologous-cultured ADMSCs can provide significant pain relief and functional improvements, the relationship between dosage and outcomes is far from straightforward. Notably, improvements were observed across a range of dosages, with some studies providing evidence to indicate that lower doses could be equally effective over time. This finding challenges the traditional notion of a direct dose–response correlation, thus emphasizing the need for a more in-depth understanding of ADMSC therapy beyond mere dosage considerations, and indicating that the benefits of ADMSC therapy on overall well-being have yet to be sufficiently clarified and accordingly warrant further investigation. The absence of a clear dose–response relationship, particularly in cases in which high doses do not always yield the best outcomes, indicates that optimal dosing may require a more detailed understanding of the mechanisms underlying the effects of ADMSC therapy.
The method of ADMSC administration can also play a key role in treatment efficacy. Whereas the primary delivery method in most studies has involved intra-articular injections, other techniques, such as scaffold-based delivery and arthroscopic implantation, have also been assessed. Intra-articular injections offer a minimally invasive approach and have been shown to reduce pain and improve function in knee OA patients [63]. However, a limitation of this approach is the potential for an uneven distribution of cells within the joint, which could influence overall therapeutic outcomes [64]. Comparatively, scaffold-based delivery systems, such as fibrin scaffolds, provide a more structured environment for ADMSCs, promoting better cell retention and cartilage regeneration at the injury site [65]. Studies have indicated that compared with simple injection or implantation without a scaffold, using a fibrin scaffold in conjunction with ADMSCs can enhance cartilage regeneration [44, 45] thereby emphasizing the importance of cell engraftment and survival at the site of lesion [43, 44]. Although arthroscopic implantation can facilitate precise cell placement, potentially improving outcomes in advanced OA cases, the invasiveness of this process limits its use to specific patient groups. These insights underline the need for further research to optimize ADMSC application methods and fully understand their therapeutic potential in the treatment of OA.
Whereas both ADMSC and SVF therapies can significantly improve pain relief and cartilage regeneration, differences in administration methods, dosages, and preparatory protocols are likely to contribute to outcome variations. In contrast to ADMSCs, which are pre-cultured and administered in controlled dosages, SVFs are applied immediately after extraction, resulting in a less controlled composition of cell populations. These factors might influence therapeutic efficacy, particularly regarding the speed of pain relief and the extent of cartilage regeneration. Accordingly, standardizing dosages and delivery methods is essential to enable optimization of the efficacies of both therapies.
Safety profile
Our findings in this review indicate that the safety profiles of both ADMSC and SVF therapies are generally favorable, with most adverse effects being minor, such as pain and swelling at the site of injection. These effects are typically transient and resolve without additional intervention, which is consistent with the findings of previous systematic reviews that have reported no serious adverse effects from ADMSC therapy [22, 66]. With regard to the serious adverse effects of death and a case of gastric cancer reported in one study [52], the insights provided by two orthopedic surgeons performing stem cell therapy are enlightening. They posited that death from stroke within 14 days of injection could potentially be associated with thrombosis as an adverse effect, although linking the cause of death at 23 months, as reported in the article, to stem cell therapy is less clear-cut. Similarly, establishing a direct association between the case of gastric cancer and stem cell therapy is challenging, making it difficult to categorize an adverse effect of the therapy.
No severe adverse events were observed among the studies included in this review, but theoretical risks, including immune responses or cellular over-proliferation, have been discussed in the literature [67]. Although not evident in our analysis, these concerns warrant further investigation, particularly in the context of long-term and large-scale studies, to establish the safety profiles of these regenerative therapies fully.
Strengths and limitations
This systematic review provides an in-depth analysis of ADMSCs in knee OA, highlighting the influence of different cell processing methods. However, despite the comprehensive nature of this review, several limitations should be acknowledged, given the high heterogeneity in study designs, injection doses, and outcome measures, thereby making it difficult to generalize study outcomes. The lack of standardized dosing criteria and variability further complicates effective comparison, thus emphasizing the need for dose standardization to optimize the use of ADMSCs in clinical settings. Additionally, diverse control interventions may have contributed to the variability in reported outcomes. Future research should accordingly focus on standardizing methods, assessing longer-term outcomes, and conducting larger RCTs to integrate these therapies into clinical practice better.
Conclusion
Our findings in this study indicate that autologous-cultured ADMSCs and SVFs can significantly improve knee function, reduce pain, and enhance cartilage repair in knee OA patients. Whereas ADMSCs may provide more rapid pain relief, both treatments have good long-term efficacy in terms of functional improvement and cartilage regeneration. The favorable safety profile of ADMSCs supports their use in clinical settings. However, further research is required to refine application methods, clarify dose–response relationships, and establish standardized protocols to maximize the therapeutic potential of both therapies in treating knee OA.
Supplementary Information
Artificial intelligence
The authors declare that they have not use AI-generated work in this manuscript.
Abbreviations
- ADMSCs
Adipose-derived mesenchymal stem cells
- OA
Osteoarthritis
- QoL
Quality of life
- RCT
Randomized controlled trial
- MD
Mean difference
- CI
Confidence interval
- SMD
Standardized mean difference
- VAS
Visual analog scale
- WOMAC
Western ontario and mcmaster universities osteoarthritis index
- WORMS
Whole-organ magnetic resonance imaging score
- MOCART
Magnetic resonance observation of cartilage repair tissue
- IKDC
International knee documentation committee
- ICRS
International cartilage repair society
- SD
Standard deviation
- SF-36
Short form (36) health survey
- SF-12
Short form (12) health survey
- SVF
Stromal vascular fraction
Author contributions
H. Lee, and S–H. Lee designed the study. H. Lee and Y. Lim abstracted the data and performed the analysis and drafted the manuscript. S–H. Lee supervised the study. All authors read and approved the final manuscript.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alkan BM, et al. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod Rheumatol. 2014;24(1):166–71. [DOI] [PubMed] [Google Scholar]
- 2.Ma W, et al. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(49): e23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkely G, et al. Do nonsteroidal anti-inflammatory drugs have a deleterious effect on cartilage repair? A systematic review. Cartilage. 2021;13(1):326S-341S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Aziem AA, et al. Effect of a physiotherapy rehabilitation program on knee osteoarthritis in patients with different pain intensities. J Phys Ther Sci. 2018;30(2):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng C-Y, et al. Benefits and mechanisms of exercise training for knee osteoarthritis. Front Physiol. 2021;12: 794062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (adscs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci. 2019;20(10):2523. 10.3390/ijms20102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruppert KA, et al. Human mesenchymal stromal cell-derived extracellular vesicles modify microglial response and improve clinical outcomes in experimental spinal cord injury. Sci Rep. 2018;8(1):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed MR, et al. Combination of ADMSCs and chondrocytes reduces hypertrophy and improves the functional properties of osteoarthritic cartilage. Osteoarthr Cartil. 2014;22(11):1894–901. [DOI] [PubMed] [Google Scholar]
- 9.Bernacki SH, Wall ME, Loboa EG. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 2008;86:257–78. [DOI] [PubMed] [Google Scholar]
- 10.Koh YG, et al. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308–16. [DOI] [PubMed] [Google Scholar]
- 11.Koh YG, et al. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109. [DOI] [PubMed] [Google Scholar]
- 12.Mikłosz A, Nikitiuk BE, Chabowski A. Using adipose-derived mesenchymal stem cells to fight the metabolic complications of obesity: Where do we stand? Obes Rev. 2022;23(5): e13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurul AA, et al. Mesenchymal stem cells: current concepts in the management of inflammation in osteoarthritis. Biomedicines. 2021;9(7):785. 10.3390/biomedicines9070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A-T, et al. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: a concise review. World J Stem Cells. 2019;11(4):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, et al. Intra-articular injection of mesenchymal stem cells after high tibial osteotomy in osteoarthritic knee: two-year follow-up of randomized control trial. Stem Cells Transl Med. 2022;11(6):572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota N, et al. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am J Sports Med. 2019;47(11):2577–83. [DOI] [PubMed] [Google Scholar]
- 17.Vargel İ, et al. Autologous adipose-derived tissue stromal vascular fraction (AD-tSVF) for knee osteoarthritis. Int J Mol Sci. 2022;23(21):13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, et al. Stromal vascular fraction: a regenerative reality? Part 2: mechanisms of regenerative action. J Plast Reconstr Aesthet Surg. 2016;69(2):180–8. [DOI] [PubMed] [Google Scholar]
- 19.Goncharov EN, et al. Analyzing the clinical potential of stromal vascular fraction: a comprehensive literature review. Medicina. 2024;60(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiryaki T, et al. Adipose-derived stromal vascular matrix (SVM): a new paradigm in regenerative medicine. Cell. 2021;R4(9):e3060. [Google Scholar]
- 21.Muthu S, et al. Is culture expansion necessary in autologous mesenchymal stromal cell therapy to obtain superior results in the management of knee osteoarthritis?—meta-analysis of randomized controlled trials. Bioengineering. 2021;8(12):220. 10.3390/bioengineering8120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadelkarim M, et al. Safety and efficacy of adipose-derived mesenchymal stem cells for knee osteoarthritis: a systematic review and m-analysis. Joint Bone Spine. 2022;89(5): 105404. [DOI] [PubMed] [Google Scholar]
- 23.Issa MR, et al. The role of adipose-derived mesenchymal stem cells in knee osteoarthritis: a meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis. 2022;14:1759720x221146005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, DG Altman Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions: Cochrane book series, p. 187–241 (2008).
- 26.Higgins JP et al., Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions, p. 205–228. (2019)
- 27.Lin L, Aloe AM. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat Med. 2021;40(2):403–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandler J, Cumpston M, Li T. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019. [Google Scholar]
- 29.Ren B, et al. Clinical phase I/II trial of SVF therapy for cartilage regeneration: a cellular therapy with novel 3D MRI imaging for evaluating chondral defect of knee osteoarthritis. Front Cell Dev Biol. 2023;11:1106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prizov A, et al. Differences in synovial cytokine profile associated with long-term clinical outcomes in patients with knee osteoarthritis undergoing corrective osteotomy with platelet-rich plasma or stromal vascular fraction post-treatments. Int J Mol Sci. 2022;23(21):12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevela EY, et al. Comparative efficacy of the stromal-vascular fraction cells of lipoaspirate and hyaluronic acid in the treatment of gonarthrosis: results of an interim analysis. Bull Exp Biol Med. 2022;174(1):131–6. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, et al. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: a minimum 5-year follow-up study. Stem Cell Res Ther. 2022;13(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. The effect of autologous adipose-derived stromal vascular fractions on cartilage regeneration was quantitatively evaluated based on the 3d-fs-spgr sequence: a clinical trial study. Biomed Res Int. 2022;2022:2777568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, et al. The clinical efficacy of arthroscopic therapy with knee infrapatellar fat pad cell concentrates in treating knee cartilage lesion: a prospective, randomized, and controlled study. J Orthop Surg Res. 2021;16(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garza JR, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med. 2020;48(3):588–98. [DOI] [PubMed] [Google Scholar]
- 36.Kim YS, et al. Comparative matched-pair cohort analysis of the short-term clinical outcomes of mesenchymal stem cells versus hyaluronic acid treatments through intra-articular injections for knee osteoarthritis. J Exp Orthop. 2020;7(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran TDX, et al. Time- and Kellgren–Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells. 2019;8(4):308. 10.3390/cells8040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YS, Koh YG. Comparative matched-pair analysis of open-wedge high tibial osteotomy with versus without an injection of adipose-derived mesenchymal stem cells for varus knee osteoarthritis: clinical and second-look arthroscopic results. Am J Sports Med. 2018;46(11):2669–77. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PD, et al. Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl Med. 2017;6(1):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koh YG, et al. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30(11):1453–60. [DOI] [PubMed] [Google Scholar]
- 41.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–7. [DOI] [PubMed] [Google Scholar]
- 42.Hong Z, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial. Int Orthop. 2019;43(5):1123–34. [DOI] [PubMed] [Google Scholar]
- 43.Tsubosaka M, et al. Comparison of clinical and imaging outcomes of different doses of adipose-derived stromal vascular fraction cell treatment for knee osteoarthritis. Cell Transplant. 2021;30:9636897211067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YS, et al. Comparative matched-pair analysis of the injection versus implantation of mesenchymal stem cells for knee osteoarthritis. Am J Sports Med. 2015;43(11):2738–46. [DOI] [PubMed] [Google Scholar]
- 45.Kim YS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176–85. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho Schweich-Adami L, et al. The intra-articular injection of adipose-derived stem cells decreases pain and reduces inflammation in knee osteoarthritis, with or without the addition of platelet-rich plasma also improves functionality. J Tissue Eng Regen Med. 2022;16(10):900–12. [DOI] [PubMed] [Google Scholar]
- 47.Venosa M, et al. Platelet-rich plasma and adipose-derived mesenchymal stem cells in association with arthroscopic microfracture of knee articular cartilage defects: a pilot randomized controlled trial. Adv Orthop. 2022;2022:6048477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiao Z, et al. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: a Phase IIa trial. Regen Med. 2020;15(1):1193–214. [DOI] [PubMed] [Google Scholar]
- 49.Freitag J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30. [DOI] [PubMed] [Google Scholar]
- 50.Lee WS, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokota N, et al. Clinical safety and effectiveness of adipose-derived stromal cell versus stromal vascular fraction injection for treatment of knee osteoarthritis: 2-year results of parallel single-arm trials. Am J Sports Med. 2022;50(10):2659–68. [DOI] [PubMed] [Google Scholar]
- 53.Song Y, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. [DOI] [PubMed] [Google Scholar]
- 54.Jo CH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–83. [DOI] [PubMed] [Google Scholar]
- 55.Pers YM, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jo CH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66. [DOI] [PubMed] [Google Scholar]
- 57.Lv X, et al. Comparative efficacy of autologous stromal vascular fraction and autologous adipose-derived mesenchymal stem cells combined with hyaluronic acid for the treatment of sheep osteoarthritis. Cell Transplant. 2018;27(7):1111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index: reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Scand J Rheumatol. 1999;28(4):210–5. [DOI] [PubMed] [Google Scholar]
- 59.Pan T, Hennrikus WL. Outcomes of surgical treatment of symptomatic bipartite patella in teenage athletes. J Pediatric Orthop B. 2022;31(4):371–5. [DOI] [PubMed] [Google Scholar]
- 60.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9(10):584–94. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Shah KM, Luo J. Strategies for articular cartilage repair and regeneration. Front Bioeng Biotechnol. 2021;9: 770655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, et al. Development and prospect of intra-articular injection in the treatment of osteoarthritis: a review. J Pain Res. 2020;13:1941–55. 10.2147/JPR.S260878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou D, et al. Intra-articular nanodrug delivery strategies for treating osteoarthritis. Drug Discov Today. 2023;28(3): 103482. [DOI] [PubMed] [Google Scholar]
- 65.Liang J, et al. Biomaterial-based scaffolds in promotion of cartilage regeneration: recent advances and emerging applications. J Orthop Transl. 2023;41:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muthu S, et al. Comparative effectiveness of adipose-derived mesenchymal stromal cells in the management of knee osteoarthritis: a meta-analysis. World J Orthop. 2023;14(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. 2021;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.