ABSTRACT
Campylobacter jejuni and Campylobacter coli represent the leading causes of bacterial gastroenteritis in humans, and infections can produce post-infectious irritable bowel syndrome (PI-IBS). Rhesus macaques (Macaca mulatta) (RM) are similarly susceptible to acute campylobacteriosis and represent a potential model of PI-IBS. We characterized the Campylobacter species circulating in an RM breeding colony using culture, qPCR, and whole genome sequencing (WGS). We also compared the C. jejuni and C. coli prevalence in RM as detected with qPCR versus culture and identified risk factors for bacteria presence and intestinal disease. Culture of 275 samples yielded C. coli (103) and C. jejuni (8), of which 21.6% were resistant to quinolones and 3.6% were resistant to macrolides. Multidrug-resistant isolates were obtained exclusively from animals exhibiting diarrhea or with histologically confirmed chronic enterocolitis. WGS revealed a non-clonal population of Campylobacter spp. Genotypic predictions of resistance were excellent except for aminoglycosides. All sequenced isolates contained genes for all subunits of cytolethal distending toxin. qPCR detected a prevalence of 45.9% for C. coli and 29.6% for C. jejuni. The quantity of either bacteria was significantly higher (P < 0.05) in animals with intestinal disease compared to healthy animals, though only young age was significantly associated with the presence of Campylobacter sp. or intestinal disease. Significantly more C. jejuni positive animals were detected with qPCR than with culture. These results provide a comprehensive characterization of Campylobacter spp. circulating in a breeding colony of RM in the United States and suggest that qPCR is superior for the detection of C. jejuni in RM.
IMPORTANCE
Gastrointestinal disease is one of the most common reasons for hospitalization in non-human primate colonies and accounts for over one-third of non-research related euthanasia. In rhesus macaques, this manifests as both acute diarrhea and chronic enterocolitis (CE), a syndrome of chronic diarrhea resulting in poor weight gain or weight loss which is minimally responsive to treatment. Campylobacter spp. are major causes of acute enterocolitis in rhesus macaques and may predispose individuals to the development of CE, similar to post-infectious irritable bowel syndrome in humans. Despite these concerns, there are few studies characterizing Campylobacter in rhesus macaque colonies, in particular utilizing whole genome sequencing and assessing findings with respect to the health status of the host. Our findings provide insight into Campylobacter strains circulating in rhesus macaque colonies, which can improve clinical monitoring, assist in treatment decisions, and provide new avenues of investigation into campylobacteriosis as a catalyst for CE.
KEYWORDS: Campylobacter, rhesus macaque, Campylobacter jejuni, Campylobacter coli, chronic enterocolitis, post-infectious irritable bowel, macaque, diarrhea
INTRODUCTION
Campylobacter are Gram-negative, microaerophilic, spiral-shaped bacteria that can be commensal or pathogenic in the gastrointestinal tract of birds and mammals, with C. jejuni and C. coli representing the leading causes of bacterial gastroenteritis in humans worldwide (1–3). Campylobacter jejuni is a known pathogen of rhesus macaques (Macaca mulatta) but there is some debate over the pathogenicity of C. coli in this species (4–7). Prevalence estimates of Campylobacter-infected rhesus macaques range between 45% and 97% of individuals, with most identifiable isolates classified as C. coli and fewer as C. jejuni and C. lari (5, 8–10). Campylobacteriosis in rhesus macaques is classically associated with acute bacterial enterocolitis. However, Campylobacter infections in humans are notorious for resulting in post-infectious sequelae, including Guillain-Barré syndrome, reactive arthritis, and post-infectious irritable bowel syndrome (PI-IBS) (11–14). PI-IBS is a syndrome of chronic, low-grade intestinal inflammation resulting in abdominal pain and stool disturbances, with up to 20% of individuals developing PI-IBS following an episode of Campylobacter-associated enteritis (2, 3, 15). Numerous host factors play a role in whether an individual develops PI-IBS following infection, but some studies suggest Campylobacter strain-specific differences which are more likely to produce PI-IBS. Toxigenic strains in particular have been implicated, and the B subunit of cytolethal distending toxin (CdtB) has been proposed to be a catalyst for disease as a molecular mimic of the host protein vinculin (16–18). Whole genome sequencing (WGS) also demonstrated strains with specific variations in the expression of genes associated with bacterial stress response and core biosynthetic pathways to be more likely to result in PI-IBS (19).
Gastrointestinal disease in rhesus macaques is one of the most common reasons for hospitalization and accounts for up to 33% of non-research related euthanasias (20). In addition to acute enterocolitis, often Campylobacter-associated, rhesus macaques experience a syndrome termed chronic enterocolitis (CE), otherwise known as idiopathic chronic diarrhea. CE is a syndrome of chronic diarrhea resulting in poor weight gain and failure to thrive and often leads to euthanasia due to welfare concerns (4, 13, 21–24). There is growing suspicion that Campylobacter spp. infections may predispose rhesus macaques to the development of CE, similar to PI-IBS in humans (23, 25). Despite this, few studies have surveyed the characteristics of Campylobacter spp. circulating in rhesus macaque colonies, and even fewer have used WGS (5, 8, 10, 26, 27). Defining the role of Campylobacter spp. in the development of CE and characterizing the role of C. coli in both acute and chronic disease in rhesus macaques may facilitate the identification of controllable risk factors and therapeutic targets. This would result in the reduction of morbidity and mortality associated with both acute colitis and CE (5, 28). Detailed characterization could also support the utilization of CE in rhesus macaques as a natural model for PI-IBS in humans.
Diagnosis of campylobacteriosis in rhesus macaques is typically limited to culture methods. However, for many bacteria, molecular methods of detection such as quantitative PCR (qPCR) are superior to culture, particularly when animals are shedding low numbers of bacteria (29, 30). qPCR may also provide a rapid method for quantifying bacterial loads which could be associated with the disease status of the host. In this study, we validate the use of qPCR for C. coli and C. jejuni detection in rectal swabs from rhesus macaques. The qPCR assays, traditional culture methods, and WGS of obtained isolates were used to compare the Campylobacter spp. affecting healthy rhesus macaques and those with acute colitis and CE. These findings will provide clinicians with a rapid, reliable diagnostic technique for C. jejuni and C. coli in rhesus macaques and lend insight into antimicrobial susceptibility, bacterial quantity, and strain differences which may play a role in the health status of the host animal.
RESULTS
We collected 277 samples from 266 animals with five animals sampled at multiple time points. Of those, 240 samples had paired culture and PCR swabs, 35 samples had only a culture swab, and 2 had only a qPCR swab. Fifty-eight samples from 50 individuals were obtained from animals with intestinal disease at the time of sample collection. Animals with known intestinal disease included animals with acute colitis, CE, colonic carcinoma, and intestinal amyloidosis. The age, sex, and disease status of sampled animals are shown in Table 1.
TABLE 1.
Population summary of sampled animals by age, sex, and health status at the time of first sampling
| Health status | Juvenilea | Peripubertala | Young adulta | Adulta | Total | |
|---|---|---|---|---|---|---|
| Male | Healthy | 11 | 17 | 11 | 16 | 55 |
| Acute colitis | 1 | 0 | 0 | 0 | 1 | |
| CE | 9 | 2 | 2 | 4 | 17 | |
| Developed CEb | 0 | 1 | 0 | 0 | 1 | |
| Other | 1 | 0 | 1 | 1 | 3 | |
| Total male | 22 | 20 | 14 | 21 | 77 | |
| Female | Healthy | 11 | 35 | 69 | 43 | 158 |
| Acute colitis | 4 | 2 | 0 | 1 | 7 | |
| CE | 6 | 3 | 0 | 6 | 15 | |
| Developed CEb | 0 | 3 | 0 | 1 | 4 | |
| Other | 0 | 0 | 0 | 5 | 5 | |
| Total female | 21 | 43 | 69 | 56 | 189 | |
| Total | 43 | 63 | 83 | 77 | 266 |
Juvenile, ≤2 years of age; peripubertal, 3–4 years of age; young adult, 4–9 years of age; adult, ≥10 years of age.
Animals developed CE following date of sampling.
Culture results
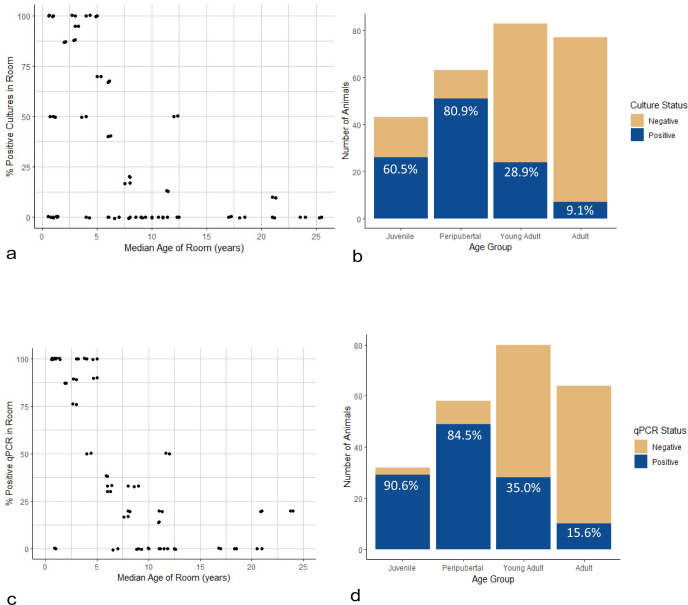
Culture of 275 rectal swabs yielded 115 Campylobacter sp. isolates (41.8% prevalence). However, three isolates were not frozen for further analysis and one isolate could not be recovered following freezing, resulting in 111 Campylobacter sp. isolates available for further study and the reduction of the overall sample size to 271 for further prevalence calculations. Eighty-six isolates were from apparently healthy animals, 21 were from animals with known intestinal disease at the time of sample collection, and 4 were from animals that developed CE within 1 year of sampling, yielding a total of 25 isolates from animals that had or developed intestinal disease. Culture-positive results were grouped distinctly by animal room of residence and age group (Fig. 1). Juvenile and peripubertal age groups contained the largest proportion of positive cultures. Prevalence data, age, and sampling characteristics for each room are shown in Table S1. Matrix-assisted, laser desorption and ionization, time-of-flight (MALDI-TOF) species identification of the 111 isolates yielded 103 C. coli isolates (38%, 103/271) and 8 C. jejuni isolates (2.95%, 8/271). Of the samples from animals with intestinal disease at the time of sample collection, 23 isolates were C. coli and 2 were C. jejuni. Both C. jejuni isolates from symptomatic animals were obtained from animals diagnosed with CE. Sixteen of the C. coli isolates from symptomatic animals were obtained from animals diagnosed with CE, two were from animals with acute colitis, and one was from an animal with colon carcinoma. Four C. coli isolates were from animals diagnosed with CE following sample collection.
Fig 1.
Distribution of Campylobacter spp. culture or qPCR positive animals. (a) Each data point represents a unique room location, plotted against the median age of the room and Campylobacter spp. prevalence using culture in the room. (b) Number of animals culture positive for either C. jejuni or C. coli separated by age group. (c) Each data point represents a unique room location, plotted against the median age of the room and Campylobacter spp. prevalence using qPCR in the room. (d) Number of animals qPCR positive for either C. jejuni or C. coli separated by age group. Juvenile animals are 2 or less years old, peripubertal animals are 3 or 4 years old, young adult animals are 5 years old, and adult animals are 10 or more years old.
A summary of the antimicrobial resistance profiles and associated minimum inhibitory concentration (MIC) data is shown in Table 2. Eighty-three of the isolates (74.7%) were sensitive to all antimicrobials tested and 28 (25.2%) were resistant to at least one antibiotic class. A total of 21.6% of the isolates displayed resistance to quinolones, 7.2% to tetracyclines, 3.6% to macrolides, and 3.6% to lincosamides. There was no disagreement between drugs of the same class. None of the isolates were resistant to amphenicols or aminoglycosides. Isolates that displayed resistance to macrolides or lincosamides were invariably also resistant to quinolones and tetracyclines and were considered multidrug resistant (MDR). MDR strains were obtained only from animals with intestinal disease at the time of sample collection.
TABLE 2.
Summary of MALDI-TOF species identification, antimicrobial susceptibility phenotypes, and MIC data from the obtained 111 Campylobacter sp. isolatesa,b
| Species (n) and characteristic | Antimicrobial class susceptibility | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Susceptiblec | MDR | Quinolone | Macrolide | Amphenicol | Aminoglycoside | Lincosamide | Tetracycline | |||
| Ciprofloxacin | Nalidixic acid | Azithromycin | Erythromycin | Florfenicol | Gentamicin | Clindamycin | Tetracycline | |||
| % of all isolates | 74.70% | 3.60% | 78.38% | 78.38% | 96.40% | 96.40% | 0% | 0% | 96.40% | 92.79% |
| C. coli (103) | ||||||||||
| % of isolates | 74.80% | 3.80% | 77.70% | 77.70% | 96.20% | 96.20% | 0% | 0% | 96.20% | 93.30% |
| MIC range tested | NAd | NA | 0.015–64 | 4–64 | 0.015–64 | 0.03–64 | 0.03–64 | 0.12–32 | 0.03–16 | 0.06–64 |
| Resistance breakpoint | NA | NA | ≥1 | ≥32 | ≥1 | ≥32 | ≥8 | ≥4 | ≥2 | ≥16 |
| MIC range of isolates | NA | NA | 0.06 - ˃64 | 8 - > 64 | 0.03 - > 64 | 0.12 - > 64 | 0.5–2 | 0.25–1 | 0.12–16 | 0.12 - > 64 |
| MIC mode | NA | NA | 0.12 | ≤4 | 0.06 | 1 | 1 | 0.5 | 0.25 | 0.25 |
| MIC50 | NA | NA | 0.12 | ≤4 | 0.06 | 0.5 | 1 | 0.5 | 0.25 | 0.25 |
| MIC90 | NA | NA | 64 | 64 | 0.12 | 1 | 1 | 1 | 0.5 | 1 |
| C. jejuni (8) | ||||||||||
| % of isolates | 75% | 0% | 87.50% | 87.50% | 0% | 0% | 0% | 0% | 0% | 87.50% |
| MIC range | NA | NA | 0.015–64 | 4–64 | 0.015–64 | 0.03–64 | 0.03–64 | 0.12–32 | 0.03–16 | 0.06–64 |
| Resistance breakpoint | NA | NA | ≥1 | ≥32 | ≥0.5 | ≥32 | ≥8 | ≥4 | ≥1 | ≥16 |
| MIC range of isolates | NA | NA | 0.06–8 | ≤4 - > 64 | ≤0.015–0.03 | 0.12–0.25 | 0.5–1 | 0.25–0.5 | 0.016–0.12 | 0.06–32 |
| MIC mode | NA | NA | 0.06 | 4 | 0.03 | 0.25 | 0.5 | 0.5 | 0.12 | 0.12 |
| MIC50 | NA | NA | 0.06 | 4 | 0.03 | 0.25 | 0.5 | 0.5 | 0.12 | 0.12 |
| MIC90 | NA | NA | 8 | 64 | 0.03 | 0.25 | 1 | 0.5 | 0.12 | 32 |
Percentages are expressed as percent of isolates of the indicated Campylobacter species.
All MIC data are reported in μg/mL.
Susceptible to all tested antimicrobials.
Abbreviations: MALDI-TOF, matrix assisted laser desorption ioniziation time of flight; MDR, multidrug resistant; MIC, minimum inhibitory concentration; NA, not applicable.
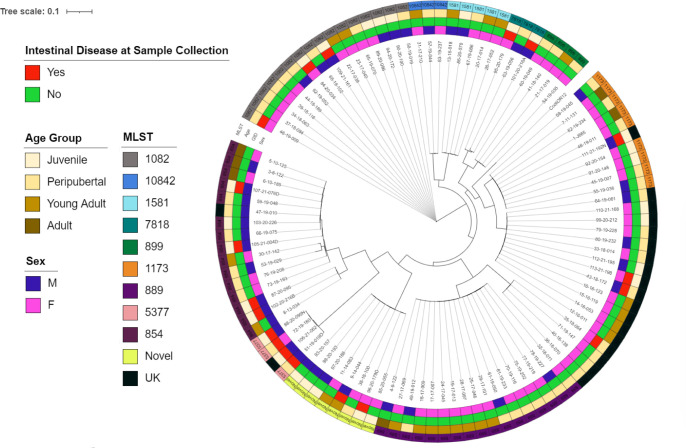
WGS of the isolates yielded Campylobacter genome sequences between 1.6 Mbp and 1.8 Mbp. Characterization of the sequences is available in Bacon et al. (31). Examination of phylogenetic relationships (Fig. 2) revealed a non-clonal population with some clustering by animal room (data not shown), age, sex, and clinical status of the animal. Two novel multi-locus sequence types (MLST) were identified (Table S2). Seven sequence types (STs) across 22 isolates were not identified by automated methods; however, using phylogenetic analysis, three STs grouped identically with known MLSTs, and the remainder were across two distinct groups. The vast majority of MLSTs were classified as host generalists. One isolate (MLST 10842) has been primarily reported in poultry. The C. coli MLST 5377 group contained all the multidrug-resistant isolates and was isolated from animals with intestinal disease. Three of four of these animals had a history of treatment with multiple antibiotics prior to sampling, though one animal had no record of antibiotic treatment. Three of these isolates, including the isolate from the animal with no history of antibiotic treatment, were clonal isolates, with the fourth very closely related as shown in Fig. 2. C. coli isolates from MLST groups 889, 899, 1581, and 10842 were only obtained from healthy individuals.
Fig 2.
Phylogenetic tree of obtained C. coli isolates rooted to the representative C. coli OR12 (GenBank accession no. CP013733) strain with annotations for the age group, sex of the animal, clinical status of the animal at the time of sample collection, and MLST of the isolate. UK, unknown MLST as determined by automated methods; GID, intestinal disease at the time of sample collection.
Regarding genotypic antimicrobial resistance profiles (Table S2), 13 isolates were eliminated from this portion of the analysis due to low coverage, though all isolates were assessed for known point mutations conferring resistance. Two genes for beta-lactam antibiotic resistance were detected, with blaOXA-193 detected in both C. jejuni and C. coli and blaOXA-489 detected only in C. coli. Two genes for aminoglycoside resistance were detected, with aadE-Cc found in C. coli only and aph(3′)-III found in both species. The tet(O) gene for tetracycline resistance was detected in both species. The 23s r.2075A>G point mutation which confers macrolide resistance was detected only in C. coli, the gyrA_2 p.T861 point mutation which confers quinolone resistance was detected in both species, and the gyrA_2 p.C861 which also confers quinolone resistance was only detected in C. jejuni. Agreement between phenotypic resistance profiles and genotypic predictions of resistance was 100% for ciprofloxacin resistance, 99.1% for erythromycin with one isolate phenotypically resistant but lacking the associated point mutation, and 98.0% for tetracycline resistance with two isolates displaying phenotypic resistance but lacking the tet(O) gene. The greatest disagreement was with regards to gentamicin with only 79.6% match between phenotypic and genotypic resistance profiles. All isolates were phenotypically susceptible to gentamicin, but twenty isolates contained one of two identified resistance genes. The minimum, maximum, and most frequently reported MIC were identical between isolates that carried aminoglycoside resistance genes and those that did not.
Ninety-seven virulence genes were identified across the 111 isolates. However, the only virulence gene known to play a role in PI-IBS development is Cdt (16, 17, 32, 33). All eight C. jejuni isolates had all three (A, B, and C) subunits of Cdt. The full virulence factor data set for the C. jejuni isolates is available in Table S3. Virulence factor assessment of C. coli isolates was hampered as the virulence factor database (VFDB) uses C. jejuni as the reference genome. Manual, individualized sequence evaluation revealed a 99.6% to 100% sequence match of known C. coli Cdt subunit A, B, and C sequences to each of the 103 C. coli isolates, indicating the presence of the genes for each subunit of Cdt (34).
qPCR results
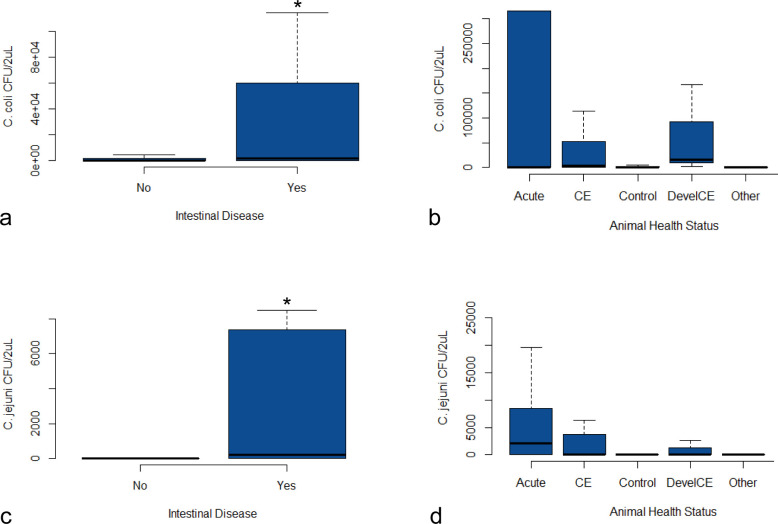
Two hundred and forty-one samples were tested with qPCR. All tested samples had adequate extraction control (Xeno Internal Control) and sampling control (OSM) Ct values and were included for further analysis. Four samples had discordant results over two rounds of qPCR for either C. jejuni or C. coli and were removed from the referable portion of the analysis. Only the first swab taken from each animal was included, resulting in 236 samples analyzed. Similar to the culture results, animals positive for either C. coli or C. jejuni using qPCR were grouped by room and by age, though with slightly more mixed results using qPCR. Again, the majority of positive results were obtained from juvenile and peripubertal animals (Fig. 1; Table S1). The C. coli qPCR prevalence was 45.9%, and the C. jejuni qPCR prevalence was 29.6%. 24.9% of samples were qPCR positive for both species. The quantity of bacteria detected was significantly higher (P < 0.05) for both C. coli and C. jejuni in animals with intestinal disease at the time of sample collection compared to animals that were healthy at collection, including those that developed CE following sampling (Fig. 3). The number of individuals in separate disease categories was too low to determine significant differences between intestinal disease groups. However, in addition to increases in animals with acute colitis or CE, animals that developed CE after sample collection also had increased bacterial loads compared to healthy animals (Fig. 3).
Fig 3.
Bacterial quantities, expressed as colony forming units (CFUs) per 2µL of DNA extract, compared across categories of host health status. The “Other” category includes animals with colon carcinoma and intestinal amyloidosis. Significant difference (P ˂ 0.05) is denoted by an asterisk. (a) C. coli quantity distributed by disease status at the time of sample collection, (b) Campylobacter coli quantity distributed by specific disease category (significance not assessed due to small categorical sample sizes), (c) Campylobacter jejuni quantity distributed by disease status at the time of sample collection, and (d) C. jejuni quantity distributed by specific disease category (significance not assessed due to small categorical sample sizes).
Risk factor analysis
Using logistic regression and controlling for room as a random effect, variables were tested to determine potential risk factors for the presence of Campylobacter sp. using either culture or qPCR, and to determine if Campylobacter sp. presence alone was a risk factor for the presence of intestinal disease (Table 3). The only risk factor associated with Campylobacter presence was age, with juvenile and peripubertal animals at increased risk for the presence of Campylobacter sp. using either culture or qPCR. Odds ratio (OR) for juvenile animals using culture was 19.88 (95% CI = 3.59–110.19), OR for peripubertal animals using culture was 47.04 (95% CI = 7.54–293.33), OR for juvenile animals using qPCR was 88.48 (95% CI = 10.66–734.33), and OR for peripubertal animals using qPCR was 33.89 (95% CI 7.25–158.47). Similarly, the only risk factor associated with intestinal disease was also age, with juveniles at increased risk (OR = 55.88, 95% CI = 2.93–1,067.12).
TABLE 3.
Results of bivariable and logistic regression analysis of potential risk factors for Campylobacter sp. presence via culture or PCR and potential risk factors for the presence of gastrointestinal disease at the time of sample collectiona,b
| Risk factor | Bivariable analysis, P value | Number tested | Number positive (%) | Logistic regression analysis | |||
|---|---|---|---|---|---|---|---|
| Odds ratio | LL 95%CI | UL 95%CI | P value | ||||
| Culture positive | |||||||
| Room | 6.56E−16 | RE | |||||
| Sex | 0.046 | ||||||
| Female | 189 | 69 (36.5) | Referent | ||||
| Male | 77 | 30 (50.6) | 0.109 | ||||
| Age | 2.20E−16 | ||||||
| Juvenile | 43 | 26 (60.5) | 19.88 | 3.59 | 110.19 | 0.001 | |
| Peripubertal | 63 | 51 (80.9) | 47.04 | 7.54 | 293.33 | 3.73E−05 | |
| Young adult | 83 | 24 (28.9) | 0.195 | ||||
| Adult | 77 | 7 (9.09) | Referent | ||||
| GI disease at collection | 0.332 | 48 | 16 (33.3) | NI | |||
| PCR positive Campylobacter sp. | |||||||
| Room | 1.91E−12 | RE | |||||
| Sex | 0.272 | ||||||
| Female | 168 | 79 (47.0) | NI | ||||
| Male | 66 | 37 (56.0) | NI | ||||
| Age | 2.20E−16 | ||||||
| Juvenile | 32 | 29 (90.6) | 88.48 | 10.66 | 734.33 | 3.30E−05 | |
| Peripubertal | 58 | 49 (84.5) | 33.89 | 7.25 | 158.47 | 7.57E−06 | |
| Young adult | 80 | 28 (35.0) | 0.136 | ||||
| Adult | 64 | 10 (15.6) | Referent | ||||
| GI disease at collection | 0.009 | 20 | 16 (80.0) | 0.239 | |||
| PCR positive C. coli | |||||||
| Room | 1.08E−11 | RE | |||||
| Sex | 0.352 | ||||||
| Female | 168 | 73 (43.5) | NI | ||||
| Male | 66 | 24 (51.5) | NI | ||||
| Age | 2.20E−16 | ||||||
| Juvenile | 31 | 28 (87.5) | 76.69 | 9.34 | 630.08 | 5.37E−05 | |
| Peripubertal | 58 | 44 (75.9) | 21.69 | 4.59 | 102.62 | 1.04E−04 | |
| Young adult | 80 | 25 (31.3) | 0.309 | ||||
| Adult | 64 | 10 (15.6) | Referent | ||||
| GI disease at collection | 0.013 | 20 | 15 (75.0) | 0.324 | |||
| GI disease at time of collection | |||||||
| Room | 2.20E−16 | RE | |||||
| Sex | 0.02 | ||||||
| Female | 189 | 27 (14.3) | Referent | ||||
| Male | 77 | 21 (27.3) | 4.21 | 1 | 17.74 | 0.05 | |
| Age | 3.73E−09 | ||||||
| Juvenile | 43 | 21 (48.8) | 55.88 | 2.93 | 1,067.12 | 0.008 | |
| Peripubertal | 63 | 7 (11.1) | 0.455 | ||||
| Young adult | 83 | 3 (3.6) | 1 | ||||
| Adult | 77 | 17 (22.1) | Referent | ||||
| Culture positive | 0.332 | 108 | 16 (13.8) | NI | |||
| PCR positive either | 0.009 | 116 | 16 (13.8) | 0.583 | |||
| PCR positive both | 0.008 | 55 | 10 (18.2) | 0.585 | |||
| PCR positive C. jejuni | 0.01 | 64 | 11 (17.2) | 0.867 | |||
| PCR positive C. coli | 0.013 | 107 | 15 (14.0) | 0.807 | |||
| History of antimicrobial use | 0.147 | 161 | 34 (21.1) | 0.052 | |||
LL, lower limit; UL, upper limit; GI, gastrointestinal; NI, not included in logistic regression analysis; RE, random effect.
2.2E−16 indicates <2.2E−16.
Comparison of culture and qPCR methods
Prevalence calculated using qPCR was higher than prevalence with culture across all categories, with the prevalence of C. jejuni calculated using qPCR significantly higher (P < 0.05) than that calculated using culture. Prevalence of C. coli was 38.0% using culture and 46.3% with qPCR, and prevalence of C. jejuni was only 2.5% using culture but was 29.6% with qPCR. Using the kappa statistic to compare the two tests, qPCR was in moderate agreement with culture results with respect to C. coli (ĸ = 0.67) and there was none to slight agreement between the two tests regarding C. jejuni (ĸ = 0.12).
DISCUSSION
We characterized the Campylobacter spp. circulating in a closed specific pathogen free (SPF) breeding colony of rhesus macaques in the United States using culture, WGS, and qPCR. We used these results to determine if any species or strain-specific factors could be identified as associated with acute colitis or CE. Previous non-human primate (NHP) colony surveys for Campylobacter have been limited to species level detection, limited antimicrobial susceptibility profiles, and evaluation of the presence of Cdt (5, 8, 26). WGS studies have previously been limited to single genome reports, a selection of isolate sequences without specific regard to health status, or as part of a larger phylogenetic study (5, 27, 35, 36). A rhesus macaque colony survey from Brazil in 2007 found infants had higher susceptibility to the bacteria than adults, males tended to be infected more than females, and C. jejuni and C. coli displayed sensitivity to nalidixic acid but resistance to cephalothin (8). A similar study in cynomolgus macaques (Macaca fascicularis) demonstrated variable resistance profiles in both C. jejuni and C. coli including resistance to erythromycin, tetracycline, ciprofloxacin, and amoxicillin (37). While these general characterizations are important, they do not specifically assess Campylobacter spp. with regard to the health status of the infected individual.
Of the samples tested using culture in this study, 41.8% grew either C. coli or C. jejuni with no other species isolated. Both species were obtained from healthy animals and from those with intestinal disease. Standard clinical Campylobacter sp. isolation techniques likely select for C. coli and C. jejuni as thermophilic species and may miss non-thermophilic species, many of which are involved in human disease processes (2). Due to laboratory procedures for colony selection, only one isolate per sample was obtained, though a previous study using multiple characterization methods showed pig-tailed macaques (Macaca nemestrina) can experience mixed infections, which we also observed in our rhesus macaques using qPCR (6). Regarding antimicrobial susceptibility profiles, 21.6% of isolates were resistant to quinolones and 3.6% were resistant to macrolides, similar to resistance patterns of concern in isolates from the United States human population (38–40). MDR isolates were only isolated from animals with intestinal disease. Interestingly, these MDR isolates were contained within the ST 5377 group, which has also been reported as a consistently MDR ST in antibiotic-free swine production systems (41). Three of the four animals with MDR isolates were treated with macrolide antibiotics prior to sampling; however, only one was treated prior with a quinolone antibiotic, none were treated with tetracyclines or lincosamides, and one showed no record of antibiotic treatment. These results suggest empiric use of quinolones and macrolides in rhesus macaque colonies should be limited to preserve their utility. Antibiotic therapy in animals with intestinal disease should be directed based on isolate-specific antimicrobial susceptibility testing. Additionally, quinolones and macrolides may be less likely to be useful in suspected cases of acute campylobacteriosis.
Using WGS, the isolates were further characterized with regard to their MLST, phylogenetic relationships, genotypic antimicrobial susceptibility patterns, and certain virulence factors. Thirteen MLSTs, primarily host-generalists, were identified, indicating non-clonal strains, a somewhat unexpected finding given the closed nature of the colony. Some MLSTs were found only in animals with intestinal disease, and some MLSTs were only obtained from healthy animals, though the number of isolates from each MLST was considered too small to determine if this was a statistically significant predictive pattern. This heterogeneity does suggest that determining if certain MLSTs are predictive of disease status is a valuable future avenue of investigation. Historically, epidemiologic investigations in humans have shown no particular associations between Campylobacter MLST and overall virulence, but recent investigations showed certain MLSTs may predispose to PI-IBS; this should be explored with rhesus macaques and CE (19, 42).
The genetic profiles for antimicrobial resistance tended to match the phenotypic resistance profiles for the isolates, except in the case of genes for aminoglycoside resistance. No isolates demonstrated phenotypic resistance to aminoglycosides, but an entire phylogenetic cluster did contain genes which would predict aminoglycoside resistance, either aadE-Cc or aph(3′)-III. Both genes independently are known to confer resistance in Campylobacter spp., though they may occur within the same isolate, and other studies have noted a similar mismatch (43, 44). This suggests that the genes are either not expressed or are dysfunctional/non-functional, and future studies may investigate the cause of this mismatch (45). A small number of isolates also displayed phenotypic resistance for erythromycin or tetracycline but without the associated point mutation or gene, likely explained by other known resistance mechanisms in Campylobacter sp., including the CmeABC efflux pump and altered membrane permeability (43, 46, 47).
Regarding virulence factors, previous studies suggest CdtB as important in the development of PI-IBS (16–18, 48). All isolates contained genes for all three subunits of Cdt, consistent with both C. jejuni and C. coli being pathogens, though in vitro studies are required to test the functionality of these genes. While C. jejuni and C. coli are genetically similar, work focusing on evaluating other potential virulence factor differences in C. coli strains from healthy animals versus animals with intestinal disease was hampered by the VFDB utilizing C. jejuni as the reference genome. As demonstrated by the investigations into Cdt, sequences can vary enough between the two organisms to suggest interpreting virulence factor results for C. coli with caution. Future work will investigate these potential differences individually with particular attention to genes that are known to play a role in the development of PI-IBS (19). Except for the recent work investigating C. jejuni genotypes associated with PI-IBS, whole genome sequencing of Campylobacter spp. in humans and other veterinary species has been primarily used in outbreak related epidemiologic investigations. While we did not obtain enough isolates from animals that went on to develop CE after sample collection to determine specific strain differences that may result in CE, we did demonstrate strain differences between animals with intestinal disease and healthy animals and identified future avenues of investigation which may allow the identification of novel biomarkers or therapeutic targets in rhesus macaque acute colitis and CE.
We also validated qPCR assays for the detection of C. jejuni and C. coli in rectal swabs from rhesus macaques. Using qPCR, we identified a higher prevalence of animals infected with either species when compared to culture, and co-infection with C. coli and C. jejuni was identified in 24.9% of samples. Most surprisingly, 29.6% of samples were positive for C. jejuni using qPCR, while only 2.5% of samples were positive for C. jejuni on culture. Given the large portion of coinfections on qPCR, this is likely driven by colony selection bias during the culture process in a clinical setting. In the authors’ experience, C. coli colonies tend to grow more robustly than C. jejuni colonies at 42°C, though with the same general colony morphology, and so would be more likely to be selected for speciation and subculturing. Ct values for C. coli tended to be higher than those for C. jejuni which would support this observation. This effect could be mediated by speciating and subculturing multiple potential Campylobacter colonies from the same plate over a more prolonged period, but this would significantly increase the burden on clinical diagnostic laboratories and may not be feasible in all settings. While culture and isolation are required for antimicrobial susceptibility testing and robust genomic analyses, we propose the use of qPCR as a rapid, reliable diagnostic method for campylobacteriosis in rhesus macaques. Ideally, culture and molecular methods will be used simultaneously as the information provided is complimentary. qPCR also allows bacterial quantification. We found significant increases in Campylobacter quantity in animals with intestinal disease versus healthy animals, which appears to be driven largely by the vast quantities of bacteria in some animals with acute colitis, and more moderate increases in animals with CE. The increase of Campylobacter quantities in animals with CE is likely consistent with the dysbiotic state associated with this disease process. Additionally, while sample sizes did not allow statistical evaluation, a trend of increased bacterial quantity in animals that were healthy at the time of sample collection and went on to develop CE suggests that further study could yield bacterial quantity as a potential diagnostic or even prognostic factor for CE.
The final component of this study was to determine if there were identifiable risk factors for bacteria presence or for the presence of intestinal disease at the time of sample collection. While the studied colony is primarily used for breeding and sale purposes, resulting in an overall colony distribution skewed toward older females, attempts were made to distribute sampling evenly across age groups and both sexes. The male-to-female sampling ratio was 0.4. Using generalized linear mixed model (GLMM), the only significant risk factor for the presence of Campylobacter was age, with juveniles and peripubertal animals at increased risk, and similarly, the only significant risk factor for the presence of intestinal disease at the time of sample collection was age, with juveniles at increased risk. While the increased risk was significant for these groups compared with that of adults, the CI of the odds ratios was very wide, limiting our ability to precisely quantify the magnitude of the risk. Younger age as a risk factor for campylobacteriosis in humans has been primarily related to increased environmental exposure and less robust innate immunity; however, successful vaccine studies in NHPs indicate that some degree of accumulation of acquired immunity to individual strains could play a role as well (5, 6, 28, 49–51). As the majority of the isolates in this study were C. coli, these results, in conjunction with the bacterial quantity findings, may support C. coli as a more opportunistic pathogen, though the obtained C. coli isolates did contain genes for toxin production. Similarly, co-infection of C. jejuni and C. coli was not identified as an independent risk factor for the presence of intestinal disease, though following these co-infected animals to determine if they are more likely to present later with CE will be interesting. As most of the animals with intestinal disease in this study were already diagnosed with CE, a future prospective study focusing more specifically on animals with acute colitis will be valuable. These results do suggest that animals less than 5 years old would be the most valuable targets for future studies on Campylobacter sp. in rhesus macaques and potential Campylobacter sp. mitigation efforts.
In summary, these findings provide a comprehensive characterization of Campylobacter sp. circulating in a breeding colony of rhesus macaques in the United States, including WGS data of 111 isolates, and support the utilization of qPCR for the detection of C. coli and C. jejuni in conjunction with culture for diagnosis of campylobacteriosis. Future studies will focus on using these tools in animals with acute colitis to identify if any Campylobacter-specific factors may play a role in the development of CE following an episode of acute campylobacteriosis, as seen with PI-IBS in humans.
MATERIALS AND METHODS
Animals and sample collection
The Keeling Center for Comparative Medicine and Research (KCCMR) at The University of Texas MD Anderson Cancer Center is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility where animals are cared for in accordance with the USDA Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and established Institutional Animal Care and Use Committee policies (52). Research was conducted under the oversight of an Institutional Animal Care and Use Committee (IACUC), protocols #01437-RN03 and #0804-RN03. Animals were part of a closed Indian-origin rhesus macaque (Macaca mulatta) breeding colony (n, ~1,000) SPF for Simian immunodeficiency virus, Simian retrovirus type I, Simian T-lymphotropic virus, and herpes B virus since 1991. Animals were housed in covered outdoor gang-cages or “corn crib” structures each housing a single small breeding group or a larger group of juvenile and peripubertal animals, each with a designated room number. Paired rectal swabs were obtained opportunistically from 266 animals during sedated (10 mg/kg ketamine) annual health evaluations, while hospitalized for diarrhea, or during necropsy evaluation. One swab was placed in a Cary-Blair Agar BD BBL CultureSwab Transport System (Fisher Scientific, Waltham, MA, USA) or Amies Remel BactiSwab Gel Collection and Transport Swab (Fisher Scientific, Waltham, MA, USA) for culture and the other was placed in an empty sterile microcentrifuge tube for DNA extraction and qPCR. Occasionally, samples were obtained from an individual at multiple time points or only a single swab was obtained from a single time point. If only a single swab was obtained, it was directed for either culture or qPCR. Attempts were made to distribute sampling evenly across all age groups and both sexes, but opportunistic sampling did not allow for true systematic or random selection of subjects. Animals’ health status was assigned upon review of medical records following data collection, and age groups were defined as follows: juvenile, ≤2 years old; peripubertal, 3–4 years old; young adult, 5–9 years old; and adult, ≥10 years old.
Culture methods
Rectal swabs directed for culture were processed on the day of collection. Swabs were used to inoculate Campy Blood Agar Blaser with 5% Sheep Blood and Antibiotics plates (Fisher Scientific, Waltham, MA, USA) and incubated at 41°C–43°C in a microaerophilic environment for up to 72 hours. Plates with characteristic growth were screened for Campylobacter spp. using a Gram stain and oxidase test. Positive isolates were sub-cultured into Brucella broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with 10% glycerol added and frozen at −80°C prior to transport from KCCMR to the Texas A&M Clinical Microbiology Laboratory. Isolates were revived on either BD BBL Trypticase Soy Agar with 5% Sheep Blood (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) or Blood Free Campylobacter Selectivity Agar (Himedia Laboratories Private Limited, Maharashtra, India) plates incubated at 42°C for 48 hours in a microaerophilic environment. Isolates were speciated using MALDI-TOF mass spectrometry (Biotyper, Bruker, Billerica, MA, USA). Antimicrobial susceptibility testing was performed using a commercial drug panel (Sensititre CAMPY AST Plate; Thermo Scientific, Cleveland, OH, USA) which tested for sensitivity to azithromycin, erythromycin, ciprofloxacin, nalidixic acid, clindamycin, florfenicol, gentamicin, and tetracycline according to the manufacturer’s recommendations (53). CLSI breakpoints for erythromycin and tetracycline were used (54). Categories of susceptible or resistant for the remaining tested antibiotics were determined using the National Antimicrobial Resistance Monitoring System for Enteric Bacteria guidelines (55).
For WGS, cultured isolates were processed and analyzed using the commercial QIAcube HT DNA extraction platform (Qiagen, Germantown, MD, USA), DNA Technologies XGEN Normalase DNA Library Prep Kit EZ and xGen UDI primers (Integrated DNA Technologies, Coralville, IA, USA), the Illumina MiSeq platform, and an established bioinformatics pipeline with the High-Performance Research Computing system at Texas A&M University, as described previously (31). Isolate sequences are available in NCBI under the BioProject accession number PRJNA1054170. Maximum likelihood phylogenies for the C. coli isolates were created by running assemblies through Parsnp v.1.2 and FastTree2 using the complete reference genome of a C. coli strain OR12 isolate from NCBI (GenBank accession no. CP013733) (56, 57). Individual C. coli isolate sequences were compared against known sequences for each subunit using the BV-BRC BLAST function, specifically to evaluate the presence or absence of genes for the three subunits of Cdt in C. coli (34).
DNA extraction of rectal swabs for qPCR
Rectal swabs intended for qPCR were stored at −80°C until the time of DNA extraction, with a collection to extraction interval of 1–7 months. Samples were thawed at room temperature, and DNA was extracted from each swab using a commercial kit (QIAamp Power Fecal Pro DNA Kit; Qiagen, Germantown, MD, USA). The initial CD1 reagent was added to the microcentrifuge tube containing the swab and vortexed for 1–2 minutes to loosen and homogenize the fecal material from the swab. The remaining fluid was pressed from the swab, and the swab was removed. The resulting solution was added to the provided PowerBead Pro tubes, and 4 µL VetMax Xeno Internal Positive Control (Thermo Fisher Scientific, Cleveland, OH, USA) was added to each sample as an extraction efficiency control. The remainder of the procedure was followed per the manufacturer’s instructions. An empty tube was included in each round of extractions as a negative extraction control. Eluted DNA was stored at −80°C and shipped to the Texas A&M University Clinical Microbiology laboratory where it was stored at −20°C until qPCR.
qPCR
We validated two multiplex qPCR assays for DNA extracted from rhesus macaque rectal swabs, one for the C. jejuni gyrA gene and one for the C. coli cadF gene. All qPCR was performed using the Applied Biosystems 7500 Fast Real-Time PCR System with associated 7500 Fast SDS v2.3 software (Thermo Fisher Scientific, Cleveland, OH, USA). Primers and probes were obtained from Sigma-Aldrich (St. Louis, MO, USA) and are listed in Table 4.
TABLE 4.
Quantitative PCR reagent sequences
| Gene target | Reagent | Sequence 5′−3′ | Origin |
|---|---|---|---|
| C. coli cadF | Forward primer | GAGAAATTTTATTTTTATGGTTTAGCTGGT | (58) |
| Reverse primer | ACCTGCTCCATAATGGCCAA | ||
| Probe | 6FAM]CCTCCACTTTTATTATCAAAAGCGCCTTTAGAA[BHQ2] | ||
| C. coli ceuE a | Forward primer | AAGCTCTTATTGTTCTAACCAATTCTAACA | (59) |
| Reverse primer | TCATCCACAGCATTGATTCCTAA | ||
| Probe | [6FAM]TTGGACCTCAATCTCGCTTTGGAATCATT[BHQ2] | ||
| C. coli glyA a | Forward primer | AAACCAAAGCTTATCGTGTGC | (60) |
| Reverse primer | AGTGCAGCAATGTGTGCAATG | ||
| Probe | [6FAM]CAACTTCATCCGCAAT[BHQ2] | ||
| C. jejuni gyrA | Forward primer | AAGATACGGTCGATTTTGTTCCA | (61) |
| Reverse primer | CTACAGCTATACCACTTGAACCATTTAATA | ||
| Probe | [FAM]TGATGGTTCAGAAAGCGAACCTGATGTTTT[BHQ2] | ||
| NHP OSM | Forward primer | CCTCGGGCTCAGGAACAAC | (62) |
| Reverse primer | GGCCTTCGTGGGCTCAG | ||
| Probe | [TAM]TACTGCATGGCCCAGCTGCTGGACAA[BHQ2] |
These primer/probe combinations performed poorly compared to the cadF assay and were not used in sample testing.
The C. jejuni qPCR assay leveraged methods developed for clinical use in the Texas A&M Clinical Microbiology Laboratory and previously published primer-probe sets (61). Two microliters of each DNA sample and blank was combined with 3.85 µL of nuclease-free water (Invitrogen, Waltham, MA, USA), 5 µL of Taqman Fast Virus 1-step Master Mix 4× (Thermo Fisher, Cleveland, OH, USA), 1.25 µL (500 nM) of forward and reverse primers for the C. jejuni gyrA gene, 0.4 µL (100 nM) of the probe for the C. jejuni gyrA gene, 1 µL of the VetMaxXeno Internal Positive Control Assay (Thermo Fischer Scientific, Cleveland, OH, USA) as an extraction efficiency control, and 2.5 µL (250 µM) forward and reverse primers for the NHP oncostatin M (OSM) gene, and 0.25 µL (100 nM) probe for the NHP OSM gene to confirm adequate sampling by the rectal swab collection method, for a total reaction volume of 20 µL per sample. Each set of reactions was run with a standard curve of known quantities of C. jejuni ATCC 33560 DNA as a positive control and to allow direct quantification of bacterial amounts in each sample. Two microliters of nuclease-free water was used as a no-template negative control, 2 µL of DNA extracted from rhesus macaque whole blood was used as a positive control for the OSM, and 2 µL (1,000 copies/µL) Xeno Internal Control DNA was used as a positive control for the Xeno Internal Control Assay. The reaction mixtures were subject to quantification with the following amplification cycle: 2 cycles at 95°C for 20 seconds, followed by 45 cycles of 3 seconds at 95°C and 30 seconds at 60°C. Samples were run in duplicate and those with appropriate amplification curves referable to the C. jejuni standard curve were considered positive for C. jejuni, and a bacterial quantity was calculated. Samples displaying disagreement were repeated in duplicate once. Samples continuing to display disagreement were labeled as “suspect” and removed from further analysis. The Xeno assay was considered positive if the Ct value was less than 38. OSM was considered positive if the Ct value was less than or equal to the OSM positive control, with an average control Ct value of 30.7 and an average sample Ct value of 26.4. Samples negative for either the Xeno internal positive control or OSM were removed from further analysis.
A similar referable clinical test was not available for the detection of C. coli. Initial tests for primer selection and validation were required, as well as validation of multiplexing capability with the Xeno Internal Control and OSM assays. A description of the validation efforts for the C. coli assay, resulting in the selection of the cadF C. coli target, as well as validation of testing archived samples is available in Supplemental Methods and Results, with supporting tables and figures (Tables S4 and S5; Fig. S1). The final reaction method was 2 µL of each DNA sample and blank combined with 4.35 µL of nuclease-free water (Invitrogen, Waltham, MA, USA), 5 µL of Taqman Fast Virus 1-step Master Mix 4× (Thermo Fisher, Cleveland, OH, USA), 1 µL (300 nM) of forward and reverse primers for the C. coli cadF gene, 0.4 µL (100 nM) of the probe for the C. coli cadF gene, 1 µL of Xeno Assay, and 2.5 µL (1 µM) forward and reverse primers for the NHP OSM gene, and 0.25 µL (100 nM) probe for the NHP OSM gene, for a total reaction volume of 20 µL per sample. As with the C. jejuni reaction, each set of reactions was run with a standard curve of known quantities of C. coli (ATCC 49941), 2 µL of nuclease-free water as a no-template negative control, 2 µL of DNA extracted from rhesus macaque whole blood as a positive control for the OSM, and 2 µL Xeno Internal Control DNA as a positive control for the Xeno Internal Control. The reaction parameters were identical to the C. jejuni gyrA reaction.
Statistical analysis
All statistical analyses were performed using R software (version 4.2.2) in R studio, using only results from the first sample from each animal in the case of repeated sampling (63). We calculated prevalence and explored risk factors for Campylobacter sp. presence as detected by culture or qPCR, relative to disease and host characteristics using logistic regression with GLMMs, controlling for room as a random effect. Each risk factor was tested for potential significance with χ2 or Fisher’s exact tests. Risk factors with a P value ≤0.25 in bivariable analysis were included in the logistic regression analysis. For risk factors included in logistic regression analysis and subsequently significant (P < 0.05), odds ratios, and 95% confidence intervals were calculated. Additionally, to determine if bacterial quantity as detected by qPCR was significantly associated with disease, the bacterial quantity as measured by qPCR was tested for normality using the Shapiro–Wilk test and then subjected to Wilcoxon signed-rank testing for significance. Results were considered significant if P < 0.05. For comparison of culture (considered the gold standard) and qPCR methods for detecting Campylobacter sp. presence, agreement between methods was tested using Cohen’s kappa (κ).
ACKNOWLEDGMENTS
We thank the KCCMR rhesus team for their assistance in facilitating collection of the rectal swabs, the KCCMR Clinical Microbiology Laboratory for their assistance in culturing the Campylobacter isolates, the TAMU Clinical Microbiology Laboratory for their assistance in qPCR development and antimicrobial susceptibility testing, the Norman lab for their gracious provision of sequencing resources and expertise, and the TAMU High Performance Research Computing (HPRC) for the advanced computing resources used to conduct this research.
Research support for Dr. Rebecca Bacon is provided by the NIH T32 Ruth L Kirschstein National Research Service Award (NRSA) Texas A&M AgriLife Research Institutional Training Grant (T32 OD011083). Grants from Cattlemen for Cancer Research (CCR) and the ACLAM Foundation provided funds for bacterial culture, isolation, MALDI-TOF, DNA extraction, and qPCR testing. A Texas A&M University School of Veterinary Medicine & Biomedical Sciences (VMBS) grant provided funding for a portion of the DNA extraction and whole genome sequencing processes. Funding to Dr. Sara Lawhon provided by the FDA Vet-LIRN Program through an infrastructure grant (U18FD006171) helps support clinical investigations. Funding through FDA Vet-LIRN supported the development of the C. jejuni qPCR (U18FD005013, U18FD006446, U18FD006664).
Contributor Information
Carolyn L. Hodo, Email: clhodo@mdanderson.org.
Sara D. Lawhon, Email: slawhon@cvm.tamu.edu.
Vincent B. Young, University of Michigan—Ann Arbor, Ann Arbor, Michigan, USA
ETHICS APPROVAL
Research was conducted under the oversight of an Institutional Animal Care and Use Committee (IACUC), protocols #01437-RN03 and #0804-RN03.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00560-24.
Validation of the Campylobacter real-time PCR methods; Fig. S1; Tables S4 and S5.
Details of Campylobacter species-positive animals by qPCR or culture distributed by room with median age, age range, and number of animals tested of each room.
MLST and genotypic antimicrobial resistance data in comparison to phenotypic resistance profiles for each evaluated Campylobacter isolate.
Virulence factors identified in 8 Campylobacter jejuni isolates from rhesus macaque rectal swabs.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Bolton DJ. 2015. Campylobacter virulence and survival factors. Food Microbiol 48:99–108. doi: 10.1016/j.fm.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 2. Costa D, Iraola G. 2019. Pathogenomics of emerging Campylobacter species. Clin Microbiol Rev 32. doi: 10.1128/CMR.00072-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goddard MR, O’Brien S, Williams N, Guitian J, Grant A, Cody A, Colles F, Buffet J-C, Adlen E, Stephens A, Godfray HCJ, Maiden MCJ. 2022. A restatement of the natural science evidence base regarding the source, spread and control of Campylobacter species causing human disease. Proc Biol Sci 289:20220400. doi: 10.1098/rspb.2022.0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prongay K, Park B, Murphy SJ. 2013. Risk factor analysis may provide clues to diarrhea prevention in outdoor-housed rhesus macaques (Macaca mulatta). Am J Primatol 75:872–882. doi: 10.1002/ajp.22150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quintel BK, Prongay K, Lewis AD, Raué H-P, Hendrickson S, Rhoades NS, Messaoudi I, Gao L, Slifka MK, Amanna IJ. 2020. Vaccine-mediated protection against Campylobacter-associated enteric disease. Sci Adv 6:eaba4511. doi: 10.1126/sciadv.aba4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell RG, Sarmiento JI, Fox J, Panigrahi P. 1990. Evidence of reinfection with multiple strains of Campylobacter jejuni and Campylobacter coli in Macaca nemestrina housed under hyperendemic conditions. Infect Immun 58:2149–2155. doi: 10.1128/iai.58.7.2149-2155.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westreich ST, Ardeshir A, Alkan Z, Kable ME, Korf I, Lemay DG. 2019. Fecal metatranscriptomics of macaques with idiopathic chronic diarrhea reveals altered mucin degradation and fucose utilization. Microbiome 7:41. doi: 10.1186/s40168-019-0664-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andrade MCR, Gabeira SC de O, Abreu-Lopes D, Esteves WTC, Vilardo M de CB, Thomé JD da S, Cabello PH, Lauria-Filgueiras AL. 2007. Circulation of Campylobacter spp. in rhesus monkeys (Macaca mulatta) held in captivity: a longitudinal study. Mem Inst Oswaldo Cruz 102:53–57. doi: 10.1590/s0074-02762007000100008 [DOI] [PubMed] [Google Scholar]
- 9. Kienesberger S, Perez-Perez GI, Rivera-Correa JL, Tosado-Acevedo R, Li H, Dubois A, Gonzalez-Martinez JA, Dominguez-Bello MG, Blaser MJ. 2012. Serologic host response to Helicobacter pylori and Campylobacter jejuni in socially housed rhesus macaques (Macaca mulatta). Gut Pathog 4:1–9. doi: 10.1186/1757-4749-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zang X, Pascoe B, Mourkas E, Kong K, Jiao X, Sheppard SK, Huang J. 2023. Evidence of potential Campylobacter jejuni zooanthroponosis in captive macaque populations. Microb Genom 9:001121. doi: 10.1099/mgen.0.001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barrett E, Carr D, Bell ML, Pogreba-Brown K. 2018. Post-infectious sequelae after Campylobacter enteric infection: a pilot study in Maricopa County, Arizona, USA. Pilot Feasibility Stud 4:142. doi: 10.1186/s40814-018-0335-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batz MB, Henke E, Kowalcyk B. 2013. Long-term consequences of foodborne infections. Infect Dis Clin North Am 27:599–616. doi: 10.1016/j.idc.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 13. Johnson AL, Keesler RI, Lewis AD, Reader JR, Laing ST. 2022. Common and not-so-common pathologic findings of the gastrointestinal tract of rhesus and cynomolgus macaques. Toxicol Pathol 50:638–659. doi: 10.1177/01926233221084634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riddle MS, Gutierrez RL, Verdu EF, Porter CK. 2012. The chronic gastrointestinal consequences associated with Campylobacter. Curr Gastroenterol Rep 14:395–405. doi: 10.1007/s11894-012-0278-0 [DOI] [PubMed] [Google Scholar]
- 15. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. 2000. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47:804–811. doi: 10.1136/gut.47.6.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morales W, Triantafyllou K, Parodi G, Weitsman S, Park SC, Rezaie A, Pichetshote N, Lin E, Pimentel M. 2020. Immunization with cytolethal distending toxin B produces autoantibodies to vinculin and small bowel bacterial changes in a rat model of postinfectious irritable bowel syndrome. Neurogastroenterol Motil 32:e13875. doi: 10.1111/nmo.13875 [DOI] [PubMed] [Google Scholar]
- 17. Pokkunuri V, Pimentel M, Morales W, Jee S-R, Alpern J, Weitsman S, Marsh Z, Low K, Hwang L, Khoshini R, Barlow GM, Wang H, Chang C. 2012. Role of cytolethal distending toxin in altered stool form and bowel phenotypes in a rat model of post-infectious irritable bowel syndrome. J Neurogastroenterol Motil 18:434–442. doi: 10.5056/jnm.2012.18.4.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. 2001. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis 184:606–609. doi: 10.1086/322845 [DOI] [PubMed] [Google Scholar]
- 19. Peters S, Pascoe B, Wu Z, Bayliss SC, Zeng X, Edwinson A, Veerabadhran-Gurunathan S, Jawahir S, Calland JK, Mourkas E, Patel R, Wiens T, Decuir M, Boxrud D, Smith K, Parker CT, Farrugia G, Zhang Q, Sheppard SK, Grover M. 2021. Campylobacter jejuni genotypes are associated with post-infection irritable bowel syndrome in humans. Commun Biol 4:1015. doi: 10.1038/s42003-021-02554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blackwood RS, Tarara RP, Christe KL, Spinner A, Lerche NW. 2008. Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comp Med 58:81–87. [PMC free article] [PubMed] [Google Scholar]
- 21. Ardeshir A, Oslund KL, Ventimiglia F, Yee J, Lerche NW, Hyde DM. 2013. Idiopathic microscopic colitis of rhesus macaques: quantitative assessment of colonic mucosa. Anat Rec 296:1169–1179. doi: 10.1002/ar.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howell S, White D, Ingram S, Jackson R, Larin J, Morales P, Garcia AP, Hicks C, Hopper K, Wagner J. 2012. A bio-behavioral study of chronic idiopathic colitis in the rhesus macaque (Macaca mulatta). Appl Anim Behav Sci 137:208–220. doi: 10.1016/j.applanim.2012.01.003 [DOI] [Google Scholar]
- 23. Laing ST, Merriam D, Shock BC, Mills S, Spinner A, Reader R, Hartigan-O’Connor DJ. 2018. Idiopathic colitis in rhesus macaques is associated with dysbiosis, abundant enterochromaffin cells and altered T-cell cytokine expression. Vet Pathol 55:741–752. doi: 10.1177/0300985818780449 [DOI] [PubMed] [Google Scholar]
- 24. Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. 2003. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun 71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bacon RL, Taylor L, Gray SB, Hodo CL. 2024. Analysis of cell populations in the normal rhesus macaque (Macaca mulatta) lower intestinal tract and diagnostic thresholds for chronic enterocolitis. Vet Pathol 61:303–315. doi: 10.1177/03009858231203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dassanayake RP, Zhou Y, Hinkley S, Stryker CJ, Plauche G, Borda JT, Sestak K, Duhamel GE. 2005. Characterization of cytolethal distending toxin of Campylobacter species isolated from captive macaque monkeys. J Clin Microbiol 43:641–649. doi: 10.1128/JCM.43.2.641-649.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weis AM, Storey DB, Taff CC, Townsend AK, Huang BC, Kong NT, Clothier KA, Spinner A, Byrne BA, Weimer BC. 2016. Genomic comparison of Campylobacter spp. and their potential for zoonotic transmission between birds, primates, and livestock. Appl Environ Microbiol 82:7165–7175. doi: 10.1128/AEM.01746-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Islam D, Lewis MD, Srijan A, Bodhidatta L, Aksomboon A, Gettayacamin M, Baqar S, Scott D, Mason CJ. 2006. Establishment of a non-human primate Campylobacter disease model for the pre-clinical evaluation of Campylobacter vaccine formulations. Vaccine 24:3762–3771. doi: 10.1016/j.vaccine.2005.07.023 [DOI] [PubMed] [Google Scholar]
- 29. LaLonde-Paul D, Cummings KJ, Rodriguez-Rivera LD, Wu J, Lawhon SD. 2019. Ciprofloxacin resistance among Campylobacter jejuni isolates obtained from shelter dogs in Texas. Zoonoses Public Health 66:337–342. doi: 10.1111/zph.12544 [DOI] [PubMed] [Google Scholar]
- 30. Leahy AM, Cummings KJ, Rodriguez-Rivera LD, Hamer SA, Lawhon SD. 2017. Faecal Campylobacter shedding among dogs in animal shelters across Texas. Zoonoses Public Health 64:623–627. doi: 10.1111/zph.12356 [DOI] [PubMed] [Google Scholar]
- 31. Bacon RL, Norman KN, Nickodem CA, et al. 2024. Whole-genome sequences of Campylobacter coli and Campylobacter jejuni isolates from rhesus macaques (Macaca mulatta) with and without intestinal disease. Microbiol Resour Announc:e00018000-24. doi: 10.1128/mra.00018-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pimentel M, Morales W, Rezaie A, Marsh E, Lembo A, Mirocha J, Leffler DA, Marsh Z, Weitsman S, Chua KS, Barlow GM, Bortey E, Forbes W, Yu A, Chang C. 2015. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS ONE 10:e0126438. doi: 10.1371/journal.pone.0126438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmulson M, Balbuena R, Corona de Law C. 2016. Clinical experience with the use of anti-CdtB and anti-vinculin antibodies in patients with diarrhea in Mexico. Rev Gastroenterol Mex (Eng Ed) 81:236–239. doi: 10.1016/j.rgmxen.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Olson RD, Assaf R, Brettin T, Conrad N, Cucinell C, Davis JJ, Dempsey DM, Dickerman A, Dietrich EM, Kenyon RW, et al. 2023. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res 51:D678–D689. doi: 10.1093/nar/gkac1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SG, Summage-West CV, Sims LM, Foley SL. 2021. Complete genome sequence of Campylobacter fetus subsp. venerealis P4531 from a rhesus monkey. Microbiol Resour Announc 10:e00739-21. doi: 10.1128/MRA.00739-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SG, Summage-West CV, Sims LM, Wu L, Kim J, Nho S, Foley SL. 2022. Complete genome sequence of Campylobacter coli strain P4581, a hybrid carrying Campylobacter jejuni genomic content, isolated from rhesus monkey, Macaca mulatta. Microbiol Resour Announc 11:e00847-22. doi: 10.1128/mra.00847-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koga T, Aoki W, Mizuno T, Wakazono K, Ohno J, Nakai T, Nomiya T, Fujii M, Fusegawa K, Kinoshita K, Hamada T, Ikeda Y. 2017. Antimicrobial resistance in Campylobacter coli and Campylobacter jejuni in cynomolgus monkeys (Macaca fascicularis) and eradication regimens. J Microbiol Immunol Infect 50:75–82. doi: 10.1016/j.jmii.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention NCfEaZIDN . 2021. Division of healthcare quality promotion (DHQP): 2019 AR threats report. www.cdc.gov.
- 39. Qin X, Wang X, Shen Z. 2023. The rise of antibiotic resistance in Campylobacter. Curr Opin Gastroenterol 39:9–15. doi: 10.1097/MOG.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 40. Yang Y, Feye KM, Shi Z, Pavlidis HO, Kogut M, J Ashworth A, Ricke SC. 2019. A historical review on antibiotic resistance of foodborne Campylobacter. Front Microbiol 10:1509. doi: 10.3389/fmicb.2019.01509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quintana-Hayashi MP, Thakur S. 2012. Phylogenetic analysis reveals common antimicrobial resistant Campylobacter coli population in antimicrobial-free (ABF) and commercial swine systems. PLoS ONE 7:e44662. doi: 10.1371/journal.pone.0044662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colles FM, Maiden MCJ. 2012. Campylobacter sequence typing databases: applications and future prospects. Microbiology 158:2695–2709. doi: 10.1099/mic.0.062000-0 [DOI] [PubMed] [Google Scholar]
- 43. Iovine NM. 2013. Resistance mechanisms in Campylobacter jejuni. Virulence 4:230–240. doi: 10.4161/viru.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao J, Cheng Y, Zhang W, Lu Q, Guo Y, Hu Q, Wen G, Shao H, Luo Q, Zhang T. 2023. Genetic characteristics, antimicrobial susceptibility, and virulence genes distribution of Campylobacter isolated from local dual-purpose chickens in central China. Front Cell Infect Microbiol 13:1236777. doi: 10.3389/fcimb.2023.1236777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Painset A, Day M, Doumith M, Rigby J, Jenkins C, Grant K, Dallman TJ, Godbole G, Swift C. 2020. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Campylobacter jejuni and Campylobacter coli isolated from cases of diarrhoeal disease in England and Wales, 2015-16. J Antimicrob Chemother 75:883–889. doi: 10.1093/jac/dkz539 [DOI] [PubMed] [Google Scholar]
- 46. Cagliero C, Mouline C, Cloeckaert A, Payot S. 2006. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother 50:3893–3896. doi: 10.1128/AAC.00616-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pumbwe L, Randall LP, Woodward MJ, Piddock LJV. 2004. Expression of the efflux pump genes cmeB, cmeF and the porin gene porA in multiple-antibiotic-resistant Campylobacter jejuni. J Antimicrob Chemother 54:341–347. doi: 10.1093/jac/dkh331 [DOI] [PubMed] [Google Scholar]
- 48. Pimentel M, Morales W, Pokkunuri V, Brikos C, Kim SM, Kim SE, Triantafyllou K, Weitsman S, Marsh Z, Marsh E, Chua KS, Srinivasan S, Barlow GM, Chang C. 2015. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following Campylobacter jejuni infection in a rat model. Dig Dis Sci 60:1195–1205. doi: 10.1007/s10620-014-3435-5 [DOI] [PubMed] [Google Scholar]
- 49. Lee G, Pan W, Peñataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. 2013. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 7:e2036. doi: 10.1371/journal.pntd.0002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pazzaglia G, Bourgeois AL, el Diwany K, Nour N, Badran N, Hablas R. 1991. Campylobacter diarrhoea and an association of recent disease with asymptomatic shedding in Egyptian children. Epidemiol Infect 106:77–82. doi: 10.1017/s0950268800056466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sainato R, ElGendy A, Poly F, Kuroiwa J, Guerry P, Riddle MS, Porter CK. 2018. Epidemiology of Campylobacter infections among children in Egypt. Am J Trop Med Hyg 98:581–585. doi: 10.4269/ajtmh.17-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silverman J, Suckow MA, Murthy S. 2014. The IACUC handbook. CRC Press. [Google Scholar]
- 53. Jorgensen JH, Hindler JF, Reller LB, Weinstein MP. 2007. New consensus guidelines from the clinical and laboratory standards institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin Infect Dis 44:280–286. doi: 10.1086/510431 [DOI] [PubMed] [Google Scholar]
- 54. CLSI . 2017. VET06 Methods for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria isolated from animals. 1st ed. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA. [Google Scholar]
- 55. CDC . 2019. National antimicrobial resistance monitoring system for enteric bacteria: antibiotics tested by NARMS
- 56. Price MN, Dehal PS, Arkin AP. 2010. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 5:e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Treangen TJ, Ondov BD, Koren S, Phillippy AM. 2014. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 15:524. doi: 10.1186/s13059-014-0524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toplak N, Kovač M, Piskernik S, Možina SS, Jeršek B. 2012. Detection and quantification of Campylobacter jejuni and Campylobacter coli using real-time multiplex PCR. J Appl Microbiol 112:752–764. doi: 10.1111/j.1365-2672.2012.05235.x [DOI] [PubMed] [Google Scholar]
- 59. Best EL, Powell EJ, Swift C, Grant KA, Frost JA. 2003. Applicability of a rapid duplex real-time PCR assay for speciation of Campylobacter jejuni and Campylobacter coli directly from culture plates. FEMS Microbiol Lett 229:237–241. doi: 10.1016/S0378-1097(03)00845-0 [DOI] [PubMed] [Google Scholar]
- 60. Leblanc-Maridor M, Beaudeau F, Seegers H, Denis M, Belloc C. 2011. Rapid identification and quantification of Campylobacter coli and Campylobacter jejuni by real-time PCR in pure cultures and in complex samples. BMC Microbiol 11:113. doi: 10.1186/1471-2180-11-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iijima Y, Asako NT, Aihara M, Hayashi K. 2004. Improvement in the detection rate of diarrhoeagenic bacteria in human stool specimens by a rapid real-time PCR assay. J Med Microbiol 53:617–622. doi: 10.1099/jmm.0.45607-0 [DOI] [PubMed] [Google Scholar]
- 62. Bruce AG, Bakke AM, Thouless ME, Rose TM. 2005. Development of a real-time QPCR assay for the detection of RV2 lineage-specific rhadinoviruses in macaques and baboons. Virol J 2. doi: 10.1186/1743-422X-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Team RC . 2016. R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. Available from: http://www.R-project org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of the Campylobacter real-time PCR methods; Fig. S1; Tables S4 and S5.
Details of Campylobacter species-positive animals by qPCR or culture distributed by room with median age, age range, and number of animals tested of each room.
MLST and genotypic antimicrobial resistance data in comparison to phenotypic resistance profiles for each evaluated Campylobacter isolate.
Virulence factors identified in 8 Campylobacter jejuni isolates from rhesus macaque rectal swabs.