Abstract
Sarcopenia is a prevalent condition in patients with chronic kidney disease (CKD), intricately linked to adverse prognoses, heightened cardiovascular risks, and increased mortality rates. Extensive studies have found a close and complex association between gut microbiota, kidney and muscle. On one front, patients with CKD manifest disturbances in gut microbiota and alterations in serum metabolites. These abnormal microbiota composition and metabolites in turn participate in the development of CKD. On another front, altered gut microbiota and its metabolites may lead to significant changes in metabolic homeostasis and inflammation, ultimately contributing to the onset of sarcopenia. The disturbance of gut microbial homeostasis, coupled with the accumulation of toxic metabolites, exerts deleterious effects on skeletal muscles in CKD patients with sarcopenia. This review meticulously describes the alterations observed in gut microbiota and its serum metabolites in CKD and sarcopenia patients, providing a comprehensive overview of pertinent studies. By delving into the intricate interplay of gut microbiota and serum metabolites in CKD-associated sarcopenia, we aim to unveil novel treatment strategies for ameliorating their symptoms and prognosis.
Keywords: Chronic kidney disease, Sarcopenia, Gut microbiota, Metabolomics
Introduction
Chronic kidney disease (CKD) is a condition associated with various metabolic disorders that can affect energy, protein, and muscle metabolism, and is considered a major risk factor for sarcopenia [1]. Meanwhile, with the increasing global prevalence of CKD, sarcopenia, as a prevalent condition in CKD, is also receiving increasing attention. According to the diagnostic criteria of the European Working Group on Sarcopenia in Older People (EWGSOP) 2018, sarcopenia is characterized by low muscle mass, diminished muscle strength, and poor physical function [2]. A relevant study has indicated that the prevalence of sarcopenia in CKD patients ranges from 5–62.5% [3]. This variability of prevalence is mainly related to various factors such as the differences in study population (age, gender, race, stage of CKD), diagnostic criteria (such as those from EWGSOP and Asian Working Group for Sarcopenia), and methods used to assess muscle mass (such as dual energy X-ray absorptiometry, bioimpedance analysis and so on). In individuals with CKD, sarcopenia is frequently associated with poor physical activity performance, elevated risk of disability, increased incidence of cardiovascular events, and higher mortality [4–6]. Our previous research has found that low skeletal muscle density at the first lumbar vertebral level assessed by computed tomography, as an independent risk factor for both cardiac and all-cause death in CKD patients undergoing initial-dialysis [7]. Additionally, diaphragm dysfunction assessed by ultrasound has been shown to predict clinical outcomes in CKD patients receiving hemodialysis (HD) [8, 9]. Therefore, it is essential to investigate the factors contributing to the high prevalence of sarcopenia in CKD patients and to explore effective interventions.
Currently, our research along with findings from other teams suggest that the presence of hormonal imbalances, chronic inflammation, uremic toxin accumulation, metabolic acidosis, and related mechanical factors in CKD patients collectively contribute to a decrease in muscle synthesis and an increase in catabolism. This results in an unfavorable protein homeostasis balance, ultimately leading to the development of sarcopenia in patients [1, 10–12]. Notably, recent studies have also identified the significant role of gut microbiota disturbances in the development and progression of CKD with sarcopenia [13–15].
Altered gut microbiota has been observed in CKD patients experiencing sarcopenia [14]. Furthermore, there is evidence of the accumulation of gut microbiota-associated uremic toxins in the mice model of CKD with sarcopenia [16]. We hypothesized that gut microbiota could significantly contribute to the pathogenesis of sarcopenia in CKD. This contribution may occur through the destruction of gut barrier, accumulation of toxic metabolites, reduction of short-chain fatty acid (SCFA) and the induction of systemic low-grade inflammation [15, 17]. However, the underlying molecular mechanisms and signaling pathways remain inadequately understood. Therefore, further in-depth search and exploration of the changes and related mechanisms of gut microbiota and its serum metabolites in CKD patients with sarcopenia are needed, which in turn will provide new interventions for the prevention or amelioration of sarcopenia in CKD within a clinical setting.
In this review, we provide an overview of the current status of research landscape regarding gut microbiota disturbances in CKD patients, as well as a thorough examination of the alterations in gut microbiota and its metabolites associated with sarcopenia. Subsequently, we analyse the potential correlations among the gut microbiota, its serum metabolites and CKD with sarcopenia, proposing that the regulation of gut microbiota and its metabolites could emerge as a promising novel strategy for preventing or ameliorating sarcopenia in CKD.
Interaction between CKD and gut microbiota disturbances
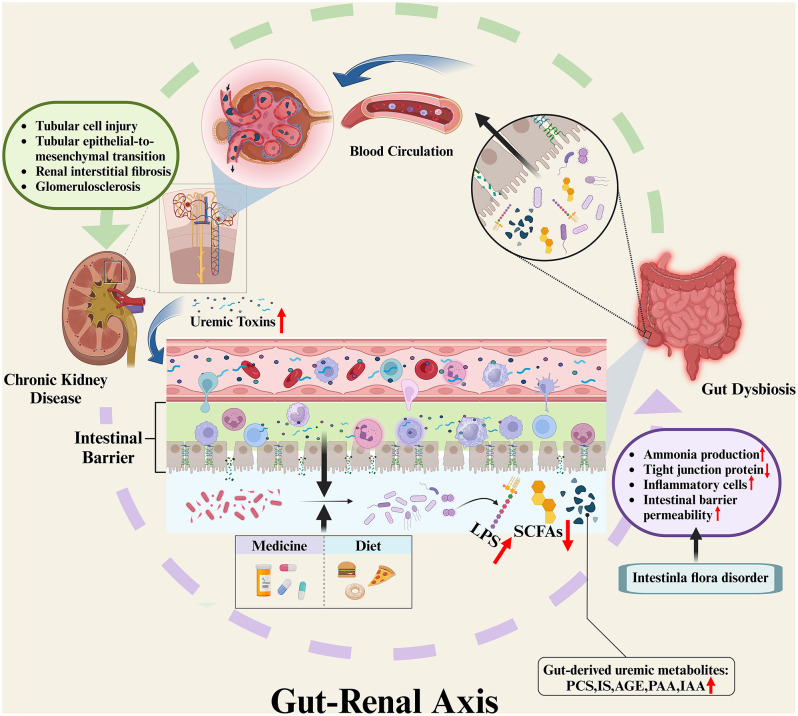
It has long been observed that there is a complex interaction between the gut microbiota and the kidney, termed the gut-renal axis (Fig. 1). As CKD progresses, there are discernible changes in both the quantity and quality of gut microbiota changes, which are accompanied by alterations to the gut barrier, and an increase in gut permeability [18–20]. Primarily, shifts in the internal environment of CKD patients contribute to alterations in the gut microbiota. Increased levels of circulating urea in patients with progressive renal failure promotes the colonization and proliferation of urea-utilizing microbiota. Concurrently, the expansion of uricase-containing microbiota may result from increased colonic secretion of uric acid and oxalic acid, leading to gut microbiota disturbances [18, 21]. In addition to the above, reduced dietary fiber intake, increased constipation, diminished protein absorption, and frequent use of antibiotics and iron therapy are also involved in the development of gut microbiota disturbances in CKD patients [22]. Subsequently, changes in the gut microbiota give rise to heightened ammonia production in the intestinal lumen, and cause changes in intestinal pH [18, 22], thereby increasing gut permeability by modifying the tight junctions of intestinal epithelial cells [19]. Histological studies of the colon in CKD patients have revealed significant reductions in mucosal tight junction proteins, infiltration of inflammatory cells such as monocytes in the lamina propria, and thickening of the colon wall [20].
Fig. 1.
Interaction between CKD and gut microbiota disturbance (Gut-Renal Axis). Increased levels of uremic toxins in the circulation of CKD patients lead to dysbiosis of the gut microbiota, while dietary intake and medication use of CKD patients are also involved in the process of dysbiosis. This leads to increased ammonia production in the intestinal lumen, which in turn leads to a decrease in tight junction proteins in the intestinal mucosa, infiltration of inflammatory cells in the lamina propria, and ultimately an increase in the permeability of the intestinal barrier. In turn, intestinal flora disorders also lead to accumulation of bacterial products including LPS and reduction in SCFAs levels, which are also involved in the process of impairment of intestinal mucosal barrier function. This makes it easier for gut-derived uremic metabolites, such as PCS, IS, AGEs, PAA, IAA, which accumulate in the intestinal lumen, to enter the systemic circulation. These uremic toxins reach the kidneys through the bloodstream, activate the corresponding pathways, and induce an inflammatory response that further causes tubular cell injury, tubular epithelial-to-mesenchymal transition, and renal interstitial fibrosis and glomerulosclerosis, thereby promoting the progression of CKD. LPS, lipopolysaccharides; SCFAs, short-chain fatty acids; PCS, p-cresyl sulfate; IS, indoxyl sulfate; AGEs, advanced glycation end products; PAA, phenylacetic acid; IAA, indole-3-acetic acid. Image drawn with BioRender.com
Gut microbiome dysbiosis
Gut microbiota
In the gut microbiota of patients with CKD, there is an increase in the levels of Proteobacteria, Bacteroidetes, Clostridium, Enterobacteriaceae, Citrobacter and Coprobacillus, while the abundance of Lactobacillaceae, Lachnospiraceae, Ruminococcus, Faecalibacterium and Prevotella spp are lower [18, 23–31], as illustrated in Table 1. Concurrently, there is a shift towards production of gut-derived uremic metabolites which is associated with the changes to the gut microbiota [32–34].
Table 1.
Clinical studies of changes in gut microbiota in CKD patients
| Study | Age | Sample size | Research object | Changes of microbes in CKD population |
|---|---|---|---|---|
| Vaziri ND et al. 2013 [18] | 39–71 y | 36 | HD | Lower level of Lactobacillaceae and Prevotellaceae |
| Ye et al. 2018 [24] | 30–75 y | 153 | HD |
Lower level of Firmicutes, Lachnospiraceae, Ruminococcaceae and Faecalibacterium ; Higher level of Bacteroidetes, Proteobacteria, Clostridiales, and Enterobacteriaceae |
| Wang et al. 2020 [25] | >18 y | 292 | HD |
Lower level of Prevotella spp (mainly P. copri), Clostridium spp and several butyrate producers (Roseburia spp, Faecalibacterium prausnitzii and Eubacterium rectale); Higher level of Eggerthella lenta, Flavonifractor spp (mainly F. plautii), Alistipes spp (mainly A. finegoldii and A. shahii) and Fusobacterium spp |
| Zhang et al. 2023 [31] | 37–67 y | 668 | HD |
Lower level of Prevotella and Roseburia ; Higher level of Blautia spp., Dorea spp., and Eggerthellaceae |
| Wang et al. 2019 [28] | 30–66 y | 191 | CKD 1–5 |
Lower level of Prevotella; Higher level of Bacteroides |
| Margiotta et al. 2020 [26] | ≥ 65 y | 79 | CKD 3b-4 |
Lower level of saccharolytic and butyrate-producing bacteria (Prevotella spp., Faecalibacterium prausnitzii, Roseburia spp.); Higher level of Citrobacter, Coprobacillus |
| Sato et al. 2021 [30] | 20–90 y | 111 | CKD 1-3a |
Lower level of butyrate-producing species Roseburia inulinivorans, Ruminococcus torques and Ruminococcus lactaris ; Higher level of Bacteroides caccae and Bacteroides coprocora |
| Wang et al. 2023 [27] | >18 y | 88 | CKD 1–5 |
Lower level of SCFA-producing bacteria (R. bromii, R. callidus, R. hominis, E. rectale, F. prausnitzii, C. comes, C. eutactus, C. sporogenes, S. variabile, D. succinatiphilus, B. adolescentis, L. crispatus, A. indistinctus, and A. inops); Higher level of C. freundii, C. werkmanii, F. plautii, and A. caccae. |
| Peters et al. 2023 [29] | 44–71 y | 2438 | CKD 1–5 |
Lower level of eight species from genus Prevotella, and many species from class Clostridia within genera Eubacterium, Clostridium, Roseburia, and Ruminococcus ; Higher level of Erysipelotrichia, Clostridia, Coriobacteriia, and Fusobacteriia |
Gut-derived metabolites
Uremic metabolites have been categorized into small water-soluble molecules, protein-bound uremic toxins (PBUTs) and middle molecules based on their solubility and molecular weight. Small water-soluble molecules produced by gut microbiota, include asymmetric dimethylarginine (ADMA), which is associated with Streptomyces coelicolor, Mycobacterium tuberculosis and Pseudomonas aeruginosa, trimethylamine-N-oxide (TMAO), via Firmicutes and Proteobacteria, and urea, which is associated with E. coli are elevated in the blood of patients with CKD [35–38]. PBUTs such as p-cresyl sulfate (PCS), indoxyl sulfate (IS), indole acetic acid, kynurenines and phenylacetic acid are difficult to remove by dialysis and accumulate in CKD patients, thereby leading to systemic oxidative stress and inflammation [39–43]. In addition, advanced glycation end products (AGEs) accumulate in CKD patients and play a crucial role in CKD-associated complications (e.g. atherosclerotic cardiovascular disease, left ventricular hypertrophy, heart failure and anemia) [44]. AGEs contribute to insulin resistance and endothelial dysfunction in patients with diabetes or CKD by promoting inflammation and oxidative stress [45]. However, it is unclear whether AGEs-associated gut microbiota is altered in patients with CKD [44]. Currently, there is no evidence to support a direct link between the production or metabolism of middle molecules (including β2-microglobulin, ghrelin and parathyroid hormone, and so on) by gut microbiota. The available studies currently suggest that gut microbiota involvement is partially required for the physiological effects of most middle molecules [46].
Along with changes in gut-derived uremic metabolites, alterations in the gut microbiota of CKD patients also lead to a reduced secretion of other metabolites, such as short-chain fatty acids (SCFAs). Relevant studies have identified a reduction in SCFA-producing gut microbiota, including Faecalibacterium, Eubacterium, Roseburia, and Anaerostipes from the phylum Firmicutes and Lactobacillus in CKD patients, with a consequent reduction in serum SCFA levels [28, 46, 47]. As SCFA levels decrease and the pH in the intestinal lumen rises, pathogenic bacteria such as Enterobacteriaceae further grows [48], leading to an increase in related uremic toxin levels.
CKD progression
The relationship between gut microbiota disturbances and CKD is bidirectional. In CKD patients, the reduction in beneficial gut commensal microbiota and the proliferation of harmful gut microbiota leads to elevated levels of uremic toxins, the release of pro-inflammatory factors, a disruption of intestinal barrier, and abnormalities in the immune system [49]. These factors collectively exacerbate the progression of CKD.
Uremic toxins accumulation
The most studied gut microbiota-derived uremic toxins are PCS and IS. p-Cresol is a metabolite of tyrosine, and indole is produced by the metabolism of tryptophan. Both are absorbed through the gut and enter the liver for further metabolism to form PCS and IS, respectively, which are generally cleared and excreted by the kidneys [50]. Elevated levels of PCS and IS in the serum of CKD patients contributes to increased expression of deoxyribonucleic acid (DNA) methyltransferase [51], enhanced NADPH oxidase activity and reactive oxygen species (ROS) production [52]. Furthermore, they up-regulate the expression of macrophage chemotactic protein-1 (MCP-1) and intercellular adhesion molecule-1 (ICAM-1) in the kidney [53, 54], activate the renin-angiotensin-aldosterone system/transforming growth factor-β (TGF-β) pathway [55], and induce inflammatory responses. These responses subsequently lead to tubular cell injury, tubular epithelial-to-mesenchymal transition, renal interstitial fibrosis and glomerulosclerosis, which collectively promote CKD progression [56]. Moreover, researche has demonstrated that in the state of impaired kidney function, gut-derived uremic metabolites such as AGEs, phenylacetic acid (PAA), and indole-3-acetic acid (IAA) systemically accumulate in the body, which further contributes to inflammation and the development of CKD [57].
Disruption of intestinal barrier
Simultaneously, the impairment of gut barrier caused by gut dysbiosis is also implicated in the progression of CKD. The overgrowth of harmful gut microbiota leads to the accumulation of endotoxins, such as lipopolysaccharides (LPS). For example, LPS activates macrophages by binding to Toll-like receptor 4 (TLR4), which leads to the release of pro-inflammatory cytokines such as IL-6 and TNF-α from macrophages and promotes the expression of soluble TNF receptors, thereby contributing to altered membrane permeability [46]. Meanwhile, the levels of butyrate-producing gut microbiota decrease in CKD patients. Butyrate, a type of SCFAs, is known for its ability in promoting the production of mucoproteins and tight junction proteins, as well as its anti-inflammatory and antioxidant effects. The decrease in butyrate levels in CKD patients exacerbates inflammation, and disrupts the gut barrier, making it easier for gut-derived uremic metabolites to enter the circulation and worsen CKD [58, 59].
Gut microbiota disturbances not only accelerate the progression of CKD, but also significantly impact cardiovascular disease, cognitive abnormalities, skeletal mineral-metabolism abnormalities, and other complications [37, 38, 60–64]. In summary, current studies have identified the presence of disturbed gut microbiota in CKD patients, leading to corresponding changes in their related metabolites. Simultaneously, disturbed gut microbiota and its metabolites may also play an important role in the progression of CKD as well as in other comorbidities, underscoring the significance of the gut-kidney interaction.
Gut microbiota in patients with Sarcopenia: changes and influences
The gut microbiota may have detrimental effects on muscle mass and function by inducing anabolic imbalances, insulin resistance, promoting chronic systemic inflammation [65, 66]. This connection between gut microbiota and muscle has been termed the “gut-muscle axis”, and this hypothesis has been well validated in non-CKD animal models. Interventions targeting the “gut-muscle axis” through modulation of gut microbiota can partially reverse skeletal muscle injury and enhance muscle mass and function [67, 68]. However, studies exploring the ‘gut-muscle axis’ in humans remain limited, with only a few studies having reported associations between altered gut microbiota with sarcopenia in non-CKD patients [65, 69–73](Table 2). There were two animal studies, four clinical studies, and one study that investigated both human populations and mice. Among the animal studies, two involved microbiota transplantation, and the changes in the gut microbiota of the sarcopenic mice model were characterized by an increase in the abundance of Bacteroidaceae and Fusobacteriaceae [16, 73]. Additionally, one animal study reported a decrease in the abundance of Lactobacillus in the sarcopenic model group [16]. These five clinical observational studies included a total of 1586 human subjects, all of these subjects were elderly and older than 53 years of age. In the sarcopenia population, similar to the findings in animal studies, an increase in the abundance of Bacteroidaceae and Fusobacteriaceae was observed [69–71]. One of the studies noted a decrease in the abundance of Prevotellaceae [72], indicating that the role of gut microbiota in the development of muscle loss is a crucial area of research. However, due to the limitations of observational studies, along with the differences in race, geographic factors, and dietary conditions among the populations included, no definitive causal relationship between gut microbiota disturbances and sarcopenia has been established.
Table 2.
Changes in the composition of gut microbiota associated with sarcopenia
| Study | Research object | Age | Sample size | Changes of microbes in sarcopenia group |
|---|---|---|---|---|
| Wang et al. 2022 [70] | human | 53–81 y | 1417 | Higher level of Desulfovibrio piger, Clostridium symbiosum, Hungatella effluvii, Bacteroides fluxus, Absiella innocuum, Coprobacter secundus and Clostridium citroniae |
| Han et al. 2022 [71] | human | 65–78 y | 88 |
Lower level of Ruminococcacae, Prevotellaceae, and Akkermansiaceae ; Higher level of Bacteroidaceae and Fusobacteriaceae |
| Ticinesi et al. 2020 [65] | human | 70–86 y | 17 | Lower level of Faecalibacterium prausnitzii, Roseburia inulinivorans and Alistipes shahii |
| Picca et al. 2019 [69] | human | >70 y | 35 |
Lower level of Barnesiellaceae and Christensenellaceae ; Higher level of Oscillospira and Ruminococcus |
| Fielding et al. 2019 [72] | human | 70–82 y | 29 | Lower level of family-level Prevotellaceae, genus-level Prevotella and Barnesiella, and species-level Barnesiella intestinihominis |
| microflora colonized mice | - | 36 | ||
| Uchiyama et al. 2020 [16] | mice | 13 week | 20 |
Lower level of Lactobacillus, Lactonifactor and Tannerrella ; Higher level of Allobaculum, Clostridium cluster IV and Alistipes |
| Lee et al. 2023 [73] | mice | 9 week | 45 | Higher level of Alistipes, Lachnospiraceae, and Bacteroides. |
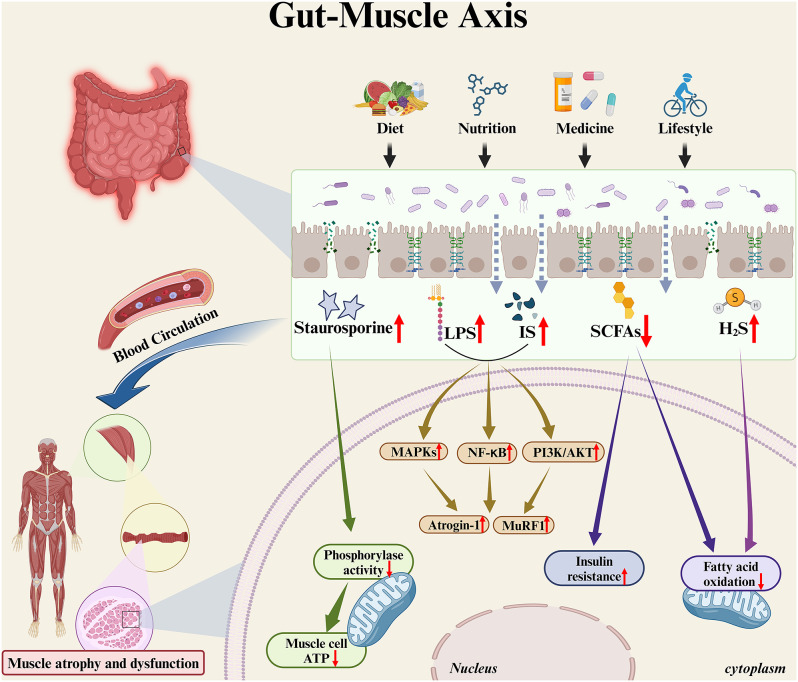
Since the composition of gut microbiota is strongly influenced by external factors such as diet, nutrition, medication and lifestyle, which are key contributors to the development of sarcopenia, it is hypothesized that the gut microbiota acts as a mediator of these external factors, thereby affecting muscle mass and function in individuals with sarcopenia [66, 74] (Fig. 2).
Fig. 2.
Influence of gut microbiota and its metabolites on muscle mass and function. In patients with sarcopenia, diet, nutrition, medication and lifestyle profoundly affect the composition of the gut microbiota. Along with their disturbed gut microbiota and impaired intestinal barrier function, bacterial products from the intestinal lumen are more likely to enter the systemic circulation and affect the overall muscle mass and function through the bloodstream. Staurosporine is a broadly specific kinase inhibitor that reduces muscle cell mitochondrial phosphorylase activity and ATP production. While both IS and LPS can upregulate the expression of muscle atrophy-related genes such as Atrogin-1 and MuRF1 by activating the PI3K/AKT, NF-κB and MAPKs signaling pathways. Reduction of SCFAs may promote insulin resistance in muscle cells and, together with the accumulation of hydrogen sulfide, reduces mitochondrial fatty acid oxidation, which further contributes to the increase of intramuscular fatty acid deposition. The above changes in gut microbiota metabolites ultimately lead to a decline in muscle mass and function through various pathways. ATP, adenosine triphosphate; IS, indoxyl sulfate; LPS, lipopolysaccharides; PI3K/AKT, phosphoinositide 3-kinase /protein kinase B; NF-κB, nuclear factor-κB; MAPKs, mitogen-activated protein kinase; SCFAs, short-chain fatty acids; H2S, hydrogen sulfide. Image drawn with BioRender.com
First, dysfunction in gut mucosal barrier may play a central role. Studies conducted on aged mice indicates that dysbiotic gut microbiota is associated with impaired gut barrier function [75]. This impairment facilitates entry of bacterial products into the systemic circulation, thereby activating inflammatory responses and inducing dysregulation of the immune system, ultimately affecting muscle mass and strength [76, 77].
Second, metabolites produced by gut microbiota are unequivocally associated with sarcopenia. A metagenomic study based on a rural Chinese population identified six gut microbiota species that were positively correlated with the severity of sarcopenia. Among them, Desulfovibrio piger is one of the most abundant and prevalent Desulfovibrio bacteria in the human digestive tract [70]. This sulphate-reducing bacterium has the capability to reduce dietary sulfites and sulfates, leading to the sulfation of mucopolysaccharides in mucins and the subsequent generation of hydrogen sulfide [78]. Hydrogen sulfide may be involved in the pathogenesis of sarcopenia by inhibiting fatty acid oxidation, thereby negatively impacting muscle energy utilization and decreased muscle endurance, and on the other hand, damaging the gut epithelium, causing systemic and chronic inflammation [79, 80]. Additionally, besides the production of hydrogen sulfide, D. piger is also associated with the biosynthesis of staurosporine [70], a broadly specific kinase inhibitor and a commonly-used stimulator of apoptosis that can reduce adenosine triphosphate (ATP) production in C2C12 mouse muscle myoblasts, thereby leading to muscle atrophy and dysfunction [81]. Furthermore, IS and LPS both trigger muscle atrophy and systemic inflammatory responses by upregulating the expression of muscle atrophy-related genes through activation of the phosphoinositide 3-kinase (PI3K) /Akt, nuclear factor kappa-B (NF-κB), and mitogen-activated protein kinases (MAPKs) signaling pathways in a CKD mouse model [82]. Alternatively, study has shown that in a mice model fed a high-fat diet, SCFAs can increase muscle fatty acid oxidation via adenosine monophosphate activated protein kinase (AMPK) and peroxisome proliferator-activated receptor-δ (PPARδ) signaling pathways. In several in vitro studies using rodent and human intestinal cell lines, SCFAs may also improve muscle insulin sensitivity by increasing the levels of the gut-derived peptides tyrosine tyrosine (PYY) and glucagon-like peptide-1 (GLP-1) [83]. The reduction in SCFA production may promote insulin resistance, diminish mitochondrial fatty acid oxidation, increase intramuscular fatty acid deposition, ultimately leading to a decline in muscle mass and function [65, 66].
In general, alterations in gut microbiota and its metabolites can induce notable changes in the anabolic-catabolic balance and inflammation, which are involved in the pathogenesis of sarcopenia. However, since both gut microbiota and muscle metabolism are influenced by diet, exercise, age, and various other relevant factors, the hypothesis of “gut-muscle axis” cannot be fully confirmed in the broader population. Substantial advancements for mechanistic research and comprehensive clinical studies need to be performed.
Renal-gut-muscle axis in CKD patients with Sarcopenia
For individuals suffering from CKD and sarcopenia, the concept of “renal-gut-muscle axis” has been proposed, which means that kidney and gut microbiota play roles in the maintenance of skeletal muscle mass, composition, and body functions. Furthermore, it suggests that disruptions in gut microbiota-derived uremic metabolites negatively affect the skeletal muscles of CKD patients [15].
Relevant animal or clinical studies
Research revealed that adenine-induced CKD-mice exhibited significantly diminished grip strength, running distance, skeletal muscle and muscle fiber size, and a reduced number of mitochondrial-rich muscle fibers compared to control mice [16]. The gut microbiota of CKD mice had reduced Lactobacillus and increased Clostridales and Erysipelotrichales. Notably, transplantation of fecal bacteria from this group of mice into healthy, germ-free mice resulted in muscle depletion similar to symptoms observed in CKD mice. At the same time, a substantial accumulation of gut-derived uremic metabolites was noted in both CKD mice and those receiving the fecal transplant. In particular, the serum concentrations of IS, hippuric acid (HA), phenyl sulfate (PhS), and indoles and phenol in feces were significantly elevated in both types of mice. This study suggests that in CKD mice, gut microbiota disturbances lead to impaired gut barrier function and the onset of a systemic inflammatory state, as well as heightened levels of associated bacterial metabolites. These factors are partly involved in the progression of sarcopenia [16].
A clinical study involving elderly non-dialysis CKD patients (with an eGFR between 10 and 45 mL/min/1.73 m2) demonstrated that individuals with sarcopenia exhibited a higher abundance of Micrococcaceae and Verrucomicrobiaceae families, and Megasphaera, Rothia, Veillonella, Akkermansia and Coprobacillus genera. Conversely, they had a lower abundance of the Gemellaceae and Veillonellaceae families, along with Acidaminococcus and Gemella genera. Although significant differences in the gut microbiota between sarcopenia and non-sarcopenia patients were identified, the study did not identify statistically significant differences for uremic toxins (indoxyl sulphate and p-cresyl sulphate) and inflammatory cytokines (tumor necrosis factor-α, interleukin-6, interleukin-17, interleukin-12 p70, monocyte chemoattractant protein-1 and fetuin-A), except for interleukin-10, which was found at higher levels in non-sarcopenia patients. However, further analysis failed to establish a specific association between interleukin-10 and the gut microbiota [14]. This cross-sectional study highlighted associations between CKD patients with sarcopenia and gut microbiota disturbances, but more pertinent clinical trials are required to validate their correlation, especially given that this cohort only included elderly non-dialysis CKD patients.
Based on these findings, it is reasonable to assume that the existence of “renal-gut-muscle axis” in CKD patients with sarcopenia. However, further experimental and theoretical studies are imperative to substantiate this assumption.
Mechanism of renal-gut-muscle axis
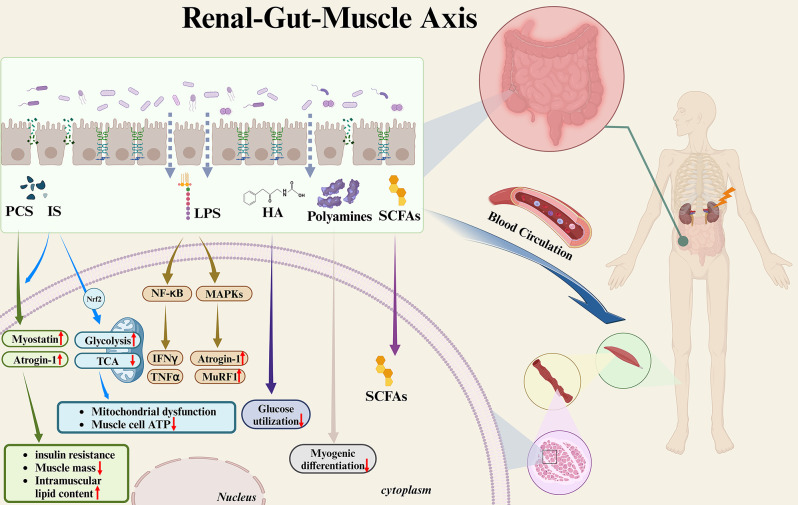
Building upon the available insights, this article delves into the impact of gut microbiota and its derived serum metabolites on skeletal muscle in CKD patients (Fig. 3).
Fig. 3.
Effects of gut microbiota-related metabolites on skeletal muscle in CKD patients. In patients with CKD, changes in the gut microbiota lead to heightened intestinal permeability. This allows gut-derived bacterial metabolites to enter the bloodstream, thereby inducing deterioration of skeletal muscle mass and function. IS and PCS accumulate in skeletal muscle, inducing the expression of the muscle atrophy markers myostatin and atrogin-1, thus leading to a reduction in muscle mass, insulin resistance, and increased intramuscular lipid content. In addition, IS can lead to increased glycolysis via Nrf2 on the one hand, and to downregulation of the TCA cycle on the other hand, thereby inducing mitochondrial dysfunction and ATP shortage in muscle cells. LPS can lead to muscle atrophy mediated by NF-κB through IFNγ/TNFα induction. LPS can also cause muscle atrophy by up-regulating atrogin-1 and MuRF1 via MAPKs. HA may contribute to the decline in muscle function by inhibiting glucose utilization in muscle cells. Metabolites of polyamines may inhibit myogenic differentiation and cause muscle atrophy. SCFAs may have a negative impact on muscle due to their reduced levels. IS, indoxyl sulfate; PCS, p-cresyl sulfate; Nrf2, nuclear factor (erythroid-2-related factor)-2; TCA, tricarboxylic acid; ATP, adenosine triphosphate; LPS, lipopolysaccharides; NF-κB, nuclear factor-κB; MAPKs, mitogen-activated protein kinase; HA, hippuric acid; SCFAs, short-chain fatty acids. Image drawn with BioRender.com
First, the altered composition and function of the gut microbiota in CKD patients leads to an increased level of urease-expressing Enterobacteriaceae, resulting in heightened permeability of intestinal epithelial cells [21, 84]. Second, alterations in gut microbiota-associated metabolites, including IS, PCS, and others, further contribute to the deterioration of skeletal muscle mass and function [56].
IS and PCS
Originating from the Enterobacteriaceae family, IS and PCS experience an upswing in the intestinal lumen of CKD patients and can enter the bloodstream due to the compromised intestinal barrier, thereby exerting a detrimental impact on skeletal muscle [56]. IS and PCS can accumulate in skeletal muscle, thereby triggering the expression of myostatin and atrogin-1, which are markers of muscle atrophy. This leads to a decrease in muscle mass, induces insulin resistance in muscle cells, and augments intramuscular lipid content [85–87]. In addition, comprehensive metabolomics revealed that IS is involved in the pathogenesis of sarcopenia by enhancing glycolysis via nuclear factor (erythroid-2-related factor)-2 on the one hand, and suppressing the tricarboxylic acid (TCA) cycle on the other, which collectively causes mitochondrial dysfunction and ATP shortage [85].
Other gut-derived bacterial metabolites
Various gut microbiota metabolites that have been linked to muscle function and physical performance in adults, such as HA, polyamines, LPS, SCFA, and others. However, the relationship between these metabolites and skeletal muscle in CKD patients remains controversial and warrants confirmation in future studies. (a) HA, originating from the degradation of aromatic compounds by intestinal bacteria, has been identified as an inhibitor of glucose utilization in muscle cells. This inhibition may contribute to muscle weakness in CKD patients [88]. (b) Alterations in the balance of polyamines, metabolites of proteins, could also contribute to the dysregulation of muscle metabolism. In CKD patients, increased degradation of spermidine and spermine to acrolein has been observed, which inhibits myogenic differentiation and causes muscle atrophy in animal models [89, 90]. (c) LPS, a metabolite of gram-negative bacteria, follows a specific signaling pathway in muscle atrophy in CKD patients, which is known as the lipopolysaccharide/toll-like receptor/ NF-κB signaling pathway. Disturbances of the gut microbiota in CKD patients causes an increase in lipopolysaccharides, further activating this pathway, leading to muscle atrophy mediated by NF-κB through interferon (IFN)γ/tumor necrosis factor (TNF)-α induction [91]. LPS can also upregulate atrogin-1 and muscle RING-finger protein-1 (MuRF1) through p38 MAPK after activating toll-like receptors, causing muscle atrophy [92]. (d) Conversely, gut microbiota-derived SCFA (including acetate, propionate, and butyrate), known for their positive effects on the renal-gut-muscle axis, are reduced in CKD subjects [28]. While animal studies also have shown a positive effect of SCFAs on young animal muscles [67, 93], its impact on muscles in patients with end-stage renal disease (ESRD) have yet to be evaluated.
In summary, there is a potential connection among the renal, muscle, and gut microbiota in CKD patients. CKD patients exhibit alterations in gut microbiota composition and function, along with diminished gut barrier function. These changes, coupled with shifts in metabolites associated with gut microbiota, further exacerbate muscle damage, ultimately fostering the development of sarcopenia, which is collectively known as the “renal-gut-muscle axis”. Consequently, research endeavors should delve into the intricate interactions among the gut, muscle and gut microbiome, as well as the associated molecular mechanisms, in order to develop innovative interventions.
Novel therapy for CKD patients with Sarcopenia: improving gut microbiota
Current therapies
Currently, clinical interventions predominantly revolve around nutritional adjustments, exercise, and medication for CKD patients with sarcopenia [1]. Nutritional intervention, constituting a fundamental therapeutic approach, emphasizes adequate energy intake. A meta-analysis revealed that oral nutritional supplements, encompassing a blend of protein, essential amino acids, and other macronutrients, can improve muscle mass in CKD patients on dialysis [94]. However, caution is advised against excessive protein intake, as it may elevate the risk of acidosis. Recommendations propose that CKD patients with a high risk of ESRD (CKD stage 4–5) should refrain from a daily protein intake exceeding 0.8 g protein/kg, while patients with a low risk of ESRD (CKD stage 3) can moderate their protein intake to 1.5 g/kg [95]. However, it’s crucial to note that the above recommendations warrant further evaluation through rigorous clinical trials.
Exercise stands out as another non-pharmacological strategy to improve the quality of life and prognosis of CKD patients with sarcopenia. In a mouse model of CKD, both resistance exercise and endurance training decreased proteolysis in skeletal muscle, which was associated with increased phosphorylation of Akt and forkhead transcription factors 1 (FoxO1) and suppressed activation of caspase-3 and ubiquitin–proteasome proteolytic system (UPS) [96]. Furthermore, a randomized controlled trial revealed that 30–45 min of aerobic and resistance exercise (alternating between treadmill, elliptical crosstrainer, Nu-Step crosstrainer, and recumbent stationary bike) three times per week could enhance muscle function, while possibly reducing oxidative stress and markers of inflammation in CKD patients by inducing the expression of antioxidant enzymes and inducing mitochondrial biogenesis [97, 98].
Finally, regarding pharmacological interventions, researchers have evaluated the efficacy of drugs such as myostatin antagonism and omega-3 polyunsaturated fatty acids (PUFAs) in addressing muscle atrophy. However, further studies are warranted to validate their efficacy and safety. Myostatin antagonism demonstrated the reversal of muscle mass loss in a mice model of CKD [99]. Nonetheless, a clinical trial in the Duchenne muscular dystrophy population was prematurely terminated due to potential adverse effects of myostatin antagonism, including epistaxis and capillary dilatation, as well as a lack of evidence of therapeutic efficacy [100]. In another clinical trial involving maintenance hemodialysis CKD patients, PUFAs supplementation administered to the test group for 12 weeks reduced proteolysis in their forearm muscles [101], suggesting its feasibility for further investigation in CKD patients with sarcopenia. Besides the aforementioned therapeutic approaches, regulating the gut microbiota and its metabolites emerges as a promising therapy for CKD patients with sarcopenia.
Emerging therapies
Current approaches to improving the disturbances of gut microbiota and its metabolites in CKD patients with sarcopenia primarily involve dietary intervention, prebiotic and probiotic therapy. These strategies aim to decrease uremic toxins, mitigate oxidative stress, and attenuate systemic inflammatory responses. In addition, fecal microbiota transplantation (FMT) and adsorption therapy have also demonstrated positive outcomes in both clinical and animal studies (Table 3) [68, 93, 102–109].
Table 3.
Clinical and animal studies related to the improvement of gut microbiota and its metabolites
| Study | Study design | Research object | Sample size | Intervention | Main result |
|---|---|---|---|---|---|
| Cancello et al. 2019 [102] | Longitudinal intervention study | Human, elderly obese women | 20 | Hypocaloric Mediterranean diet |
Akkermansia and Parabacteroides as well as of SCFAs producers were improved; Lowered the abundance of Collinsella, typically associated with obesity. |
| Buigues et al. 2016 [103] | RCT | Human, people aged 65 and older | 60 | Prebiotic (composed of a mixture of inulin plus fructooligosaccharides) | The overall rate of frailty was not significantly modified, but exhaustion and handgrip strength were significantly improved. |
| Huang et al. 2019 [104] | RCT | Human, healthy people aged 20–30 y | 54 | Probiotics (Lactobacillus plantarum TWK10) |
Body fat significantly decreased; Muscle mass and endurance performance significantly increased. |
| Yamamoto et al. 2015 [105] | Longitudinal observational study | Human, HD | 20 | AST-120 | IS, PCS and phenyl sulfate continued to decrease. |
| Okamoto et al. 2019 [106] | - | Animal, young, healthy mice | - | High-fiber diet |
Fecal SCFAs were increased; Muscle mass and treadmill endurance capacity were increased. |
| Yang et al. 2018 [107] | - | Animal, CKD mice | - | Prebiotic fiber xylooligosaccharide (XOS) |
Six out of the nine bacterial genera enriched in CKD were significantly reduced; Cecal SCFA production increased and blood PCS were decreased. |
|
Ni et al. 2019 [68] |
- | Animal, C57BL/6 mice (10 months) | 46 | Probiotics (Lacticaseibacillus casei LC122 or Bifidobacterium longum BL986) |
Gut barrier function was improved; Muscle strength and function were enhanced. |
|
Scheiman et al. 2019 [93] |
- | Animal, C57BL/6 mice | 32 | Probiotics (Veillonella atypica) | Running time of extreme treadmill and the circulating SCFAs concentration were increased. |
|
Liu et al. 2022 [108] |
- | Animal, CKD rat | 35 | FMT |
Lactobacillus johnsonii and Lactobacillus intestinalis were improved; PBUTs accumulation was reduced. |
| Nishikawa et al. 2015 [109] | - | Animal, CKD mice | 48 | AST-120 |
Plasma IS levels were significantly ameliorated; Exercise capacity and mitochondrial biogenesis of skeletal muscle were improved. |
Dietary habits
The high variability of the gut microbiota is closely related to dietary habits, as long-term dietary patterns shape the gut microbiota [110]. Consuming foods rich in dietary fiber may alter the gut microbiota to a fermentation pattern favorable to SCFA production [15]. This is achieved by modifying the carbohydrate/protein ratio and reducing the duration of amino acid fermentation. Consequently, this shifts reduces the circulating levels of gut-derived uremic metabolites, maintaining the function and integrity of the intestinal mucosa, and improves muscle mass and physical functioning in young animals [102, 106, 111]. It has also been demonstrated that dietary fiber supplementation can improve grip strength in the elderly [103]. Therefore, increasing the intake of dietary fiber, particularly soluble fiber, in the diets of CKD patients may positively impact skeletal muscle, as soluble fiber is fermented by gut microbiota into SCFAs, which further inhibit the growth of pathogenic bacteria [112].
Probiotics and prebiotics
Probiotics and prebiotics play a pivotal role in fostering the growth of beneficial flora by selectively increasing glycolytic bacteria (responsible for breaking down dietary fiber) and decreasing proteolytic bacteria in the colon, consequently reducing the production of uremic toxins [107]. Probiotics are defined by the World Health Organization as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [113]. Currently utilized probiotics include Bifidobacterium, Lactobacillus and Streptococcus. Prebiotics are non-digestible food components selectively utilized by the gut microbiota that may contribute to restoring the integrity of the colonic barrier and modulating the composition of the microbiota, with examples such as inulin, resistant starch, oligofructose, galactooligosaccharide, among others. Studies indicate that oligofructose can boost the number of Bifidobacteria in the gut, leading to an increase in muscle proportion and a decrease in fat proportion [114]. Probiotics may ameliorate muscle loss symptoms in CKD by regulating energy metabolism [68, 93, 104, 115]. However, both probiotic and prebiotic treatments have relatively limited research regarding their application in CKD patients with sarcopenia. There is substantial variability in the survival of these strains within the human body, as well as in the nature of the prebiotic species. Therefore, further research is necessary to comprehensively explore their therapeutic potential.
Fecal microbiota transplantation
FMT may play a role in improving intestinal barrier permeability and mitigating gut microbiota-associated uremic toxins by reconstituting the gut microbiota in CKD patients with sarcopenia. Analogous to organ transplantation, FMT involves the collection and processing feces from a healthy donor, subsequently transplanted into the recipient through procedures such as colonoscopy or enema. In recent years, oral methods of FMT mediated by enteric capsules have also emerged, which improves patient compliance [116]. Some investigators have transplanted fecal contents from sham-operated donors into the intestines of CKD rats disinfected with a mixture of antibiotics, and it was observed that FMT significantly reduced serum levels of uremic toxins, thereby reducing renal injury and inflammatory status [108]. However, there is currently insufficient evidence to advocate for the widespread implementation of FMT in CKD patients with sarcopenia, though FMT remains a promising therapeutic avenue.
Oral adsorbents
Oral adsorbents may exhibit efficacy in CKD patients with sarcopenia by binding to uremic toxins. The currently employed oral adsorbent, AST-120, consists of microspheres made from porous carbon material that reduce serum IS levels and the incidence of end-stage renal disease in a dose-dependent manner [117, 118]. In a CKD rat model, AST-120 improved intestinal barrier function and mitigated plasma endotoxin levels and oxidative stress [109, 119]. Furthermore, AST-120 also reduced serum levels of IS, PCS, phenyl sulfate levels and oxidative stress in CKD patients on hemodialysis [105]. Thus, applying this approach to decrease concentrations of gut microbiota-associated uremic toxins may partially ameliorate skeletal muscle atrophy in CKD patients. Further validation through pertinent animal experimental studies is required.
In conclusion, the improvement of gut microbiota and its derived serum metabolites could prove to be an effective strategy for improving symptoms and prognosis of CKD patients with sarcopenia in clinical practice. Nevertheless, interventions aimed at improving outcomes for CKD patients dealing with sarcopenia remain relatively understudied, with the involved mechanisms yet to be thoroughly elucidated.
Conclusions and perspectives
Sarcopenia is closely related to the unfavorable prognosis of CKD patients. Those with sarcopenia often face an elevated risk of death and adverse cardiovascular events. The gut microbiota is a crucial factor that influences physiological function. A complex interaction interplay exists between disturbances of gut microbiome and CKD patients with sarcopenia. The gut microbiota and its serum metabolites may significantly contribute to the pathogenesis of sarcopenia in CKD, including inducing muscle atrophy such as IS, PCS, etc., and exerting a positive effect on the renal-gut-muscle axis such as SCFA. Hence, improvement of the gut microbiota and its serum metabolites could emerge as a novel and broadly promising strategy for preventing or ameliorating sarcopenia in patients with CKD. This could involve supplementation with dietary fiber, probiotics and prebiotics, FMT, and oral adsorbents. However, the lack of large-scale clinical studies investigating changes in gut microbiota and its metabolites in CKD patients with sarcopenia, coupled with an incomplete understanding of the molecular mechanisms associated with the “renal-gut-muscle axis” and the injurious effects of certain gut-derived uremic metabolites to skeletal muscle, underscores the need for further research. In this context, additional studies are imperative to unveil the alterations and effects of gut microbiota and its serum metabolites in CKD patients with sarcopenia. These studies should aim to explore the potential benefits of interventions targeting gut microbiota and gut-derived metabolites in CKD patients with sarcopenia.
Abbreviations
- CKD
Chronic kidney disease
- HD
Hemodialysis
- SCFA
Short-chain fatty acid
- PBUTs
Protein-bound uremic toxins
- ADMA
Asymmetric dimethylarginine
- TMAO
Trimethylamine-N-oxide
- PCS
P-cresyl sulfate
- IS
Indoxyl sulfate
- AGEs
Advanced glycation end products
- DNA
Deoxyribonucleic acid
- ROS
Reactive oxygen species
- MCP-1
Macrophage chemotactic protein-1
- ICAM-1
Intercellular adhesion molecule-1
- TGF-β
Transforming growth factor-β
- PAA
Phenylacetic acid
- IAA
Indole-3-acetic acid
- LPS
Lipopolysaccharides
- ATP
Adenosine triphosphate
- PI3K
Phosphoinositide 3-kinase
- NF-κB
Nuclear factor kappa-B
- MAPKs
Mitogen-activated protein kinases
- AMPK
Adenosine monophosphate activated protein kinase
- PPARδ
Peroxisome proliferator-activated receptor-δ
- PYY
Peptide tyrosine tyrosine
- GLP-1
Glucagon-like peptide-1
- HA
Hippuric acid
- PhS
Phenyl sulfate
- TCA
Tricarboxylic acid
- IFN
Interferon
- TNF
Tumor necrosis factor
- MuRF1
Muscle RING-finger protein-1
- ESRD
End-stage renal disease
- FoxO
Forkhead transcription factors
- UPS
Ubiquitin–proteasome proteolytic system
- PUFAs
Polyunsaturated fatty acids
- FMT
Fecal microbiota transplantation
Author contributions
GZ and JC contributed to the conception of the work and drafted the manuscript. XW, WH and BW revised the manuscript. All authors contributed to the critical reading and approving the final version.
Funding
This study was funded by the Young Scholars of Yangtze River Scholar Professor Program (2024 WB); the National Natural Science Foundation of China (82100615 and 82100721); the China Postdoctoral Science Foundation (2022M710686); the Foundation of Jiangsu Commission of Health (M2021048); and the Project of Taizhou Clinical Medical School of Nanjing Medical University (TZKY20220209 and TZKY20230101).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guohao Zheng and Jingyuan Cao contributed equally to this work.
Contributor Information
Wei He, Email: bingbing_he@yeah.net.
Bin Wang, Email: wangbinhewei@126.com.
References
- 1.Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat Rev Nephrol. 2022;18:138–52. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto SM, Picard K, Klein M. Sarcopenia and sarcopenic obesity in chronic kidney disease: update on prevalence, outcomes, risk factors and nutrition treatment. Curr Opin Clin Nutr. 2022;25:371–7. [DOI] [PubMed] [Google Scholar]
- 4.Wathanavasin W, Banjongjit A, Avihingsanon Y, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, et al. Prevalence of Sarcopenia and its impact on cardiovascular events and mortality among dialysis patients: a systematic review and meta-analysis. Nutrients. 2022;14:4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro HS, Neri S, Oliveira JS, Bennett PN, Viana JL, Lima RM. Association between Sarcopenia and clinical outcomes in chronic kidney disease patients: a systematic review and meta-analysis. Clin Nutr. 2022;41:1131–40. [DOI] [PubMed] [Google Scholar]
- 6.Lai S, Muscaritoli M, Andreozzi P, Sgreccia A, De Leo S, Mazzaferro S, et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition. 2019;62:108–14. [DOI] [PubMed] [Google Scholar]
- 7.Sheng MJ, Cao JY, Hou SM, Li M, Wang Y, Fang Q, et al. Computed tomography-determined skeletal muscle density predicts 3-year mortality in initial-dialysis patients in China. J Cachexia Sarcopenia Muscle. 2023;14:2569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Yin Q, Wang SY, Wang YY, Xiao JJ, Tang TT, et al. Ultrasound-assessed diaphragm dysfunction predicts clinical outcomes in hemodialysis patients. Sci Rep-Uk. 2022;12:16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Yin Q, Wang YY, Tu Y, Han Y, Gao M, et al. Diaphragmatic dysfunction associates with dyspnoea, fatigue, and hiccup in haemodialysis patients: a cross-sectional study. Sci Rep-Uk. 2019;9:19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gungor O, Ulu S, Hasbal NB, Anker SD, Kalantar-Zadeh K. Effects of hormonal changes on Sarcopenia in chronic kidney disease: where are we now and what can we do? J Cachexia Sarcopenia Muscle. 2021;12:1380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/AKT pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17:1388–94. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Wang B, Hassounah F, Price SR, Klein J, Mohamed T, et al. The impact of senescence on muscle wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2023;14:126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Del-Campo F, Avesani CM, Stenvinkel P, Lindholm B, Cueto-Manzano AM, Cortés-Sanabria L. Gut microbiota disturbances and protein-energy wasting in chronic kidney disease: a narrative review. J Nephrol. 2023;36:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margiotta E, Caldiroli L, Callegari ML, Miragoli F, Zanoni F, Armelloni S, et al. Association of Sarcopenia and gut microbiota composition in older patients with advanced chronic kidney disease, investigation of the interactions with uremic toxins, inflammation and oxidative stress. Toxins. 2021;13:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustgarten MS. The kidney-gut-muscle axis in end-stage renal disease is similarly represented in older adults. Nutrients. 2020;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchiyama K, Wakino S, Irie J, Miyamoto J, Matsui A, Tajima T, et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol Dial Transpl. 2020;35:1501–17. [DOI] [PubMed] [Google Scholar]
- 17.Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34:1347–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–15. [DOI] [PubMed] [Google Scholar]
- 19.Yin L, Li X, Ghosh S, Xie C, Chen J, Huang H. Role of gut microbiota-derived metabolites on vascular calcification in CKD. J Cell Mol Med. 2021;25:1332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hy. 2012;21:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cigarran GS, González PE, Cases AA. Gut microbiota in chronic kidney disease. Nefrologia. 2017;37:9–19. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Ning X, Liu B, Dong R, Bai M, Sun S. Specific alterations in gut microbiota in patients with chronic kidney disease: an updated systematic review. Ren Fail. 2021;43:102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirong YE, Minjie Z, Lixin YU, Junsheng YE, Lin Y, Lisha S. Gut microbiota in renal transplant recipients, patients with chronic kidney disease and healthy subjects. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Yang S, Li S, Zhao L, Hao Y, Qin J, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. 2020;69:2131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margiotta E, Miragoli F, Callegari ML, Vettoretti S, Caldiroli L, Meneghini M, et al. Gut microbiota composition and frailty in elderly patients with chronic kidney disease. PLoS ONE. 2020;15:e228530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Ainiwaer A, Song Y, Qin L, Peng A, Bao H, et al. Perturbed gut microbiome and fecal and serum metabolomes are associated with chronic kidney disease severity. Microbiome. 2023;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Lv D, Jiang S, Jiang J, Liang M, Hou F, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci. 2019;133:1857–70. [DOI] [PubMed] [Google Scholar]
- 29.Peters BA, Qi Q, Usyk M, Daviglus ML, Cai J, Franceschini N, et al. Association of the gut microbiome with kidney function and damage in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Gut Microbes. 2023;15:2186685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato N, Kakuta M, Hasegawa T, Yamaguchi R, Uchino E, Murashita K, et al. Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol Dial Transpl. 2021;36:1675–84. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Wang X, Li S, Cao X, Zou J, Fang Y, et al. Metagenome-wide analysis uncovers gut microbial signatures and implicates taxon-specific functions in end-stage renal disease. Genome Biol. 2023;24:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22:1769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rysz J, Franczyk B, Ławiński J, Olszewski R, Ciałkowska-Rysz A, Gluba-Brzózka A. The impact of CKD on uremic toxins and gut microbiota. Toxins. 2021;13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Zhuang J, Tang P, Li J, Xiong X, Deng H. The role of the gut microbiota in coronary heart disease. Curr Atheroscler Rep. 2020;22:77. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Wang H, Guo D, Man X, Liu J, Li J, et al. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren Fail. 2021;43:1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, et al. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study). Clin Chem. 2007;53:273–83. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–25. [DOI] [PubMed] [Google Scholar]
- 39.Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-Cresyl Sulfate. Toxins. 2017;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qayed M, Michonneau D, Socié G, Waller EK. Indole derivatives, microbiome and graft versus host disease. Curr Opin Immunol. 2021;70:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelante T, Puccetti M, Giovagnoli S, Romani L. Regulation of host physiology and immunity by microbial indole-3-aldehyde. Curr Opin Immunol. 2021;70:27–32. [DOI] [PubMed] [Google Scholar]
- 43.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57:523–35. [DOI] [PubMed] [Google Scholar]
- 44.Yabuuchi J, Ueda S, Yamagishi SI, Nohara N, Nagasawa H, Wakabayashi K, et al. Association of advanced glycation end products with Sarcopenia and frailty in chronic kidney disease. Sci Rep-Uk. 2020;10:17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur J Clin Nutr. 2013;67:239–48. [DOI] [PubMed] [Google Scholar]
- 46.Bhargava S, Merckelbach E, Noels H, Vohra A, Jankowski J. Homeostasis in the gut microbiota in chronic kidney disease. Toxins. 2022;14:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung S, Barnes JL, Astroth KS. Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. Adv Nutr. 2019;10:888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorbara MT, Dubin K, Littmann ER, Moody TU, Fontana E, Seok R, et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J Exp Med. 2019;216:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transpl. 2015;30:924–33. [DOI] [PubMed] [Google Scholar]
- 50.Ondrussek-Sekac M, Navas-Carrillo D, Orenes-Piñero E. Intestinal microbiota alterations in chronic kidney disease and the influence of dietary components. Crit Rev Food Sci. 2021;61:1490–502. [DOI] [PubMed] [Google Scholar]
- 51.Sun CY, Chang SC, Wu MS. Suppression of klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83:582–92. [DOI] [PubMed] [Google Scholar]
- 53.Shimizu H, Bolati D, Higashiyama Y, Nishijima F, Shimizu K, Niwa T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Life Sci. 2012;90:525–30. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu H, Yisireyili M, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-κB and p53 in proximal tubular cells. Life Sci. 2013;92:143–8. [DOI] [PubMed] [Google Scholar]
- 55.Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE. 2012;7:e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-Cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Hou Y, Wang G, Zheng X, Hao H. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interplay. Trends Endocrin Met. 2020;31:818–34. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez A, Krieg R, Massey HD, Carl D, Ghosh S, Gehr T, et al. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol Dial Transpl. 2019;34:783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikee R, Sasaki N, Yasuda T, Fukazawa S. Chronic kidney disease, gut dysbiosis, and constipation: a burdensome triplet. Microorganisms. 2020;8:1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephro. 2009;4:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, et al. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007;71:738–43. [DOI] [PubMed] [Google Scholar]
- 62.Chiang CK, Tanaka T, Inagi R, Fujita T, Nangaku M. Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab Invest. 2011;91:1564–71. [DOI] [PubMed] [Google Scholar]
- 63.Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durgan DJ, Zubcevic J, Vijay-Kumar M, Yang T, Manandhar I, Aryal S, et al. Prospects for leveraging the microbiota as medicine for hypertension. Hypertension. 2024;81:951–63. [DOI] [PubMed] [Google Scholar]
- 65.Ticinesi A, Mancabelli L, Tagliaferri S, Nouvenne A, Milani C, Del RD, et al. The gut-muscle axis in older subjects with low muscle mass and performance: a proof of concept study exploring fecal microbiota composition and function with shotgun metagenomics sequencing. Int J Mol Sci. 2020;21:8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. 2019;11:1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11:eaan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, et al. Lactobacillus and bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res. 2019;63:e1900603. [DOI] [PubMed] [Google Scholar]
- 69.Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Junior HJ, et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the biosphere study. Nutrients. 2019;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zhang Y, Lane NE, Wu J, Yang T, Li J, et al. Population-based metagenomics analysis reveals altered gut microbiome in Sarcopenia: data from the Xiangya Sarcopenia Study. J Cachexia Sarcopenia Muscle. 2022;13:2340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han DS, Wu WK, Liu PY, Yang YT, Hsu HC, Kuo CH, et al. Differences in the gut microbiome and reduced fecal butyrate in elders with low skeletal muscle mass. Clin Nutr. 2022;41:1491–500. [DOI] [PubMed] [Google Scholar]
- 72.Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. 2019;127:110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SY, Kim JH, Lee DY, Hur SJ. Characterization of gut microbiota in mouse models of aging and sarcopenia. Microbiol Res. 2023;275:127462. [DOI] [PubMed] [Google Scholar]
- 74.Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del RD, et al. Aging gut microbiota at the cross-road between nutrition, physical frailty, and Sarcopenia: is there a gut-muscle axis? Nutrients. 2017;9:1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep-Uk. 2019;9:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buford TW, Carter CS, VanDerPol WJ, Chen D, Lefkowitz EJ, Eipers P, et al. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gibson GR. Physiology and ecology of the sulphate-reducing bacteria. J Appl Bacteriol. 1990;69:769–97. [DOI] [PubMed] [Google Scholar]
- 79.Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455–9. [DOI] [PubMed] [Google Scholar]
- 80.Bekfani T, Bekhite EM, Derlien S, Nisser J, Westermann M, Nietzsche S, et al. Skeletal muscle function, structure, and metabolism in patients with heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. Circ-Heart Fail. 2020;13:e7198. [DOI] [PubMed] [Google Scholar]
- 81.Hilder TL, Carlson GM, Haystead TA, Krebs EG, Graves LM. Caspase-3 dependent cleavage and activation of skeletal muscle phosphorylase b kinase. Mol Cell Biochem. 2005;275:233–42. [DOI] [PubMed] [Google Scholar]
- 82.Liu C, Cheung WH, Li J, Chow SK, Yu J, Wong SH, et al. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 84.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep-Uk. 2016;6:36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Enoki Y, Watanabe H, Arake R, Sugimoto R, Imafuku T, Tominaga Y, et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep-Uk. 2016;6:32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spustová V, Dzúrik R, Geryková M. Hippurate participation in the inhibition of glucose utilization in renal failure. Czech Med. 1987;10:79–89. [PubMed] [Google Scholar]
- 89.Lee NK, MacLean HE. Polyamines, androgens, and skeletal muscle hypertrophy. J Cell Physiol. 2011;226:1453–60. [DOI] [PubMed] [Google Scholar]
- 90.Chen HJ, Wang CC, Chan DC, Chiu CY, Yang RS, Liu SH. Adverse effects of acrolein, a ubiquitous environmental toxicant, on muscle regeneration and mass. J Cachexia Sarcopenia Muscle. 2019;10:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma JF, Sanchez BJ, Hall DT, Tremblay AK, Di Marco S, Gallouzi IE. STAT3 promotes IFNγ/TNFα-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. Embo Mol Med. 2017;9:622–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Passey SL, Bozinovski S, Vlahos R, Anderson GP, Hansen MJ. Serum amyloid a induces toll-like receptor 2-dependent inflammatory cytokine expression and atrophy in C2C12 skeletal muscle myotubes. PLoS ONE. 2016;11:e146882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu Y, Wang YJ, Lu Q. The effect of oral nutritional supplement on muscle fitness of patients undergoing dialysis: a systematic review and meta-analysis. J Adv Nurs. 2021;77:1716–30. [DOI] [PubMed] [Google Scholar]
- 95.Isaka Y. Optimal protein intake in pre-dialysis chronic kidney disease patients with Sarcopenia: an overview. Nutrients. 2021;13:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang XH, Du J, Klein JD, Bailey JL, Mitch WE. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009;76:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ikizler TA, Robinson-Cohen C, Ellis C, Headley S, Tuttle K, Wood RJ, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocr Metab. 2001;86:355–62. [DOI] [PubMed] [Google Scholar]
- 99.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. Faseb J. 2011;25:1653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55:458–64. [DOI] [PubMed] [Google Scholar]
- 101.Deger SM, Hung AM, Ellis CD, Booker C, Bian A, Chen G, et al. High dose omega-3 fatty acid administration and skeletal muscle protein turnover in maintenance hemodialysis patients. Clin J Am Soc Nephro. 2016;11:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cancello R, Turroni S, Rampelli S, Cattaldo S, Candela M, Cattani L, et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients. 2019;11:3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016;17:932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang WC, Lee MC, Lee CC, Ng KS, Hsu YJ, Tsai TY, et al. Effect of lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients. 2019;11:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamoto S, Kazama JJ, Omori K, Matsuo K, Takahashi Y, Kawamura K, et al. Continuous reduction of protein-bound uraemic toxins with improved oxidative stress by using the oral charcoal adsorbent AST-120 in haemodialysis patients. Sci Rep-Uk. 2015;5:14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol-Endoc M. 2019;316:E956–E966. [DOI] [PubMed] [Google Scholar]
- 107.Yang J, Li Q, Henning SM, Zhong J, Hsu M, Lee R, et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol Nutr Food Res. 2018;62:e1800014. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Zhang M, Wang X, Liu P, Wang L, Li Y, et al. Fecal microbiota transplantation restores normal fecal composition and delays malignant development of mild chronic kidney disease in rats. Front Microbiol. 2022;13:1037257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nishikawa M, Ishimori N, Takada S, Saito A, Kadoguchi T, Furihata T, et al. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transpl. 2015;30:934Y–942. [DOI] [PubMed] [Google Scholar]
- 110.Almoosawi S, Winter J, Prynne CJ, Hardy R, Stephen AM. Daily profiles of energy and nutrient intakes: are eating profiles changing over time? Eur J Clin Nutr. 2012;66:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y. Linking the microbiota, chronic disease, and the immune system. Trends Endocrin Met. 2016;27:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fielding RA, Lustgarten MS. Impact of a whole-food, high-soluble fiber diet on the gut-muscle axis in aged mice. Nutrients. 2024;16(9):1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastro Hepat. 2014;11:506–514. [DOI] [PubMed] [Google Scholar]
- 114.Collins KH, Hart DA, Smith IC, Issler AM, Reimer RA, Seerattan RA, et al. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiol Rep. 2017;5:e13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang G, Du Y, Jia JS, Yuan WJ. The influence of gut microbiota on skeletal muscle metabolism in patients with chronic kidney disease and intervention strategies. Zhonghua Nei Ke Za Zhi. 2020;59:326–328. [DOI] [PubMed] [Google Scholar]
- 116.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. Jama-J Am Med Assoc. 2017;318:1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47:565–577. [DOI] [PubMed] [Google Scholar]
- 118.Su PY, Lee YH, Kuo LN, Chen YC, Chen C, Kang YN, et al. Efficacy of AST-120 for patients with chronic kidney disease: a network meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:676345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol. 2013;37:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.