ABSTRACT
Candida glabrata exhibits innate resistance to azole antifungal drugs but also has the propensity to rapidly develop clinical drug resistance. Azole drugs, which target Erg11, is one of the major classes of antifungals used to treat Candida infections. Despite their widespread use, the mechanism controlling azole-induced ERG gene expression and drug resistance in C. glabrata has primarily revolved around Upc2 and/or Pdr1. Phylogenetic and syntenic analyses revealed that C. glabrata, following a whole genome duplication event, maintained HAP1A and HAP1B, whereas Saccharomyces cerevisiae only retained the HAP1A ortholog, HAP1. In this study, we determined the function of two zinc cluster transcription factors, Hap1A and Hap1B, as direct regulators of ERG genes. In S. cerevisiae, Hap1, an ortholog of Hap1A, is a known transcription factor controlling ERG gene expression under aerobic and hypoxic conditions. Interestingly, deleting HAP1 or HAP1B in either S. cerevisiae or C. glabrata, respectively, showed altered susceptibility to azoles. In contrast, the strain deleted for HAP1A did not exhibit azole susceptibility. We also determined that the increased azole susceptibility in a hap1BΔ strain is attributed to decreased azole-induced expression of ERG genes, resulting in decreased levels of total ergosterol. Surprisingly, Hap1A protein expression is barely detected under aerobic conditions but is specifically induced under hypoxic conditions, where Hap1A is required for the repression of ERG genes. However, in the absence of Hap1A, Hap1B can compensate as a transcriptional repressor. Our study shows that Hap1A and Hap1B is utilized by C. glabrata to adapt to specific host and environmental conditions.
IMPORTANCE
Invasive and drug-resistant fungal infections pose a significant public health concern. Candida glabrata, a human fungal pathogen, is often difficult to treat due to its intrinsic resistance to azole antifungal drugs and its capacity to develop clinical drug resistance. Therefore, understanding the pathways that facilitate fungal growth and environmental adaptation may lead to novel drug targets and/or more efficacious antifungal therapies. While the mechanisms of azole resistance in Candida species have been extensively studied, the roles of zinc cluster transcription factors, such as Hap1A and Hap1B, in C. glabrata have remained largely unexplored until now. Our research shows that these factors play distinct yet crucial roles in regulating ergosterol homeostasis under azole drug treatment and oxygen-limiting growth conditions. These findings offer new insights into how this pathogen adapts to different environmental conditions and enhances our understanding of factors that alter drug susceptibility and/or resistance.
KEYWORDS: Candida glabrata; zinc cluster transcription factors; azole antifungal drugs; drug resistance mechanisms; gene regulation; hypoxia; ergosterol pathway; ERG11; ERG3; Hap1; HAP1A (ZCF4, MAR1); HAP1B (ZCF27, HAP1)
INTRODUCTION
Invasive and drug-resistant fungal infections are significant public health issues, and new estimates indicate that life-threatening fungal infections affect over 6.5 million people globally each year (1). Among these global invasive fungal infections, more than 70% are caused by invasive Candida species, which include Candida albicans and other non-albicans (NAC) Candida species, such as C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis (2–5). Of the NAC species listed, Candida glabrata is considered the second or third most commonly isolated NAC Candida species, with C. albicans being the most commonly isolated (2, 4–6). The traditional genus Candida is a paraphyletic group, and C. glabrata is more closely related to Saccharomyces cerevisiae than to other common human pathogens, including C. albicans (7). The last common ancestor (LCA) of C. glabrata and C. albicans existed ~250 million years ago (Mya), whereas the LCA of C. glabrata and S. cerevisiae occurred ~50 Mya (8). C. glabrata is considered the major pathogenic species of the post-whole genome duplication (WGD) Saccharomycetaceae group, with immunosuppressed patients (e.g., those with diabetes mellitus, cancer, or organ transplants) and/or elderly patients being particularly susceptible to these infections (6, 9–12).
C. glabrata is also a non-CTG clade Candida species that is known for its intrinsic resistance to azole drugs and ability to develop clinical azole drug resistance (7, 13, 14). Azole drugs target and inhibit the enzyme lanosterol 14-α-demethylase (Erg11), which is an essential enzyme to produce ergosterol in fungi (15–17). Mechanisms of acquiring clinical azole drug resistance have been extensively documented across Candida species and include mutations in ERG11, ERG3, UPC2, and/or PDR1 (14, 18–25). Among these genes, gain of function (GOF) mutations in the zinc cluster transcription factors Upc2 and Pdr1 result in increased expression of ERG11 and/or the ABC drug transporter CDR1, respectively (24, 26–30). As for C. glabrata clinical drug-resistant isolates, Pdr1 GOF mutations are considered the predominant cause for clinical drug resistance (28, 31).
In addition to Upc2 and Pdr1, several known and/or putative zinc cluster factors (Zcfs) are critical transcriptional regulators involved in stress response in fungi and amoeba (32, 33). Interestingly, 17 of the 41 C. glabrata ZCF genes when deleted show enhanced azole susceptibility as indicated by MIC and/or plate-based growth assays (34). This observation underscores the importance and need to further investigate the role of these zinc cluster transcription factors in C. glabrata. However, with the exception of Upc2A (Zcf5), Pdr1 (Zcf1), Stb5 (Zcf24), and Hap1A (Mar1, Zcf4), little research has been done to understand the mechanistic role of other C. glabrata Zcf proteins during azole treatment conditions and/or hypoxic growth (30, 35–39).
In this report, we show for the first time that S. cerevisiae strains deleted for HAP1 exhibit azole hypersusceptibility when compared to a FY2609 WT strain containing a WT copy of the HAP1 gene. Interestingly, S288C strains, including the commonly used BY4741 and BY4742, exhibit similar azole susceptibility to hap1Δ strains due to a partially disrupted hap1 gene by a Ty1 element (hap1-Ty1 mutation). From syntenic and phylogenetic analysis, C. glabrata contains two ohnologs (gene duplicates originating from whole genome duplication), Hap1A (Zcf4, Mar1) and Hap1B (Zcf27, Hap1), which are homologs of S. cerevisiae Hap1. Based on these observations, we hypothesized that deletion of C. glabrata HAP1 ohnologs would also have a similar azole susceptible phenotype. However, only deletion of C. glabrata HAP1B, but not HAP1A, showed an azole hypersusceptible phenotype. Upon further investigation, we established that altered azole susceptibility of the hap1BΔ strain is attributed to a decrease in azole-induced ERG gene expression, resulting in a subsequent reduction in total ergosterol levels. Moreover, azole hypersusceptibility of the hap1BΔ strain was alleviated when complemented with a plasmid expressing HAP1B or when exogenous ergosterol was introduced into the growth media, but not when the AUS1 sterol transporter was deleted. Interestingly, unlike Hap1B, Hap1A protein levels were nearly undetectable under both untreated and azole-treated conditions. However, under hypoxic conditions, Hap1A was highly induced, while the expression of Hap1B remained unchanged. Moreover, the hap1AΔ strain showed a growth defect that correlated with a failure to repress ERG genes under hypoxic conditions, while the hap1BΔ strain grew similar to Cg2001 WT and maintained ERG gene repression. Interestingly, the hypoxic growth defect and failure to repress ERG genes is further exacerbated in a hap1AΔhap1BΔ double-deletion strain, suggesting Hap1B can compensate for the loss of Hap1A. Additionally, our studies demonstrated that Hap1B and Hap1A can associate with promoters of ERG genes, and their enrichment at these sites is further enhanced upon azole treatment or hypoxic conditions, respectively. Overall, we have discovered that C. glabrata maintains two Hap1 ohnologs to regulate ergosterol homeostasis. Specifically, Hap1B aids in facilitating azole-mediated gene activation, while Hap1A mediates hypoxia-induced gene repression.
RESULTS
Hap1 alters azole susceptibility in S. cerevisiae
In S. cerevisiae, there are three zinc cluster transcription factors Upc2, Ecm22, and Hap1 that are known to regulate the expression of ergosterol gene expression for sterol homeostasis (40–44). In addition, Upc2 and Ecm22 are also known to mediate azole susceptibility in S. cerevisiae (45, 46). However, until now, the role of Hap1 in altering azole susceptibility has not been determined. To test this hypothesis, the hap1-Ty1 mutation was deleted in S288C strains BY4741 and FY2609 to generate BY4741 hap1Δ (this study) and FY2611 hap1Δ (40), respectively (see Table S3). The indicated strains were tested for growth in liquid cultures and through serial-dilution spot assays with and without 16 µg/mL fluconazole (Fig. 1A). Interestingly, the BY4741 strain exhibited a slight increase in fluconazole susceptibility compared with the BY4741 hap1Δ strain (Fig. 1A and B). We suspect that the enhanced azole susceptibility in the BY4741 strain is because of a known insertion of an in-frame Ty1 sequence at the 3′ end of the HAP1 open reading frame (ORF), resulting in the expression of a mutated HAP1 that lacks 13 amino acids from its C-terminus and contains an additional 32 amino acids encoded from the Ty1 sequence. The insertion of the Ty1 element does not seem to affect the growth of BY4741 (hap1-Ty1 mutation) versus FY2609 (HAP1 WT) under untreated conditions (Fig. 1A through C; Table S1). In contrast, deletion of HAP1 (FY2611 hap1Δ) showed a hypersusceptible phenotype compared with FY2609 when grown on agar plates or in liquid culture containing 16 µg/mL fluconazole (Fig. 1A and C). In addition, both the FY2611 hap1Δ and BY4741 hap1Δ strains have a similar doubling time in the presence and absence of fluconazole (Fig. 1A through C; Table S1). To our knowledge, this is the first observation that Hap1 contributes to azole susceptibility in S. cerevisiae. We suspect that this phenotype has not been observed until now because earlier functional genomics screens used the BY4741 and BY4742 parental and deletion strain collections (47).
Fig 1.
Zinc cluster transcription factor Hap1 in S. cerevisiae alters fluconazole susceptibility. (A) Fluconazole susceptibility of BY4741, BY4741 hap1Δ, FY2609, and FY2611 hap1Δ of S. cerevisiae S288C strains. Fivefold serial dilution assays of indicated strains grown on SC plates with and without 16 µg/mL fluconazole and incubated at 30°C for 48 h. A minimum of three biological replicates were performed. (B and C) Growth curve of indicated strains grown in SC liquid media with or without 16 µg/mL fluconazole. Error bars represent SD.
Sequence comparison, phylogenetics, and synteny of Hap1 homologs
Two proteins that are encoded by CAGL0B03421g or HAP1A (aliases MAR1 or ZCF4) and CAGL0K05841g or HAP1B (aliases HAP1 or ZCF27) in C. glabrata share a similar domain structure with S. cerevisiae Hap1 (Fig. 2A). The three domain structures conserved across all three proteins include the N-terminal DNA-binding domain (DBD), a repression module (RPM), and the C-terminal activation domains (ACTs) (Fig. 1A). In addition, Hap1 contains seven heme-responsive motifs (HRMs) characterized by a (K/R)CP(V/L)DH sequence motif (48, 49), whereas Hap1A has five HRMs, and Hap1B has six HRMs (Fig. 2A). Amino acid sequence comparisons indicate that Hap1A shows a higher percent sequence identity and similarity to Hap1 than to Hap1B (Fig. 2B). Additionally, a phylogenetic tree was constructed to investigate the evolutionary relationships between Hap1, Hap1A, and Hap1B, which shows that Hap1A groups more closely with Hap1 compared to Hap1B (Fig. 2C). Furthermore, synteny information provided by the Yeast Gene Order Browser (http://ygob.ucd.ie/) (50) indicates that Hap1 and Hap1A are syntenic (Fig. 2D). Taken together, our evolutionary analysis indicates that Hap1, Hap1A, and Hap1B evolved by whole genome duplication and that Hap1 and Hap1A are orthologs. In addition, the Hap1B ortholog was lost in Saccharomyces by reductive evolution at some point in an ancestor of the genus, returning the gene family to a single copy status in S. cerevisiae.
Fig 2.
Sequence and Evolutionary analysis of Hap1. (A) Schematic of conserved domains found in S. cerevisiae Hap1, C. glabrata, Hap1A, and C. glabrata Hap1B. (B) Table indicating the percent identity and similarity between Hap1, Hap1A, and Hap1B. (C) Gene phylogeny of Hap1 homologs. The phylogeny was rooted based on the species tree, and branch values represent ultrafast bootstrap support. Red color indicates species that evolved following the whole genome duplication event in their last common ancestor, and black color indicates outgroup species. (D) Syntenic analysis between S. cerevisiae HAP1 (YLR256W) and C. glabrata Hap1A (CAGL0B03421) and Hap1B (CAGL0K05841) provided by the Yeast Gene Order Browser (http://ygob.ucd.ie/). Red asterisks mark the position of the HAP1A (CAGL0B03421g) and HAP1B (CAGL0K05841g).
Hap1B, rather than, Hap1A alters azole susceptibility in C. glabrata
Because deletion of HAP1 in S. cerevisiae altered azole susceptibility, we wanted to determine if C. glabrata (Cg) strains lacking Hap1A and Hap1B have a similar susceptibility to azole drugs. To test this hypothesis, we deleted HAP1A and HAP1B in the C. glabrata CBS138 (ATCC Cg2001) WT strain and performed liquid growth and serial-dilution spot assays with and without 32 µg/mL fluconazole (Fig. 3A through C). In the untreated conditions, both hap1BΔ and hap1AΔ strains grew similar to the Cg2001 WT strain on agar plates and liquid cultures (Fig. 3A through C). We also did not observe any differences in doubling times (Table S2). However, in the presence of fluconazole, the hap1BΔ strain showed an azole hypersusceptibility phenotype on agar plates along with a growth delay and longer doubling times when cultured in liquid media, whereas hap1AΔ strain grew like the Cg2001 WT strain (Fig. 3A and C; Table S2). Furthermore, a hap1AΔhap1BΔ double-deletion strain showed azole susceptibility similar to a hap1BΔ strain (Fig. 3A). To confirm that our observed azole hypersusceptible phenotype was due to the loss of HAP1B, complementation assays were performed using a C. glabrata (ATCC 200989 or Cg989 WT) strain. The full-length HAP1B open-reading frame with its endogenous promoter were cloned in the pGRB2.0 plasmid and transformed into a Cg989 hap1BΔ deletion strain (Tables S3 and S4). The pGRB2.0 vector was also transformed into the Cg989 WT and hap1BΔ strains as controls. The HAP1B plasmid construct was able to rescue azole susceptibility as shown by a serial-dilution spot assay (Fig. 3D), while the hap1BΔ strain expressing the plasmid only (Vector) construct remain hypersusceptible (Fig. 3D). In addition, gene expression analysis also confirmed that HAP1B and HAP1A were not expressed in their respective deletion strains (Fig. S1A and B). In addition, we confirmed that the genes upstream and downstream of HAP1B were expressed in hap1BΔ similar to the Cg2001 WT strain (Fig. S2A and B). Finally, we also deleted the upstream (CAGL0K05819g) and downstream (CAGL0K05863g) genes and observed little to no change in azole susceptibility (Fig. S2C). Overall, our data show that Hap1B, rather than Hap1A, plays a specific role in mediating azole susceptibility.
Fig 3.
C. glabrata Hap1B, rather than, Hap1A alters fluconazole susceptibility. (A) Fivefold serial dilution spot assays of Cg2001 WT, hap1BΔ, and hap1AΔ strains plated on SC plates with and without 32 µg/mL fluconazole. A minimum of three biological replicates were performed. (B) Liquid growth curves of the indicated C. glabrata strains grown in SC media with or without 32 µg/mL fluconazole. Error bars represent SD. (C) Fivefold serial dilution assays of Cg989 WT and hap1BΔ transformed with plasmids expressing HAP1B from its endogenous promoter or empty vector spotted on SC-Ura plates with and without 32 µg/mL fluconazole at 30°C for 48 h. Error bars represent SD. A minimum of three biological replicates were performed.
Expression of CYC1 depends on Hap1B, but not Hap1A, because of differences in protein expression
In S. cerevisiae. Hap1 is known to regulate the expression of the CYC1 gene (48, 49, 51–53). To determine if Hap1B and/or Hap1A also controls the expression of C. glabrata CYC1 gene, Cg2001 WT, hap1BΔ, and hap1AΔ strains were grown in the presence and absence of azole treatment, and qRT-PCR transcript analysis was performed. Interestingly, CYC1 transcript analysis revealed that the loss of HAP1B, but not HAP1A, resulted in a 50% decrease in CYC1 expression, irrespective of drug treatment (Fig. 4A and B). To determine if this difference was a consequence of transcript levels of HAP1B and HAP1A, qRT-PCR analysis was performed on Cg2001 WT cells treated with or without 64 µg/mL fluconazole for three or 6 h. Both HAP1B and HAP1A transcript levels were expressed with no significant differences between untreated and fluconazole treated conditions (Fig. 4C through E; Table S7). Furthermore, HAP1B transcript levels are not altered in hap1AΔ strain and vice versa, indicating they are independent of each other (Fig. S1A and B). Because there were similar transcript levels between HAP1A and HAP1B (Fig. 4E), we checked for differences in protein levels between Hap1B and Hap1A. In order to do this, we constructed, and PCR confirmed 3×FLAG-tagged strains where the 3×FLAG-tag was genomically inserted at the C-terminus of HAP1B and HAP1A. We also observed no significant changes in growth between WT and 3×FLAG-tagged strains when treated with and without 32 µg/mL azole (Fig. 4F and G). For protein extraction of the indicated strains, HAP1B-3×FLAG and HAP1A-3×FLAG tagged strains were grown with or without 64 µg/mL fluconazole for 3 or 6 h. Western blot analysis indicated that the Hap1B-3×FLAG protein expression remained nearly constant with and without drug treatment (Fig. 4H). Unexpectedly, we observed virtually no expression of Hap1A-3×FLAG protein regardless of drug treatment (Fig. 4H, Short Exp). Even with longer exposure times, barely detectable levels of Hap1A were observed (Fig. 4H, Long Exp), suggesting that Hap1A is regulated at the post-transcriptional level. Due to essentially undetectable levels of Hap1A protein, we suspect that this is why a hap1AΔ strain does not alter CYC1 gene expression or show hypersusceptibility to azoles.
Fig 4.
Transcript and protein analysis of CYC1, HAP1B, and HAP1A. (A and B) Transcript levels of CYC1 from the indicated strains treated with and without 64 µg/mL fluconazole for 3 h. (C and D) Transcript levels of HAP1B and HAP1A from the indicated strains treated with and without 64 µg/mL fluconazole for 3 h. For panels A–D, transcript levels were set relative to WT and normalized to RDN18 mRNA levels. (E) Transcript level comparison of HAP1A and HAP1B from untreated WT. Data were analyzed from three or more biological replicates with three technical replicates. Statistics were determined using the GraphPad Prism Student t-test, version 9.5.1. Error bars represent SD. ns, P > 0.05; **P < 0.01. (F and G) Fivefold serial dilution spot assays of Cg2001 WT, hap1BΔ, hap1AΔ, HAP1B-3×FLAG, and HAP1A-3×FLAG strains plated on SC plates with and without 32 µg/mL fluconazole. A minimum of three biological replicates were performed. (H) Western blot analysis of Hap1B-3× FLAG and Hap1A-3×FLAG with and without treatment with 64 µg/mL fluconazole for 3 and 6 h. Western blots showing Short (Short Exp) and long (Long Exp) chemiluminescence exposure. Histone H3 was used as the loading control.
Hap1B is dispensable for expression of drug efflux pumps but is needed for azole-induced expression of ergosterol (ERG) genes
Because the hap1BΔ strain showed altered azole susceptibility (Fig. 3A through C), we wanted to identify the mechanism mediating this phenotype. A common mechanism of altering azole resistance in C. glabrata involves the upregulation of drug efflux pumps, such as CDR1, PDH1, and SNQ2, facilitated by the zinc cluster transcription factor Pdr1 (28, 29, 31, 54, 55). To determine if expression of drug efflux pumps is altered in the hap1BΔ strain in the presence or absence of 64 µg/mL fluconazole, the expression levels of the known azole transporters CDR1, PDH1, and SNQ2 as well as the transcriptional regulator PDR1 were analyzed by qRT-PCR analysis. Our transcript analysis revealed no significant difference in the expression of any of the genes encoding ABC transporters in the hap1BΔ strain compared with the Cg2001 WT strain (Fig. 5A and B; Fig. S3A and B), indicating that altered expression of known azole drug efflux pumps under these conditions is not the main reason for azole hypersusceptibility for the hap1BΔ strain.
Fig 5.
Transcript analysis of drug efflux pump and ergosterol (ERG) genes. (A and B) Transcript levels of drug efflux pumps in Cg2001 WT and hap1BΔ strains treated with or without 64 µg/mL fluconazole for 3 h. (C–F) Transcript levels of ERG genes in Cg2001 WT and hap1BΔ strains treated with or without 64 µg/mL fluconazole for 3 h. For panels A–F, all strains were treated with or without 64 µg/mL fluconazole for 3 h. Transcript levels were set relative to the untreated WT and normalized to RDN18 mRNA levels. Data were analyzed from four biological replicates with three technical replicates each. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05; **, P < 0.01. Error bars represent the SD.
In S. cerevisiae, Hap1 is known to regulate steady state transcript levels of ergosterol biosynthesis genes, such as ERG11, ERG3, ERG5, and ERG2 (40, 41, 44, 49, 52, 56, 57). In addition, altered ERG11 gene expression in C. glabrata is also a mechanism that can lead to azole hypersusceptibility phenotypes (24, 58, 59). To determine if altered ERG gene expression was a mechanism for the observed azole hypersusceptibility of the hap1BΔ strain, Cg2001 WT and hap1BΔ strains were treated with and without 64 µg/mL fluconazole, and ERG11, ERG3, ERG5, and ERG2 transcript levels were analyzed by qRT-PCR. In the absence of the drug, with the exception of ERG3, no significant difference in the expression levels of ERG11, ERG5, or ERG2 was observed between the Cg2001 WT and hap1BΔ strains (Fig. 5C through F). However, upon treatment with fluconazole, all four ERG genes failed to induce to wild-type levels in the hap1BΔ strain (Fig. 5C through F). Furthermore, a hap1AΔ strain did not have altered ERG11 and ERG3 expression, which coincides with its lack of expression and azole hypersusceptible phenotype (Fig. S3C and D). Altogether, our data indicate that in addition to Upc2A, Hap1B serves as another critical transcription factor for the azole-induced expression of the late ergosterol pathway genes.
Hap1B-3×FLAG is enriched at ERG gene promoters
Because our data show decreased expression of ergosterol genes in the hap1BΔ strain upon azole treatment (Fig. 5C through F), we suspect that Hap1B is a direct transcription factor for the ERG genes. To determine if Hap1B directly targets the promoter of the ERG11 gene, chromatin immunoprecipitation (ChIP) assays were performed using anti-FLAG monoclonal antibodies and chromatin isolated from untagged Cg2001 WT and Hap1B-3×FLAG strains, treated with or without fluconazole. ChIP-qPCR fluorescent probes were designed to recognize a promoter distal region (PDR) and promoter proximal region (PPR) of the ERG11 promoter (Fig. 6A). Using these probes, a significant enrichment of Hap1B was detected at both ERG11 promoter regions compared to the untagged control (Fig. 6B and C; Table S8). In addition, Hap1B was further enriched at the promoter of ERG11 upon azole treatment (Fig. 6B and C; Table S8) supporting the importance of Hap1B in azole-induced gene expression. No significant enrichment of Hap1B was detected at the 3′ UTR of ERG11 regardless of treatment (Fig. S4), indicating specific enrichment at the promoter region.
Fig 6.
Chromatin immunoprecipitation analysis of Hap1B at ERG gene promoters. (A) Schematic of ERG11 and ERG3 promoter distal region (PDR) and promoter proximal region (PPR). (B–E) ChIP analysis of Cg2001 WT (untagged), Hap1B-3×FLAG at two ERG11 promoter regions (PDR and PPR), and two ERG3 promoter regions (PDR and PPR) when treated with or without 64 µg/mL fluconazole for 3 h. ChIP analysis was normalized to DNA input samples and set relative to untagged WT. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001. Error bars represent SD for three biological replicates with three technical replicates.
We also examined Hap1B localization status on the ERG3 promoter by ChIP analysis (Fig. 6D and E). To determine if Hap1B binds to the promoter of ERG3, two ChIP-qPCR fluorescent probes were designed to recognize the promoter distal region (PDR) and promoter proximal region (PPR) of the ERG3 promoter (Fig. 6A). Similar to the ERG11 promoter, Hap1B was detected at the ERG3 distal promoter region and was further enriched upon fluconazole treatment (Fig. 6D; Table S8). However, we did not detect any Hap1B enrichment at the proximal promoter region (Fig. 6E; Table S8) regardless of azole treatment. Overall, our data demonstrate that Hap1B directly targets the promoters of ERG11 and ERG3 to help facilitate the proper expression of ERG genes and maintenance of ergosterol homeostasis during azole treatment.
The hap1BΔ strain has altered azole susceptibility due to decreased ergosterol levels, which can be suppressed by exogenous sterols and active sterol import
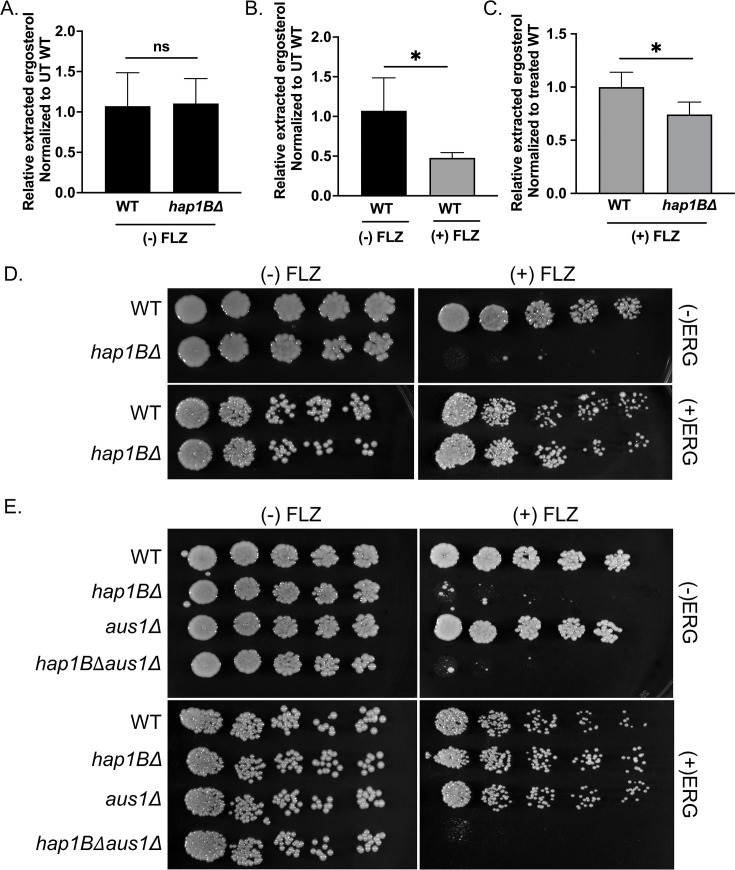
Because azole-induced ERG gene expression is diminished in the hap1BΔ strain, we would expect an additional decrease in ergosterol levels in this strain, which would explain why a hap1BΔ strain has an increase in azole susceptibility. To ascertain whether total endogenous ergosterol levels differed between Cg2001 WT and hap1BΔ strains upon azole treatment, non-polar lipids were extracted from both strains in the presence and absence of 64 µg/mL fluconazole. Total ergosterol level was measured by high-performance liquid chromatography (HPLC) analysis, and cholesterol was used as an internal standard control. No significant difference was observed between Cg2001 WT and hap1BΔ strains in the untreated conditions (Fig. 7A), concurring with our gene expression analysis showing no significant difference in expression of multiple ERG genes without azole treatment. However, upon fluconazole treatment, the Cg2001 WT strain demonstrated the expected decrease in ergosterol levels (Fig. 7B), whereas the hap1BΔ strain exhibited an additional 30% reduction in total ergosterol compared with the treated Cg2001 WT strain (Fig. 7C).
Fig 7.
Disruption of ergosterol levels in a hap1BΔ strain alters azole susceptibility (A–C) HPLC analysis of the total ergosterol extracted from the Cg2001 WT and hap1BΔ strains treated with or without 64 µg/mL fluconazole for 3 h. The figure represents a ratio between ergosterol and cholesterol compared with treated and untreated WT samples. Data were generated from four biological replicates. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05. Error bars represent the SD. (D and E) Fivefold dilution spot assays of the indicated C. glabrata strains grown on SC plates with and without 32 µg/mL fluconazole and/or with and without 20 µg/mL ergosterol. A minimum of three biological replicates were performed.
Due to this observation, we hypothesized that the decrease in ergosterol content contributes to azole hypersensitivity and reasoned that exogenous supplementation with ergosterol would suppress the azole hypersensitive phenotype observed for the hap1BΔ strain. To test this hypothesis, Cg2001 WT and hap1BΔ strains were plated on synthetic complete (SC) media supplemented with or without exogenous ergosterol and/or fluconazole. In support of our hypothesis, serial-dilution spot assays showed that the addition of exogenous ergosterol completely suppressed the azole hypersensitive phenotype of the hap1BΔ strain, whereas hap1BΔ strain without ergosterol retained the hypersensitive phenotype (Fig. 7D). Because ergosterol is solubilized in the presence of Tween 80–ethanol solution, we wanted to determine if this suppression was specific to ergosterol. Thus, Cg2001 WT and hap1BΔ strains were plated on SC media supplemented with a Tween 80–ethanol solution with or without fluconazole. As indicated in Fig. S5, Tween 80–ethanol did not suppress hap1BΔ azole hypersusceptible phenotype (Fig. S5) indicating that suppression was mediated by exogenous ergosterol uptake.
Based on these observations, we also expected that deletion of the only known sterol importer AUS1 would prevent sterol uptake by hap1BΔ strains (60–62). To determine this, we constructed an aus1Δ strain and a hap1BΔaus1Δ double deletion strain and performed serial-dilution spot assays on agar plates supplemented with or without exogenous ergosterol in the presence and/or absence of fluconazole (Fig. 7E). As anticipated, the hap1BΔaus1Δ strain remained hypersensitive to fluconazole with or without exogenous ergosterol (Fig. 7E). However, growth of the hap1BΔ strain was not altered by fluconazole and/or exogenous ergosterol and grew similar to the Cg2001 WT strain (Fig. 7E). Overall, our data elucidate the mechanistic basis and pathway underlying the hypersensitive phenotype observed in the hap1BΔ strain. Because Hap1A is not expressed, it is unclear what role it plays, if any, under azole treatment. In summary, our findings represent the first characterization of Hap1B as direct transcription factor for regulating ergosterol genes and ergosterol homeostasis in response to azole drug treatment.
Hap1A is induced upon hypoxic exposure
In aerobic conditions, S. cerevisiae Hap1 functions as a transcriptional activator of CYC1 and ERG genes (40, 41, 44, 48, 49, 51–53, 56, 57). Furthermore, our presented data suggest that Hap1B operates similarly to Hap1, by regulating the corresponding conserved genes in C. glabrata. Interestingly, in S. cerevisiae, Hap1 functions also as a transcriptional repressor to shut down ERG genes under hypoxia by recruiting a corepressor complex containing Set4, Tup1, and Ssn6 corepressors (40, 57, 63, 64). Currently, it is not known if Hap1B, Hap1A, or another transcription factor functions to repress C. glabrata ERG genes under hypoxic conditions.
Due to our observed phenotype for the hap1BΔ strain, but not for the hap1AΔ strain under azole treated conditions, C. glabrata Cg2001 WT, hap1BΔ, and hap1AΔ strains were serially diluted fivefold on agar plates and grown under aerobic or hypoxic conditions (Fig. 8A). Interestingly, under hypoxic conditions, hap1AΔ and hap1AΔhap1BΔ but not hap1BΔ strains exhibited a statistically significant slow growth defect, as determined by colony size (Fig. 8A). We also did not observe any significant changes in growth difference between WT and 3×FLAG-tagged strains when grown under hypoxic conditions (Fig. 8B). Measuring the colony diameter revealed an approximate 40% decrease in the size of hap1AΔ when compared to the Cg2001 WT and the hap1BΔ colonies, suggesting a potential function for Hap1A (Fig. 8C). Interestingly, hap1AΔhap1BΔ colonies showed a ~60% decrease in size relative to the Cg2001 WT and a ~38% decrease in size when compared with the hap1AΔ colonies (Fig. 8C), indicating that Hap1B has a compensatory function when Hap1A is absent. Due to the significant differences in protein expression observed between Hap1B and Hap1A under aerobic conditions, we also evaluated the transcript and protein expression levels of Hap1A and Hap1B under hypoxic conditions. Using qRT-PCR analysis, a fourfold increase in HAP1A transcript levels was detected after two hours under hypoxic conditions while HAP1B transcript levels remained unaltered from aerobic to hypoxic conditions (Fig. 8D and E). In addition, we assessed the protein levels of Hap1A-3×FLAG and Hap1B-3×FLAG-tagged strains using Western blot analysis. Remarkably, we detected robust levels of Hap1A protein under hypoxic conditions, while Hap1B protein levels remained the same from aerobic to hypoxic conditions (Fig. 8F and G). Taken together, we have identified Hap1A as the first hypoxia-inducible transcription factor in C. glabrata. Given that S. cerevisiae Hap1 is required for repressing ERG genes under hypoxic conditions, we anticipate that Hap1A is hypoxia-induced to function in a similar manner.
Fig 8.
Phenotypic and expression analysis of C. glabrata strains under hypoxic conditions (A and B) Fivefold serial dilution spot assays of Cg2001 WT, hap1BΔ, hap1AΔ, HAP1B-3×FLAG, and HAP1A-3×FLAG strains grown on YPD plates under aerobic and hypoxic conditions. The fourth dilution of the hypoxic plate (A) was enlarged for enhanced visibility. A minimum of three biological replicates were performed. (C) Graphical representation of colony sizes of the indicated strains when grown under hypoxic conditions. Colony sizes were measured using ImageJ, version 1.51. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; ***, P < 0.001. (D and E) Transcript analysis of HAP1A and HAP1B of the Cg2001 WT strain when grown under hypoxic conditions over a time course of 0, 2, 4, 6, and 8 h. The relative transcript levels were set to Cg2001 WT before hypoxic exposure (0 hr) and normalized to RDN18. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05. (F and G) Western blot analysis of Hap1A-3×FLAG and Hap1B-3×FLAG protein levels over a time course of 0, 2, 4, 6, and 8 h of hypoxic exposure. Cg2001 WT (untagged) strain was used as a negative control for both aerobic (Aer) and hypoxic (Hyp) conditions. Histone H3 was used as a loading control.
Ergosterol genes are downregulated upon hypoxic conditions
In S. cerevisiae, it is well established that exposure to hypoxia leads to the repression of the ERG pathway (40, 57, 63). To determine if hypoxia-mediated repression of ERG genes is conserved and robust in C. glabrata, as observed in S. cerevisiae, we performed transcript analysis of multiple ERG genes involved in the late ergosterol biosynthesis pathway, namely, ERG11, ERG3, ERG2, and ERG5. When comparing the indicated ERG gene transcript levels under aerobic versus hypoxic conditions, we observed a significant decrease of 70%–90% in expression under hypoxic conditions (Fig. 9A through D). These findings confirm that a conserved mechanism between S. cerevisiae and C. glabrata is maintained for shutting down ergosterol biosynthesis in response to hypoxic conditions.
Fig 9.
Ergosterol biosynthesis genes in C. glabrata are repressed upon hypoxic exposure. (A–D) The expression of ERG11, ERG3, ERG2, and ERG5 was analyzed in C. glabrata WT cells under both aerobic and hypoxic conditions. Transcript analysis was set relative to aerobic WT and normalized to RDN18 as the internal control. Data were collected from a minimum of three biological replicates, each with three technical replicates. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05. Error bars represent the SD.
Hap1A represses genes from ergosterol pathway under hypoxic conditions while Hap1B plays a compensatory role
In S. cerevisiae, it is known that following exposure to hypoxia ERG genes are repressed by a WT copy of HAP1 but not by hap1-Ty1 expressed in S288C strains (40, 57, 63). To determine if Hap1B and/or Hap1A shares the same function as Hap1 under hypoxic conditions, qRT-PCR analysis on ERG genes was performed. Surprisingly, our transcript analysis did not detect any significant differences in the transcript levels of ERG11, ERG3, ERG5, and ERG2 between the Cg2001 WT and hap1BΔ strain under hypoxic conditions (Fig. 10A through D). In contrast, we observed a significant increase in the transcript levels of ERG11, ERG3, and ERG5 genes in the hap1AΔ strain compared with Cg2001 WT strain (Fig. 10A, B and D), indicating Hap1A acts as a transcriptional repressor. ERG2 showed no significant difference in the transcript levels upon hypoxic exposure in either hap1BΔ or hap1AΔ strain (Fig. 10C), despite being repressed upon hypoxic exposure (Fig. 9C). Interestingly, all four ERG genes show significant increase in transcript levels in the hap1AΔhap1BΔ double-deletion strain when compared with the Cg2001 WT and hap1AΔ strains, suggesting that Hap1B plays a compensatory role to repress ERG genes (Fig. 10A through D). Overall, our findings suggest that Hap1A is directly or indirectly involved in hypoxia-induced ERG gene repression and suggest that Hap1B can play a compensatory role.
Fig 10.
ERG genes are repressed by Hap1A rather than Hap1B upon hypoxic conditions. (A–D) After 8 h of hypoxic exposure, transcript levels of ERG11, ERG3, ERG5, and ERG2 from the indicated strains were determined by qRT-PCR analysis. The levels of ERG genes were set relative to WT and normalized to RDN18. Data were generated from a minimum of three biological replicates. Statistics were determined using the GraphPad prism student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001. Error bars represent the SD.
Both Hap1A-3×FLAG and Hap1B-3×FLAG are enriched on ERG11 and ERG3 gene promoter upon hypoxic exposure
Because we determined that Hap1B was enriched at the promoter sequences of ERG11 and ERG3 under aerobic azole conditions, we wanted to assess the direct binding of Hap1B and Hap1A at ERG gene promoters under hypoxic conditions. To determine this, ChIP assays were performed using anti-FLAG monoclonal antibodies and chromatin isolated from untagged Cg2001 WT, Hap1B-3×FLAG, and Hap1A-3×FLAG strains grown for 8 h under hypoxic conditions. The same ChIP-qPCR fluorescent probes used under azole-treated conditions were utilized to assess the enrichment of Hap1B and Hap1A at the ERG11 and ERG3 promoter distal region (PDR) and promoter proximal region (PPR) (Fig. 11A). At the ERG11 promoter, Hap1B was not enriched at the more distal promoter region but showed 3.5-fold enrichment at the proximal promoter region (Fig. 11B and C). Interestingly, this differs from our observations under azole-treated conditions, where Hap1B was more enriched at the distal promoter region than the proximal promoter region (Fig. 6B and C). For Hap1A, we observed a fivefold enrichment at the ERG11 distal promoter region compared with untagged Cg2001 WT strain, but no enrichment was observed at the proximal region region (Fig. 11D and E). In addition, Hap1B and Hap1A enrichment was specific to the promoter of ERG11 since no significant enrichment was observed at the 3′ UTR of ERG11 (Fig. S6A and B). At the ERG3 promoter, Hap1B was not enriched at the distal promoter region but showed a threefold enrichment at the proximal promoter region (Fig. 11E and F). Again, this differs from our observations under azole-treated conditions where Hap1B enriches exclusively at the ERG3 distal promoter region but not at the proximal promoter region (Fig. 6D and E). In contrast, under hypoxic conditions, Hap1A was 20-fold enriched at the ERG3 distal promoter region, but twofold enriched at the proximal promoter region, suggesting that Hap1A occupies both sites but prefers the distal proximal regions (Fig. 11G and H). Altogether, our data suggest that both Hap1A and Hap1B can bind to the ERG gene promoters under hypoxic conditions; however, each ERG gene may have a similar but also distinct mechanism for gene repression.
Fig 11.
Chromatin immunoprecipitation analysis of Hap1B and Hap1A at ERG gene promoters under hypoxic conditions. (A) Schematic of ERG11 and ERG3 promoter distal region (PDR) and promoter proximal region (PPR). (B–I) ChIP analysis of Cg2001 WT (untagged), Hap1B-3×FLAG and Hap1A-3×FLAG at two ERG11 promoter regions (PDR and PPR) and two ERG3 promoter regions (PDR and PPR) after 8 h of hypoxic treatment. ChIP analysis was normalized to DNA input samples and set relative to untagged WT. Data were generated from three biological replicates, with three technical replicates each. Statistics were determined using the GraphPad Prism Student t test, version 9.5.1. ns, P > 0.05; *, P < 0.05; **, P < 0.01. Error bars represent the SD.
DISCUSSION
In this study, the roles of the C. glabrata zinc cluster transcription factor ohnologs, Hap1A and Hap1B, were investigated in response to azole drug treatment and hypoxic conditions. Our data suggest that Hap1B functions similarly to S. cerevisiae Hap1 under aerobic conditions, regulating the conserved genes CYC1 and ERG3 under untreated conditions. Additionally, we found that loss of HAP1B, but not HAP1A, impacts azole susceptibility due to the inability to adequately induce ERG genes under azole drug treatment and maintain ergosterol homeostasis. Furthermore, we discovered that HAP1A transcript is upregulated, and protein levels are specifically detected in response to hypoxia, allowing it to function as a repressor of ERG genes. Overall, our study revealed that C. glabrata utilizes Hap1A and Hap1B to control gene expression and mediate proper ergosterol homeostasis in response to both azole drug treatment and hypoxic conditions (see model Fig. 12A and B).
Fig 12.
Models depicting the role of Hap1B and Hap1A in response to azole drug treatment and hypoxic conditions. (A) Under aerobic conditions with fluconazole treatment, Hap1B (yellow) binds to the ERG11 distal promoter region (PDR), aiding in transcriptional activation of ERG11. Upc2A (green) binds to the ERG11 proximal promoter region (PPR) and is the commonly known transcription factor for ERG11. Hap1A is not depicted or control expression of ERG11 because it is not expressed, as determined by our data. Under hypoxic conditions, Hap1A (blue) is induced and highly expressed where it prefers to bind to the PDR of ERG11 to repress ERG11 and to prevent binding of Hap1B in the PDR. Hap1B binds to the PPR region and could help in repression. (B) Under aerobic conditions with fluconazole treatment, Hap1B (yellow) binds to the ERG3 distal promoter region (PDR), aiding Upc2A in transcriptional activation of ERG3. Upc2A (green) is known to bind to the ERG3 proximal promoter region and a known transcription factor for ERG3. Again, Hap1A is not shown to control the expression of ERG3 because it is not expressed. Under hypoxic conditions, Hap1A is induced and highly expressed where it prefers to bind the PDR. As indicated, both Hap1A and Hap1B bind to the PPR regions of ERG3 and could help in repression. (C) Based on hypoxic gene expression data, Hap1A and Hap1B equally contribute to the repression of ERG2. Overall, these models indicate utilization of three zinc cluster transcription factors for direct and distinct promoter control of ERG genes in response to azole treatment and hypoxic conditions. Upc2A was not depicted in the hypoxic diagrams since it is unclear if Upc2A is present or absent at the repressed ERG genes. However, we would expect that Upc2A is the main transcriptional activator when Hap1A and Hap1B are not present. For panels A–C, arrows represent activation, while bars indicate inhibition. The dotted lines and question marks denote unknown interactions or regulatory mechanisms.
Even though phylogenetic and syntenic analyses indicate that S. cerevisiae Hap1 is orthologous to C. glabrata Hap1A, we determined that C. glabrata Hap1B, but not Hap1A, alters azole susceptibility. Although Upc2A is the major transcription factor associated with azole-mediated induction of ERG genes, our study provides new insights into an additional transcriptional regulator besides Upc2A that is needed for azole-induced expression of ERG genes. Additional genetic and biochemical studies will be needed to determine the mechanism by which Hap1B and Upc2A operate together in response to azole drugs. Nonetheless, we speculate that Hap1B could mediate either a direct or indirect cooperative event that assists Upc2A in fully inducing ergosterol genes (see model, Fig. 12A and B). Additionally, in S. cerevisiae, deleting both Upc2 and its paralog Ecm22 further alters azole drug susceptibility, resistance to amphotericin B, and ERG gene expression (42, 43, 45, 63). Thus, Upc2A and Hap1B may be operating in an analogous manner. However, there exists a distinct possibility that other yet-to-be identified zinc cluster transcription factors could be involved in regulating ERG gene expression. Identifying additional transcription factors besides Hap1B and Upc2A will be important to fully understand what contributes to azole susceptibility and/or clinical drug resistance.
In contrast to Hap1B, Hap1A protein levels were nearly undetectable under aerobic and/or azole-treated conditions, with significant induction observed only under hypoxic conditions. This explains why the HAP1A deletion strain lacks an azole hypersensitive phenotype or any alteration in ERG gene expression. Based on our data, Hap1A protein levels are likely being regulated by an unknown post-transcriptional mechanism. Although we have not identified the regulatory mechanism governing Hap1A protein levels, we suspect that it is degraded via a specific ubiquitin ligase. Hap1A may also be regulated in a manner similar to human HIF-1α (65, 66). To our knowledge, Hap1A represents the first identified hypoxia-induced zinc cluster transcription factor and understanding the precise mechanism of protein degradation would be of interest.
Hap1A was previously named Mar1 (Multiple Azole Resistance 1) because deletion of Hap1A in the C. glabrata KUE100 strain was shown to alter azole susceptibility when treated with high concentrations of azoles, we have not been able to confirm this with our studies using the C. glabrata 2001 strain (34, 39). Currently, the reason behind these discrepancies is unclear, but there could be differences in C. glabrata strains or conditions where Hap1A is expressed at higher levels than what we have observed. However, the findings by Gale et al., utilizing a C. glabrata BG14 strain and employing a Hermes transposon approach to screen for fluconazole susceptibility, provided support for our observations (67). In their study, they identified several genes that when disrupted, altered azole drug susceptibility, including Hap1B but not Hap1A (67). More studies will be needed to completely understand the role of Hap1A in azole susceptibility, if any, and how it is regulated at the transcriptional and post-transcriptional level. Nonetheless, our results are clear and consistent where Hap1A and Hap1B can play hypoxia-specific roles in repressing ERG genes (see model, Fig. 12A through C). We suspect that Hap1A and/or Hap1B, similar to Hap1 in S. cerevisiae, operates with a corepressor complex to repress ERG genes (57, 63, 64). Hypoxia-induced expression of Hap1A and the growth defect observed in the hap1AΔ and hap1AΔhap1BΔ strains under hypoxic conditions highlight the importance of these transcription factors in metabolic adaptation and survival in oxygen-limited environments. In addition, it is likely Hap1A hypoxia-specific induction plays additional roles for C. glabrata to survive and propagate under low oxygen while within the humans.
Overall, this study expands our understanding of the transcriptional regulation of ergosterol biosynthesis in C. glabrata. This is significant because targeting ergosterol and/or enzymes involved in ergosterol biosynthesis have yielded highly useful and effective antifungals (64, 68, 69). Thus, studies focused on the regulatory mechanisms of this pathway could lead to the development of targeted antifungal therapies and help in overcoming the challenge of azole resistance in clinical settings. Because zinc cluster transcription factors are unique to fungi and not found in humans (32), there could be an opportunity to explore them as drug targets. Overall, our findings reveal a novel regulatory mechanism where Hap1A and Hap1B are differentially employed by C. glabrata to manage ergosterol biosynthesis and maintain membrane integrity under varying environmental conditions. Our findings provide some of the first insights into the functional role of two zinc cluster transcription factors. We suspect that further studies on these and similar factors will enhance our understanding of the pathophysiology and drug resistance mechanisms of C. glabrata.
MATERIALS AND METHODS
Plasmids and yeast strains
All plasmids and yeast strains are described in Table S3 and Table S4. The S288C BY4741 S. cerevisiae strain was obtained from Open Biosystems. The S288C strain containing the HAP1-Ty1 sequence was corrected with a wild-type copy of HAP1 (FY2609), and the HAP1 deletion strain (FY2611) was kindly provided to us by Dr. Fred Winston, Department of Genetics, Harvard Medical School (40). C. glabrata 2001 (CBS138, ATCC 2001) and C. glabrata 989 (ATCC 200989) were acquired from the American Type Culture Collection (70). For Hap1B complementation assays, a genomic fragment containing the HAP1B promoter, 5′ UTR, open reading frame (ORF), and 3′ UTR was PCR-amplified and cloned into the pGRB2.0 plasmid (Addgene) (71) using restriction enzymes BamHI and SacII. For endogenous C-terminal epitope tagging, a 3×FLAG-NatMX cassette was PCR-amplified from pYC46 plasmid (Addgene) and inserted at the C-terminus of HAP1B and HAP1A (72, 73). All C. glabrata strains were created using the CRISPR-Cas9 RNP system as previously described (72). Briefly, for generating deletion strains, two CRISPR gRNAs were designed near the 5′ and 3′ ORFs of the gene of interest. Drug-resistant selection markers were PCR-amplified using Ultramer DNA Oligos (IDT) from pAG32-HPHMX6 (hygromycin) or pAG25-NATMX6 (nourseothricin). For 3×FLAG epitope tagging, one CRISPR gRNA was designed in the 3′ UTR of the gene of interest. Cells were then electroporated with the CRISPR-RNP mix and the drug resistance cassette.
Serial-dilution spot and liquid growth assay
For serial-dilution spot assays, yeast strains were grown to saturation overnight in synthetic complete (SC) media at 30°C. Cells were diluted to OD600 of 0.1 and allowed to grow to exponential phase with continuous shaking at 30°C. Each strain was then spotted in fivefold dilution starting at an OD600 of 0.01 on untreated SC agar plates or plates containing 16 µg/mL fluconazole (Cayman) for S. cerevisiae and 32 µg/mL fluconazole (Cayman) for C. glabrata. Plates were grown at 30°C for 2 days. For liquid growth assay, the yeast strains were inoculated in SC media and grown to saturation overnight. The cultures were then diluted to an OD600 of 0.1 and grown to log phase with shaking at 30°C. Upon reaching log phase, the strains were diluted to an OD600 of 0.001 in a 96-well round bottom plate containing 100 µL of SC media with and without 16 µg/mL fluconazole for S. cerevisiae and 32 µg/mL fluconazole for C. glabrata. Fluconazole, a triazole antifungal commonly used to treat Candida infections, was dissolved in sterile purified water for use in these studies. Cells were grown in liquid culture for 50 h with shaking at 30°C, and the OD600 was measured every 15 min using a Bio-Tek Synergy four multimode plate reader. For spot assays under hypoxia, YPD plates were placed inside the BD Gaspak EZ anaerobe gas generating pouch system with indicator (BD 260683) after spotting and incubated for up to 7 days. Hypoxic cell collection for qRT-PCR, Western blot, and ChIP assays was performed by growing the indicated yeast strains in YPD media for 8 h using the BD GasPak EZ anaerobe gas-generating pouch system (BD 260683). Cells were immediately spun down for 1 min and flash frozen to maintain the hypoxic state.
Phylogenetic analysis
For the phylogenetic analysis, the multiple sequence alignment for the full HAP1 gene family was downloaded from the Yeast Gene Order Browser (http://ygob.ucd.ie/) (50). This file included homologous genes for 19 species: 11 united by a whole genome duplication event in their last common ancestor (S. cerevisiae, S. kudriavzevii, S. mikatae, C. glabrata, Kazachstania africana, Kazachstania naganishii, Naumovozyma castellii, Naumovozyma dairenensis, Tetrapisispora blattae, Tetrapisispora phaffii, and Vanderwaltozyma polyspora) and eight outgroups (Eremothecium cymbalariae, Eremothecium gossypii, Kluyveromyces lactis, Lachancea kluyveri, Lachancea thermotolerans, Lachancea waltii, Torulaspora delbrueckii, and Zygosaccharomyces rouxii). Additionally, we identified Hap1 homologs in Nakaseomyces bacillisporus and Nakaseomyces delphensis, two species related to C. glabrata, by querying the Hap1 sequence against both genomes in JGI Mycocosm using the built in BLAST tool (74). Combined, all sequences were realigned using Muscle5 v 5.1 using default settings (75). The aligned sequences were then used to generate a maximum-likelihood phylogenetic tree with IQ-TREE version 2.2.0, using the built-in ModelFinder to determine the best-fit nucleic acid substitution model and 1,000 ultrafast bootstrap replicates (76). The tree was visualized using ETE v3 (77).
Quantitative real-time PCR analysis
RNA was isolated from C. glabrata strains grown in SC or YPD treated with or without 64 µg/mL fluconazole using standard acid phenol purification method, and 1 µg RNA was reverse transcribed to cDNA using the All-in-One 5× RT Mastermix kit (ABM). Gene expression primers were designed using Primer Express 3.0 software and are listed in Table S5. Quantitative real-time polymerase chain reaction (qRT-PCR) values are indicated in Tables S7 and S9. At least three biological replicates, including three technical replicates, were performed for all samples. Data were analyzed by the comparative CT method (2–ΔΔCT) where RDN18 (18S rRNA) was used as an internal control. All samples were normalized to untreated untagged wild-type strain. GraphPad Prism version 9.5.1 was used to determine the unpaired t-test for determining statistical significance.
Yeast extraction and Western blot analysis
The indicated C. glabrata strains were grown in SC or YPD media under aerobic conditions with or without 64 µg/mL of azoles or hypoxic conditions. Yeast whole cell extraction and Western blot analysis to detect Hap1A-3×FLAG, Hap1B-3×FLAG, and histone H3 were performed as previously described (78). The monoclonal FLAG M2 mouse antibody (F1804, Sigma-Aldrich) was used at a 1:5,000 dilution to detect Hap1A-3×FLAG and Hap1B-3×FLAG. as previously described (57). The histone H3 rabbit polyclonal antibody (PRF&L) was used at a 1:100,000 dilution as previously described (72). HRP-conjugated anti-rabbit or anti-mouse IgG was used as secondary antibody.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed using ZipChIP as previously described (79). Briefly, 50 mL cultures of indicated yeast strains were grown to exponential phase (OD600 of 0.6) in SC or YPD media with or without shaking at 30°C under aerobic or 8 h of hypoxic condition, respectively. Cells grown in SC media under aerobic condition were treated with 64 µg/mL fluconazole (Cayman) for 3 h and collected. Cells were then formaldehyde cross-linked for 15 min and harvested as previously described (79). The cells were lysed by bead-beating with glass beads, and lysate was separated from beads. Upon separation, cell lysates were transferred to Diagenode Bioruptor Pico microtubes and sonicated with a Diagenode Bioruptor Pico at the high frequency setting for 30 s ON and 30 s OFF for 20 cycles. After sonication, cell lysates were pre-cleared with 5 µL of unbound protein G magnetic beads (10004D, Invitrogen) for 30 min with rotation at 4°C. Then, 300 µL of precleared lysate was immunoprecipitated with 10 µL of protein G- magnetic beads (10004D, Invitrogen) conjugated to 1 µL of M2 FLAG antibody (F1804, Sigma-Aldrich). Probe and primer sets used for qPCR analysis are described in Table S6, and qPCR values are indicated in Tables S8 and S10.
Ergosterol extraction
Ergosterol was extracted from indicated strains as previously described (58, 80). Cultures were grown overnight in SC minimal media. Saturated cultures were back diluted to OD600 of 0.1 and were grown at 30°C to exponential phase (OD600 of 0.6), with or without 64 µg/mL fluconazole treatment. Sterols were extracted from yeast using 4 M potassium hydroxide in 70% (vol/vol) ethanol at 85°C for 1 h. After extraction, nonpolar lipids were separated by washing with methanol twice. Nonpolar sterols were crystallized after evaporating the n-hexane and dissolved in 100% methanol. Samples were analyzed by HPLC using a C18 column with a flow rate of 1 mL/min of 100% methanol. Ergosterol was detected at 280 nm, and cholesterol, used as an internal control for extraction, was detected at 210 nm.
ACKNOWLEDGMENTS
This publication was supported by grants from the NIH National Institute of Allergy and Infectious Diseases to S.D.B. (AI136995) and to J.B.G (T32AI148103). Funding support was also provided from Purdue University AgSEED Crossroads program to support Indiana’s Agriculture and Rural Development (to S.D.B), Purdue Institute for Cancer Research (Grant P30CA023168: Bindley Metabolite Profiling Facility), Department of Biochemistry Bird Stair Fellowship (to D.S.), and NIFA 1007570 (to S.D.B).
Contributor Information
Scott D. Briggs, Email: sdbriggs@purdue.edu.
Aaron P. Mitchell, University of Georgia, Athens, Georgia, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00524-24.
Figures S1–S6 and Tables S1–S10.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Denning DW. 2024. Global incidence and mortality of severe fungal disease. Lancet Infect Dis 24:e428–e438. doi: 10.1016/S1473-3099(23)00692-8 [DOI] [PubMed] [Google Scholar]
- 2. Fang W, Wu J, Cheng M, Zhu X, Du M, Chen C, Liao W, Zhi K, Pan W. 2023. Diagnosis of invasive fungal infections: challenges and recent developments. J Biomed Sci 30:42. doi: 10.1186/s12929-023-00926-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delaloye J, Calandra T. 2014. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5:161–169. doi: 10.4161/viru.26187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunke S, Hube B. 2013. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cell Microbiol 15:701–708. doi: 10.1111/cmi.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Husni R, Bou Zerdan M, Samaha N, Helou M, Mahfouz Y, Saniour R, Hourani S, Kolanjian H, Afif C, Azar E, El Jisr T, Mokhbat J, Abboud E, Feghali R, Abboud E, Matta H, Karayakouboglo G, Matar M, Moghnieh R, Daoud Z. 2023. Characterization and susceptibility of non-albicans Candida isolated from various clinical specimens in Lebanese hospitals. Front Public Health 11:1115055. doi: 10.3389/fpubh.2023.1115055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner SA, Butler G. 2014. The Candida pathogenic species complex. Cold Spring Harb Perspect Med 4:a019778. doi: 10.1101/cshperspect.a019778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, et al. 2004. Genome evolution in yeasts. Nature New Biol 430:35–44. doi: 10.1038/nature02579 [DOI] [PubMed] [Google Scholar]
- 8. Crunden JL, Diezmann S. 2021. Hsp90 interaction networks in fungi-tools and techniques. FEMS Yeast Res 21:foab054. doi: 10.1093/femsyr/foab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flevari A, Theodorakopoulou M, Velegraki A, Armaganidis A, Dimopoulos G. 2013. Treatment of invasive candidiasis in the elderly: a review. Clin Interv Aging 8:1199–1208. doi: 10.2147/CIA.S39120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. 2018. Invasive candidiasis. Nat Rev Dis Primers 4:18026. doi: 10.1038/nrdp.2018.26 [DOI] [PubMed] [Google Scholar]
- 11. Fidel PL, Vazquez JA, Sobel JD. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev 12:80–96. doi: 10.1128/CMR.12.1.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurtzman CP. 2003. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res 4:233–245. doi: 10.1016/S1567-1356(03)00175-2 [DOI] [PubMed] [Google Scholar]
- 13. Gabaldón T, Martin T, Marcet-Houben M, Durrens P, Bolotin-Fukuhara M, Lespinet O, Arnaise S, Boisnard S, Aguileta G, Atanasova R, et al. 2013. Comparative genomics of emerging pathogens in the Candida glabrata clade. BMC Genomics 14:623. doi: 10.1186/1471-2164-14-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. 2016. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol 7:2173. doi: 10.3389/fmicb.2016.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lass-Flörl C. 2011. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs (Abingdon Engl) 71:2405–2419. doi: 10.2165/11596540-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 16. Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. 1995. Mode of action and resistance to azole antifungals associated with the formation of 14 alpha-methylergosta-8,24(28)-dien-3 beta,6 alpha-diol. Biochem Biophys Res Commun 207:910–915. doi: 10.1006/bbrc.1995.1272 [DOI] [PubMed] [Google Scholar]
- 17. Warrilow AGS, Martel CM, Parker JE, Melo N, Lamb DC, Nes WD, Kelly DE, Kelly SL. 2010. Azole binding properties of Candida albicans sterol 14-alpha demethylase (CaCYP51). Antimicrob Agents Chemother 54:4235–4245. doi: 10.1128/AAC.00587-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanglard D, Ischer F, Koymans L, Bille J. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother 42:241–253. doi: 10.1128/AAC.42.2.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White TC. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14alpha demethylase in Candida albicans. Antimicrob Agents Chemother 41:1488–1494. doi: 10.1128/AAC.41.7.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, Schumacher U, Einsele H. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett 400:80–82. doi: 10.1016/s0014-5793(96)01360-9 [DOI] [PubMed] [Google Scholar]
- 21. Watson PF, Rose ME, Ellis SW, England H, Kelly SL. 1989. Defective sterol C5-6 desaturation and azole resistance: a new hypothesis for the mode of action of azole antifungals. Biochem Biophys Res Commun 164:1170–1175. doi: 10.1016/0006-291x(89)91792-0 [DOI] [PubMed] [Google Scholar]
- 22. Flowers SA, Barker KS, Berkow EL, Toner G, Chadwick SG, Gygax SE, Morschhäuser J, Rogers PD. 2012. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical isolates of Candida albicans. Eukaryot Cell 11:1289–1299. doi: 10.1128/EC.00215-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunkel N, Liu TT, Barker KS, Homayouni R, Morschhäuser J, Rogers PD. 2008. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot Cell 7:1180–1190. doi: 10.1128/EC.00103-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whaley SG, Caudle KE, Vermitsky JP, Chadwick SG, Toner G, Barker KS, Gygax SE, Rogers PD. 2014. UPC2A is required for high-level azole antifungal resistance in Candida glabrata. Antimicrob Agents Chemother 58:4543–4554. doi: 10.1128/AAC.02217-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simonicova L, Moye-Rowley WS. 2020. Functional information from clinically-derived drug resistant forms of the Candida glabrata Pdr1 transcription factor. PLoS Genet 16:e1009005. doi: 10.1371/journal.pgen.1009005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoot SJ, Smith AR, Brown RP, White TC. 2011. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob Agents Chemother 55:940–942. doi: 10.1128/AAC.00995-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heilmann CJ, Schneider S, Barker KS, Rogers PD, Morschhäuser J. 2010. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob Agents Chemother 54:353–359. doi: 10.1128/AAC.01102-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vermitsky JP, Edlind TD. 2004. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother 48:3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrari S, Ischer F, Calabrese D, Posteraro B, Sanguinetti M, Fadda G, Rohde B, Bauser C, Bader O, Sanglard D. 2009. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog 5:e1000268. doi: 10.1371/journal.ppat.1000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ni Q, Wang C, Tian Y, Dong D, Jiang C, Mao E, Peng Y. 2018. CgPDR1 gain-of-function mutations lead to azole-resistance and increased adhesion in clinical Candida glabrata strains. Mycoses 61:430–440. doi: 10.1111/myc.12756 [DOI] [PubMed] [Google Scholar]
- 31. Tsai H-F, Krol AA, Sarti KE, Bennett JE. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob Agents Chemother 50:1384–1392. doi: 10.1128/AAC.50.4.1384-1392.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacPherson S, Larochelle M, Turcotte B. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev 70:583–604. doi: 10.1128/MMBR.00015-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Bürglin TR, et al. 2013. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol 14:R11. doi: 10.1186/gb-2013-14-2-r11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klimova N, Yeung R, Kachurina N, Turcotte B. 2014. Phenotypic analysis of a family of transcriptional regulators, the zinc cluster proteins, in the human fungal pathogen Candida glabrata. G3 (Bethesda) 4:931–940. doi: 10.1534/g3.113.010199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ollinger TL, Vu B, Murante D, Parker JE, Simonicova L, Doorley L, Stamnes MA, Kelly SL, Rogers PD, Moye-Rowley WS, Krysan DJ. 2021. Loss-of-function ROX1 mutations suppress the fluconazole susceptibility of upc2AΔ mutation in Candida glabrata, implicating additional positive regulators of ergosterol biosynthesis. mSphere 6:e00830-21. doi: 10.1128/msphere.00830-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vu BG, Stamnes MA, Li Y, Rogers PD, Moye-Rowley WS. 2021. The Candida glabrata Upc2A transcription factor is a global regulator of antifungal drug resistance pathways. PLoS Genet 17:e1009582. doi: 10.1371/journal.pgen.1009582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagi M, Nakayama H, Tanabe K, Bard M, Aoyama T, Okano M, Higashi S, Ueno K, Chibana H, Niimi M, Yamagoe S, Umeyama T, Kajiwara S, Ohno H, Miyazaki Y. 2011. Transcription factors CgUPC2A and CgUPC2B regulate ergosterol biosynthetic genes in Candida glabrata. Genes Cells 16:80–89. doi: 10.1111/j.1365-2443.2010.01470.x [DOI] [PubMed] [Google Scholar]
- 38. Noble JA, Tsai HF, Suffis SD, Su Q, Myers TG, Bennett JE. 2013. STB5 is a negative regulator of azole resistance in Candida glabrata. Antimicrob Agents Chemother 57:959–967. doi: 10.1128/AAC.01278-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pais P, Galocha M, Califórnia R, Viana R, Ola M, Okamoto M, Chibana H, Butler G, Teixeira MC. 2022. Characterization of the Candida glabrata transcription factor CgMar1: role in azole susceptibility. JoF 8:61. doi: 10.3390/jof8010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hickman MJ, Winston F. 2007. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol Cell Biol 27:7414–7424. doi: 10.1128/MCB.00887-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davies BSJ, Rine J. 2006. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174:191–201. doi: 10.1534/genetics.106.059964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davies BSJ, Wang HS, Rine J. 2005. Dual activators of the sterol biosynthetic pathway of Saccharomyces cerevisiae: similar activation/regulatory domains but different response mechanisms. Mol Cell Biol 25:7375–7385. doi: 10.1128/MCB.25.16.7375-7385.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vik A Rine J. 2001. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol 21:6395–6405. doi: 10.1128/MCB.21.19.6395-6405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tamura K-I, Gu Y, Wang Q, Yamada T, Ito K, Shimoi H. 2004. A hap1 mutation in a laboratory strain of Saccharomyces cerevisiae results in decreased expression of ergosterol-related genes and cellular ergosterol content compared to sake yeast. J Biosci Bioeng 98:159–166. doi: 10.1016/S1389-1723(04)00260-9 [DOI] [PubMed] [Google Scholar]
- 45. Marie C, Leyde S, White TC. 2008. Cytoplasmic localization of sterol transcription factors Upc2p and Ecm22p in S. cerevisiae. Fungal Genet Biol 45:1430–1438. doi: 10.1016/j.fgb.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barker KS, Pearson MM, Rogers PD. 2003. Identification of genes differentially expressed in association with reduced azole susceptibility in Saccharomyces cerevisiae. J Antimicrob Chemother 51:1131–1140. doi: 10.1093/jac/dkg217 [DOI] [PubMed] [Google Scholar]
- 47. Giaever G, Nislow C. 2014. The yeast deletion collection: a decade of functional genomics. Genetics 197:451–465. doi: 10.1534/genetics.114.161620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Creusot F, Verdière J, Gaisne M, Slonimski PP. 1988. CYP1 (HAP1) regulator of oxygen-dependent gene expression in yeast. I. Overall organization of the protein sequence displays several novel structural domains. J Mol Biol 204:263–276. doi: 10.1016/0022-2836(88)90574-8 [DOI] [PubMed] [Google Scholar]
- 49. Fytlovich S, Gervais M, Agrimonti C, Guiard B. 1993. Evidence for an interaction between the CYP1(HAP1) activator and a cellular factor during heme-dependent transcriptional regulation in the yeast Saccharomyces cerevisiae. EMBO J 12:1209–1218. doi: 10.1002/j.1460-2075.1993.tb05762.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Byrne KP, Wolfe KH. 2005. The yeast gene order browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res 15:1456–1461. doi: 10.1101/gr.3672305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guarente L, Mason T. 1983. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell 32:1279–1286. doi: 10.1016/0092-8674(83)90309-4 [DOI] [PubMed] [Google Scholar]
- 52. Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne J-B, Reynolds DB, Yoo J, Jennings EG, Zeitlinger J, Pokholok DK, Kellis M, Rolfe PA, Takusagawa KT, Lander ES, Gifford DK, Fraenkel E, Young RA. 2004. Transcriptional regulatory code of a eukaryotic genome. Nature New Biol 431:99–104. doi: 10.1038/nature02800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gaisne M, Bécam AM, Verdière J, Herbert CJ. 1999. A “natural” mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1). Curr Genet 36:195–200. doi: 10.1007/s002940050490 [DOI] [PubMed] [Google Scholar]
- 54. Whaley SG, Zhang Q, Caudle KE, Rogers PD. 2018. Relative contribution of the ABC transporters Cdr1, Pdh1, and Snq2 to azole resistance in Candida glabrata. Antimicrob Agents Chemother 62:e01070-18. doi: 10.1128/AAC.01070-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Castanheira M, Deshpande LM, Davis AP, Carvalhaes CG, Pfaller MA. 2022. Azole resistance in Candida glabrata clinical isolates from global surveillance is associated with efflux overexpression. J Glob Antimicrob Resist 29:371–377. doi: 10.1016/j.jgar.2022.05.004 [DOI] [PubMed] [Google Scholar]
- 56. Turi TG, Loper JC. 1992. Multiple regulatory elements control expression of the gene encoding the Saccharomyces cerevisiae cytochrome P450, lanosterol 14 alpha-demethylase (ERG11). J Biol Chem 267:2046–2056. [PubMed] [Google Scholar]
- 57. Serratore ND, Baker KM, Macadlo LA, Gress AR, Powers BL, Atallah N, Westerhouse KM, Hall MC, Weake VM, Briggs SD. 2018. A novel sterol-signaling pathway governs azole antifungal drug resistance and hypoxic gene repression in Saccharomyces cerevisiae Genetics 208:1037–1055. doi: 10.1534/genetics.117.300554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baker KM, Hoda S, Saha D, Gregor JB, Georgescu L, Serratore ND, Zhang Y, Cheng L, Lanman NA, Briggs SD. 2022. The set1 histone H3K4 methyltransferase contributes to azole susceptibility in a species-specific manner by differentially altering the expression of drug efflux pumps and the ergosterol gene pathway. Antimicrob Agents Chemother 66:e0225021. doi: 10.1128/aac.02250-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vu BG, Moye-Rowley WS. 2022. Azole-resistant alleles of ERG11 in Candida glabrata trigger activation of the Pdr1 and Upc2A transcription factors. Antimicrob Agents Chemother 66:e02098–21. doi: 10.1128/aac.02098-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nakayama H, Tanabe K, Bard M, Hodgson W, Wu S, Takemori D, Aoyama T, Kumaraswami NS, Metzler L, Takano Y, Chibana H, Niimi M. 2007. The Candida glabrata putative sterol transporter gene CgAUS1 protects cells against azoles in the presence of serum. J Antimicrob Chemother 60:1264–1272. doi: 10.1093/jac/dkm321 [DOI] [PubMed] [Google Scholar]
- 61. Zavrel M, Hoot SJ, White TC. 2013. Comparison of sterol import under aerobic and anaerobic conditions in three fungal species, Candida albicans, Candida glabrata, and Saccharomyces cerevisiae. Eukaryot Cell 12:725–738. doi: 10.1128/EC.00345-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li QQ, Tsai HF, Mandal A, Walker BA, Noble JA, Fukuda Y, Bennett JE. 2018. Sterol uptake and sterol biosynthesis act coordinately to mediate antifungal resistance in Candida glabrata under azole and hypoxic stress. Mol Med Rep 17:6585–6597. doi: 10.3892/mmr.2018.8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jordá T, Barba-Aliaga M, Rozès N, Alepuz P, Martínez-Pastor MT, Puig S. 2022. Transcriptional regulation of ergosterol biosynthesis genes in response to iron deficiency. Environ Microbiol 24:5248–5260. doi: 10.1111/1462-2920.16157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jordá T, Puig S. 2020. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes (Basel) 11:795. doi: 10.3390/genes11070795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Masoud GN, Li W. 2015. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389. doi: 10.1016/j.apsb.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fong GH, Takeda K. 2008. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ 15:635–641. doi: 10.1038/cdd.2008.10 [DOI] [PubMed] [Google Scholar]
- 67. Gale AN, Sakhawala RM, Levitan A, Sharan R, Berman J, Timp W, Cunningham KW. 2020. Identification of essential genes and fluconazole susceptibility genes in Candida glabrata by profiling Hermes transposon insertions. G3 10:3859–3870. doi: 10.1534/g3.120.401595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. 2014. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med 5:a019752. doi: 10.1101/cshperspect.a019752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee Y, Robbins N, Cowen LE. 2023. Molecular mechanisms governing antifungal drug resistance. NPJ Antimicrob Resist 1:5. doi: 10.1038/s44259-023-00007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kitada K, Yamaguchi E, Arisawa M. 1995. Cloning of the Candida glabrata TRP1 and HIS3 genes, and construction of their disruptant strains by sequential integrative transformation. Gene 165:203–206. doi: 10.1016/0378-1119(95)00552-h [DOI] [PubMed] [Google Scholar]
- 71. Zordan RE, Ren Y, Pan S-J, Rotondo G, De Las Peñas A, Iluore J, Cormack BP. 2013. Expression plasmids for use in Candida glabrata. G3 (Bethesda) 3:1675–1686. doi: 10.1534/g3.113.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gregor JB, Gutierrez-Schultz VA, Hoda S, Baker KM, Saha D, Burghaze MG, Briggs SD. 2023. Expanding the toolkit for genetic manipulation and discovery in Candida species using a CRISPR ribonucleoprotein-based approach. bioRxiv:2023.06.16.545382. doi: 10.1101/2023.06.16.545382 [DOI] [Google Scholar]
- 73. Yáñez-Carrillo P, Orta-Zavalza E, Gutiérrez-Escobedo G, Patrón-Soberano A, De Las Peñas A, Castaño I. 2015. Expression vectors for C-terminal fusions with fluorescent proteins and epitope tags in Candida glabrata. Fungal Genet Biol 80:43–52. doi: 10.1016/j.fgb.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 74. Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, Riley R, Salamov A, Zhao X, Korzeniewski F, Smirnova T, Nordberg H, Dubchak I, Shabalov I. 2014. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res 42:D699–704. doi: 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Edgar RC. 2022. Muscle5: high-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat Commun 13:6968. doi: 10.1038/s41467-022-34630-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. doi: 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huerta-Cepas J, Serra F, Bork P. 2016. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33:1635–1638. doi: 10.1093/molbev/msw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mersman DP, Du H-N, Fingerman IM, South PF, Briggs SD. 2012. Charge-based interaction conserved within histone H3 lysine 4 (H3K4) methyltransferase complexes is needed for protein stability, histone methylation, and gene expression. J Biol Chem 287:2652–2665. doi: 10.1074/jbc.M111.280867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harmeyer KM, South PF, Bishop B, Ogas J, Briggs SD. 2015. Immediate chromatin immunoprecipitation and on-bead quantitative PCR analysis: a versatile and rapid ChIP procedure. Nucleic Acids Res 43:e38. doi: 10.1093/nar/gku1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. South PF, Harmeyer KM, Serratore ND, Briggs SD. 2013. H3K4 methyltransferase Set1 is involved in maintenance of ergosterol homeostasis and resistance to Brefeldin A. Proc Natl Acad Sci U S A 110:E1016–25. doi: 10.1073/pnas.1215768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S6 and Tables S1–S10.