Abstract
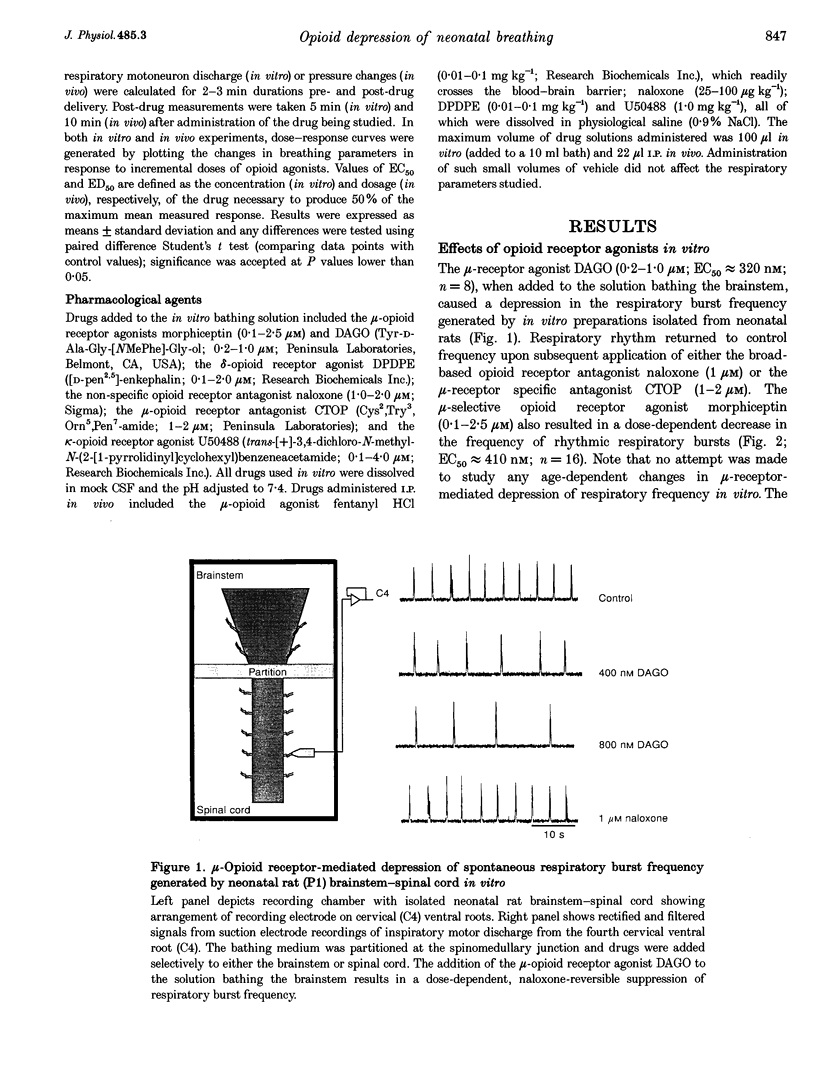
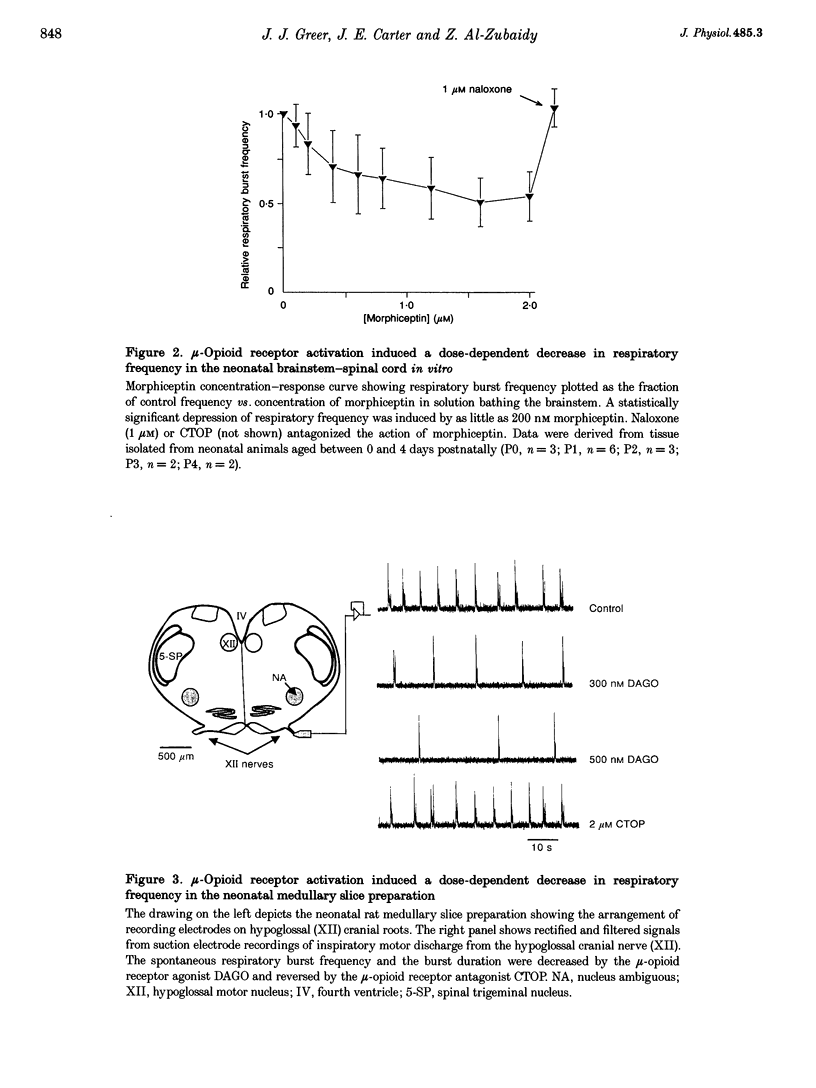
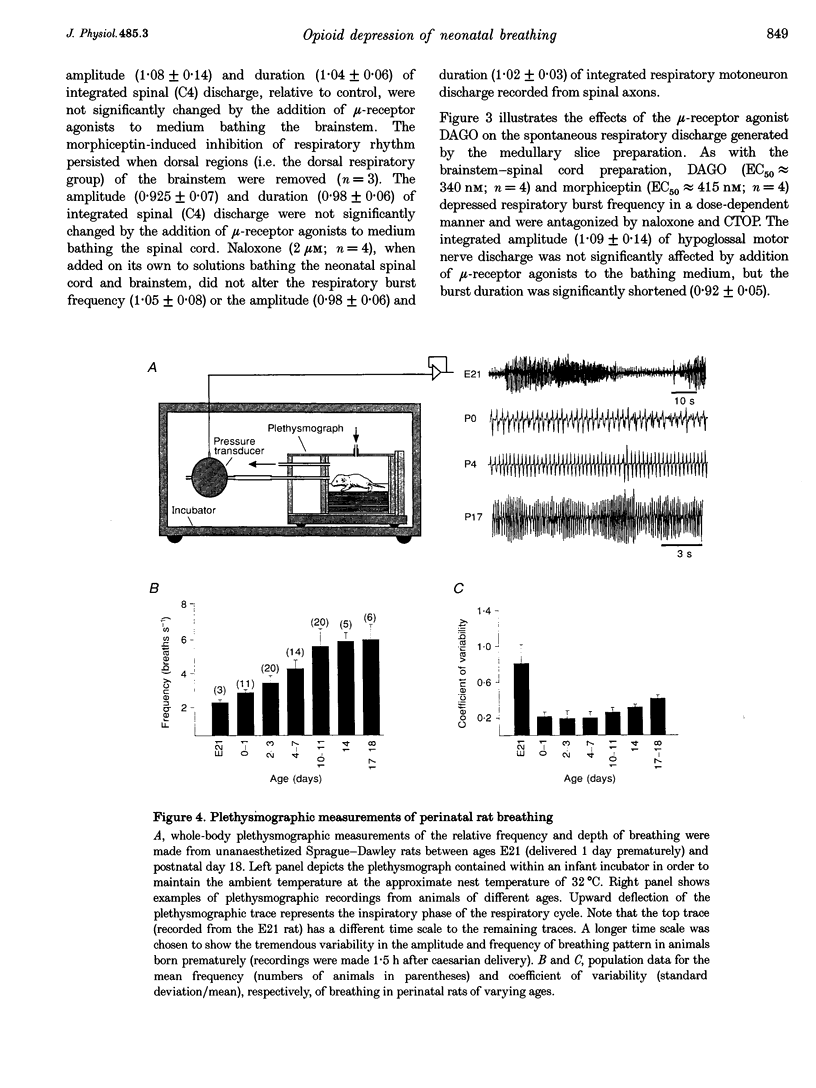
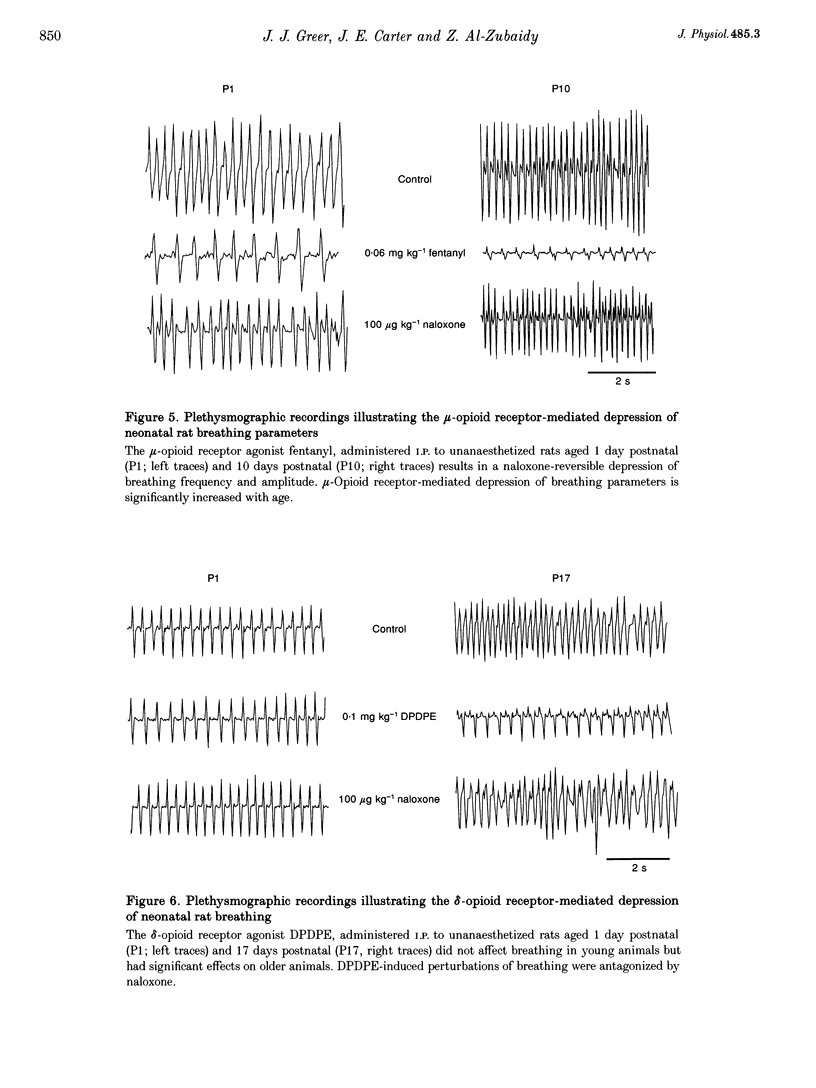
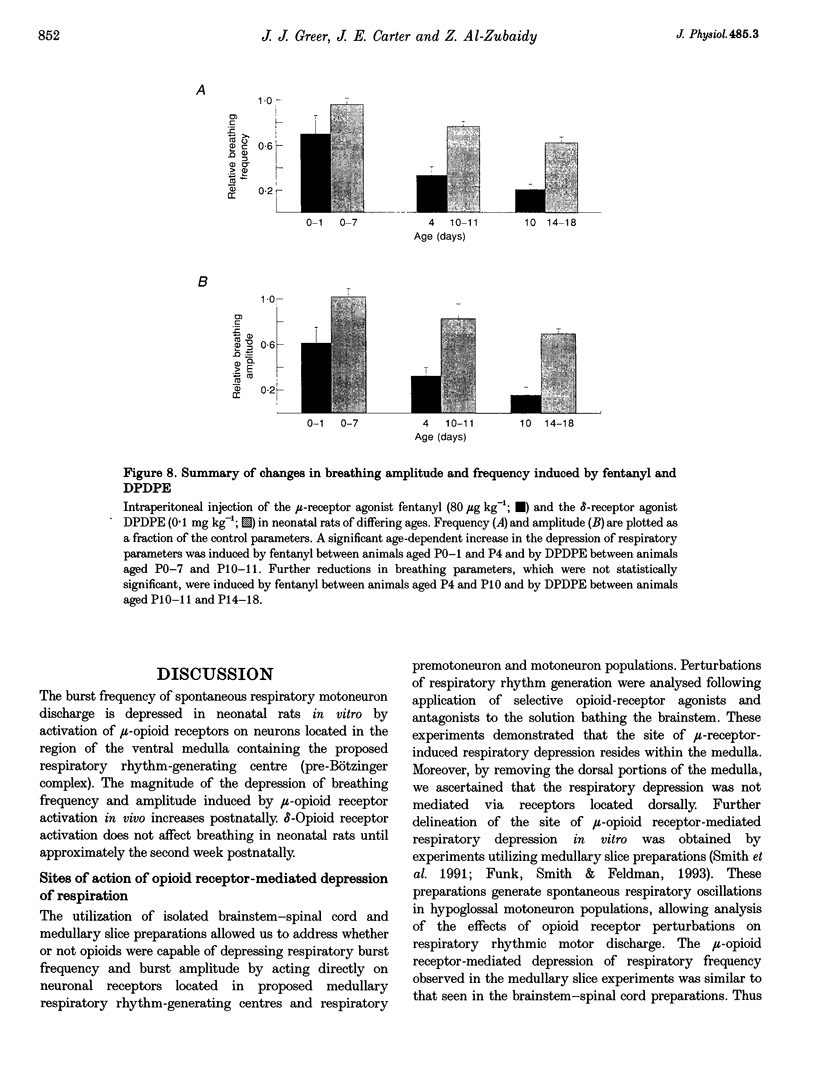
1. The effects of opioid receptor agonists and antagonists on the breathing pattern of neonatal rats were studied. Three experimental approaches were taken. In the first approach, the effects of opioid agonists and antagonists on the spontaneous respiratory neural activity generated by brainstem-spinal cords isolated from neonatal rats aged 0-4 days postnatal (P0-4) maintained in vitro were studied. Secondly, similar studies were performed utilizing medullary slice preparations consisting of respiratory rhythm-generating regions (pre-Bötzinger complex). Thirdly, whole-body plethysmographic recordings were obtained from unanaesthetized neonatal (P0-18) rats before and after I.P. administration of opioid-receptor agonists and antagonists. 2. The mu-receptor agonists morphiceptin and DAGO (Tyr-D-Ala-Gly-[NMePhe]-Gly-ol), when added either to the solutions bathing the brainstems of neonatal rat brainstem-spinal cord preparations or bathing the medullary slice preparations, resulted in a naloxone-reversible, dose-dependent decrease in the frequency of respiratory rhythmic discharge. 3. The respiratory burst frequency and amplitude in vitro were unaffected by the addition of the delta-opioid receptor agonist DPDPE ([D-pen2,5]-enkephalin) and the kappa-opioid receptor agonist U50488 (trans-[+]-3,4-dichloro-N-methyl-N-(2-[1- pyrrolidinyl]cyclohexyl) benzene-acetamide) or the opioid receptor antagonist naloxone. 4. Intraperitoneal administration of the mu-opioid receptor agonist fentanyl resulted in a naloxone-reversible, dose-dependent decrease in the frequency and amplitude of breathing of unanaesthetized neonatal rats (P0-P10). I.P. administration of the delta-opioid receptor agonist DPDPE did not affect breathing of neonatal rats until the second week postnatally. 5. We conclude that opioids suppress the frequency of neonatal rat respiration by acting via mu-opioid receptors located within regions of the ventral medulla containing respiratory rhythm-generating centres (the pre-Bötzinger complex). delta-Opioid receptor activation does not affect breathing in neonatal rats until approximately the second week postnatally.
Full text
PDF

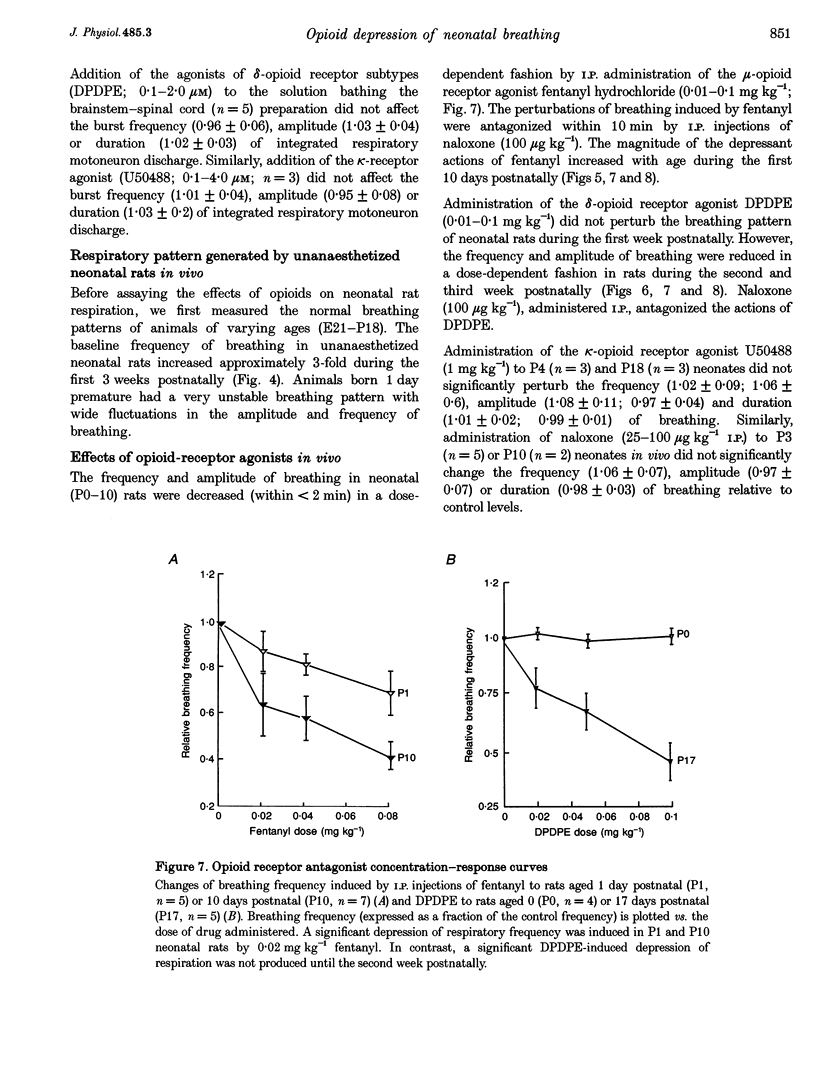



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Nishimura M., Kobayashi S., Yoshioka A., Yamamoto M., Miyamoto K., Kawakami Y. Effects of naloxone on the sensation of dyspnea during acute respiratory stress in normal adults. J Appl Physiol (1985) 1993 Feb;74(2):590–595. doi: 10.1152/jappl.1993.74.2.590. [DOI] [PubMed] [Google Scholar]
- Attali B., Saya D., Vogel Z. Pre- and postnatal development of opiate receptor subtypes in rat spinal cord. Brain Res Dev Brain Res. 1990 Apr 1;53(1):97–102. doi: 10.1016/0165-3806(90)90128-l. [DOI] [PubMed] [Google Scholar]
- Capogna M., Gähwiler B. H., Thompson S. M. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993 Oct;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C. J., Ostrea E. M., Jr, Stryker J. C., Smialek Z. Sudden infant death syndrome among infants of drug-dependent mothers. J Pediatr. 1979 Sep;95(3):407–409. doi: 10.1016/s0022-3476(79)80517-x. [DOI] [PubMed] [Google Scholar]
- Chernick V., Madansky D. L., Lawson E. E. Naloxone decreases the duration of primary apnea with neonatal asphyxia. Pediatr Res. 1980 Apr;14(4 Pt 1):357–359. doi: 10.1203/00006450-198004000-00021. [DOI] [PubMed] [Google Scholar]
- Coquerel A., Buser M., Tayot J., Pfaff F., Matray F., Proust B. Beta-endorphin and neurotensin in brainstem and cerebrospinal fluid in the sudden infant death syndrome. Neurochem Int. 1992 Jan;20(1):97–102. doi: 10.1016/0197-0186(92)90131-a. [DOI] [PubMed] [Google Scholar]
- Crook T. J., Kitchen I., Hill R. G. Effects of the delta-opioid receptor antagonist naltrindole on antinociceptive responses to selective delta-agonists in post-weanling rats. Br J Pharmacol. 1992 Oct;107(2):573–576. doi: 10.1111/j.1476-5381.1992.tb12785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denavit-Saubié M., Champagnat J., Zieglgänsberger W. Effects of opiates and methionine-enkephalin on pontine and bulbar respiratory neurones of the cat. Brain Res. 1978 Oct 20;155(1):55–67. doi: 10.1016/0006-8993(78)90305-0. [DOI] [PubMed] [Google Scholar]
- Funk G. D., Smith J. C., Feldman J. L. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993 Oct;70(4):1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Greer J. J., Smith J. C., Feldman J. L. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991 Jun;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H. C., Ottoson C. K., White W. F. Three-dimensional distribution of 3H-naloxone binding to opiate receptors in the human fetal and infant brainstem. J Comp Neurol. 1990 Jan 1;291(1):55–78. doi: 10.1002/cne.902910106. [DOI] [PubMed] [Google Scholar]
- Kitchen I., McDowell J. Development of delta-opioid receptors in rat brain characterised by [3H]-[D-Pen2,D-Pen5] enkephalin binding. NIDA Res Monogr. 1986;75:67–68. [PubMed] [Google Scholar]
- Leibstein A. G., Dermietzel R., Willenberg I. M., Pauschert R. Mapping of different neuropeptides in the lower brainstem of the rat: with special reference to the ventral surface. J Auton Nerv Syst. 1985 Nov;14(3):299–313. doi: 10.1016/0165-1838(85)90118-3. [DOI] [PubMed] [Google Scholar]
- McCann E. M., Lewis K. Control of breathing in babies of narcotic- and cocaine-abusing mothers. Early Hum Dev. 1991 Dec;27(3):175–186. doi: 10.1016/0378-3782(91)90193-7. [DOI] [PubMed] [Google Scholar]
- Morin-Surun M. P., Boudinot E., Fournie-Zaluski M. C., Champagnat J., Roques B. P., Denavit-Saubie M. Control of breathing by endogenous opioid peptides: possible involvement in sudden infant death syndrome. Neurochem Int. 1992 Jan;20(1):103–107. doi: 10.1016/0197-0186(92)90132-b. [DOI] [PubMed] [Google Scholar]
- Myer E. C., Morris D. L., Adams M. L., Brase D. A., Dewey W. L. Increased cerebrospinal fluid beta-endorphin immunoreactivity in infants with apnea and in siblings of victims of sudden infant death syndrome. J Pediatr. 1987 Nov;111(5):660–666. doi: 10.1016/s0022-3476(87)80239-1. [DOI] [PubMed] [Google Scholar]
- Olson E. B., Jr Naloxone accelerates the rate of ventilatory acclimatization to hypoxia in awake rats. Life Sci. 1987 Jul 13;41(2):161–167. doi: 10.1016/0024-3205(87)90489-9. [DOI] [PubMed] [Google Scholar]
- Sales N., Riche D., Roques B. P., Denavit-Saubie M. Localization of mu- and delta-opioid receptors in cat respiratory areas: an autoradiographic study. Brain Res. 1985 Oct 7;344(2):382–386. doi: 10.1016/0006-8993(85)90820-0. [DOI] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Zheng D., McCleskey E. W. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991 Jan;6(1):13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- Shook J. E., Watkins W. D., Camporesi E. M. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis. 1990 Oct;142(4):895–909. doi: 10.1164/ajrccm/142.4.895. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Feldman J. L. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987 Oct;21(2-4):321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Greer J. J., Liu G. S., Feldman J. L. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990 Oct;64(4):1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Spain J. W., Roth B. L., Coscia C. J. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa). J Neurosci. 1985 Mar;5(3):584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrook R. A., Feldman H. A., Fencl V., Forte V. A., Jr, Gabel R. A., Leith D. E., Weinberger S. E. Naloxone does not affect ventilatory responses to hypoxia and hypercapnia in rats. Life Sci. 1984 Feb 27;34(9):881–887. doi: 10.1016/0024-3205(84)90205-4. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988 Nov;8(11):4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpey T. L., Chavkin C. Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron. 1991 Feb;6(2):281–289. doi: 10.1016/0896-6273(91)90363-5. [DOI] [PubMed] [Google Scholar]