Abstract
Objective
To investigate the effects of zoledronic acid on muscle metabolism in mice with osteoporosis and sarcopenia and elucidate the possible underlying mechanism.
Methods
Twenty-four 8-week-old male C57BL/6J mice were randomly divided into four groups: non-suspension (N-SUS), suspension (SUS), suspension + zoledronic acid (ZA), and suspension + PTH(PTH) groups. Equal doses of saline, zoledronic acid, and PTH were administered subcutaneously. After 4 weeks, the mice were sacrificed, and body weight and muscle mass (gastrocnemius and soleus) were measured, the right tibia of mice was taken for micro-CT examination, and the muscle specimens were analyzed using HE staining, ATPase staining, western blotting, and real-time PCR.
Results
Compared with the N-SUS group, the bone mineral density (BMD), trabecular bone relative volume (BV/TV) and trabecular bone number (Tb.N) were significantly decreased in the SUS group (P < 0.01), the trabecular bone separation(Tb.Sp)was significantly increased (P < 0.01), which was reversed in ZA and PTH group (P < 0.01).Compared to the SUS group, the body and muscle weights of the ZA and PTH groups were significantly increased. Compared to the SUS group, the muscle structure was less damaged, the proportion of type I muscle fibers was increased, and the protein expression of β-catenin and AKT were upregulated in the ZA and PTH groups(P < 0.05). In addition, the mRNA expression levels of Wnt3a, Wnt16, Myf5, and PI3K were significantly increased (P < 0.05), where as those of Myogenic Differentiation Antigen(MyoD )and Myogenin (MyoG) were significantly decreased (P < 0.05). No significant differences were observed between the ZA and PTH groups.
Conclusions
Zoledronic acid can reduce muscle loss and damage by upregulating the mRNA expression of Wnt and PI3K and the protein expression of β-catenin and AKT.Our results provide a novel basis for the development of drugs for the treatment of osteoporosis combined with sarcopenia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-08054-0.
Keywords: Zoledronic acid, Sarcopenia, Muscle metabolism, Osteoporosis
Introduction
Sarcopenia is a disease characterized by the progressive and widespread loss of muscle mass, strength, and function associated with increased age, leading to limited activity, falls accidents, and even death [1]. Meanwhile, osteoporosis is a systemic metabolic bone disease characterized by reduced bone mass, disrupted bone microstructure, increased bone brittleness, decreased bone strength, and fractures. Studies have suggested that decreased muscle content, strength, and function can significantly increase the risk of osteoporosis, a main risk factor for fall accidents and fractures, and that decreased bone strength can also significantly increase the prevalence of sarcopenia. Sarcopenia and osteoporosis are often accompanied by and interact with each other, jointly increasing the disability and mortality rates of the elderly population [2, 3]. Current prevention and treatment measures for sarcopenia include nutritional, exercise, and drug therapies. Currently, drug therapy mainly includes vitamin D, androgens, selective androgen receptor modulators, growth hormones, and myostatin inhibitors [4]. However, there are no guidelines for the treatment of sarcopenia, and the development of new treatments is urgently needed. Zoledronic acid, a commonly used drug against osteoporosis, has been found to enhance muscle function. Hassouneh et al. [5] found that zoledronic acid could increase the sensitivity of skeletal muscle tissue sensitivity to insulin action at aged rats. Thus, it increases the glucose uptake capacity and enhances muscle activity. In addition, Hain [6] and Essex [7] both found that zoledronic acid not only reduced bone mass loss, but also improved muscle mass and strength in chemotherapy-induced mice. At the same time, some scholars [8] have also found that zoledronic acid can improve the appendicular skeletal muscle mass and appendicular skeletal muscle mass index in patients with osteoporosis. However, Haeri et al. [9] found no increase in limb muscle mass in older women treated with zoledronate, although bone mineral density at the hip and spine increased. so the exact efficacy of zoledronic acid remain to be verified. And its specific mechanisms of action remain unclear. In this study, we aimed to explore the effect of zoledronic acid on muscle metabolism in mice with osteoporosis and sarcopenia, analyze its possible mechanism of action, and provide ideas and basis for the development of new drugs for the treatment of sarcopenia.
In view of this, we first need to establish a simple and effective animal model of osteoporosis combined with sarcopenia. At present, there are various animal models of sarcopenia at home and abroad. These included aging animal models [10], aging + high-fat diet animal models [11], accelerated aging animal models [12], and hindlimb unloading animal models [13], Gene knockout animal model [14]. Although the pathology of accelerated aging model is similar to that of geriatric pathology, the cost is high, and gene knockout model cannot be widely used as an animal model of sarcopenia at present. Therefore, aging + high-fat diet and hindlimb unloading animal models are commonly used modeling methods with short time and low cost. In addition, some scholars [15] have found that the tail suspension method (hindlimb unloading) removes the weight of the rat’s hind limbs, BMD and BV/TV of tibial subchondral bone decreased significantly, and TbSp increased significantly, which was consistent with the manifestation of osteoporosis. At the same time, studies [16] have shown that after 4 weeks of hindlimb unloading in rats, 50% loss of myofibrillar protein could be observed in soleus muscle, and the contraction tension and mass of soleus and plantaris muscle were reduced by about 50%. These macroscopic and microscopic changes of skeletal muscle are consistent with the characteristics of skeletal muscle changes in sarcopenia.All these evidences support that hindlimb unloading can be used as an animal model of osteoporosis combined with sarcopenia. And greatly increase the simplicity of operation.
Methods
Experimental animals
Twenty-four healthy 8-week-old, specific pathogen-free male C57BL/6J mice (body weight,25 ± 3 g) were purchased from Shanghai Jihui Experimental Animal Feeding Co. LTD.(animal production license number: SCXK (Shanghai): 2017-0012). The mice were housed at a constant temperature of 22 ± 2 °C, 50–60% humidity, alternating light and dark every 12 h, and 15–20 air changes hourly. They were randomly divided into four groups according to the number table method: non-suspension group (N-SUS), suspension group (SUS), suspension + zoledronic acid group (ZA), and suspension + PTH group (PTH).
Establishment of an animal model of osteoporosis combined with sarcopenia
After the mouse tail was picked, the tail was wrapped by a circle of gauze, and then multiple circles of breathable tape were wrapped to make a hanging point at the midpoint of the tail, connect the fish line to hang, and the other end of the fish line was fixed to the appropriate part of the cage top, adjust the length of the fish line to ensure that the hind limbs hanging just out of the horizontal, the body, and the horizontal surface at an approximately 30 ° angle.The mice could still rotate and feed freely.The set-up was maintained for 4 weeks [15, 16].
Medical treatment
Treatment was administered at the beginning of modeling. A single dose of zoledronic acid100 µg/kg (Novartis, JiangSu, China) was administered to the ZA group via subcutaneous injection.PTH (1–34) 40 µg/kg (Mimotopes Peptides, WuXi, China), was administered to the PTH group 5 days a week, with 2 days interval, for 4 weeks.The N-SUS and SUS groups received equal doses of saline [17].
Weight measurement
After 4 weeks of treatment, mice weight in each group were recorded using an electronic scale. Mice were euthanized by intraperitoneal injection of 2 milliliters of 0.3% concentration pentobarbital, then sacrificed by neck removal. The right tibia of the mice was taken, and the tibia of the mice was fixed with 4% paraformaldehyde. The calves were separated, and the intact gastrocnemius and soleus muscles were removed, rinsed with cold saline, trimmed clean of visible fat and connective tissues, and then weighed.
Micro computed tomography (Micro-CT) examination
The Skyscan1176 scanning software on the microCT (MCT-III, ZKKS, China) was used to set the following scanning parameters: voltage 45KeV, electric current 555uA, power consumption 25 W, exposure time 48ms, resolution 35 μm (actual resolution: 34.92 μm), scanning Angle 180° (2 pictures were captured every 0.7°), serial scanning of 9 μm per slice were done. After scanning, a fixed volume of interest (VOI) was selected in the center of the specimen for data analysis. Then, the three-dimensional images of the tibia were reconstructed by NRecon software. The on-board CTvox 3D imaging software was used to draw 3D stereoscopic images.
Hematoxylin-eosin staining
Washing the calf muscles to remove glycerin, fixed in 4% paraformaldehyde at 4 for 48 h, washed with normal saline, dehydrated gradually with alcohol, treated with xylene transparent, embedded in paraffin, stained with hematoxylin-eosin, and systematically photographed to observe the number and shape of muscle fibers.
ATPase staining
Muscle specimens were fixed in4% paraformaldehyde for 24 h; rinsed with normal saline, followed by 10%, 20%, and 30% sucrose solutions; and then dehydrated. The surrounding liquid was blotted using an absorbent paper. Muscle tissues were embedded in optimal cutting temperature(OCT) compound, The 10 μm thickness muscle was frozen, serially sliced and dried. Subsequently, the muscle fiber staining was performed to observe changes in the number of different types of muscle fibers.
Western blot
Muscle tissue samples were collected. Proteins were extracted with RIPA lysis buffer(Beyotime, ShangHai, China), and protein concentration was determined using a BCA protein quantification kit(Beyotime, ShangHai, China). The proteins were separated using 10% SDS-PAGE and then electrotransferred to PVDF membranes(GE, USA). The PVDF membranes were blocked in TBST solution containing 5% skim milk for 1 h. Primary antibodies (β-catenin, Akt) were added and incubated overnight at 4 °C, and then secondary antibodies were added and incubated for 1 h at 16–20 °C.Finally, the membranes were washed with TBST(Applygen, BeiJing, China) for 30 min. Chemiluminescence was measured using an ECL chemiluminescence solution(Clinx, ShangHai, China).
Real-time polymerase chain reaction (PCR)
Genes related to muscle metabolism (Wnt3a, Wnt16,Myf5, PI3K, MyoD, and MyoG) were selected to investigate the effects of zoledronic acid on muscle metabolism. Briefly, total RNA from each sample was extracted using the TRIzol one-step method and reverse-transcribed into cDNA using an RT-PCR kit, according to the manufacturer’s instructions. SYBR real-time PCR was performed using an ABI7500 real-time fluorescent quantitative PCR instrument. Relative expression was calculated using the 2•Ct method.
Statistical analysis
The SPSS 20.0 software was used for the data analysis. One-way ANOVA was used to analyze measurements between multiple groups that conformed to a normal distribution and the chi-square test.The Tukey test was used for further two-way comparison between groups.The Dunnett’s T3 test or independent sample t-test was used to analyze measurements that conformed to a normal distribution but the variance was not chi-square.The Kruskal-Wallis H-test was used for data that were not normally distributed. The level of significance was set at α = 0.05. All data are expressed as mean ± standard deviation, and P < 0.05 was considered statistically significant.
Results
Body, hallux valgus, and gastrocnemius muscle weight measurements
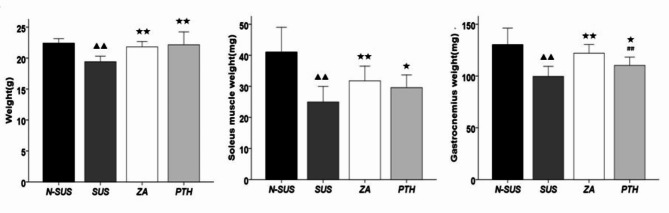
As shown in Fig. 1, mice body weight, as well as the soleus muscle and gastrocnemius weights in the SUS group decreased significantly compared with the N-SUS group (P < 0.01).Meanwhile, these weight measurements significantly increased in the ZA and PTH groups, compared with that of the SUS group (P < 0.05 or P < 0.01).
Fig. 1.
Body weight, and soleus and gastrocnemius muscle weights of mice in each group. Compared with the non-suspension group: ▴P < 0.05, ##▴ ▴P < 0.01. Compared with the suspension group: *P < 0.05, **P < 0.01. Compared with the suspension + zoledronic acid group: #P < 0.05, ##P < 0.01
Micro-CT
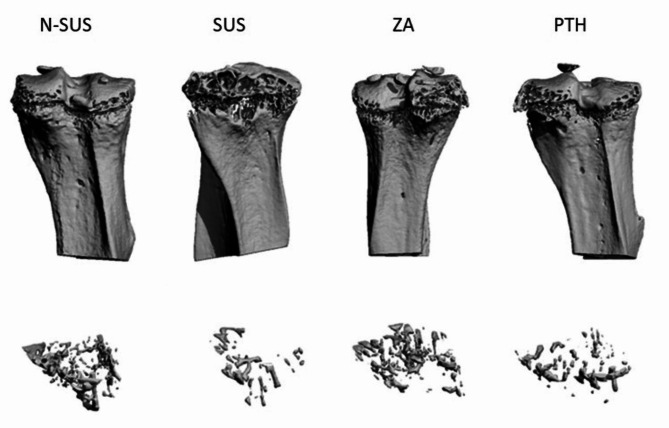
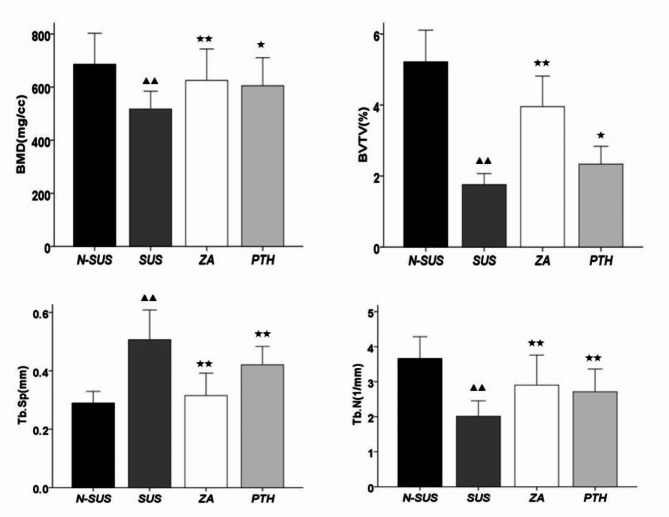
As shown in Figs. 2 and 3, the tibial bone mineral density (BMD), trabecular relative volume (BV/TV), and trabecular number (Tb.N) in the SUS group significantly decreased(P < 0.01), and the trabecular separation(Tb.Sp) degree significantly increased, compared to that in the N-SUS group, while. Compared with the SUS group, the tibial BMD(P < 0.01), BV/TV(P < 0.05),Tb.N(P < 0.01) in the ZA and PTH groups were significantly increased, and Tb.Sp was significantly decreased (P < 0.01).
Fig. 2.
CT scan of the tibia in each mouse group
Fig. 3.
Changes in the tibial CT parameters in each mouse group. Compared with the non-suspension group: ▴P < 0.05, ▴▴P < 0.01. Compared with the suspension group: *P < 0.05, **P < 0.01
HE staining of gastrocnemius tissue
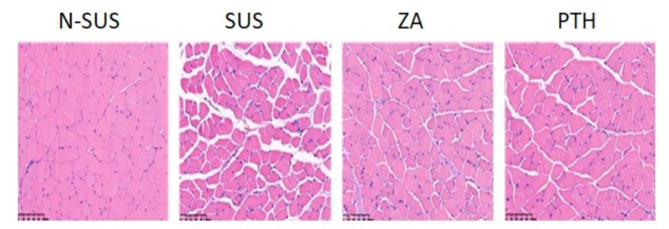
The gastrocnemius tissue was stained with HE and scored as follows: 0, normal tissue without damage;1, slight tissue damage;2, slight tissue damage and slight enlargement of tissue space;3, moderate tissue damage and moderately enlarged interstitial space; and4, severe tissue damage and severely enlarged tissue space.As shown in Fig. 4; Table 1, the gastrocnemius muscle tissue structure of mice in the N-SUS group was complete, the muscle fibers were closely arranged, and no damage was observed. Meanwhile, that of mice in the SUS group was seriously damaged, observed with muscle fiber atrophy, and had a decreased cross-sectional area and an increased gap in muscle fiber. Muscle fiber atrophy and increased muscle space in the gastrocnemius muscle of the ZA and PTH groups were alleviated.
Fig. 4.
Histopathological changes in the gastrocnemius of mice in each group (200×,100 μm)
Table 1.
HE staining score of the gastrocnemius muscle tissue
| Group | HE Score |
|---|---|
| N-SUS | 0.33 ± 0.52 |
| SUS | 3.67 ± 0.52▴▴## |
| ZA | 1.50 ± 0.55**## |
| PTH | 1.67 ± 0.52**## |
Compared with the non-suspension group: ▴P < 0.05, ▴▴P < 0.01. Compared with the suspension group: *P < 0.05, #**P < 0.01
ATPase staining
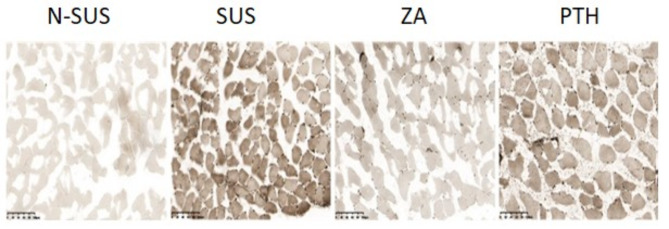
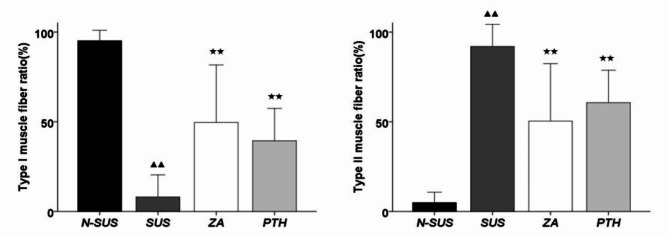
Compared to the N-SUS group, the proportion of types I and II muscle fibers in the SUS group were significantly decreased and increased, respectively (P < 0.01;Figs. 5 and 6). Meanwhile, compared to the SUS group, that of types I and II muscle fibers in the ZA and PTH groups were significantly increased and decreased, respectively (P < 0.01).
Fig. 5.
Mice muscle fiber typing and staining (200×, 100 μm)
Fig. 6.
The proportion of types I and II muscle fibers in each group. Compared with the non-suspension group: ▴P < 0.05, ▴▴ɛP < 0.01. Compared with the suspension group: *P < 0.05, **P < 0.01
Western blot
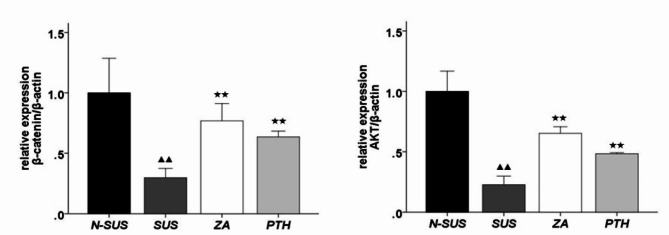
As shown in Fig. 7, the expression levels of β-catenin and AKT in the gastrocnemius muscle tissue of the SUS group were significantly decreased (P < 0.01),compared to those of the N-SUS group. Meanwhile, those of the ZA and PTH groups were significantly increased (P < 0.01), compared to those of the SUS group.
Fig. 7.
Protein expression of β-catenin and AKT protein in the gastrocnemius muscle tissue. Compared with the non-suspension group: ▴P < 0.05, ▴▴P < 0.01. Compared with the suspension group: *P < 0.05, **P < 0.01
Real-timePCR
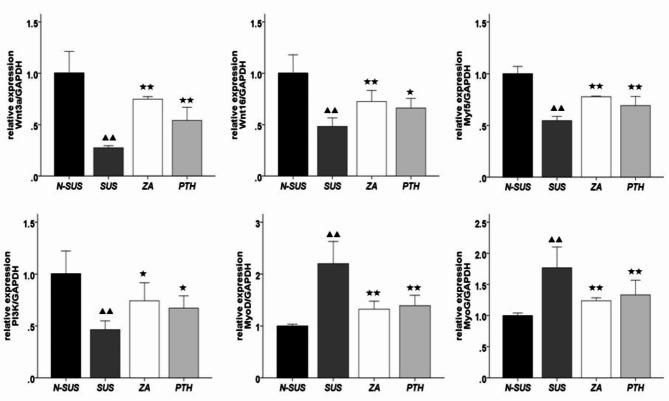
The primer sequences are shown in Table 2, searched through NCBI and artificially synthesized by Kangwei Century Biotechnology Co.Ltd. Compared to those of the N-SUS group, the mRNA expression levels of Wnt3a, Wnt16, Myf5, and PI3K in the gastrocnemius muscle tissue of the SUS group were significantly decreased (P < 0.01), where as those of MyoD and MyoG were significantly increased (P < 0.01) (Fig. 8). Compared to the SUS group, the mRNA expression levels of Wnt3a, Wnt16, Myf5, and PI3Kwere significantly increased in the ZA and PTH groups (P < 0.05 or P < 0.01), while those of MyoD and MyoG were significantly decreased (P < 0.01).
Table 2.
Primer sequence
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse Wnt3a | CCCGTGCTGGACAAAGCT | TCTGCACATGAGCGTGTCACT |
| Mouse Wnt16 | CAGGGCAACTGGATGTGGTT | CTCGTGTCGGAACTGGCTTC |
| Mouse Myf5 | CACCACCAACCCTAACCAGAG | AGGCTGTAATAGTTCTCCACCTG |
| Mouse PI3K | ACACCACGGTTTGGACTATGG | GGCTACAGTAGTGGGCTTGG |
| Mouse MyoD | ACGGCATGATGGACTACAGC | AGGCAGTCGAGGCTCGACA |
| Mouse MyoG | AGGGGATCATCTGCTCCCAG | ATCCCGGCAGACAATCTCAG |
| Mouse GAPDH | TGTCGTGGAGTCTACTGGTG | GCATTGCTGACAATCTTGAG |
Fig. 8.
mRNA expression in the gastrocnemius muscle tissue. Compared with the non-suspension group: ▴P < 0.05, ▴▴P < 0.01. Compared with the suspension group: *P < 0.05, **P < 0.01
Discussion
Patients with sarcopenia frequently develop osteoporosis. As a first-line anti-osteoporosis drug, zoledronic acid not only reduces bone resorption and improves bone mass, but it also increases muscle mass [8]. This provides a new approach for the treatment of sarcopenia. However, the specific mechanism involved remains unclear. Zoledronic acid was used to treat osteoporosis and sarcopenia in mice. Results showed that it could increase the protein expression of β-catenin and AKT protein in the gastrocnemius tissue of mice by upregulating the expression of Wnt3a, Wnt16, PI3K, Myf5, and other genes, thereby increasing muscle weight and improving muscle fiber damage.
Sarcopenia and osteoporosis are closely associated. Studies have found that they share common signaling pathways involved in regulating the metabolic processes in muscle and bone cells. Among them, the Wnt/β-catenin signaling pathway is not only involved in the regulation of osteoblast activity, but it is also related to muscle regeneration. Osteocytes can secrete sclerostin, Wnt3a, and prostaglandins, regulate the Wnt/β-catenin pathway, and affect bone and muscle metabolism [18]. Muscle contraction is an important mechanical stimulus for bones that can activate the Wnt/β-catenin pathway in osteocytes by enhancing fluid flow shear stress, thereby regulating the activity of osteoblasts. Another signaling pathway closely related to the muscle and bone is the PI3K/Akt (intracellular phosphatidylinositol kinase/protein kinase B) pathway, through which insulin-like growth factor-1 can promote muscle and bone anabolism [19]. In the present study, we conducted a preliminary investigation of the proteins and genes involved in these two pathways. The results showed that compared with the SUS group, the weight and muscle mass of the ZA group significantly increased, the degree of gastrocnemius muscle tissue injury was reduced, and the mRNA expression of Wnt and PI3K, and protein expression β-catenin and Akt were significantly upregulated. Fujimaki [20] et al. found that PTH increased the grip strength and maximum running speed of osteoporotic mice by regulating the proliferation, migration, and differentiation of myoblasts through the Wnt signaling pathway. Both the zoledronic acid group and PTH group in this experiment have the effect of enhancing muscle function in mice, and there is no significant difference between the two groups.It is speculated that zoledronic acid may increase the muscle mass of mice with sarcopenia and osteoporosis through the Wnt/β-catenin and PI3K/Akt signaling pathways. Similarly, scholars found that fermented Tenebrio molitor larvae extract could offset the negative effects on muscle tissue and improve grip strength in rats treated with dexamethasone through PI3K-Akt-mTOR signaling pathway [21]. Deng et al. found that CILP2 inhibitors can improves glucose metabolism and mitochondrial dysfunction in sarcopenia via Wnt/β-catenin signalling pathway and its downstream cascade [22]. However, this needs to be further verified at the cellular and molecular levels.However, this needs to be further verified at the cellular and molecular levels.
Further, zoledronic acid was administered to sarcopenic mice and results showed that it significantly increases body weight as well as muscle mass and fiber density and strength. Essex et al. [7]found that zoledronic acid increased muscle mass by 12% and muscle strength by 42% in mice subjected to chemotherapy. Haeri et al. [9]found that limb muscle mass did not increase in elderly women administered with zoledronic acid, although the bone mineral density of the hip joint and spine increased. The present study verified that zoledronic acid could significantly increase muscle fiber content and improve the basic structure of muscle tissue using animal experiments, which was different from the study of Haeri et al. [9]. Similarly, other clinical trials have suggested that zoledronic acid can improve muscle strength and exercise capacity in postmenopausal women [8, 23]. The contradicting results may be because the follow-up period in the study of Haeri et al. [9] was 2 years, and zoledronic acid 5 mg was administered as a single dose. The other studies have tested the muscle strength and quality of postmenopausal women once a year for 3 years after treatment with zoledronic acid, and observed different results. Although the dosage and frequency of the drug administered to the mice in this experiment were similar to those used performed by Haeri et al. [9], the differences may be due to the complex structure of the human body. Moreover, there are many uncontrollable factors in clinical trials that lead to differences in the results.
Furthermore, we observed that the types I and II muscle fibers in the SUS group significantly decreased and increased compared to those in the N-SUS group, respectively. Moreover, compared to the SUS group, the ZA and PTH groups had significantly increased and decreased proportion of types I and II muscle fibers, respectively. Each muscle contains muscle fibers with different rates of contraction and strength, which are mainly divided into type I (slow twitch) and II muscle (fast twitch) fibers.Type I muscle fibers contain a large amount of oxidases (a large number of mitochondria), are surrounded by capillary vessels, and have a higher concentration of myoglobin than any other fiber, the key component(Oxidase enzyme) that provides type I fibers with a large capacity for aerobic metabolism and a strong resistance to fatigue. Meanwhile, type II muscle fibers have a strong explosive force and high contraction speed; however, they are prone to fatigue. In the present study, fatigue resistance of the hind leg muscles of mice significantly worsen after suspension. Further, the ability to maintain a stable balance posture was decreased, which can also explain why elderly individuals with sarcopenia are prone to fall accidents. Moreover, the use of zoledronic acid can significantly increase the proportion of type I muscle fibers in the gastrocnemius muscle, thereby increasing the fatigue resistance of sarcopenic mice and maintaining a comfortable and stable posture.
Myogenin (MyoG) and myogenic regulatory factors (MyoD and Myf5) are important genes involved in muscle formation and differentiation. MyoD and Myf5 are homologous determinants that play important roles in the process of muscle formation, whereas MyoG plays a key role in the terminal differentiation of myoblasts. In the present study, the expression of Myf5 in the ZA group was significantly higher than that in the SUS group, suggesting that zoledronic acid promotes myoblast differentiation by promoting Myf5 gene expression. However, the expression of MyoD in the ZA and PTH groups were significantly lower than that in the SUS group. One possible reason may be the selective mRNA expression of MyoD and MyoG in fast(Type II) and slow(Type I) muscle fibers [24]. Hughes et al. [25] found that MoyD and MyoG are highly expressed in fast and slow muscle fibers, respectively. Therefore, it is possible that although zoledronate and PTH can promote the gene expression of MyoD, these drug can promote the conversion of type II muscle fibers (fast muscle) to type I muscle fibers (slow muscle). We found that MyoD expression is lower in the SUS group than in the control group. It is also possible that MyoD and Myf5 have complementary redundant functions [26]; therefore, when Myf5 expression is increased, MyoD expression is not markedly detected. As a slow muscle regulatory gene, the expression level of MyoG in each experimental group was not consistent with that in the in vivo experiment, which is similar to the results of He [17]. This gene is considered downstream of MyoD and Myf5, and it may indirectly affect muscle metabolism through other tissues (such as the bone).
However, this study has the following certain limitations. Firstly, this study demonstrated that zoledronic acid could reduce muscle damage and loss in sarcopenic mice, it was not clear whether zoledronic acid would affect grip strength and motor performance in sarcopenic mice due to the lack of measurements. Secondly, The observation time for this experiment is only 4 weeks, and the long-term efficacy still needs to be observed for a longer period of time.
Conclusion
Zoledronic acid can increase muscle mass, and improve muscle fiber atrophy and damage in mice with osteoporosis combined with sarcopenia by upregulating the mRNA expression of Wnt, PI3K, Myf5,as well as the protein expression of β-catenin and AKT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Ningbo University School of Medicine for providing experimental venue.
Abbreviations
- ZA
Zoledronic Acid
- PTH
Parathyroid Hormone
- HE
Hematoxylin-Eosin
- ATPase
Adenosine Triphosphatase
- RT-PCR
Reverse Transcription-Polymerase Chain Reaction
- PI3K
Phosphatidylinositol-3-kinase
- AKT
Serine threonine protein kinase
- MyoG
Myogenin
- MyoD
Myogenic Differentiation Antigen
- Myf5
Myogenic factor 5
- Micro-CT
Micro Computed Tomography
- BMD
Bone Mineral Density
- BV/TV
Bone Volume Fraction
- Tb.Th
Trabecular Thickness
- Tb.N
Trabecular Number
- Tb.Sp
Trabecular Separation
Author contributions
All authors contributed to the conception, design, and data analysis of this study. Weilong Li and Ming Xu contributed to the acquisition and interpretation of data and drfted the manuscript. Weilong Li and Ming Xu wrote the manuscript and contributed equally to this work. All authors approved the final version of the manuscript. Xuchao Shi and Jie Gu contributed to the design of the study, performed statistical analyses, and critically revised the manuscript. Jian Guo and Yuanlin Xu contributed to the data collection, interpretation, and provided critical feedback on the manuscript.BoDai contributed to the interpretation of data and provided significant revisionsto the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work, ensuring that any questions related to the accuracy or integrity of the study are appropriately addressed and resolved.
Funding
This work was supported by Ningbo Health Technology Project (No:2021Y42).
Data availability
The data that support the findings of this study are available from the principal investigator upon reasonable request dbblrmyy@163.com.
Declarations
Ethics approval and consent to participate
All operations were carried out in accordance with animal ethics(approved by the Experimental Animal Ethics Committee of Ningbo University, China), and the approval number is 10985, The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:S990–1. [DOI] [PubMed] [Google Scholar]
- 2.Heng MWY, Chan AWD, Man REK, et al. Individual and combined associations of sarcopenia, osteoporosis and obesity with frailty in a multi-ethnic asian older adult population. BMC Geriatr 2023; 23: 802. [DOI] [PMC free article] [PubMed]
- 3.Li WJ, Wang XL, Chu YR, et al. Association of sarcopenia and vitamin D deficiency with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Clin Rheumatol 2023. [DOI] [PubMed]
- 4.Miyazaki S, Tamaki A, Wakabayashi H, et al. Definition, diagnosis, and treatment of respiratory sarcopenia. Curr Opin Clin Nutr Metab Care 2023. [DOI] [PubMed]
- 5.Hassouneh LKM, Timofiychuk OA and Babenko NA.Acid sphingomyelinase inhibitors, imipramine and zoledronic acid, increase skeletal muscle tissue sensitivity to insulin action at old age. General physiology and biophysics, 2018; 37:163–174. [DOI] [PubMed]
- 6.Hain BA, Jude B, Xu H, et al. Zoledronic Acid Improves Muscle Function in Healthy Mice Treated with Chemotherapy. J Bone Miner Res 2020; 35: 368–381. [DOI] [PubMed]
- 7.Essex AL, Pin F, Huot JR, et al. Bisphosphonate Treatment Ameliorates Chemotherapy-Induced Bone and Muscle Abnormalities in Young Mice. Front Endocrinol (Lausanne) 2019;10: 809. [DOI] [PMC free article] [PubMed]
- 8.Huang CF, Shiao MS,Mao TY. Retrospective Study of the Effects of Zoledronic Acid on Muscle Mass in Osteoporosis Patients. Drug Des Devel Ther 2021; 15: 3711–3715. [DOI] [PMC free article] [PubMed]
- 9.Haeri NS, Perera S, Greenspan SL. Does Zoledronic Acid Improve Appendicular Lean Mass in Older Women with Osteoporosis? A Sub-Analysis of a Randomized Clinical Trial. J Frailty Aging 2022; 11: 420–425. [DOI] [PMC free article] [PubMed]
- 10.Potsch P MS, Tschirner A, Palus S, et al., et al. The anabolic catabolic transforming agent (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J Cachexia Sarcopenia Muscle. 2014;5:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fellner C, Schick F, Kob R, et al. Diet-induced and age-related changes in the quadriceps muscle: MRI and MRS in a rat model of Sarcopenia. Gerontology. 2014;60:530–8. [DOI] [PubMed] [Google Scholar]
- 12.Chiba Y, Shimada A, Kumagai N, et al. The senescence-accelerated mouse (SAM): a higher oxidative stress and age-dependent degenerative diseases model. Neurochem Res. 2009;34:679–87. [DOI] [PubMed] [Google Scholar]
- 13.Wronski TJ and Morey-Holton ER. Skeletal response to simulated weightlessness: a comparison of suspension techniques. Aviat Space Environ Med, 1987; 58: 63–68. [PubMed]
- 14.Romanick M, Thompson LV and Brown-Borg HM, Murine models of atrophy, cachexia, and sarcopenia in skeletal muscle.Biochim Biophys Acta, 2013;1832: 1410–1420. [DOI] [PMC free article] [PubMed]
- 15.He ZN, Nie PF, Gu J, et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Joint Res 2020; 9: 731–741. [DOI] [PMC free article] [PubMed]
- 16.Tsika RW, Herrick RE, Baldwin KM. Effect of anabolicsteroids on skeletal muscle mass during hindlimb suspension. J Appl Physiol. 1987;63:2122–7. [DOI] [PubMed] [Google Scholar]
- 17.He YH. The preliminary research of PLC—independent PKC signaling pathway in PTH peptide on bone muscle metabolism[D]. GuangZhou.Southern Medical University, 2018.
- 18.Wannenes F, Papa V, Greco EA, et al. Abdominal Fat and Sarcopenia in Women Significantly Alter Osteoblasts Homeostasis In Vitro by a WNT/ beta -Catenin Dependent Mechanism. Int J Endocrinol 2014. 2014: 278316. [DOI] [PMC free article] [PubMed]
- 19.Yang SY, Hoy M, Fuller B, et al. Pretreatment with insulin-like growth factor I protects skeletal muscle cells against oxidative damage via PI3K/Akt and ERK1/2 MAPK pathways. Lab Invest 2010; 90: 391–401. [DOI] [PubMed]
- 20.Fujimaki T, Ando T, Hata T, et al. Exogenous parathyroid hormone attenuates ovariectomy-induced skeletal muscle weakness in vivo. Bone. 2021;151:116029. [DOI] [PubMed] [Google Scholar]
- 21.Han JS, Choi SY, Choi RY, et al. Anti-muscle atrophy effect of fermented Tenebrio molitor larvae extract by modulating the PI3K-Akt-mTOR/FoxO3α pathway in mice treated with dexamethasone.Biomedicine & Pharmacotherapy, 2024; 178: 1–11. [DOI] [PubMed]
- 22.Deng Zb, Song C, Chen L, et al. Inhibition of CILP2 Improves Glucose Metabolism and Mitochondrial Dysfunction in Sarcopenia via the Wnt Signalling Pathway. J Cachexia Sarcopenia Muscle, 2024. [DOI] [PubMed]
- 23.Miedany YE, Gaafary ME, Toth M, et al. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol 2021;40: 4225–4232. [DOI] [PubMed]
- 24.Simon MH, JMT, Stephen JT, et al. Selective accumulation of MyoD and Myogenin mRNAs in fast and slowadult skeletal muscle is controlled by innervation and hormones. Development 1993; 118: 1137–1147. [DOI] [PubMed]
- 25.Simon MH, KK, Michael R, et al. MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mechanisms of Development 1997; 61: 151–163. [DOI] [PubMed]
- 26.Sophie van Doorslaer de Ten Ryen, Warnier G, Gnimassou O, et al. Higher strength gain after hypoxic vs normoxic resistance training despite no changes in muscle thickness and fractional protein synthetic rate. FASEB J 2021; 35: e21773. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the principal investigator upon reasonable request dbblrmyy@163.com.