Abstract
Indian Mustard (Brassica juncea L.) is a globally cultivated winter oilseed crop of the rapeseed-mustard group. It is predominantly grown in the semi-arid northwest agroclimatic zone of India, characterized by high soil salinity. Enhancing tolerance to salt stress in B. juncea is therefore crucial for sustaining its production in this region. Long non-coding RNAs (lncRNAs) play critical roles in coordinating gene expression under various abiotic stresses, including salt stress, but their involvement in the salt stress response in B. juncea remains largely unknown. In this study, we conducted RNA-seq analysis on control, salt-stressed, and salt-shocked young leaves of the salt-tolerant B. juncea cv CS-52. We identified a total of 3,602 differentially expressed transcripts between stress versus control and shock versus control samples. Among these, 61 were identified as potential lncRNAs, with 21 specific to salt stress and 40 specific to salt shock. Of the 21 lncRNAs specific to salt stress, 15 were upregulated and six were downregulated, while all 40 lncRNAs unique to salt shock were downregulated. Chromosomal distribution analysis of the lncRNAs revealed their uneven placement across 18 chromosomes in B. juncea. RNA-RNA interaction analysis between salt stress-upregulated lncRNAs and salt stress-related miRNAs identified 26 interactions between 10 lncRNAs and 23 miRNAs and predicted 13 interactions between six miRNAs and 13 mRNAs. Finally, six lncRNA-miRNA-mRNA interaction networks were established, involving five lncRNAs, 13 miRNAs, and 23 mRNAs. RT-qPCR analysis revealed the upregulation of four out of five lncRNAs, along with their target mRNAs, supporting their involvement in the salt stress response in B. juncea.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-024-10964-1.
Keywords: Brassica juncea, lncRNA, eTMs, Salt stress, Salt shock
Introduction
Salinity poses a global environmental constraint, leading to reduced crop productivity and quality [1]. It induces extensive physiological dysfunctions in plants, with the severity depending on the genotype, plant growth phase, and ionic strength of the salt source [2]. Gradual exposure to increasing salt concentrations or sustained exposure to low salinity levels results in salt stress, while the instant imposition of high salinity levels causes salt shock [3]. Salt stress is a recurring challenge for crops in arid and semi-arid regions, but salt shock, though less common, can have severe consequences. Salt shock typically occurs during rare events such as tsunamis, where seawater floods cultivated land. The increasing frequency of such events, exacerbated by rising sea levels due to climate change, poses a growing threat to coastal agricultural soils. Sudden soil salinization driven by these factors represents a significant risk to agricultural productivity in coastal regions. Areas particularly vulnerable to this phenomenon include the Middle East, North Africa, Latin America, the Caribbean, Sub-Saharan Africa, and East Asia. The potential impacts on food security and local economies in these regions highlight the urgent need for strategies to mitigate the effects of salt shock and enhance resilience in coastal agriculture [4, 5].
Indian Mustard (Brassica juncea L.) (AABB; 2n = 36) is a globally cultivated winter oilseed crop. It dominates the rapeseed-mustard group in India, covering over 90% of the total acreage [6]. Second only to groundnut in the oilseed economy, it contributes 20% to total oilseed production. The distribution of B. juncea is concentrated in the semi-arid northwest agroclimatic zone of India, characterized by elevated salinity levels in groundwater and moderate to high sodicity issues [7]. Understanding and improving its tolerance to salt stress are crucial to boost and sustain B. juncea production in India.
In higher organisms, less than 3% of genetic transcripts encode proteins. The remaining transcriptome consists of ncRNA transcripts, previously considered as transcriptional noise. However, recent high-throughput sequencing and experimental validation have substantiated the participation of ncRNAs in various gene regulation processes, including epigenetic, transcriptional, and post-transcriptional [8]. Classified as housekeeping or regulatory based on origin, biogenesis, and mode of action, ncRNAs play crucial roles in plant growth, development, and abiotic stress responses. Among regulatory ncRNAs, lncRNAs (transcripts longer than 200 nucleotides) account for a significant share and can impact diverse biological and metabolic processes, aiding plants in surviving abiotic and biotic challenges [9].
A number of lncRNAs are reported in several plant species with their regulatory mechanisms partially revealed in stress response, phosphate homeostasis, and male sterility [10, 11]. Salt stress-related lncRNAs have been identified in various plants, such as Arabidopsis, Gossypium hirsutum, Medicago truncatula, Triticum aestivum, Glycine max, Pistacia vera, and Sorghum bicolor [12–18]. Notably, lncRNAs like DRIR in Arabidopsis and Mulnc1 in mulberry are reported to be associated with salt stress response [19, 20]. While research in this area has advanced for several plant species, there is a significant gap in lncRNA research under elevated salt stress environments in B. juncea.
This study analyzed RNA-seq data from control, salt-stressed, and salt-shocked leaf samples of B. juncea cv CS-52 and identified a total of 61 differentially expressed lncRNAs under salt stress and shock conditions. Of these 61 differentially expressed lncRNAs, only 15 lncRNAs specific to salt stress were upregulated, while the remaining lncRNAs were downregulated. Furthermore, we identified miRNAs and mRNAs targets for the 15 salt stress-upregulated lncRNAs. The constructed lncRNA-miRNA-mRNA networks, involving functionally annotated targets, revealed the involvement of lncRNAs in the regulation of multiple processes. Additionally, we predicted five salt-stress-related lncRNAs as eTMs for six miRNAs and conducted co-expression analysis of these lncRNAs and their mRNA targets under salt stress conditions. The concurrent upregulation of lncRNAs and target mRNAs under salt stress suggests that lncRNAs may play a role in the salt stress response in B. juncea. The findings of this study are anticipated to contribute to a deeper understanding of the complex regulatory framework involving lncRNAs in salt tolerance, laying the foundation for targeted interventions and functional validations to enhance salt stress tolerance in B. juncea.
Materials and methods
Plant materials
We used CS-52, a pure line selection from DIRA-343, in this study. CS-52 is a highly salt-tolerant Indian mustard cultivar that can withstand salinity levels of up to 9 dS/m without significantly compromising seed yield [21]. This salinity-tolerant cultivar was chosen to better understand the mechanisms of salt stress tolerance, which can be more effectively studied in a tolerant cultivar than in a susceptible one.
RNA-seq data acquisition and reference-based transcriptome assembly
We obtained raw RNA-seq reads from the NCBI Sequence Read Archive (SRA) database, generated from control and salt-stressed [21] as well as salt-shocked [22] young leaves of B. juncea cv CS-52 harvested 23 days after sowing. The quality of the raw reads was assessed using FastQC v0.11.9 software [23]. Trimmomatic v0.39 software [24] was used to remove adaptor contamination and low-quality reads, with an average length (nucleotides) and quality score cut-off set at 36 and 25, respectively. HISAT2 v2.1.1 software [25] was used with default parameters to align the clean reads to the B. juncea reference genome (Braju_tum_V2.0) downloaded from the BRAD v3.0 database (http://brassicadb.org) [26]. The genome was indexed using the hisat2-build command available in the HISAT2 v2.1.1 software. The output SAM file from HISAT2 was sorted using SAMtools v1.16 software [27]. Cufflinks v2.2.1 software [28] was used to assemble aligned reads into full-length transcripts, quantify expression levels for each transcript, and calculate expression differences among control, salt-stressed, and salt-shocked leaf samples. Transcripts with FPKM values > 0.5 and lengths > 200 nucleotides were considered for further downstream analysis [29].
Identification of differentially expressed transcripts
Differentially expressed transcripts between salt stress versus control and salt shock versus control samples were analysed using edgeR [30]. Differentially expressed transcripts with P values ≤ 0.05 and log2 fold-changes ( ≥ + 2 and ≤ -2) were considered significantly differentially expressed. The Venny v2.1.0 software [31] was used to identify common and unique differentially expressed transcripts between stress versus control and shock versus control samples.
Functional annotation of differentially expressed transcripts
The functional annotation of differentially expressed transcripts was conducted using the Blast2GO pipeline within OmicsBox v2.1.14 software [32]. To identify homologs, transcript query sequences were compared against the NCBI Viridiplantae protein sequence database using blastx-fast, with an e-value threshold of 1.0E-3, a percent identity of ≥ 95%, and a hit number threshold of 20. Subsequently, the blast results were used for GO mapping [33] and annotation. To improve annotation quality, the integration of GO terms from InterProScan was combined with GO annotations obtained from GO mapping, ensuring the inclusion of reliable plant-specific GO terms. To unveil plant-related metabolic and biological pathways associated with the differentially expressed transcripts, OmicsBox v2.2.14 software was used to determine KEGG pathways [34] using the KEGG pathway tool with default parameters.
In silico prediction and genomic localization of lncRNAs
All the differentially expressed transcripts were subjected to a blast search against the NCBInr database. Transcript sequences lacking blast hit in the NCBInr database were then analyzed using the PLEK v1.2 [35] and the CPC2 [36] software to identify ncRNAs. Venny v2.1.0 software was used to identify common and unique ncRNAs predicted by both software. The common ncRNAs predicted by PLEK and CPC2 were further subjected to an hmmscan search against the Pfam database to eliminate transcripts with probable coding potential. Transcripts lacking Pfam hits were then searched against the Rfam database to filter out small ncRNAs (miRNA, snRNA, tRNA, rRNA, and snoRNA). The remaining ncRNAs were considered potential lncRNAs and were annotated using the RNAcentral database. The genomic location of each lncRNA was determined through a BLASTn search against the B. juncea reference genome. The MapChart v2.32 software was used to plot the chromosome-wise position of the lncRNAs [37].
Analysis of lncRNA-miRNA interaction and prediction of eTMs
We retrieved 547 Brassicaceae-specific miRNAs (Arabidopsis thaliana − 11; Brassica campestris − 8; Brassica napus − 237; Brassica oleracea − 11; Brassica rapa − 168; and Camelina sativa− 112) from miRBase (https://www.mirbase.org/) (Supplementary Table S1). The interaction between salinity-related lncRNAs and Brassicaceae-specific miRNAs was predicted using the plant small RNA target (psRNATarget) analysis server [38]. psRNATarget was executed with the default parameters: expectation = 5, penalty for the G: U pair = 0.5, penalty for other mismatches = 1, extra weight in seed region = 1.5, seed region = 2–13 nucleotides, HSP size = 19, penalty for opening gap = 2, penalty for extending gap = 0.5, translation inhibition range = 10–11 nucleotides, and bulges (gap) -allowed.
Prediction of eTM targets
All the miRNAs showing interaction with the salinity-related lncRNAs were subjected to the identification of their mRNA targets using the psRNATarget analysis server. The psRNATarget analysis was conducted with standard parameters, including selecting the top 200 targets, setting the expectation value ≤ 3.0, assigning a penalty of 0.5 for G: U pairs, a penalty of 1 for other mismatches, an extra weight of 1.5 in the seed region, specifying the seed region from the 2nd to the 13th nucleotide, and establishing a translation inhibition range spanning the 10th and 11th nucleotides [38].
Imposition of salt stress and sample collection
Seeds of B. juncea cv CS-52 were surface sterilized in a 2.5% sodium hypochlorite solution containing 0.1% Tween-20 for 30 min, followed by three washes for 10 min each with autoclaved double-distilled water [21]. The in vivo germination of sterilized seeds involved placing them on two layers of autoclaved water-moistened Whatman No.1 filter paper in 9 cm Petri dishes. Subsequently, the dishes were incubated at 25 °C in the dark for 72 h. Following germination, the seedlings were transferred to a continuous air-bubbling hydroponic floating raft system (12 trays, each with 15 seedlings) within a Conviron plant growth chamber maintained at 22 ± 2 °C, with a 16-hour light/8-hour dark photoperiod at a light intensity (photon flux density) of 100 µmole m − 2 s − 1. The seedlings were nurtured using the standard Hoagland solution [39] for 15 days. Subsequently, the 12 trays were divided into two groups. The first group, which served as controls, continued to receive the standard Hoagland solution. The second group was subjected to salinity stress. The salinity stress was imposed by gradually increasing NaCl concentration in the Hoagland solution as follows: 50 mM for two days, 75 mM for two days, 100 mM for two days, and 125 mM for two days. After eight days of exposure to gradually increasing salinity, the seedlings in this group were subjected to an application of 150 mM NaCl. Throughout the experiment, we replaced the Hoagland solution every 24 h. Leaf samples were collected from the salt-stressed plants three hours after the 150 mM NaCl treatment. In parallel, leaf samples were also collected from the control group. The harvested samples were cleaned with autoclaved double-distilled water and immediately frozen in liquid nitrogen.
RT-qPCR-based quantitative expression analysis of salt stress-related lncRNAs and mRNA targets
To compare the expression patterns of salt stress-related lncRNAs with their target mRNAs in B. juncea, RT-qPCR was used. Forward and reverse primers were designed using the IDT oligo analyzer tool (https://www.idtdna.com/calc/Analyzer), following the MIQE guidelines [40] (Supplementary Table S2). Total RNA from three biological replicates of the control and salt-stressed leaf samples at three hours post-salt stress was isolated using the RNeasy Plant Mini Kit (Qiagen, Germany). The amount, integrity, and quality of the isolated RNA were confirmed using a Nanodrop spectrophotometer and electrophoresis on a 1.2% agarose gel, respectively. Total RNA was converted to cDNA using the SuperScript™ III First-Strand Synthesis Kit (Invitrogen, USA). RT-qPCR was performed in optical 96-well plates (Applied Biosystems) on a StepOnePlus RT-qPCR System (Applied Biosystems, USA) using the SYBR Master Mix (Applied Biosystems, USA). The PCR program included an initial denaturation step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s, and a melt cycle from 60 to 95 °C in 0.5-s increments. The efficiencies of the RT-qPCR primers were calculated using cDNA template dilution series, and amplification specificity was verified using melting-curve analysis. The B. juncea ubiquitin gene served as the internal reference gene for both lncRNA and target mRNA expression studies [41]. Relative expression levels between different samples were calculated using the 2–ΔΔCT method [42].
Results
Transcriptome data processing and reference-based assembly
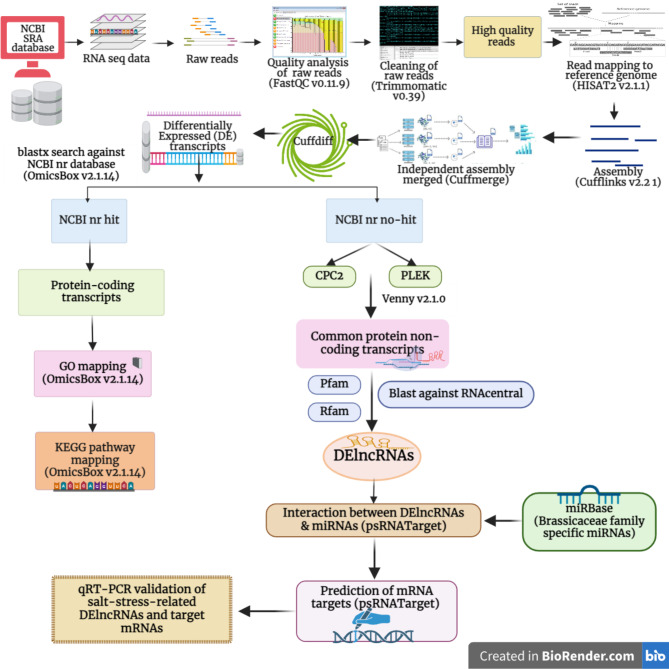
A total of 388,580,960 raw reads were retrieved from the SRA database. Cleaning the raw reads for adaptors and low-quality reads resulted in 347,040,952 clean reads. Among these, 320,268,780 (92.30%) reads were successfully aligned to the B. juncea reference genome using HISAT2 v2.1.1 software (Supplementary Table S3). Using Cufflinks v2.2.1 software, reference-based assembly of clean reads yielded 100,829 transcripts. For further analysis, 50,717 transcripts > 200 nucleotides with FPKM values > 0.5 were used. Fig. 1 depicts the pipeline used for analysing the data. Differentially expressed transcripts between salt stress versus control and salt shock versus control samples were analyzed using edgeR with P value cut-offs < 0.05 and log2 fold-change ≥ 2 and ≤ -2. A total of 3,602 differentially expressed transcripts were identified between salt stress versus control and salt shock versus control samples. Among these, 1,621 (45%) and 1,827 (50.7%) transcripts were unique to stress versus control and shock versus control samples, respectively, while 154 (4.3%) transcripts were common between them (Fig. 2).
Fig. 1.
Workflow for analysing RNA-seq data
Fig. 2.
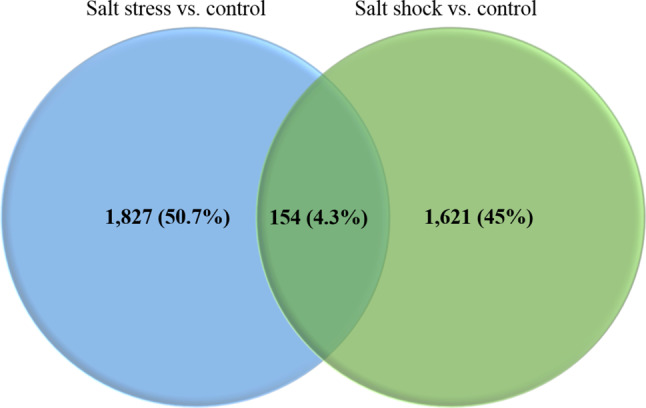
VENN diagram showing the number of differentially expressed transcripts between salt stress versus control and salt shock versus control samples
Identification of lncRNAs
We annotated the differentially expressed transcripts by performing blastx searches against the NCBInr database. A total of 89.5% (3,224) differentially expressed transcripts exhibited a blast hit against the NCBInr database, while the remaining 10.5% (378) showed no substantial similarity to known proteins. Transcripts without a blast hit in the NCBInr database were used to identify ncRNAs using PLEK and CPC2 software. The transcript analysis, using both software, commonly identified 73 non-coding transcripts. An hmmscan search against the Pfam database was used to further eliminate 12 transcripts with coding potential. The blastn searches against the Rfam database using the remaining 61 ncRNAs as queries did not reveal any significant hits or similarity to other ncRNAs (rRNA, miRNA, siRNA, snRNA, tRNA, snoRNA, etc.). Consequently, we considered all 61 ncRNAs as potential lncRNAs (Supplementary Table S4). Out of the 61 differentially expressed potential lncRNAs, 21 were specific to salt stress, while 40 were specific to salt shock. Fifteen of the 21 salt-stress-specific lncRNAs were upregulated, while six were downregulated. All 40 salt-shock-specific lncRNAs showed a downregulation.
In silico characterization of lncRNAs
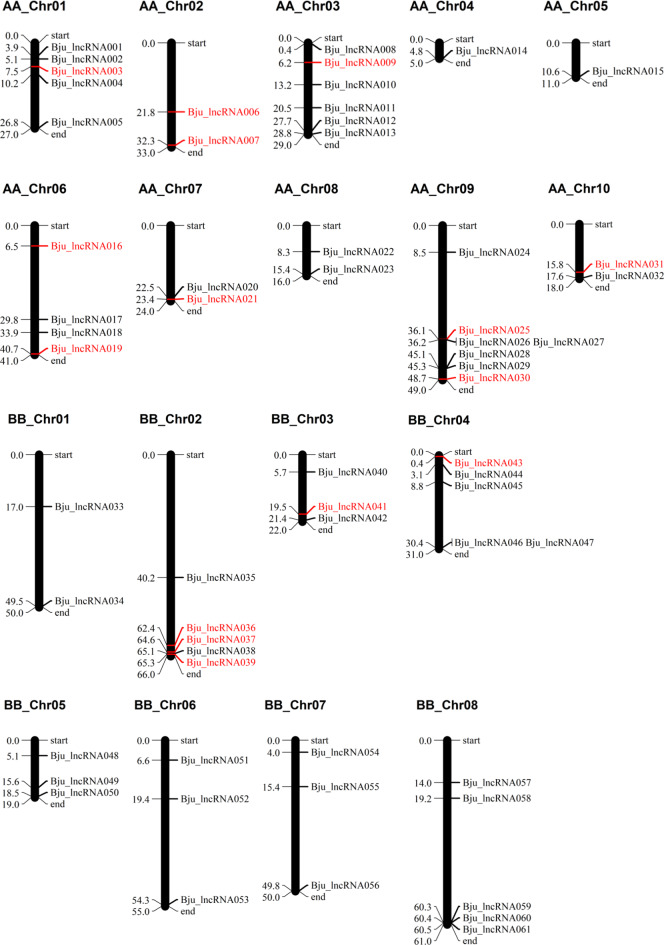
The analysis of the chromosomal location of the 61 salinity-related lncRNAs revealed their uneven distribution across the 18 chromosomes of B. juncea (A01–A10, B01–B08) (Fig. 3; Supplementary Table S5). Chromosome A09 contained the highest number (07), while chromosomes A04 and A05 contained the lowest number (01) of lncRNAs. The lncRNAs varied in length from 201 to 435 bp, with an average of 268.38 bp. A total of 32 (52.5%) of the lncRNAs had sizes ranging between 200 and 250 bp.
Fig. 3.
Chromosomal location of the salinity-related lncRNAs in B. juncea
Annotation of salinity-related differentially expressed transcripts and lncRNAs
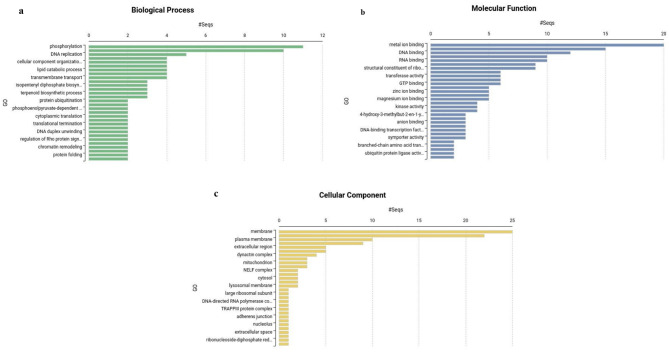
Based on GO annotation using the Blast2GO pipeline, GO terms were assigned to 638 (19.8%) out of 3,224 differentially expressed protein coding transcripts, and they were classified into three main categories: biological process (BP) with 205 (32.1%), molecular function (MF) with 309 (48.4%), and cellular components (CC) with 124 (19.4%) transcripts. In the BP category, phosphorylation (5.4%), translation (4.9%), and DNA replication (2.4%) accounted for the majority of the transcripts (Fig. 4a), while in the MF category, metal ion binding (6.5%), ATP binding (4.9%), and DNA binding (3.9%) (Fig. 4b), and in the CC gategory membrane (20.2%), cytoplasm (17.7%), plasma membrane (8.1%), and nucleolus (7.2%) accounted for the maximum number of transcripts (Fig. 4c). Using the RNAcentral database, all the 61 salinity-related potential lncRNAs were annotated as “lncRNA” (Supplementary Table S4). A total of 25 lncRNAs were identified in Brassica spp., and the rest were found in various plant species such as Arabidopsis thaliana (6), Actinidia chinensis (5), Malus domestica (4), Capsella spp. (3), Populus spp. (2), Panax ginseng (2), Musa acuminata (2), Gossypium spp. (2), Fragaria vesca (2), Coffea arabica (2), Vitis vinifera (1), Setaria italica (1), Lactuca sativa (1), Eucalyptus grandis (1), Carica papaya (1), and Ananas comosus (1).
Fig. 4.
Functional classification of GO term assigned differentially expressed trancripts into (a) biological process (b), molecular functions, and (c), cellular components
Analysis of lncRNA-miRNA interactions and prediction of eTMs and their mRNA targets
Using psRNATarget software, pairing analyses between 15 salt stress-upregulated lncRNAs and 547 Brassicaceae-specific miRNAs were conducted. As a result, a total of 26 interactions with expectation values ≥ 5 were identified between 10 lncRNAs and 23 miRNAs (Supplementary Table S6). Using psRNATarget software, all 23 miRNAs were selected for predicting mRNA targets, revealing a total of 13 interactions between six miRNAs and 13 mRNAs with expectation values ≤ 2.5 (Supplementary Table S7). Finally, a total of six eTMs, involving five lncRNAs, six miRNAs, and 13 target mRNAs, were predicted based on lncRNA-miRNA-mRNA interaction analysis (Table 1).
Table 1.
Salt-stress-related eTMs identified in B. juncea
| LncRNA | miRNA | Target mRNA | Target gene annotation | Function of target gene | Reference |
|---|---|---|---|---|---|
| Bju_lncRNA006 | bra-miR-396-5p | BjuVA01G16450 | MADS-box protein AGL24 | Salt tolerance | [43] |
| Bju_lncRNA019 | bna-novel-12-5p | BjuVB04G12640 | LRR receptor-like serine/threonine-protein kinase | Salt tolerance | [44] |
| Bju_lncRNA025 | ath-miR-854a | BjuVA06G46870 | 3-ketoacyl-CoA synthase 20 | Germination/seeding-stage salt tolerance | [45] |
| BjuVB04G12370 | |||||
| BjuVA08G30140 | Purine permease 14-like | Cytokinin transport | [46] | ||
| Bju_lncRNA031 | cas-miR-156i | BjuVB05G13850 | Putative cysteine-rich receptor-like protein kinase 23 | Disease resistance | [47] |
| Bju_lncRNA031 | cas-miR-159c-5 | BjuVB03G12830 | Chaperone protein dnaJ 11 | Drought tolerance | [48] |
| BjuVA10G03050 | 7-hydroxymethyl chlorophyll a reductase | ROS tolerance | [49] | ||
| Bju_lncRNA043 | cas-miR-858 | BjuVB02G28640 | MYB59 | Salt tolerance | [50] |
| BjuVB02G56810 | MYB111 | Regulates flavonoid biosynthesis | [51] | ||
| BjuVB06G37680 | MYB13 | Salt/drought tolerance |
[52] [50] |
||
| BjuVA07G33590 | MYB20 | Salt tolerance | [53] | ||
| BjuVA07G14640 | MYB3 | Salt tolerance |
[54] [50] |
Experimental validation
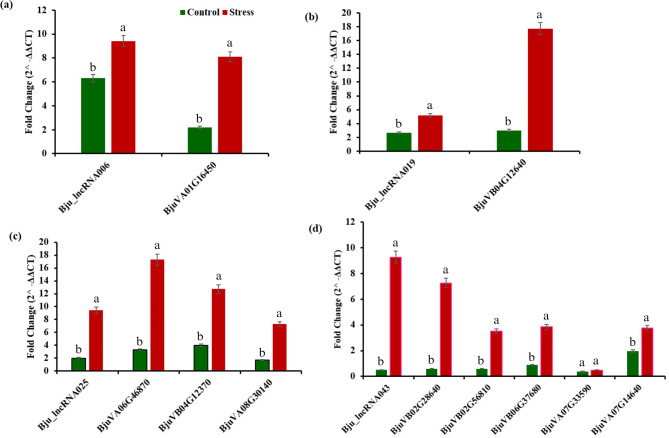
We used RT-qPCR to quantitatively assess the expression levels of five lncRNAs identified as potential target mimics for six salt-stress-related miRNAs, as well as their corresponding target mRNAs. Under salt stress conditions, four lncRNAs—Bju_lncRNA006, Bju_lncRNA019, Bju_lncRNA025, and Bju_lncRNA043—acting as potential target mimics for four miRNAs—bra-miR-396-5p, bna-novel-12-5p, ath-miR-854a, and cas-miR-858—exhibited significant upregulation, with fold changes ranging from 5.2 (Bju_lncRNA019) to 9.4 (Bju_lncRNA006 and Bju_lncRNA025). Concurrently, the expression levels of the corresponding target mRNAs also showed significant upregulation (Fig. 5). The concurrent upregulation of lncRNA (Bju_lncRNA006) and target mRNA (BjuVA01G16450) (Fig. 5a), lncRNA (Bju_lncRNA019) and target mRNA (BjuVB04G12640) (Fig. 5b), lncRNA (Bju_lncRNA025) and target mRNAs (BjuVA06G46870; BjuVB04G12370; BjuVA08G30140) (Fig. 5c), and lncRNA (Bju_lncRNA043) and target mRNAs (BjuVB02G28640; BjuVB02G56810; BjuVB06G37680; BjuVA07G33590; BjuVA07G14640) (Fig. 5d) provides compelling evidence suggesting that lncRNAs may be involved in salt stress responses in B. juncea.
Fig. 5.
RT-qPCR expression analysis, (a) lncRNA (Bju_lncRNA006) and target mRNA (BjuVA01G16450), (b) lncRNA (Bju_lncRNA019) and target mRNA (BjuVB04G12640), (c) lncRNA (Bju_lncRNA025) and target mRNAs (BjuVA06G46870; BjuVB04G12370; BjuVA08G30140), and (d) lncRNA (Bju_lncRNA043) and target mRNAs (BjuVB02G28640; BjuVB02G56810; BjuVB06G37680; BjuVA07G33590; BjuVA07G14640) under salt-stress conditions in B. juncea. Variables with the same letter have no statistically significant difference in their means
Discussion
Soil salinity is one of the most detrimental abiotic stressors affecting the yield and quality of the mustard crop worldwide [21]. Hence, enhancing salt tolerance in the mustard crop is crucial for its cultivation in salt-affected areas [55]. Salt tolerance is a complex trait governed by numerous genes regulated by an array of regulatory elements, including ncRNAs [56]. Among the ncRNAs, lncRNAs are emerging as key regulators of gene expression. Previous research has characterized many lncRNAs and proposed several mechanisms of their action. The most fundamental mechanism involves sequestering regulatory biomolecules, including miRNAs, which negatively regulate the expression of the target genes at the post-transcriptional level [57]. In this sense, the present research primarily aimed at the identification and functional validation of the lncRNA-miRNA-mRNA target networks affecting salt tolerance responses in B. juncea.
In this study, we used 388,580,960 raw RNA-seq reads obtained from the NCBI SRA database. These reads were derived from both control and salt-stressed, as well as salt-shocked, young leaves of B. juncea. Further, the reads were processed into 347,040,952 clean reads. Impressively, 92.30% of the clean reads effectively aligned to the reference genome of B. juncea, indicating that the RNA-seq data used in the present study was of sufficiently good quality [58]. The empirical analysis of the RNA-seq data identified 3,602 differentially expressed transcripts. Among these transcripts, 1,621 (45%) were specific to salt stress, 1,827 (50.7%) were specific to salt shock, and 154 (4.3%) were common to both. These results indicate that the patterns of gene expression vary in response to salt stress and salt shock and signify the method of salt application in genetic and molecular studies [5].
Under salt stress conditions, lncRNAs have been implicated in mediating both osmotic and ionic stress responses in plants [59]. Consistent with the broader role of lncRNAs as key regulators in plant stress responses [60], our study identified 61 lncRNAs that were differentially expressed under salt-stress and shock conditions in B. juncea. Of these, 21 lncRNAs were specific to salt stress, and 40 lncRNAs were specific to salt shock. Notably, all 40 salt-shock-specific lncRNAs were downregulated, corroborating their role in repressing gene expression during sudden stress events [61]. Moreover, among the 21 salt-stress-specific lncRNAs, six were downregulated, and 15 were upregulated. This differential expression pattern highlights the intricate regulatory dynamics of lncRNAs in modulating responses to varying stress intensities, underscoring their importance in fine-tuning plant adaptive strategies [61].
MicroRNAs are recognized as key players in enhancing plant resilience against abiotic stressors [62]. Under salt-stress conditions, the intricate interplay between miRNAs and lncRNAs orchestrates a regulatory network that profoundly influences the expression of salt-stress-responsive genes. LncRNAs actively participate in competitive interactions within the competing endogenous RNA (ceRNA) network [51]. In this study, we identified 26 interactions involving 10 salt-stress-specific lncRNAs and 23 crucifer miRNAs. Additionally, a total of six eTMs, involving five lncRNAs, six miRNAs, and 13 target mRNAs, were predicted based on lncRNA-miRNA-mRNA interaction analysis. All identified miRNA targets were found to be directly involved in salt-stress responses in various crucifers. Most of these miRNA targets encode transcription factors or transporters, suggesting that the identified lncRNAs may sequester salt stress-related miRNAs. This sequestration likely reduces miRNA-mediated suppression, allowing the associated mRNAs to be expressed more robustly and contributing to enhanced plant response to salt stress. Remarkably, among the six eTMs identified, which included five lncRNAs, six miRNAs, and 13 mRNAs, only four lncRNAs exhibited upregulation along with their corresponding mRNA targets. The upregulation of these specific lncRNAs under salt stress, along with the concurrent upregulation of their target mRNAs, indicates a critical role for lncRNAs in the plant’s adaptive response to salt stress. These lncRNAs likely function as eTMs, sequestering salt-stress-related miRNAs and preventing them from binding to their mRNA targets. By mitigating miRNA-mediated suppression, these lncRNAs facilitate enhanced expression of stress-responsive mRNAs, which may encode proteins involved in key physiological processes essential for salt tolerance. This mechanism likely contributes to the plant’s ability to dynamically modulate gene expression in response to salt stress, thereby promoting cellular homeostasis, osmotic balance, and overall adaptation to adverse environmental conditions. The coordinated upregulation of lncRNAs and their target mRNAs under salt stress highlights the importance of the lncRNA-miRNA-mRNA regulatory network in fine-tuning the plant’s transcriptomic landscape to optimize survival and function in high-salinity environments. Similar co-expression analyses of lncRNAs with target mRNAs have been conducted in several other crops, implicating lncRNAs in the regulation of transcripts encoding stress-related transcription factor families, such as WRKY, NAC, MYB-related, ERF, C2H2, bZIP, and bHLH [18, 63]. These findings substantiate the emerging roles of the lncRNA-miRNA-mRNA regulatory network in governing the salt stress response in crop plants. Overall, this research lays the groundwork for exploring the molecular complexities of salt stress response in B. juncea, offering avenues for functional validation, targeted manipulation, and the potential development of salt-tolerant varieties.
Conclusion
The present study highlights the regulatory significance of lncRNAs in orchestrating the molecular intricacies of the salt stress response in B. juncea. Using RNA-seq analysis, we identified 3,602 transcripts that were differentially expressed in response to salt stress and shock. Among these, 61 were identified as potential lncRNAs, with 21 specific to salt stress and 40 specific to salt shock. This differential expression pattern emphasizes the nuanced variations in gene regulation under distinct stress conditions, highlighting the importance of salt application methods in genetic and molecular investigations. Mapping lncRNAs onto the B. juncea reference genome revealed an uneven chromosomal distribution across 18 chromosomes, suggesting potential genomic regulatory hotspots. Additionally, RNA-RNA interaction analysis between salt stress-upregulated lncRNAs, salt stress-related miRNAs, and their target mRNAs uncovered intricate networks involving 10 lncRNAs, 23 miRNAs, and 13 mRNAs, elucidating the interconnected regulatory dynamics underlying salt stress adaptation. Notably, RT-qPCR validation corroborated the upregulation of a subset of lncRNAs under salt stress. Overall, these findings provide a genome-wide overview of salt stress/shock-responsive lncRNAs in B. juncea. Moreover, the lncRNAs with putative salt stress/shock-specific miRNA binding motifs, their salt stress/shock-modulated expression, and the concurrent upregulation of the cognate mRNAs serve as preliminary annotations, guiding detailed functional analysis of these lncRNAs in regulating salt stress/shock-specific responses in B. juncea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely acknowledge the Director, ICAR - Indian Institute of Agricultural Biotechnology, Ranchi 834 003, Jharkhand, India, for providing financial support and the facilities to carry out this research work.
Abbreviations
- ncRNA
Non-coding RNA
- lncRNA
Long non-coding RNA
- miRNA
microRNA
- eTM
endogenous Target Mimic
- RT-qPCR
Real-Time quantitative PCR
- GO
Gene Ontology
Author contributions
BKS conceived the project and wrote the manuscript. BKS, KUT, BP, and AP designed the experiments. MS, TM, KUT performed the experiments. KUT, TM, SK, and BS analyzed the data. AP coordinated the project. All the authors read and approved the manuscript.
Funding
This study was funded by the ICAR - Indian Institute of Agricultural Biotechnology, Ranchi, 834 003, Jharkhand, India.
Data availability
The data supporting the findings of this study are openly available in NCBI under BioProject IDs PRJNA1039958 and PRJNA276704.
Declarations
Ethics approval and consent to participate
This study was conducted following all ethical guidelines and principles and was approved by the competent authority of the Institute. All participants provided informed consent before participating in the study.
Consent for publication
All authors have agreed to the publication of this manuscript. The authors declare that they have no competing interests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kishor U. Tribhuvan and M. Shivakumaraswamy contributed equally to this work.
References
- 1.Hassani A, Azapagic A, Shokri N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat Commun. 2021;12:6663. 10.1038/s41467-021-26907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao C, Zhang H, Song C, Zhu JK, Shabala S. Mechanisms of plant responses and adaptation to soil salinity. Innovation. 2020;1:100017. 10.1016/j.xinn.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shavrukov Y. Salt stress or salt shock: which genes are we studying? J Exp Bot. 2013;64(1):119–27. 10.1093/jxb/ers316. [DOI] [PubMed] [Google Scholar]
- 4.Gupta B, Huang B. Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. 2024;1:701596. 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazhar S, Pellegrini E, Contin M, Bravo C, De Nobili M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils - a review. Front Environ Sci. 2022;10. 10.3389/fenvs.2022.909415.
- 6.Chand S, Patidar OP, Chaudhary R, Saroj R, Chandra K, Meena VK, Limbalkar OM, Patel MK, Pardeshi P, Vasisth P. Rapeseed-mustard breeding in India: scenario, achievements and research needs. Brassica Breed Biotechnol. 2021;174. 10.5772/intechopen.96319.
- 7.Singh A, Panda S. Effect of saline irrigation water on mustard (Brassica Juncea) crop yield and soil salinity in a semi-arid area of North India. Exp Agric. 2012;48:99–110. 10.1017/S0014479711000780. [Google Scholar]
- 8.Gao N, Li Y, Li J, Gao Z, Yang Z, Li Y, Liu H, Fan T. Long non-coding RNAs: the gegulatory mechanisms, research strategies, and future directions in cancers. Front Oncol. 2020;10. 10.3389/fonc.2020.598817. [DOI] [PMC free article] [PubMed]
- 9.Gonzales LR, Blom S, Henriques R, Bachem CWB, Immink RGH. LncRNAs: the art of being influential without protein. Trends Plant Sci. 2024;29(7):770–85. 10.1016/j.tplants.2024.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Zhang A, Pi W, Wang Y, Li Y, Wang J, Liu S, Cui X, Liu H, Yao D, Zhao R. Update on functional analysis of long non-coding RNAs in common crops. Front Plant Sci. 2024;15. 10.3389/fpls.2024.1389154. [DOI] [PMC free article] [PubMed]
- 11.Wunderlich M, Groß-Hardt R, Schöffl F. Heat shock factor HSFB2a involved in gametophyte development of Arabidopsis thaliana and its expression is controlled by a heat-inducible long non-coding antisense RNA. Plant Mol Biol. 2014;85. 10.1007/s11103-014-0202-0. [DOI] [PMC free article] [PubMed]
- 12.Di C, Yuan J, Wu Y, Li J, Lin H, Hu L, Zhang T, Qi Y, Gerstein MB, Guo Y, Lu ZJ. Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J. 2014;80:848–61. 10.1111/tpj.12679. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Niu QW, Wu HW, Liu J, Ye J, Yu N, Chua NH. Analysis of non-coding transcriptome in rice and maize uncovers roles of conserved lncRNAs associated with agriculture traits. Plant J. 2015;84:404–16. 10.1111/tpj.13018. [DOI] [PubMed] [Google Scholar]
- 14.Deng F, Zhang X, Wang W, Yuan R, Shen F. Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol. 2018;18:23. 10.1186/s12870-018-1238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Li M, Zhang H, Duan L, Sun X, Jiang Q. Continuous salt stress-induced long non-coding RNAs and DNA methylation patterns in soybean roots. BMC Genomics. 2019;20(1):1–2. 10.1186/s12864-019-6101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jannesar M, Seyedi SM, Moazzam Jazi M, Niknam V, Ebrahimzadeh H, Botanga C. A genome-wide identification, characterization and functional analysis of salt-related long non-coding RNAs in non-model plant Pistacia vera L. using transcriptome high throughput sequencing. Sci Rep. 2020;10(1):1–23. 10.1038/s41598-020-62108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shumayla SS, Taneja M, Tyagi S, Singh K, Upadhyay SK. Survey of high throughput RNA-seq data reveals potential roles for lncRNAs during development and stress response in bread wheat. Front Plant Sci. 2020;9:8. 10.3389/fpls.2017.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Zheng H, Li J, Liu L, Zhang X, Sui N. Comparative transcriptome analysis reveals new lncRNAs responding to salt stress in sweet sorghum. Front Bioeng Biotechnol. 2020;15:8. 10.3389/fbioe.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin T, Zhao H, Cui P, Albesher N, Xiong L. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017;175(3):1321–36. 10.1104/pp.17.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gai YP, Yuan SS, Zhao YN, Zhao HN, Zhang HL, Ji XL. A novel lncRNA, MuLnc1, associated with environmental stress in mulberry (Morus multicaulis). Front Plant Sci. 2018;9:669. 10.3389/fpls.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh BK, Singh S, Shekhawat K, Rathore S, Pandey A, Kumar S, Singh D, Choudhry S, Kumar S, Singh D. Comparative analysis for understanding salinity tolerance mechanism in Indian mustard (Brassica juncea L). Acta Physiol Plant. 2019;41. 10.1007/s11738-019-2894-x.
- 22.Sharma R, Mishra M, Gupta B, Parsania C, Singla-Pareek SL, Pareek A. De novo assembly and characterization of stress transcriptome in a salinity-tolerant variety CS52 of Brassica juncea. PLoS ONE. 2015;10(5):e0126783. 10.1371/journal.pone.0126783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews S. 2016. FASTQC. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 24.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–15. 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Wang T, He X, Cai X, Lin R, Liang J, Wu J, King G, Wang X. BRAD V3. 0: an upgraded Brassicaceae database. Nucleic Acids Res. 2022;50(D1):D1432–41. 10.1093/nar/gkab1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. [DOI] [PMC free article] [PubMed]
- 28.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–5. 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das A, Nigam D, Junaid A, Tribhuvan KU, Kumar K, Durgesh K, Singh NK, Gaikwad K. Expressivity of the key genes associated with seed and pod development is highly regulated via lncRNAs and miRNAs in pigeonpea. Sci Rep. 2019;9:18191. 10.1038/s41598-019-54340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveros JC. (2007–2015). Venny: An interactive tool for comparing lists with Venn’s Diagrams. https://bioinfogp.cnb.csic.es/tools/venny/index.html
- 32.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35. 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conesa A, Gotz S. Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics. 2008;619832. 10.1155/2008/619832. [DOI] [PMC free article] [PubMed]
- 34.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–62. 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li A, Zhang J, Zhou Z. PLEK: a tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinformatics. 2014;15:311. 10.1186/1471-2105-15-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang YJ, Yang DC, Kong L, Hou M, Meng YQ, Wei L, Gao G. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45(W1):W12–6. 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93(1):77–8. 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 38.Dai X, Zhuang Z, Zhao PX. psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018;46(W1):W49–54. 10.1093/nar/gky316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. California Agricultural Experiment Station, 347 (2nd edition); 1950. 10.1016/j.gpb.2015.09.006
- 40.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 41.Chandna R, Augustine R, Bisht NC. Evaluation of candidate reference genes for gene expression normalization in Brassica juncea using real time quantitative RT-PCR. PLoS ONE. 2012;7(5):e36918. 10.1371/journal.pone.0036918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Castelán-Muñoz N, Herrera J, Cajero-Sánchez W, Arrizubieta M, Trejo C, García-Ponce B, Sánchez MP, Álvarez-Buylla ER, Garay-Arroyo A. MADS-Box genes are key components of genetic regulatory networks involved in abiotic stress and plastic developmental responses in plants. Front Plant Sci. 2019;10:853. 10.3389/fpls.2019.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam Md M, Sharmin S, Nabi Z, Mondal SI, Islam Md S, Nayeem S, Shoyaib M, Khan H. A putative leucine-rich repeat receptor-like kinase of jute involved in stress response. Plant Mol Biol Rep. 2010;28:394–402. 10.1007/s11105-009-0166-4. [Google Scholar]
- 45.Yang Z, Yang X, Dong S, Ge Y, Zhang X, Zhao X, Han N. Overexpression of β- ketoacyl-CoA synthase from Vitis vinifera L. improves salt tolerance in Arabidopsis thaliana. Front Plant Sci. 2020;11:564385. 10.3389/fpls.2020.564385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin W, Xiao Y, Niu M, Meng W, Li L, Zhang X, Tong H. ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate Cytokinin distribution in rice. Plant Cell. 2020;32(7):2292–306. 10.1105/tpc.19.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quezada EH, García GX, Arthikala MK, Melappa G, Lara M, Nanjareddy K. Cysteine-rich receptor-like kinase gene family identification in the Phaseolus genome and comparative analysis of their expression profiles specific to mycorrhizal and rhizobial symbiosis. Genes. 2019;10(1):59. 10.3390/genes10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan F, Yang X, Cheng Y, Kang Y, Chai X. The DnaJ gene family in pepper (Capsicum annuum L.): comprehensive identification, characterization and expression profiles. Front Plant Sci. 2017;8:689. 10.3389/fpls.2017.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piao W, Han SH, Sakuraba Y, Paek NC. Rice 7-hydroxymethyl chlorophyll a reductase is involved in the promotion of chlorophyll degradation and modulates cell death signaling. Mol Cells. 2017;40(10):773. 10.14348/molcells.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukumaran S, Lethin J, Liu X, Pelc J, Zeng P, Hassan S, Aronsson H. Genome- wide analysis of MYB transcription factors in the wheat genome and their roles in salt stress response. Cells. 2023;12(10):1431. 10.3390/cells12101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Fan R, Guo S, Wang P, Zhu X, Fan Y, Song CP. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ Exp Bot. 2019;166:103807. 10.1016/j.envexpbot.2019.103807. [Google Scholar]
- 52.Huang Y, Zhao H, Gao F, Yao P, Deng R, Li C, Chen H, Wu Q. A R2R3-MYB transcription factor gene, FtMYB13, from Tartary buckwheat improves salt/drought tolerance in Arabidopsis. Plant Physiol Biochem. 2018;132:238–48. 10.1016/j.plaphy.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Cui MH, Yoo KS, Hyoung S, Nguyen HTK, Kim YY, Kim HJ, Ok SH, Yoo SD, Shin JS. An Arabidopsis R2R3-MYB transcription factor, AtMYB20, negatively regulates type 2 C serine/threonine protein phosphatases to enhance salt tolerance. Febs Lett. 2013;587(12):1773–8. 10.1016/j.febslet.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 54.Kim D, Jeon SJ, Yanders S, Park SC, Kim HS, Kim S. MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep. 2022;41(7):1549–60. 10.1007/s00299-022-02878-7. [DOI] [PubMed] [Google Scholar]
- 55.Wani AS, Ahmad A, Hayat S, Tahir I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol Biochem. 2019;135:385–94. 10.1016/j.plaphy.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Ma M, Sun Y, Lu P, Shi H, Guo Z, Zhu H. Comparative physiological and transcriptome profiles uncover salt tolerance mechanisms in alfalfa. Front Plant Sci. 2022;13:931619. 10.3389/fpls.2022.931619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom Proteom Bioinform. 2016;14(1):42–54. 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tribhuvan KU, Singh DK, Pradhan B, Bishi SK, Pandey A, Kumar S, Bhati J, Mishra DC, Das A, Sharma TR, Pattanayak A, Singh BK. Sequencing and de novo transcriptome assembly for discovering regulators of gene expression in Jack (Artocarpus heterophyllus). Genomics. 2022;114(3):110356. 10.1016/j.ygeno.2022.110356. [DOI] [PubMed] [Google Scholar]
- 59.Nejat N, Mantri N. Emerging roles of long non-coding RNAs in plant response to biotic and abiotic stresses. Crit Rev Biotechnol. 2018;38(1):93–105. 10.1016/j.stress.2023.100265. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, Wang H, Chua NH. Long noncoding RNA transcriptome of plants. Plant Biotechnol J. 2015;13(3):319–28. 10.1111/pbi.12336. [DOI] [PubMed] [Google Scholar]
- 61.Liu T, Huang Y, Chen J, Chi H, Yu Z, Wang J, Chen C. Attenuated ability of BACE1 to cleave the amyloid precursor protein via silencing long noncoding RNA BACE1–AS expression. Mol Med Rep. 2014;10(3):1275–81. 10.3892/mmr.2014.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaur S, Kumar S, Mohapatra T, MicroRNA. Noncoding but still coding, another example of self-catalysis. Funct Integr Genom. 2022;23(1):4. 10.1007/s10142-022-00926-9. [DOI] [PubMed] [Google Scholar]
- 63.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available in NCBI under BioProject IDs PRJNA1039958 and PRJNA276704.