Abstract
The advent of immune checkpoint inhibitors (ICIs) has significantly improved cancer treatment. With the increasing use of ICIs, ICI-related myocarditis has been recognized. However, an evidence-based therapeutic strategy has not been established because of the limited knowledge on ICI-related myocarditis. Here, we present four cases of ICI-related fulminant myocarditis (FM). Three of the four cases resulted in fatal outcomes despite aggressive treatment with mechanical circulatory support and immunosuppressive therapy with corticosteroids. Given the poor prognosis of ICI-FM, the establishment of rapid and adequate therapeutic interventions on the basis of clinical and pathological evaluation is imperative.
Keywords: Immune checkpoint inhibitors,; Immune-related adverse events; Fulminant myocarditis; Mechanical circulatory support; Cardiogenic shock; Ventricular arrhythmia
Background
Advances in cancer treatment have dramatically decreased cancer-related mortality. On the other hand, treatment-related side effects have gained increased importance among cancer survivors [1]. Immune checkpoint inhibitors (ICIs) have emerged as highly effective therapies for many cancers [2, 3]. ICIs are now used not only in advanced or metastatic diseases but also in the early stages of cancers for the prevention of recurrence and improvement of cure rate [2, 4]. Leveraging the immune system by targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death-1 (PD-1), or programmed cell death ligand-1 (PD-L1) leads to side effects, termed immune-related adverse events (irAEs) [5]. These irAEs occur in 50–90% of patients, and all organs can be affected [5, 6]. The frequency of ICI-related myocarditis is reportedly 0.06–1.1%, and its mortality rate is as high as 50% [6–8]. Here, we describe the characteristics and clinical course of four cases of ICI-related fulminant myocarditis (FM) complicated by hemodynamic and electrical instability requiring inotropes and mechanical circulatory support (MCS), three of which resulted in fatal outcomes (Table 1).
Table 1.
Patient Characteristics and Clinical Course
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sex | Male | Male | Male | Female |
| Age | 72 | 69 | 63 | 76 |
| Pre-hospital symptom | Presyncope | Fever, Chest pain | Fever, Chest pain | Fatigue, Dyspnea |
| Cancer | Renal cell carcinoma | Prostate cancer | Lung cancer | Lung cancer |
| Metastasis | Liver, Lung | Bone | - | - |
| ICI |
Avelumab (anti-PD-L1) Nivolumab (anti-PD-1) |
Pembrolizumab (anti-PD-1) | Atezolizumab (anti-PD-L1) | Atezolizumab (anti-PD-L1) |
| Comorbidities | Hypertension, Diabetes | Hypertension | None | Hypertension, IHD |
| Time between last ICI administration and onset of myocarditis | 18 days | 893 days | 13 days | 11 days |
| Decompensated heart failure | No | Yes | Yes | Yes |
| Cardiogenic shock | Yes | Yes | Yes | Yes |
| Arrythmia | VT | Complete AVB, VT, PAF | Complete AVB | VT, PAF |
| Associated other irAE | Hepatitis, myositis | None | None | None |
| QRS width, ms | 148 | 147 | 172 | 80 |
| LVEF (on admission) | 40 | 17 | 10 | 10 |
| Laboratory data | ||||
| CK (on admission), U/L | 5724 | 2450 | 3859 | 101 |
| CK (peak), U/L | 5724 | 5103 | 3859 | 101 |
| CK-MB (on admission), U/L | 96 | 365 | 61 | 5 |
| CK-MB (peak), U/L | 141 | 428 | 61 | 7 |
| Troponin T (on admission), ng/mL | 1.871 | 48.118 | 11 | 0.226 |
| Troponin T (peak), ng/mL | 5.343 | 48.118 | 15.561 | 0.358 |
| BNP (on admission), pg/mL | 456.2 | 921.7 | 388.9 | 1357.3 |
| CRP (on admission), mg/dL | 1.09 | 9.32 | 9.47 | 0.59 |
| Endomyocardial biopsy |
CD3+ T-cells (CD8 > CD4) CD68+ histiocytes A few CD20+ B-cells A few eosinophils High Tenascin C High PD-L1 |
CD3+ T cell (CD8 > CD4) CD68+ histocytes A few CD20+ B-cells Neutrophils / Eosinophils High Tenascin C High PD-L1 |
CD3+ T-cells (CD4 = CD8) CD68+ histiocytes A few CD20+ B-cells Moderate Tenascin C Moderate PD-L1 |
CD3+ T-cells (CD8 > CD4) Rare CD68+ histiocytes Rare CD20+ B-cells Low Tenascin C Low PD-L1 |
| Treatment | ||||
| MCS | None | VA-ECMO, IABP | VA-ECMO, Impella CP | VAV-ECMO, Impella CP |
| Immunosuppressants |
Pulse MP (1000 mg, 3 days) Followed by PRD (1 mg/kg) |
Pulse MP (1000 mg, 3 days) Followed by PRD (10 mg) |
Pulse MP (1000 mg, 3 days) |
Pulse MP (1000 mg, 3 days) Followed by PRD (1 mg/kg) |
| Outcome | Death | Death | Death | Alive |
| Cause of death | VT | Heart failure / MOF | Intracerebral hemorrhage | - |
AVB atrioventricular block, BNP B-type natriuretic peptide, CD cluster of differentiation, CK creatine kinase, CRP C-reactive protein, ECMO extracorporeal membrane oxygenation, IABP intra-aortic balloon pump, ICI immune check point inhibitor, IHD ischemic heart disease, irAE immune-related adverse effect, LVEF left ventricular ejection fraction, MCS mechanical circulatory support, MOF multiple organ failure, MP methylprednisolone, PAF paroxysmal atrial fibrillation, PD-L1 programmed cell death ligand 1, PRD prednisolone, VA veno-arterial, VAV veno-arterial-venous, VT ventricular tachycardia
Case presentation
Case 1
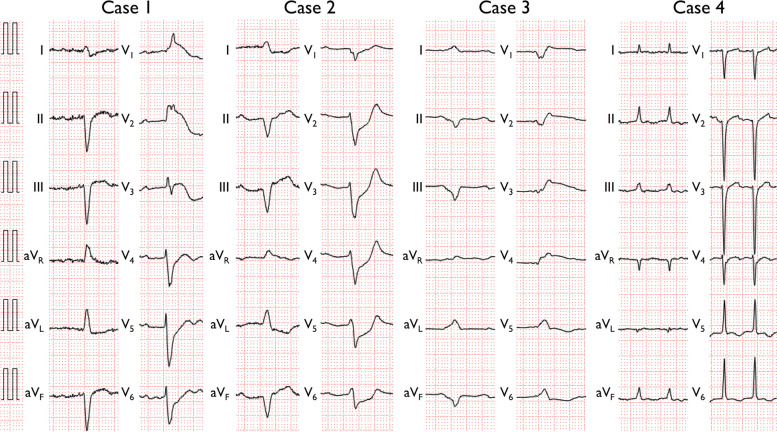
A 72-year-old man with renal cell carcinoma with lung and liver metastasis was hospitalized for asymptomatic newly developed trifascicular block with elevated troponin T (TnT) levels. He underwent right nephrectomy 8 years prior and was treated with avelumab (ICI), axitinib (tyrosine kinase inhibitor), and cabozantinib (multikinase inhibitor) for relapse. Although lung metastasis was stable, treatment with nivolumab (ICI) was started 1 month prior because of progressive liver metastasis (Table 2). He had no recent history of antecedent infection, vaccinations, or exposure to new medications other than anticancer drugs. He developed sustained ventricular tachycardia (VT) on day 7 and was subsequently transferred to our hospital. Electrocardiography (ECG) revealed a wide QRS complex and ST elevation in leads V1-V3 (Fig. 1). Echocardiography revealed left ventricular (LV) wall motion abnormalities with LV ejection fraction (LVEF) of 40%. Coronary angiography revealed no stenotic lesions. Endomyocardial biopsy (EMB) revealed severe infiltration of lymphocytes, histiocytes, and a few eosinophils with myocardial necrosis. Immunohistochemistry revealed T cells (CD3, CD8 > CD4), CD68+ histiocytes, and a few CD20+ B cells. Tenascin C, a known marker of myocarditis [9], was diffusely expressed, and the myocardium was strongly stained with PD-L1 (Fig. 2A and Fig. 3A). Pulse corticosteroid (CS) therapy with methylprednisolone (MP) followed by high-dose prednisolone (PRD) was started immediately. The TnT level decreased, and VT ceased temporarily; however, the TnT level re-escalated, and VT recurred 6 days later. The implementation of MCS and additional immunosuppressants was considered, but the patient and his family declined further invasive treatments. The patient fell into an electrical storm and died on day 9.
Table 2.
Detailed treatment history for cancersa
| Agents | Drug classes | Route of administration | Doses | Courses | First administration to onset of FM [days] | Last administration to onset of FM [days] |
|---|---|---|---|---|---|---|
| Case 1: Renal cell carcinoma | ||||||
| Avelumab | ICI | IV | 600 mg | 3 | 1158 | 1113 |
| 600 mg | 24 | 1029 | 679 | |||
| Axitinib | Tyrosine kinase inhibitor | PO | 10 mg/day | 1158 | 1113 | |
| 6 mg/day | 1085 | 677 | ||||
| Cabozantinib | Multikinase inhibitor | PO | 40 mg/day | 676 | 43 | |
| Nivoumab | ICI | IV | 240 mg | 2 | 33 | 18 |
| Case 2: Prostate adenocarcinoma | ||||||
| Leuprorelin | LH-RH analog | SC |
22.5 mg once every 24 weeks |
2881 | 155 | |
| Bicalutamide | Androgen receptor antagonist | PO | 80 mg/day | 2851 | 1246 | |
| IMRT | External beam radiation | Radiation | 76 Gy (total) | 38 | 2699 | 2644 |
| Abiraterone | Androgen synthesis inhibitor | PO | 1000 mg/day | 1226 | 1061 | |
| Enzalutamide | Androgen receptor antagonist | PO | 160 mg/day | 1020 | 886 | |
| Pembrolizumab | ICI | IV | 200 mg | 7 | 1020 | 893 |
| Docetaxel | Microtubule inhibitor | IV | 70 mg/m2 | 9 | 876 | 554 |
| Radium-223 chloride | Alpha emitter | IV | 55 kBq/kg/month | 6 | 407 | 267 |
| Ethinylestradiol | estrogen analog | PO | 1 mg/day | 386 | 359 | |
| 0.5 mg/day | 358 | 211 | ||||
| Cabazitaxel | Microtubule inhibitor | IV | 25 mg/m2 | 2 | 50 | 18 |
| Case 3: Lung cancer (poorly differentiated carcinoma) | ||||||
| Carboplatin | Platinum compound | IV | 540 mg (AUC 6) | 4 | 692 | 581 |
| Nab-Paclitaxel | Microtubule inhibitor | IV | 100 mg/m2 | 4 | 692 | 581 |
| Atezolizumab | ICI | IV | 1200 mg | 31 | 692 | 13 |
| Case 4: Lung cancer (adenocarcinoma) | ||||||
| Cisplatin | Platinum compound | IV | 60 mg/m2 | 3 | 119 | 58 |
| Vinorelbine | Microtubule inhibitor | IV | 20 mg/m2 | 3 | 119 | 58 |
| Atezolizumab | ICI | IV | 1200 mg | 1 | 11 | 11 |
AUC area under the concentration–time curve, FM fulminant myocarditis, ICI immune checkpoint inhibitor, IMRT intensity modulated radiation therapy, IV intravenous, LH-RH luteinizing hormone-releasing hormone, NA not available, PO per os, SC subcutaneous
aThe cancer therapeutics were sequenced in early order of start
Fig. 1.
12-Lead electrocardiography of patients with immune checkpoint inhibitor-related fulminant myocarditis on admission. Note the marked widening of the QRS complex and the deviation of the ST segment in Cases 1–3
Fig. 2.
Histopathology and immunohistochemistry of the endomyocardial biopsy. Hematoxylin‒eosin (H-E) staining and immunohistochemistry for the lymphocyte markers, tenascin C (TNC), and programmed cell death ligand 1 (PD-L1) in Cases 1 (A), 2 (B), 3 (C), and 4 (D) are shown. The bar indicates 100 µm
Fig. 3.
Quantitative analysis of the infiltrating inflammatory cells including CD3-, CD4-, CD8-, CD20-, and CD68-positive cells in the myocardium of Cases 1 (A), 2 (B), 3 (C), and 4 (D) are shown
Case 2
A 69-year-old man with prostate cancer (adenocarcinoma) with bone metastasis underwent chemotherapy with docetaxel (microtubule inhibitor), cabazitaxel (microtubule inhibitor) and pembrolizumab (ICI), radiation therapy, and androgen deprivation therapy (Table 2). The last administration of pembrolizumab was 2 years prior, and cabazitaxel was added for progressive bone metastasis 18 days prior. He presented to our hospital for chest pain, vomiting, and fever. He had no recent history of antecedent infection, vaccinations, or exposure to new other medications. ECG revealed complete atrioventricular block (CAVB), wide QRS, ST depression in the leads I, aVL, and V1-V6, and ST elevation in the aVR lead. (Fig. 1). Echocardiography revealed diffuse LV hypokinesis with LVEF of 17%. Since the patient was in cardiogenic shock, an intra-aortic balloon pump (IABP) and a temporary transvenous pacemaker were implemented. Coronary angiography was normal. EMB revealed dense infiltration of lymphoplasmacytes, neutrophils and eosinophils associated with myocytic damage and interstitial fibrosis. Immunohistochemistry revealed T cells (CD8 > CD4), CD68+ histiocytes, and a few CD20+ B cells. PD-L1 was highly expressed in the myocardium. (Fig. 2B and Fig. 3B). Pulse CS therapy was initiated immediately. On the same day, the patient experienced a VT storm, and a venoarterial extracorporeal membrane oxygenation (VA-ECMO) was instituted. Since the hemodynamics stabilized, the patient was weaned from VA-ECMO on day 6. Despite the decrease in TnT levels, cardiac function did not improve satisfactorily thereafter, and the patient experienced cardiogenic shock. An IABP was instituted again on day 13. He died on day 47 from multiple organ failure.
Case 3
A 63-year-old man with lung cancer (poorly differentiated carcinoma) had been receiving chemotherapy with carboplatin (platinum compound) plus paclitaxel (microtubule inhibitor), and atezolizumab (ICI) for 2 years (Table 2). The patient showed good response to the treatment and a long survival was anticipated. He was hospitalized for fever and chest pain 2 weeks after the last administration of atezolizumab. He had no recent history of antecedent infection, vaccinations, or exposure to new medications other than anticancer drugs. ECG revealed a wide QRS complex and ST elevation in the leads V1-V4 (Fig. 1). Echocardiography revealed diffuse severe LV hypokinesis with LVEF of 10%. Coronary angiography revealed no steno-occlusive lesions. Because of hemodynamic instability, a VA-ECMO and an IABP were instituted, and the patient was transferred to our hospital on day 3. An Impella CP was implemented instead of the IABP. EMB revealed diffuse lymphocytic infiltration associated with myocyte dropout and interstitial fibrosis. Immunohistochemistry revealed numerous T cells (CD4 = CD8) mixed with CD68+ histiocytes and CD20+ B cells. PD-L1 and TNC were moderately expressed in the myocardium (Fig. 2C and Fig. 3C). Pulse CS therapy was started immediately. The following day, dilated pupils were observed, and head computed tomography revealed extensive cerebral hemorrhage in the left frontoparietal lobes associated with ventricular perforation and uncal herniation. Surgery was not indicated because the coma was deemed irreversible. Although cardiac function gradually improved, the patient succumbed to intracerebral hemorrhage complicated with uncal herniation on day 13. The head CT scan of the previous day showed no metastatic lesions or hematomas. The relationship between brain hemorrhage and irAEs is uncertain.
Case 4
A 76-year-old woman underwent left upper lobectomy for lung cancer (adenocarcinoma) and received adjuvant chemotherapy with cisplatin (platinum compound) and vinorelbine (microtubule inhibitor). Atezolizumab was started thereafter for the prevention of recurrence (Table 2). She was hospitalized because of anorexia and fatigue 2 weeks after the start of atezolizumab. She had no recent history of antecedent infection, vaccinations, or exposure to new other medications. The following day, the patient developed pulmonary edema and cardiogenic shock and was transferred to our hospital. ECG revealed poor R-wave progression in leads V1-V4 and flat T-waves in all leads, with a normal QRS width (Fig. 1). Echocardiography revealed diffuse LV hypokinesis with LVEF of 10%. The increase in TnT levels was modest. Coronary angiography revealed no significant stenosis. There were no characteristic electrocardiographic or echocardiographic findings characteristic for Takotsubo cardiomyopathy. The patient did not develop giant negative T-waves during the disease course. Sustained VT occurred, but it was terminated by the infusion of amiodarone. EMB confirmed moderate inflammation of inflammatory cells with mild interstitial fibrosis. Immunohistochemically, infiltration of T cells (CD3, CD8 > CD4) was observed, but CD20+ B cells and CD68+ histiocytes were scarce. The myocardial expression of TNC and PD-L1 was weak (Fig. 2D and Fig. 3D). Because of hemodynamic instability, a VA-ECMO and an Impella CP were implemented. Pulse CS therapy followed by high-dose PRD was initiated. Since she developed alveolar hemorrhage, the ECMO configuration was switched to veno-arterial-venous (VAV)-ECMO on day 2. Cardiac function recovered after the treatment with CS, and the circuit configuration was switched to VV-ECMO on day 7. The patient was weaned from ECMO on day 17. LV function recovered to LVEF of 63%. She was transferred to a rehabilitation hospital on day 35 and discharged home 3 months later.
Discussion
Here, we present four cases of ICI-FM. Three of the four resulted in fatal outcomes, indicating a poor prognosis, which is compatible with previous reports. To our knowledge, this is the first case series describing the clinical and histopathological characteristics of ICI-FM. The mortality rate of ICI-related myocarditis is reportedly as high as 50% [6]. Once the disease progresses to the fulminant phenotype, almost all cases are fatal [10–13]. Only one study reported a survival case of an ICI-FM patient treated with VA-ECMO [14]. irAE events start within the first few weeks to months after treatment but can occur anytime, even after discontinuation of the treatment (ranging from 1 to 84 weeks) [3, 15]. In fact, the onset of irAE myocarditis ranged from weeks to years after the first treatment in our patients. In Case 2, the last administration of ICI (pembrolizumab) was 2.4 years prior to the onset of FM, and the patient developed just 18 days after the start of cabazitaxel. Taxoid-related myocarditis is very uncommon, and there is only one case report of paclitaxel-induced myocarditis [16]. In the report, however, the diagnosis of myocarditis was based on cardiac magnetic resonance imaging and the histological evidence was lacking unlike our cases. It is difficult to determine whether pembrolizumab alone provoked late myocarditis, cabazitaxel alone induced myocarditis, or cabazitaxel triggered ICI-related myocarditis in Case 2. In the pooled analysis of ICI-related myositis and myocarditis, patients with early-onset irAEs after the treatment with ICIs showed tenfold higher mortality than those with late-onset irAEs [17]. Interestingly, three fatal cases (Cases 1–3) had late-onset myocarditis, whereas the surviving patient (Case 4) had early-onset myocarditis in the present case series. Different background of the patients and the history of oncological treatment with other drugs may explain this discrepancy.
The American Society of Clinical Oncology guidelines recommend the initiation of CS therapy within 24 h for grade 2 or higher ICI-related myocarditis [15]. Early diagnosis of ICI-related myocarditis is challenging because of mild and nonspecific symptoms at the early stage; in fact, our patinets received CS therapy after developing cardiogenic shock. Interestingly, despite the devastating phenotype with cardiogenic shock and VT requiring ECMO, our only survivor (Case 4) showed mild elevation of TnT, narrow QRS, minimal ST-T change, and mild infiltration of inflammatory cells and low expression of TNC and PD-L1 in the myocardium, whereas three other fatal patients presented high TnT, wide QRS, and dense infiltration of inflammatory cells and high expression of TNC and PD-L1 in the myocardium. We could not conclude whether the high expression of PD-L1 in the myocardium was a cause or consequence (upregulation as a protective negative feedback mechanism) of myocarditis; another possibility is that PD-L1-dependent necroptosis induced by ICIs, at least in part, contributes to cytotoxicity, given the findings of a previous study in hepatocytes [18]. In the above mentioned pooled analysis of irAE myositis and myocarditis, arrhythmia and conduction disturbances were significantly associated with high mortality, consistent with our observation [17]. Power et al. reported that the patients with ICI-myocarditis presented with ECG changes such as new conduction blocks, decreased voltage, and repolarization abnormalities [19]. Another report demonstrated that the cardiac troponin level is associated with major adverse cardiomyotoxic events [20]. Although have been no large studies investigating the determinants for the prognosis of the patients with fulminant form of ICI-myocarditis associated with electrical storm and/or hemodynamic instability requiring MCS, TnT level, QRS width, and EMB findings may predict the outcome of patients.
Although the guidelines suggest the use of second-line immunosuppressants such as mycophenolate, infliximab, anti-thymocyte globulin, tocilizumab, abatacept, alemtuzumab, or tofacitinib in refractory cases [1, 15], the maximum doses of corticosteroid or the combination with other immunosuppressive therapies did not show the significant difference in mortality [17]. The development of curative therapeutic strategy against refractory ICI-related myocarditis is an urgent issue in addition to the early diagnosis and rapid intervention. Figure 4 illustrates a proposed algorithm for the management of ICI-related fulminant myocarditis based on our observation.
Fig. 4.
A proposed management algorithm for immune checkpoint inhibitors-related fulminant myocarditis. CS, corticosteroid; ECG, electrocardiography; ICI, immune checkpoint inhibitor; ICU, intensive care unit; MCS, mechanical circulatory support
In conclusion, early diagnosis and immediate therapeutic intervention on the basis of clinical and histopathological characteristics are necessary to improve the prognosis of patients with ICI-FM.
Acknowledgements
None.
Abbreviations
- ICI
Immune checkpoint inhibitor
- FM
Fulminant myocarditis
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death ligand-1
- irAE
Immune-related adverse event
- MCS
Mechanical circulatory support
- IHD
Ischemic heart disease
- TnT
Troponin T
- VT
Ventricular tachycardia
- ECG
Electrocardiography
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- EMB
Endomyocardial biopsy
- CD
Cluster of differentiation
- CS
Corticosteroid
- MP
Methylprednisolone
- PRD
Prednisolone
- CAVB
Complete atrioventricular block
- IABP
Intra-aortic balloon pump
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
- VAV-ECMO
Veno-arterial-venous extracorporeal membrane oxygenation
Authors’ contributions
RI and TH analyzed and interpreted the patient data and provided first draft. TM, MH, YT, TI provided images and revision. TH, HK, KI, WO, SA, SY, KM, TF, KS, SM, KH, SK, and SK provided revisions. YO and KA supervised and provided revisions. All authors read and approved the final manuscript.
Funding
This study did not receive any specific funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lyon AR, Lopez-Fernandez T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi J, Lichtman AH, Sharpe AH, et al. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131:e145186. [DOI] [PMC free article] [PubMed]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 4.Vansteenkiste J, Wauters E, Reymen B, et al. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30:1244–53. [DOI] [PubMed] [Google Scholar]
- 5.Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022;43:4458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. [DOI] [PubMed] [Google Scholar]
- 7.Palaskas N, Lopez-Mattei J, Durand JB, et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9:e013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morimoto S, Imanaka-Yoshida K, Hiramitsu S, et al. Diagnostic utility of tenascin-C for evaluation of the activity of human acute myocarditis. J Pathol. 2005;205:460–7. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Takahashi K, Ito K, et al. An autopsy case of lung adenocarcinoma with immune checkpoint inhibitor-induced pneumonia and fulminant myocarditis following pembrolizumab administration: a case report. Int Cancer Conf J. 2024;13:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcante L, Chandana S, Lakhani N, et al. Case report of fatal immune-mediated myocarditis following treatment with davoceticept (ALPN-202), a PD-L1-dependent CD28 costimulator and dual PD-L1/CTLA-4 checkpoint inhibitor, in combination with pembrolizumab. J Immunother Cancer. 2024;12:e009475. [DOI] [PMC free article] [PubMed]
- 12.Nishimura T, Ninomiya K, Nakashima M, et al. Fulminant myocarditis for non-small-cell carcinoma of the lung with nivolumab and ipilimumab plus chemotherapy. Intern Med. 2023;62:1319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naganuma K, Horita Y, Matsuo K, et al. An autopsy case of late-onset fulminant myocarditis induced by nivolumab in gastric cancer. Intern Med. 2022;61:2867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramayya T, Mitchell JD, Hartupee JC, et al. Delayed diagnosis and recovery of fulminant immune checkpoint inhibitor-associated myocarditis on VA-ECMO support. JACC CardioOncol. 2022;4:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–126. [DOI] [PubMed] [Google Scholar]
- 16.Johnson D, Weisleder H, Yuan H and Carrozzi G. Paclitaxel-induced myocarditis presenting as new-onset heart failure. BMJ Case Rep. 2024;17:255646. [DOI] [PMC free article] [PubMed]
- 17.Boutros A, Bottini A, Rossi G, et al. Neuromuscular and cardiac adverse events associated with immune checkpoint inhibitors: pooled analysis of individual cases from multiple institutions and literature. ESMO Open. 2023;8:100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo Y, Winarski KL, Sajib MS, et al. Atezolizumab Induces Necroptosis and Contributes to Hepatotoxicity of Human Hepatocytes. Int J Mol Sci. 2023;24:11694. [DOI] [PMC free article] [PubMed]
- 19.Power JR, Alexandre J, Choudhary A, et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. 2021;144:1521–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann LH, Heckmann MB, Bailly G, et al. Cardiomuscular biomarkers in the diagnosis and prognostication of immune checkpoint inhibitor myocarditis. Circulation. 2023;148:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.