Abstract
Background
The Blood-Brain Barrier (BBB) is a complex and dynamic interface that regulates the exchange of molecules and cells between the blood and the central nervous system. It undergoes structural and functional throughout oxidative stress and inflammation, which may compromise its integrity and contribute to the pathogenesis of neurodegenerative diseases.
Main body
Maintaining BBB integrity is of utmost importance in preventing a wide range of neurological disorders. NRF2 is the main transcription factor that regulates cellular redox balance and inflammation-related gene expression. It has also demonstrated a potential role in regulating tight junction integrity and contributing to the inhibition of ECM remodeling, by reducing the expression of several metalloprotease family members involved in maintaining BBB function. Overall, we review current insights on the role of NRF2 in addressing protection against the effects of BBB dysfunction, discuss its involvement in BBB maintenance in different neuropathological diseases, as well as, some of its potential activators that have been used in vitro and in vivo animal models for preventing barrier dysfunction.
Conclusions
Thus, emerging evidence suggests that upregulation of NRF2 and its target genes could suppress oxidative stress, and neuroinflammation, restore BBB integrity, and increase its protection.
Keywords: NRF2, Blood-Brain Barrier, Inflammation, Oxidative stress, Neurological disorders, Tight junction, Phytochemicals
Background
The Blood-Brain Barrier (BBB) forms a multicellular vascular unit that separates the central nervous system (CNS) from the peripheral blood circulation. This structure is composed of endothelial cells, pericytes, smooth muscle cells, astrocytes, and neurons [1], which together control the selective passage of small molecules, metabolites, and cells to the brain parenchyma, maintaining an environment suitable for brain homeostasis [2, 3]. It also provides protection from pathogens, inflammation, injury and disease [4]; as well as regulating the cerebral blood flow (CBF) [1]. All these cell types constitute the neurovascular unit (NVU), a term often used to refer to the functional unit of the BBB, enabling paracrine regulations [5]. Maintenance of the CBF is key to the clearance of toxic by-products of brain activity, such as metabolic waste [6, 7].
The BBB is not an impermeable barrier, but due to the presence of several specialized and diverse transport and channels, it can mediate the transport of ions, glucose, amino acids, and well-defined substrates [5]. BBB limits the entry of plasma components and leukocytes under healthy conditions. However, upon endothelial damage, the integrity of the BBB is compromised, allowing for the entry of said cells. Therefore, it’s key to protect brain endothelial cells (BEC) to maintain barrier function [5]. The BBB also releases a series of diffusible signals (nitrogen monoxide (NO), prostanoids, adenosine, ions, etc.) to regulate cerebral blood flow [1]. Loss of these barrier properties is highly linked to neurological diseases, including stroke, multiple sclerosis, traumas, and neurodegenerative disorders [3, 4].
NRF2 (Nuclear factor erythroid-related Factor 2) is the main regulator of cellular defense against oxidative and inflammatory stress [8, 9]. It regulates the expression of ~ 250 genes that present regulatory sites termed Antioxidant Response Elements (ARE). Many of the ARE-containing genes are involved in maintaining the glutathione and thioredoxin antioxidant defense systems, iron and drug metabolism, the detoxification of oxidants that could alter the redox states, and genes implicated in the repair of damaged tissue [10, 11]. In addition, the NRF2 pathway is involved in proliferation, immune cell migration, and anti-inflammatory processes [12–14].
Lack of NRF2 transcriptional activity compromises BBB integrity and increases inflammatory markers, including vascular adhesion molecules and pro-inflammatory cytokines, while also disrupting the redox and metabolic functions of endothelial cells [10, 15, 16]. In vitro models, have demonstrated a BBB alteration in NRF2-knockout samples, along with reduced expression of tight junction (TJ) proteins such as Occludin and Claudin-5 [17, 18] and adherens junction proteins such as VE-Cadherin. Furthermore, vivo models also provide evidence that in Nrf2-deficient mice, there is compromised BBB integrity, leading to leakage and an increased extravasation of plasma constituents from to vessel to into the brain parenchyma. This is also associated with an exacerbated neuroinflammatory response, showing an increased number of activated microglia and upregulation of inflammatory mediators (e.g., IL-1β) [19].
Conversely, the activation of the NRF2/ARE system can potentially prevent/reduce BBB impairment and, consequently, decrease some types of brain injury in astrocytes and BEC, which resulted in moderate peroxynitrite generation, protecting against BBB disruption [20]. In this manner, NRF2 can also serve as a potent therapeutic agent to diminish CNS injury-induced brain damage upon increasing BBB integrity. Thus, in this review, we discuss the role of NRF2 in protecting and maintaining against BBB disruption for the prevention of neurological disorders.
Features of the Blood-Brain Barrier
BBB blood vessels are comprised of several cell types, endothelial cells (EC) which make up the walls of blood vessels, mural cells, which sit on the abluminal surface of the EC layer, astrocytes and neurons (Fig. 1). The properties of BBB are mainly derived from the ECs but are induced and maintained by the interaction with astrocytes, smooth muscle cells, pericytes, and neural cells [4].
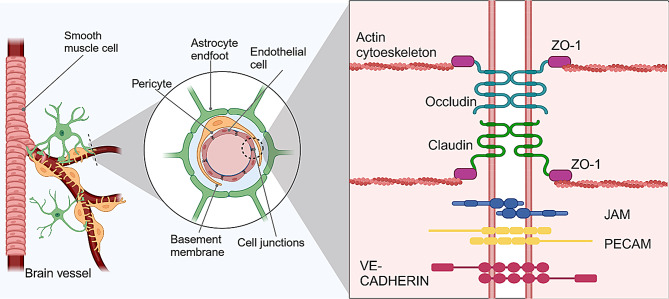
Fig. 1.
Structure of the BBB. The BBB is composed of several cell types, the smooth muscle cells are present in vessels with higher diameters, astrocytes bind to the vessel through their feet, and pericytes enclose the vessel and are surrounded by a basement membrane. The endothelial cells are tightly bound together through several cell junction proteins, which include the TJs (Claudin and Occludin) which connect to the actin cytoskeleton through ZO-1, JAM, PECAM, and adherens junctions (such as VE-Cadherin)
One of the distinguishing features of the BBB is the unique properties of its endothelial cells [21] which form a tightly sealed monolayer [5] and exhibit characteristics that differ from endothelial cells in other tissues, ensuring proper substance movement and maintaining barrier function [4]. Key differences include the presence of continuous intercellular TJ and adherens junctions (AJs), the absence of fenestrations, and low rates of transcytosis [22, 23]. These features limit both paracellular and transcellular movement of molecules [2, 4], while selective transporters and receptors facilitate regulated transport.(Fig. 1) [24–26]. Additionally, endothelial cells at the BBB secrete vasodilators and vasoconstrictors to regulate vascular tone [27].
BBB TJs allow for a high-resistance paracellular barrier to molecules and ions, polarizing the luminal and abluminal sides [4]. These TJs are present in the apical lateral membrane [28] and are linked to the cytoskeleton through interactions with cytoplasmic adaptors, creating paracellular barriers that, depending on local transport requirements, differ on the level of permeability (Fig. 1) [29]. TJ are protein complexes composed of integral membrane proteins, cytoplasmic plaque proteins, and cytoskeletal proteins, among these TJ-associated transmembrane proteins, claudins, occludins, JAMS, and ZO proteins are directly involved in the formation of TJ strands in endothelial cells [3, 30]. Claudin-5 is highly expressed and plays a key role in vertebrate BBB [31], tightening the barrier for many physiological active substances [25], which includes most physiologically active substances [28]. Occludin, a TJ-associated MARVEL protein (TAMP), is found ubiquitously in the TJ [32] and interacts with claudins to establish the integrity of the endothelium [33]. JAMs, part of the immunoglobulin superfamily [34], help to establish the correct localization of other junctional protections [35].
TJs also interact with basal AJs, connecting all ECs, and are composed of vascular endothelial (VE)-Cadherin and platelet EC adhesion molecules (PECAM1), linked to the cytoskeleton by catenins [5, 21, 23]. VE-Cadherin is essential for endothelial integrity in quiescent vessels and may regulate endothelial functions by activating directly signaling pathways related to survival and actin cytoskeleton as Phosphoinositide 3-Kinases (PI3K) [36].
For the establishment of the BBB, the transport of materials through transcellular pathways must be suppressed completely, therefore needing specialized transport systems in the plasma membranes of endothelial cells for the uptake of even small molecules such as glucose and amino acids by the CNS [37]. Moreover, the paracellular route is tightly sealed thanks to the barrier formed by the TJs [25]. So, by limiting the passive passage of molecules through the BBB, its exchange needs to be strictly regulated using particular transporters. For instance, Slc2a1, also called Glucose transporter 1 (GLUT1), has been largely studied for its crucial in supplying glucose to the CNS, with nutrient concentration gradients regulated according to the CNS’s metabolic needs. Other transporters are involved in adaptive responses for nutrient uptake and removing waste from the CNS to the blood [4].
BBB damage is a consequence of several pathological processes of the brain [28], and can contribute to the progression of the disease [38]. Most common brain disorders can lead to BBB disruption, which shares an impaired TJ network, imbalance of transporters and fluxes, and increased extravasation of blood cells [39].
Role of NRF2 in Blood-Brain Barrier integrity
NRF2 is a key transcription factor in the antioxidant and anti-inflammatory response [8]. It mediates the induction of phase II antioxidant defense enzymes, activating transcription through the ARE sequence present in the promoter region of target genes [40]. NRF2 possesses a bZIP-containing C-terminal domain, capable of heterodimerizing with small Musculoaponeurotic Fibrosarcoma (sMAF) proteins [41]. The heterodimers can recognize AREs located in the upstream regulatory regions of the target genes [42], which can initiate the transcription of roughly 250 genes related to cytoprotection [43]. These genes encode a network of cooperating enzymes that participate in phase I, II, and III biotransformation reactions and antioxidant processes. These processes include the generation of NADPH, glutathione (GSH), and thioredoxin; lipid and iron catabolism; and interactions with other transcription factors, among other functions [10]. NRF2 has also been shown to regulate the expression of various proteasome subunits and autophagy-related genes, highlighting its role in the control of proteostasis [44–46]. Some key NRF2 target genes related to antioxidant enzymes include those encoding NAD(P)H quinone oxidoreductase-1 (NQO1)), Heme oxygenase 1 (HO-1), catalytic and modulatory subunits of γ-glutamyl synthase (GCLM, GCLC), and the glutamate-cystine transporter (SLC7A11) [8, 47] playing a key role in the cellular defense system against oxidative stress [48].
NRF2 activity control mechanisms
NRF2 levels are kept low under physiological conditions with the possibility of suddenly increase them upon oxidative or inflammatory stress and this is achieved by the constitutive synthesis and degradation of NRF2 [49]. The main proteins responsible for maintaining NRF2 levels low are Kelch-like-ECH-Associated Protein 1 (KEAP1) and β-transducin repeat-containing protein (β-TrCP) [8]. KEAP1 is an E3 ligase adapter that, in unstressed conditions, degrades NRF2 through ubiquitination by recruiting the Ringbox1-Cullin 3 complex (Fig. 2). KEAP1 presents redox-active cysteine residues that react under oxidative conditions, with several electrophiles or Reactive Oxygen Species (ROS), preventing KEAP1 from degrading NRF2, leading to the stabilization and accumulation of NRF2 [50]. The main alternative pathway to KEAP1 regulation is the GSK3-β-TrCP axis. Glycogen synthase kinase (GSK-3) is a serine-threonine protein kinase that has been demonstrated to inhibit NRF2 through the ubiquitin ligase β-TrCP by phosphorylating NRF2 and forming a phosphodegron which is recognized by β-TrCP, which recruits the Ringox1-Cullin 1 ubiquitination system (Fig. 2) [51–53]. Thus, the constitutive degradation of NRF2 is inhibited, which allows NRF2 to accumulate within the cell, translocate to the nucleus, and regulate cytoprotective gene expression [54].
Fig. 2.
Regulatory pathways of NRF2. NRF2 levels are kept low in physiological conditions through two main mechanisms. KEAP1 binds to NRF2 and promotes its ubiquitination and proteasomal degradation, upon ROS, oxidative stress or electrophiles these molecules are going to interact with a series of electrosensitive cysteines present in KEAP1. This would lead to a change in conformation which results in KEAP1 being unable to ubiquitinate NRF2. The other main pathway is through GSK3-β-TrCP. GSK3-β phosphorylates NRF2 when is not inhibited by AKT phosphorylation which is activated in the presence of growth or other signaling factors. Upon NRF2 phosphorylation, this site acts as a phosphodegron which is recognized by β-TrCP, which recruits an E-3 ubiquitin ligase complex for its degradation. When NRF2 is not degraded it is able to travel to the nucleus, binding to the ARE sequence of its target genes and promoting an antioxidant and anti-inflammatory response. Regarding BBB, it also projects by downregulation of MMP expression and signaling and promotes cell junctions and BBB key transporters expression
NRF2 regulation of inflammation and oxidative stress
Multiple studies have noted that imbalanced ROS levels have been implicated in endothelial vessel integrity by acting as signaling molecules that promote angiogenesis through proliferation, cell migration, and tube formation. This phenomenon involves cells losing junctions and, thereby, losing barrier properties [55]. ROS can function as low-level signaling intermediates and regulate fundamental cell activities including growth and adaptive responses [56]. However, at higher concentrations, ROS can cause cell injury and death in endothelial cell dysfunction and loss of BBB integrity [57]. Furthermore, oxidative stress has been consistently reported to occur in most neurodegenerative conditions [58], correlating with the development of cognitive and motor disturbances [59, 60].
Neuroinflammation plays a physiological role in phagocytosis and the clearance of cellular debris, pathogens, and aberrant proteins, which is beneficial for maintaining brain homeostasis unless it becomes chronic. For instance, microglial activation promotes phagocytosis of astrocytic perivascular end-feet and reduces the expression of two TJ proteins: Occludin and Claudin-5. These changes support leukocyte infiltration across the BBB and further exacerbate neuroinflammation and neurodegeneration [61]. As such, increased BBB permeability following prolonged systemic inflammation may contribute to cognitive dysfunction. In contrast, microglia can protect the BBB from inflammatory attacks by elevating the levels of anti-inflammatory factors, such as IL-10, IL-4, IL-13, and transforming growth factor-β (TGF-β) [62, 63]. Thus, depending on the perivascular microenvironment, the highly dynamic switch of microglial phenotype between pro- and anti-inflammatory states is closely linked to their impact on BBB integrity.
In addition, other key components of BBB such as astrocytes can modulate BBB development and function under physiological conditions by secreting a series of molecules that regulate immune responses. Astrocytes are also able to release extracellular vesicles containing inflammation-related proteins and miRNAs that help support a healthy BBB integrity [64]. However, chronic inflammation can cause injury to the CNS, highlighting the importance of regulating the neuroinflammatory process [65, 66]. Triggered by pathophysiological conditions, activated astrocytes disrupt the BBB components by secreting matrix metalloproteinases 9 (MMP-9) and proinflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-α (TNFα), which leads to BBB leakage due to exacerbated inflammation [67, 68]. Moreover, neuroinflammation contributes to BBB disruption by damaging TJs, allowing leukocytes to infiltrate the brain when the barrier is compromised [69, 70].
Persistent inflammation is a hallmark of several diseases in which NRF2 mediates, likely because inflammation increases local and systemic pathologic formation of ROS [71]. In addition, ROS stimulates inflammatory response through the activation of nuclear factor k-light-chain enhancer of activated B cells (NF-κB) p65 subunit [71]. NRF2 has been extensively studied for its complex crosstalk with the NF-κB pathway, engaging in feedforward and feedback loops [15]. NF-κB is a transcription factor that plays a crucial role in regulating inflammation and suppressing ARE-dependent gene transcription [72]. However, it can also bind to the NFE2L2 gene promoter, activating NRF2 expression and establishing a negative feedback loop [73].
Initially, NRF2’s anti-inflammatory effects were attributed to its role in modulation of redox metabolism and interactions with NF-κB. However, NRF2 can also directly suppress the transcription of proinflammatory genes like interleukin-6 (IL-6) and interleukin-1β (IL-1β) in macrophages in response to lipopolysaccharide (LPS) stimulation [74]. Upon LPS exposure or pharmacological activation, NRF2 binds to the proximal promoters of these genes, blocking the recruitment of RNA polymerase II. This mechanism is independent of NRF2’s typical binding to the ARE enhancer [74].
Thus, NRF2 exhibits a pleiotropic functional role in the endogenous defense system, protecting against oxidative stress and inflammation [18, 40]. The defense against oxidate stress in the brain also acted by modulating microglial dynamics [75], regulating inflammatory signaling [47], and protecting neurons and astrocytes [40]. Conversely, the deletion or downregulation of NRF2 exacerbates cell susceptibility to the damage [18]. Vascular endothelial dysfunction and consequent CNS damage have been connected to ROS [76–78] and ROS-driven inflammation [79]. NRF2 activation likely preserves the BBB by maintaining ROS homeostasis, which ultimately leads to a decrease in the risk of cerebrovascular, neurodegenerative, and CNS disorders [77, 80, 81].
Several authors have reported that treatment with different NRF2-inducing compounds results in reduced inflammation due to NRF2 having an anti-inflammatory property, as seen previously in LPS-stimulated cells [72, 82, 83]. In inflammation assays with BEC, NRF2 activation is associated with antioxidative protection conferred by NQO1, alongside its anti-inflammatory effects [84]. Furthermore, astrocyte and microglia activation of NRF2 provides protection from neurotoxicity, ameliorating the oxidative stress and inflammation surrounding neurons and blood vessels [75]. Additionally, NRF2 plays a protective role in the conservation of the NVU [85–87] and, in conjunction with the BBB, upregulates the expression of TJs proteins [87–89].
NRF2 regulates intercellular binding proteins
There is strong evidence that NRF2 may regulate the imbalance that compromises BBB integrity, preventing leakage by maintaining ROS homeostasis and controlling inflammation. Further evidence supports the idea that NRF2 can also control the expression of junction proteins, as demonstrated by promoter analysis and detection of promoter activity [17, 90]. For instance, it had been found an ARE motif on the mouse Cldn5 (Claudin-5) and two electrophile response elements (EpRE) motifs have been identified on Cdh5 (VE-Cadherin) promoters. Using promoter-luciferase activity assay, they verified that both Claudin-5 and VE-Cadherin might be NRF2 direct target genes [17, 90].
TJ protections, such as occludins, claudins, and Zonula occludens-1 (ZO-1) are vulnerable to the effects of MMPs and were found in the extracellular matrix surrounding the brain endothelium after BBB disruption [91], leading to the degradation of these junctions [92]. MMPs belong to a family of enzymes implied in tissue remodeling [93]. Astrocytes can release MMPs that degrade the basal lamina proteins, exposing the TJ proteins to be hydrolyzed by MMPs contained in the blood vessels, such as MMP-9 [91]. Immune and endothelial cells can also release MMP-9 [94], to facilitate leukocyte diapedesis [95, 96]. MMP-2, -3, and − 9 are the primary MMPs present in the brain. MMP-2 and 9, compared to MMP-3, have a narrower spectrum of substrates but include basement membrane elements. MMP-2 is also expressed in astrocytic feet, supporting cerebral endothelial cells. MMP-9 is particularly harmful to BBB integrity, as it can hydrolyze extracellular matrix and TJ proteins, such as those involved in the BBB [97]. For example, after hypoxia/reperfusion MMP-9 is released by several cell types, including infiltrating neutrophils and lymphocytes, worsening the condition of the BBB integrity. Additionally, ZO-1 degradation has been linked to increased MMP activity, causing enhanced barrier permeability [98, 99].
NRF2, as an anti-inflammatory regulator, can mitigate the release of MMPs by downregulation of NF-kB and TNF-α signaling [96]. MMPs-2 and MMP-9 have been known to present a heightened activity following oxidative stress in other tissues [100], making NRF2 a key mediator in the antioxidant response. NRF2 activation also contributes to the inhibition of extracellular matrix (ECM) degradation, among them by decreasing the expression of two members of the MMP family; MMP-9 in macrophages and MMP-7 in epithelial cells of the human intestine, improving the disease state of inflammatory bowel disease [101, 102]. Consequently, during inflammatory processes, MMP production may be controlled directly by the NRF2 pathway or by the effect of NRF2 in the activation of the NF-κB pathway [101] in BBB extracellular matrix remodeling. For instance, Nrf2-deficient mice with skin damage induced by UV radiation show higher levels of MMP-9 compared to controls [103].
NRF2 regulation of transport system
Besides regulating the expression of neuroendothelial TJs, NRF2 also influences BBB endothelial glucose uptake. This transcription factor regulates metabolism by re-directing the metabolic flux through the pentose phosphate pathway [104]. Given the high energy demand of the CNS and the presence of various glucose transporters and mitochondria in the BBB endothelium [105, 106], NRF2 regulation of glucose uptake is likely critical for BBB function and integrity [18]. Impairment in endothelial glucose uptake downregulates TJ protein expression, resulting in loss of BBB integrity [107]. Therefore, NRF2 role in TJs population and glucose uptake appears to be entwined [18].
GLUT1 is one of the targets for transcriptional regulation by Hypoxia-inducible factor-1α (HIF-1α) [108], which in turn presents ARE sequences in its promoter that NRF2 can bind to promote its expression. Activation of NRF2 by metformin in tobacco smoke-induced cerebrovascular toxicity restores GLUT-1 levels [87], which correlates with increased cellular glucose uptake occurring with increased NRF2 activity [109].
NRF2 protective effects on Blood-Brain Barrier damage concerning neurological diseases
Several studies have indicated that BBB leakage contributes to neuro-disorders, related to cerebrovascular diseases, altered glycemia, neurodegeneration diseases, cigarette smoke toxicity, and sepsis, pointing out that the BBB should be considered for therapy [99, 110–112]. This is supported by the fact that both BBB loss of integrity and neurodegenerative disease share oxidative stress and inflammation as a factor of worse prognosis [58]. Thus, we discuss how NRF2 may regulate the imbalance of BBB integrity homeostasis in concerns of neurological diseases, related to neurovascular pathologies, hyperglycemia diseases, cigarette smoke toxicity, sepsis, and neurodegeneration.
NRF2 protective effects on cerebrovascular diseases
Cerebrovascular diseases include a reduced or altered blood flow to the brain, most commonly as a result of hypoperfusion and thrombosis, an ischemic stroke, hypertension, or traumatic brain injury, which subsequently leads to oxidative stress, hypoxia, and neuroinflammation which can be protected by NRF2 activation [113].
Ischemic/stroke
Ischemic/Stroke (I/S) occurs when a vessel supplying blood to the brain becomes obstructed and the brain is deprived of oxygen, glucose, and other essential nutrients. The pathogenesis of stroke is complex, with oxidative stress due to the overproduction of ROS and/or reactive nitrogen species (RNS) being central [114–116]. When the physiological balance between ROS generation and ROS clearance is disrupted and exceeds antioxidant capacity, oxidative stress occurs and is followed by cellular injury (Fig. 3).
Fig. 3.
The effect of cerebrovascular damage leads to brain pathologies. Cerebrovascular diseases can be caused by ischemia, traumatic head injury, or hypertension. These pathologies share an oxidative stress-inflammation feedback loop which results in a compromised BBB. There is a transport dysregulation coupled with cell death and loss of instability, thus altering brain homeostasis by the entry of toxins or antibodies. The BBB damage can accumulate and result in neuron loss, and alteration of the brain BF which ultimately can end in cognitive decline
Following a stroke and reperfusion, the cerebral vessels are key to resupplying blood flow, cerebral vessels are crucial for restoring blood flow and minimizing further tissue damage due to reduced oxygen levels [117]. However, reperfusion can lead to excessive production of ROS in the brain, including hydrogen peroxide and peroxynitrite [118]. This results in BBB destruction, edema, and brain parenchymal damage [119, 120]. Ischemic brain injury triggers a pathological cascade causing rapid and irreversible neuronal injury within the ischemic core, while the surrounding hypoperfused brain tissue, the penumbra region, can be salvaged if the blood flow is restored. A common mechanism is reperfusion from recanalized cerebral vessels but can cause cerebral edema, brain hemorrhage, and neuronal death [121]. The responses to the ischemia result in ROS accumulation which promotes, in turn, pro-inflammatory and pro-apoptotic mediators [40].
Hypoxia is a common phenomenon during this process, causing significant oxidative stress and ROS accumulation [77, 122], activating the NRF2 signaling pathway as a key regulator in antioxidant and anti-inflammatory processes (Fig. 3). A key player involved in the hypoxia response is HIF-1α, which induces the transcription of genes related to glucose metabolism, angiogenesis, erythropoiesis and cell survival [123]. HIF-1α levels are primarily elevated in the salvageable penumbra tissue [123] promoting angiogenesis, glycolysis, apoptosis and inflammation, crucial at first for brain healing after the stroke [124]. HIF-1α is described as a mediator of inflammation, which is needed to recruit leukocytes to the injury site and activate local immune cells [125]. NRF2 has been described to regulate HIF-1α activation since NRF2 upregulation led to an increase in HIF-1α pathway activity because the HIF-1α have a conserved functional ARE was identified in the promoter region of the HIF1A gene [126]. More specific causal relationships have been reported, where HIF-1α induction repressed the NRF2 transcription of HO-1 and interleukin-8 (IL-8) in endothelial cells; the decreased NRF2 activity was found to be due to the elevation of BTB Domain and CNC Homolog 1 (BACH1), a repressive partner of NRF2 [127]. Thus, the tight balance between the NRF2/HIF-1α axis regulates BBB preservation and health.
TJs are often altered and less expressed at the BBB during hypoxia [128–130], which increases paracellular flux, mainly by degradation and decreased expression of Claudin-5 and ZO-1 [130, 131] which can be upregulated by NRF2 to compensate it. Moreover, in in vitro BBB models, NRF2-knockdown was associated with in vitro BBB disruption evidenced by increased dextran leakage and decreased transendothelial electrical resistance [17, 18]. This was accompanied by reduced expression of TJ proteins ZO-1, Occludin, and Claudin-5 [132], and adherens junction protein VE-Cadherin [18, 133, 134]. In vascular cognitive impairment and dementia (VCID) rats, NRF2 activation increased the expression of Claudin-5 and Occludin [17]. In the stroke mice model, NRF2 could also protect against BBB disruption [20], and it was proved critical for ischemic preconditioning-afforded BBB preservation after stroke [90]. Besides, there has been reported an increase in nuclear NRF2 levels in the hippocampus following ischemia, which facilitated those responses [122], contributing to the overall neuroprotective effect of this transcription factor in this pathology [135].
Traumatic brain injury
Traumatic Brain Injury (TBI), also known as acquired intracranial injury, is caused by an external force, such as a blow, bump, or jolt to the head, or from an object suddenly impacting or piercing the skull into the brain tissue [136]. While the primary injury of trauma can cause direct damage to neuronal structures, the mechanical tissue deformation triggers secondary injury leading to BBB damage, edema, increased intracranial pressure, inflammation, and cell death [137]. Secondary injury is the progression of TBI as a long-term neurological problem by affects physically, cognitively, and emotionally which often leads to a permanent disability of the victim [138] which is characterized by a neuroinflammatory response [139]. Secondary injury is primarily due to oxidative stress causes, which include neuroinflammation, edema formation, BBB damage, cell death, and finally leads to cognitive impairments in TBI. Immediately after a brain injury, a huge quantity of inflammatory cytokines such as IL-1β, IL-6, TNF-α and TGF-β are released into the blood circulation which further exacerbates the trauma condition of the brain with oxidative stress [140, 141].
Emerging evidence suggests that oxidative stress not only contributes to TBI pathogenesis TBI but also initiates and promotes excitotoxicity, mitochondrial dysfunction, neuroinflammation, and other risks. NRF2 plays a protective role in TBI via fighting against oxidative damage and inflammatory response in TBI (Fig. 3) [142], and modulating microglial and macrophage function [139], by blocking the expression of proinflammatory cytokines [74]. NRF2 also suppresses reactive astrocyte activation and mitigates neuroinflammation and ferroptosis by inhibiting NF-κB, providing neuroprotective effects [143, 144]. Conversely, NRF2-genetic deletion delays the recovery of motor and cognitive functions post-TBI [145]. NRF2 activation plays a crucial role in mitigating ferroptosis by decreasing intracellular iron levels by upregulating the expression of ferritin heavy chain (FTH1) for iron storage and ferroportin (FPN) for iron efflux [146, 147]. Additionally, it inhibits glia-driven neuronal ferroptosis by positively regulating the Glutathione Peroxidase-4 (GPX4), a key regulator of ferroptosis, and Ferroptosis Suppressor Protein-1 (FSP1) pathways, which are also crucial for protecting against oxidative damage [148–150]. NRF2 can also be activated through the Tropomyosin-related Kinase Receptor type B (TrkB) receptor, upregulating NRF2 via the PI3K/AKT pathway, reducing ferroptosis and neuroinflammation, and partially restoring neurocognitive function impaired by injury [151]. This mechanism also helps reduce endothelial cell death, TJ protein loss, and BBB permeability [152].
Hypertension
High blood pressure has been found to alter the BBB [153] reduce resting cerebral blood flow, and suppress the neurovascular coupling and endothelium-dependent responses, leading to its dysfunction [154]. Severe hypertension is associated with reduced oxygen consumption and blood flow [155]. Hypertension’s role in cognitive decline, aside from its link to stroke, affects similar areas as other causes of cerebrovascular diseases [154, 156–158]. A key factor that contributes to the pathogenesis of hypertension in the brain is sympathoexcitation [159], which is prevalent in the hypothalamic paraventricular nucleus (PVN). PVN is the center of artery pressure control [160] and can affect the sympathetic outflow of all the CNS [161]. ROS is one main contributor to the sympathoexcitation in this area in different kinds of hypertension [162–165].
Regarding BBB damage, a physiopathological increase in blood pressure can ultimately result in NVU dysfunction [6, 156], often correlated with a framework of oxidative stress, ischemia, and inflammation (Fig. 3). the protective role of NRF2 in maintaining BBB integrity during hypertension may be linked to mitigating endothelial damage [166], shielding this cell type against oxidative stress [14] and reducing the blood pressure and heart rate in rats after activation as seen by using the naturals compound [167]. For instance, in hypertensive rats reduced NRF2 protein expression in the rostro-ventrolateral medulla neurons impairs mitochondrial biogenesis and contributes to the development of hypertension [168]. Silencing of Nrf2 in this rat model led to higher levels of ROS and increased hypertension, showing a 20–25% increase in the mean arterial pressure suggesting that the basal level of NRF2 in the normal state is crucial for restraining sympathetic outflow [169]. Upon NRF2 activation, reduced activity of the sympathetic nerve and blood pressure was observed in hypertensive rats [159], being able to be correlated with a protective mechanism of BBB integrity and health.
NRF2 protective effects on glycemic load-related diseases
Glycemic load-related diseases involve imbalances in the glucose levels in the body, which have been associated with an increased risk of diabetes mellitus, coronary heart disease, obesity, and cancer [170]. Diabetes mellitus (DM) is a chronic metabolic disease characterized by elevated levels of blood glucose (or blood sugar), leading to complications in several organs, including the heart, blood vessels, eyes, kidneys, and nerves, and increased premature death [171]. Diabetes and other hyperglycemic-related diseases such as obesity, are linked to proliferative lesions in small brain vessels [172] which is correlated with an increase in barrier permeability, also named diabetic encephalopathy [173]. Disruption of BBB was paired with changes in glucose transport rates, efflux transporter activity, BEC expression of matrix MMPs and immune cell trafficking [174]. Excessive glucose oxidation via the Krebs cycle induced oxidative stress, which provoked BBB loss of integrity and pericyte death (Fig. 4) [175]. Hyperglycemia has been shown to alter BBB permeability, while hypoglycemia has been seen to trigger GLUT-1 overexpression to compensate for low circulating blood glucose levels and the opposite was described for hyperglycemia [172, 176]. In both cases they present an effect on the TJ expression and endothelial oxidative and inflammatory responses, correlating with higher released levels of vascular endothelial growth factor (VEGF) in the cell medium [176]. BBB leakage in diabetes and obesity with concomitant type II diabetes correlates with the downregulation of Claudin-5 and 12, Occludin and ZO-1 [177, 178].
Fig. 4.

Effects of altered glucose blood levels on the BBB. A), high levels of glucose in the blood, such as in diabetes, lead to high activity in mitochondria which increases oxidative stress coupled with higher AGE levels, which can disrupt BBB integrity through the promotion of VEGF release by BEC and TFG-beta by pericytes, which, in turn, activate metalloproteinase to degrade cell junction. MMPs are also activated by the oxidative and inflammatory stress frame caused by elevated glucose levels. B), hypoglycemia also leads to BBB disruption, there is an increase of GLUT1 expression to compensate for the low levels of glucose and a reduced NRF2 expression, which in turn, is not able to downregulate MMPs and to mediate the antioxidative and anti-inflammatory response
Increased mitochondrial ROS appears to be a common mediator, linking increased glucose metabolism via the polyol pathway, accumulation of advanced glycation end products (AGEs), protein kinase C (PKC) activation, and increased activity of the hexosamine pathway with vascular damage in diabetes [179]. AGEs can increase BBB permeability, by inducing VEGF production in BEC and TFG-β in pericytes, which both then can act to induce secretion of MMP which can degrade TJ (Fig. 4) [173, 174].
Diabetic complications are associated with increased ROS generation resulting from elevated blood glucose and free fatty acids [180–182]. NRF2 counteracts high-glucose-induced damage and downstream markers of oxidative stress such as oxidized low-density lipoprotein (ox-LDL) [183], 4-hydroxynonenal (HNE) [184–186], and TGF-β [187] are elevated in diabetic patients and have all been shown to activate NRF2/ARE-linked gene transcription. Research in cultured bovine aortic endothelial cells [188] has established that high glucose and AGEs increase ROS production and NRF2-dependent HO-1 expression. As a result, certain antioxidant compounds may confer protection against AGE cytotoxicity by upregulating NRF2-linked antioxidant enzyme activity preventing oxidative stress in diabetes [188]. In human microvascular endothelial cells, although high glucose concentrations (30 mM, 6 h) do not significantly induce NRF2 translocation, activation of this pathway by NRF2-inducers significantly reduces hyperglycemia-induced ROS generation and protein glycosylation [189].
As stated before, BECs are highly vulnerable to oxidative and inflammatory stress, leading to a loss of BBB integrity through a reduced composition of TJ complexes as the main mechanism [58]. In pathologies related to altered glycemia pathologies, such as prolonged hypoglycemia, signification downregulation of NRF2 in BBB ECs has been detailed (Fig. 4) [18]. NRF2-knockdown resulted in a reduced expression of Claudin-5 and VE-Cadherin in BECs, without significant effects on Occludin expression [176]. The effects of NRF2 silencing on TJ were parallel to those observed with hypoglycemia [176], whose activation allowed for the partial restoration of TJ and prevented BBB permeability. Furthermore, VE-Cadherin was shown to positively regulate the transcription of Claudin-5 [190]. The effect on TJs may be explained by NRF2 involvement in the upregulation of RAS family member homolog A, an essential factor that promotes the reorganization of the actin cytoskeleton [191].
NRF2 protective effects on neurodegeneration diseases
Alzheimer’s disease
Alzheimer’s Disease (AD) accounts for 60–70% of all dementia cases and is generally characterized by an accumulation of amyloid-β (Aβ) in the form of senile plaques (SPs) and TAU protein in neurofibrillary tangles (NFTs) [192–194]. Additionally, AD involves low levels of acetylcholine, disrupted calcium regulation, oxidative stress, and neuro-inflammation [195]. The accumulation of SP and NFTs occurs in specific regions like the frontal cortex, hippocampus, and basal forebrain, which results in the decline of cognitive skills related to learning and memory [196]. Morphological changes have been observed in post-mortem brains of AD patients, such as basement membrane thickening, decreased microvessel density, capillary leakages, and accumulation of fibrinogen, albumin, prothrombin, and hemoglobin-derived peptide levels [197–199] indicative of BBB damage.
There is growing evidence that the BBB is compromised in the deposition of Aβ (Fig. 5) [200, 201]. Aβ causes mitochondrial dysfunction which results in the release of ROS to the cytosol, causing oxidative stress in neurons and endothelial cells, and it is seen as a causative factor for microhemorrhages within lobar regions [118, 202]. This enhanced permeability could lead to increased deposition of Aβ within AD brains, worsening the disease [203, 204]. A reduction in TJ proteins in BBB has been shown to enhance the clearance of brain Aβ into the blood [6, 205]. Angiogenic processes can be triggered by Aβ, which in turn results in the loss of the capillary TJ proteins (Claudin-1 and 5) and adherens junctions causing an increased permeability in rodent brains [206, 207]. The angiogenic process may be related to the Aβ accumulation that impairs the angiogenic signaling pathways, leading to reduced blood flow which decreases oxygen and nutrient supply in the brain [208].
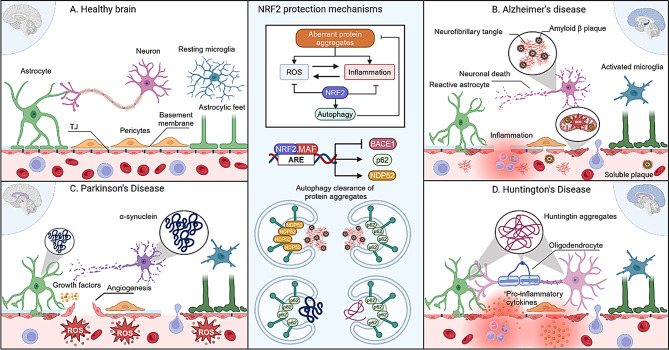
Fig. 5.
BBB landscape across different neurodegenerative diseases. A), in physiological conditions the BBB is stable, which results in an adequate transport regulation that allows the brain homeostasis. B), Alzheimer’s disease severely affects the hippocampus and cortex, in those areas we have an accumulation of amyloid β plaques and TAU neurofibrillary tangles which can be released to the bloodstream, causing inflammation and oxidative stress, which can activate microglia and astrocytes. These stresses result in a loss of the BBB integrity, where there is cell death and loss of TJ. C), Parkinson’s disease most common areas that affect in the early stages are the substancia nigra (in pink) and the basal ganglia, it is known to accumulate both in neurons and astrocytes, damaging the neurons and causing the release of growth factors that can induce an angiogenesis response from the endothelium, which needs a loss of the TJ for the cells to migrate and form novel vessels. High levels of ROS are present in the bloodstream, which contributes to the BBB damage. D), Huntington’s disease has been shown to affect the basal ganglia and the brain cortex and in the context of the BBB, it causes the release of pro-inflammatory cytokines which can damage the brain endothelium. NRF2 protects the BBB in neurodegenerative diseases through various mechanisms. Aberrant protein aggregations generate ROS and inflammation, which NRF2 can suppress while promoting autophagy pathways to clear these aggregates. NRF2 also inhibits BACE1 gene expression, reducing Aβ protein release, and activates autophagy-related genes such as p62 and NDP52 to further support the removal of harmful protein accumulations. Macroautophagy is one main pathway, which is capable of removing neurofibrillary tangles, amyloid plaques, α-synuclein and huntingtin aggregates
Particularly, the hippocampus is especially vulnerable to BBB disruption, due to the intrinsic fragility of its vascular network, which showed that BBB permeability in the hippocampus is higher compared to several other brain regions, even in human subjects with no known brain pathology [209]. The hippocampus is a region particularly reliant on TJs for proper functioning that is enriched in this cell-cell junction for mediating the transport of ions and metabolites, which ensures a balance of neurotransmitters and signaling molecules [207, 210], which is highly affected in this neurodegenerative disorder [211]. Decreased Occludin and Claudin-5 have been detailed in AD mice [210] and postmortem AD patients [205].
Development of cerebral β-amyloidosis and cognitive decline in patients with AD, with related familial cerebrovascular disorders, may be linked to defective Aβ clearance from the brain and a lack of NRF2 protection of BBB integrity [212, 213]. Animal models suggest that NRF2 plays a crucial role in mitigating Aβ cytotoxicity and clearing Aβ. For example, the 5XFAD AD-like mice model lacking NRF2 shows an increase in astrocytes and microglia and increased levels of interferon-γ (IFN-γ) and exhibits worsened cognitive deficits [214, 215]. Bahn et al. showed that NRF2 can also negatively regulate β-site Amyloid precursor protein Cleaving Enzyme 1 (BACE1) expression, which is the main secretase for Aβ peptides, through binding to ARE sites in the BACE1 promoter. These researchers showed that an NRF2 deficiency increases BACE1 expression and exacerbates Aβ plaque loads and cognitive deficits in 5XFAD mice [216], and probably a BBB injury. On the other hand, macroautophagy is the main route involved in Aβ oligomer species and Aβ plaque clearance [217] and has been linked to NRF2. Recent studies have reported that NRF2 levels are regulated by the autophagy-related adaptor protein p62 [44]. p62 shuttles ubiquitinated proteins to the proteasomal and lysosomal degradation machinery and sequesters damaged proteins into aggregates before their degradation [44]. In this context, in Nrf2-deficient mice models, increased intraneuronal Aβ aggregates were found in the hippocampus of APP/PS1ΔE9 mice when NRF2 was absent [44], which may present a compromised BBB.
It has also been described that the AD brain presents microvascular alterations, such as increased capillary tortuosity, rarefaction, and higher levels of atherosclerosis [218, 219]. In vitro, experimental evidence shows that these Aβ deposits induce cerebrovascular dysfunction in the rat brain [220] and that the Aβ peptide produces endothelial dysfunction in cerebral microvessels via ROS. This occurs when the ROS superoxide–scavenging enzyme, superoxide dismutase, prevents acetylcholine-induced endothelium-dependent vasodilation [220]. This excessive ROS production could be a primary mechanism explaining BBB disruption [221]. In this way, the activation of the NRF2 defense pathway can prevent oxidative stress and would be beneficial for preserving BBB disruption and neurological impairment during its progression, in a similar manner in other neuro disorders [213]. Conversely, an NRF2-deficiency condition would increase the intracellular ROS content, suggesting that it is important for maintaining ROS homeostasis [222].
Another component in AD progression is the accumulation of TAU oligomers in cerebral microvessels. That has also been reported in other human neuro-disorders as Lewy Body (LB) dementia, and progressive supranuclear palsy patients [223]. Microbleeds have been detected in the brains of patients affected by frontotemporal dementia [224] and cerebrovascular inflammation has been associated with TAU pathology. In these cases, areas with significant accumulation of neurofibrillary tangles exhibited upregulation of adhesion molecules, disruption of TJs, morphological alterations in brain microvessels, including thickening of the vessel wall, vessel lumen reduction as well as increased in collagen-type IV content per vessel [225, 226], and this damage can stimulate inflammation.
In AD, Blood-Brain Barrier (BBB) damage is not solely due to endothelial TJ disruption but also involves the loss of mural cells, particularly pericytes, which are crucial for maintaining BBB integrity. Pericyte loss is strongly associated with BBB disruption in the cortex and hippocampus [227]. Soluble TAU could be a contributing factor to the decrease of TJ [228] and, like Aβ, can lead to dysfunction of the BBB in the hippocampus (Fig. 5) [207, 229]. Additionally, TAU pathology has been linked to small vessel disease [230], and immune cell trafficking across the BBB also appears to be modulated by neurofibrillary pathology in Tauopathies [231].
In vitro models using primary rat BECs exposed to oligomeric TAU changed the endothelial properties of the BBB that promote the migration of immune cells from the blood into the brain [231]. This process is driven by glial cell activation, which leads to neuroinflammation in TAU pathology-affected regions and may accelerate BBB damage [232]. Activated glial cells activate and release proinflammatory cytokines, including IL-1β, TNF-α, and IL-6, which alter endothelial properties [232–234].
NRF2 gains focus on this pathology as an anti-inflammatory capacity for response through several mechanisms. It suppresses the expression of several proinflammatory cytokines secreted from microglia, macrophages, and monocytes, which can affect the neurovascular unit [74] and infiltrate into the brain parenchyma.
Several reports have suggested that misfolded or phosphorylated TAU could be degraded through the proteasome [235–237]. However, recent studies found that TAU may also be turned over by the autophagy-lysosome pathway [238–240]. Of particular interest, NRF2 activation may also reduce phosphorylated TAU protein via the autophagy-lysosome pathway through the induction of the autophagy adaptor protein NDP52 [241]. It functions similarly to p62, recognizing ubiquitinated proteins and directing them to lysosomes for degradation. Five putative ARE sequences have been identified in the promoter region of the Ndp52 gene [241], and Ndp52 mRNA levels were found to be reduced in the hippocampus of Nrf2-knockout mice [241].
Overall, these findings highlight the relevance of NRF2 in the neuronal protective process and can prevent onset and progress to neurodegenerative diseases that could go head to BBB disturbed and leaky.
Parkinson’s disease
Although previously not as extensively studied in Parkinson’s Disease (PD), evidence increasingly suggests that BBB dysfunction plays an active role in PD. PD is a neurodegenerative disease characterized by motor dysfunction and cognitive deficits, which begin in the nigrostriatal pathway and eventually spread to the cortex [242]. BBB leakage has been observed in PD patients in areas typically associated with the disease, such as the basal ganglia [243]. Furthermore, perivascular deposits of fibrinogen or fibrin, immunoglobulin G (IgG), and hemosiderin in specific regions, indicate BBB disruption [244–246]. Post-mortem samples of advanced cases have revealed capillary damage in the frontal cortex [247] and BBB permeability [6]. This suggests that BBB disruption might be implicated in the development of PD (Fig. 5).
Astrocytes, which are crucial for maintaining the BBB [248], may play a central role in BBB disruption when they become dysfunctional. These cells support the secretion of TJ proteins through the production of growth factors [249]. However, in PD, reactive astrocytes have been shown to significantly reduce growth factor expression [250], leading to TJ protein production and, consequently, BBB leakage. Although PD patients show augmented vascular density in the substantia nigra pars compacta (SNpc), in the proximity of neuronal damage, these new microvessels display impaired maturation processes and altered diameters [251].
Astrocyte-specific NRF2 expression exerts neuroprotection through several mechanisms. Primarily, NRF2 enhances metabolic efficiency and helps maintain neuronal metabolic homeostasis following mitochondrial complex II inhibition [252]. Additionally, astrocyte NRF2 mitigates oxidative stress and protects against cell death [253], maintains water and glutamate balance, and reduces excitotoxicity in cases of NRF2-deficiency [254]. Furthermore, VEGF production in astrocytes is dependent on NRF2, as demonstrated in a study using Nrf2-deficient mice [255].
α-Synuclein (α-SYN) has also been shown to impact ECs homeostasis differently (Fig. 5) [256]. In mouse models expressing the A53T mutant form of human α-SYN, TJ expression is reduced, leading to increased vascular permeability and accumulation of α-SYN in activated astrocytes, which then release VEGF. Toxic forms of α-SYN can further disrupt EC function by downregulating TJ expression and inflammatory cytokine release [257, 258].
Activation of the NRF2 pathway has protective effects on ECs, reducing oxidative stress-related damage and alleviating PD symptoms. A recent study reported that over-expression of HO-1 triggered intracellular proteasomal degradation of α-SYN [259]. HO-1 also stimulates autophagy in astrocytes, another mechanism leading to the clearance of aggregated α-SYN [260, 261]. HO-1, a heat shock protein (HSP), is part of a family that, when induced by stress, prevents protein aggregation through refolding or degradation mechanism, including α-SYN [262]. Another pathway through which α-SYN degradation occurs is via the NRF2/p62 axis, offering alternative routes for clearing α-SYN aggregates and mitigating BBB injury [263].
Besides, upregulating HO-1 in ECs via NRF2 activation exerts cytoprotective effects against oxidative stress. This enzyme degrades heme to release free iron, carbon monoxide (CO), and biliverdin, which is converted to the antioxidant bilirubin [264]. By activating antioxidant phase II enzymes, NRF2 diminishes inflammation caused by microglia in the hippocampus, reducing inducible nitric oxide synthase (iNOS) and pro-inflammatory cytokines [47, 265]. Moderately elevated CO and biliverdin generated by HO-1 may confer neuroprotection by inhibiting NADPH oxidase and scavenging nitrogen monoxide (NO), thereby protecting the neurovascular unit [265, 266].
Huntington’s disease
Huntington’s Disease (HD) is a dominantly inherited autosomal neurodegenerative disorder often diagnosed at the age of 40, although onset varies from under ten to over eighty years of age. While not traditionally considered an aging-related disease, research has revealed epigenetic age acceleration in specific brain regions of that HD [267]. Recently, morphological changes in blood vessels and BBB leakage in the caudate and putamen were observed in HD patients using magnetic resonance imaging. In HD patient samples from the putamen and striatal samples of the HD model R6/2 mice, Occludin and Claudin-5 protein levels were decreased and evidence of increased BBB permeability was found [268].
HD is distinguished by the neuropathic phenotypes in glial cells, which disrupt these cells regulatory roles and involvement in BBB breakdown (Fig. 5). Mutant huntingtin accumulates in glial cells, impairing their regulatory functions, which leads to BBB disruption [269]. Glial cell activation occurs through cytokines like TNF-α, IL-6, chemokines, iNOS, and cyclooxygenase-2 (COX-2) [270], along with NF-κB pathway activation in both neural and glial cells [271]. Activation of the NRF2 pathway may reduce the synthesis of these inflammatory and, by limiting ROS production, could help protect against oxidative stress-induced BBB damage in HD [272].
Studies using STHdhQ111/Q111 HD-like transgenic mice indicate reduced NRF2 activity and altered KEAP1 and p62 in striatal cells [273]. As NRF2 regulates macroautophagy pathways, including p62 expression, decreased p62 levels in STHdhQ111/Q111 models could impair autophagy, preventing huntingtin degradation and ultimately compromising BBB integrity [273].
NRF2 protective effects on cigarette smoke toxicity
Cigarette smoking (CS) is considered a major risk factor for several neurological disorders and neurovascular complications including stroke, small vessel ischemic disease (SVID), and cerebrovascular diseases. Research attributes these detrimental effects to the oxidative and inflammatory damage caused by a large and poorly identified number of high ROS contained in tobacco smoke (TS) [274]. The BBB is directly exposed to this host of harmful toxicants and ROS present in TS, making it a critical factor in TS-promoted CNS disorders (Fig. 6). When cigarette smoke is inhaled, many soluble and gaseous components bypass first-pass metabolism and enter the brain microvasculature via arterial circulation [274].
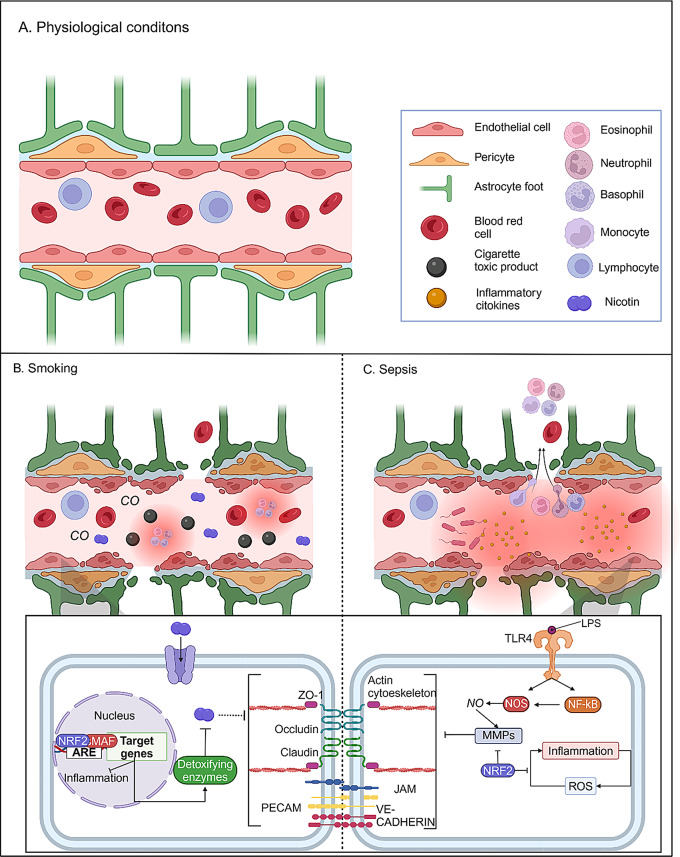
Fig. 6.
Effects of damaging substances present in the bloodstream. A), healthy conditions of a brain vessel, with all the components of the neurovascular unit. B), BBB damage caused by smoking, which causes the entry into the bloodstream of damaging gases such as CO, toxic products, and nicotine which results in inflammation, and nicotine is known to dysregulate the BEC cell junctions. C), sepsis is characterized by the elevated levels of pro-inflammatory cytokines in the bloodstream, causing an elevated inflammatory situation in the tissue, LPS present in bacteria is known to stimulate the release of nitric oxidate that activates metalloproteinases, degrading cell junctions
Despite substantial evidence linking smoking and vascular impairment, the impact of CS on the BBB has only been marginally addressed. Previous studies have disclosed that soluble TS extracts trigger strong pro-inflammatory responses at the BBB endothelial level [275]. Nicotine exposure, a key component of TS, has been found to downregulate the expression of TJ proteins such as ZO-1, occludins, and cadherins, leading to an increased BBB permeability [276, 277].
NRF2 pathway is activated in response to CS in resident macrophages, bronchial and alveolar epithelium, and lung fibroblasts of chronic smokers [278]. Following TS exposure, NRF2 translocates to the nucleus and promotes the expression of detoxification enzymes and antioxidants (Fig. 6) [88], which reduce inflammation and restore TJ protein expression [279]. However, chronic TS exposure can impair the NRF2/ARE antioxidant pathway, resulting in cerebrovascular damage and BBB breakdown, as alterations in the transcription and activation of this pathway were among the most significant changes observed in human BEC [18, 87]. Studies suggest that pharmacological activation of NRF2 reduces CS toxicity by stabilizing TJ proteins, upregulating GLUT1, and decreasing oxidative stress and inflammation [87].
NRF2 protective effects on sepsis-associated encephalopathy
Sepsis-associated encephalopathy (SAE) is a common and fatal disease, especially in critically ill patients due to systemic infection. The pathogenesis and progress of sepsis are life-threatening organ dysfunctions caused by an abnormal immune response to infection [280, 281]. This dysregulated immune response disrupts brain homeostasis and leads to a substantial risk of long-term cognitive impairment, resulting in sepsis-associated encephalopathy (SAE). SAE is characterized by excessive microglial activation, endothelial barrier dysfunction, and BBB impairment (Fig. 6), often accompanied by psychological disorders, including cognitive impairment and coma [282].
The classic model that is usually carried out in both in vitro and in vivo models is an LPS treatment, which leads to inflammation by NF-kB activation and can increase the production of ROS [48, 283, 284]. This oxidative stress damages cells, triggering neuroinflammation and the secretion of neurotransmitters such as ATP and nitric oxide (NO) [48, 65]. Moreover, ROS acts as a secondary messenger, sustaining immune activation in a feedback loop that leads to persistent inflammation and more oxidative stress. RNS can activate MMPs which trigger BBB disruption and neuroinflammation [65], and reduce the expression of TJ proteins such as ZO-1, Occludin, and Claudin-5 by stimulating the secretion of more MMPs [283].
In this context, NRF2 serves as a crucial protective factor against LPS-induced BBB injury. Studies in mouse brain microvascular endothelial cells and the hippocampus show that NRF2 protects the BBB from LPS-induced damage (Fig. 6) [48, 99]. NRF2 significantly alleviated LPS-induced BBB dysfunction both in vitro and in vivo, as evidenced by the improved TJs proteins (ZO-1 and Occludin) and the reduced expression levels of vascular cell adhesion molecule 1 (VCAM-1), an adhesion molecule that promotes BBB integrity (Fig. 6) [285, 286]. By reducing BBB permeability, NRF2 helps prevent inflammatory cytokines from infiltrating the brain, limiting microglial activation and subsequent neural cell damage [112].
Cecal ligation and puncture (CLP) is another sepsis mice model to evaluate the BBB integrity. It consists of the perforation of the cecum allowing the release of fecal material into the peritoneal cavity to generate an exacerbated immune response induced by polymicrobial infection. In a study by Yu et al., NRF2 activators in pretreated mice were associated with better cognitive outcomes, reduced BBB damage markers (e.g., decreased brain water content and dye leakage), and improved BBB integrity [287]. Nrf2-deficient mice showed greater expression of pro-inflammatory markers and oxidative stress indicators, compromising BBB stability. In contrast, wild-type mice with functional NRF2 maintained higher levels of TJ proteins like VE-Cadherin and ZO-1 in the cortex, protecting against BBB injury in SAE [287].
NRF2 inducers are protective of the Blood-Brain Barrier integrity
From a clinical perspective, the main asset of NRF2 is its potential as a pharmacological target for patient therapy [8]. Several NRF2 activators are plant-derived phytochemicals, including isothiocyanates, triterpenoids, curcumin, resveratrol, and lycopene [41], or synthetic sourced compounds [48, 288]. Many of these activators can prevent the KEAP1-mediated proteasomal degradation of NRF2, the main regulator of NRF2, thus enhancing the stabilization of the protein. Other NRF2 activators mediate the pathway through signaling cascades, further increasing its protective effects [289, 290].
This review highlights the potential of NRF2 activators, particularly phytochemicals, in maintaining BBB integrity across various neurological disorders (see Summary in Table 1).
Table 1.
Summary of NRF2 inducers involved in BBB integrity protection
| Compound | Source | Regulatory pathways | Physiopathologic model |
Reference |
|---|---|---|---|---|
| Oridonin | Chinese herbal medicine (Isodon rubescens) | JNK signaling pathway and export to the nucleus |
Ischemic/stroke (Mouse) |
[293] |
| Rhein | Medicinal plants (Rheum palmatum, Cassia tora, Polygonum multiflorum, and Aloe barbadensis) | Undescribed / Unknown |
Ischemic/stroke (Rat) |
[298] |
| Kinsenoside | Medicinal plants of the genus Anoectochilus (Anoectochilus roxburghii) | ERK signaling pathway and dissociation to KEAP1 |
Ischemic/stroke (Mouse) |
[301] |
| Nomilin | Citrus fruits including lemons, limes, oranges, grapefruits, mandarins | KEAP1 pathway |
Ischemic/stroke (Rat) |
[304] |
| Procyanidin B2 | Highest concentration in cocoa powder, chocolate, and broad beans (Vicia faba) and the lowest concentration in strawberries (Fragaria X ananassa), rubus (blackberry, raspberry), and cloudberries (Rubus chamaemorus). | SIRT1 pathway |
Ischemic/stroke (Rat) |
[307] |
| Dimethyl fumarate | Not biologic (Synthetic compound) | KEAP1 pathway |
Ischemic/stroke (Mouse) |
[316] |
| Sulforaphane | Naturally derived from the Brassica vegetable family (cauliflower, kohlrabi, broccoli, etc.) | KEAP1 pathway |
Traumatic brain injury (Rat) |
[152] |
| Allyl isothiocyanate | Natural cruciferous vegetables (family Brassicaceae) such as horseradish, mustard, radish, and wasabi | KEAP1 pathway |
Traumatic brain injury (Mouse) |
[325] |
| Fisetin | Flavonoid presents in vegetables and fruits such as apples, strawberries, grapes, cucumbers and persimmon | PI3K/AKT signaling pathway and dissociation to β-TrCP1 |
Traumatic brain injury (Mouse) |
[332] |
| Metformin | Medicinal plant known as French lilac or goat’s rue (Galega officinalis) | AMPK pathway and dissociation to β-TrCP2 |
Cigarette smoking toxicity (Mouse) |
[87] |
| Rosiglitazone | Is not biologic (Synthetic compound) | PI3K/AKT signaling pathway and dissociation to β-TrCP1 |
Cigarette smoking toxicity (Cell culture) |
[279] |
| Liensinine | Bisbenzylisoquinoline alkaloid found in various parts of the lotus (Nelumbo nucifera Gaertn) including seeds | KEAP1 pathway |
Sepsis (Mouse) |
[350] |
| Astragaloside IV | Chinese medicine herb derived from Astragalus membranaceus |
Dual regulation: SIRT1 pathway and PI3K/AKT signaling pathway and dissociation to β-TrCP1 |
Sepsis (Mouse) |
[132] |
| Baicalin | Natural flavonoids extracted from the roots of Oroxylum indicum (L.) Kurz and Scutellaria baicalensis Georgi | KEAP1 pathway |
Sepsis (Mouse) |
[99] |
| GYY4137 | Is not biologic (Synthetic compound) | KEAP1 pathway |
Sepsis (Mouse) |
[112] |
| Fenretinide | Synthetic compound derivative of retinoic acid | KEAP1 pathway |
Sepsis (Mouse) |
[48] |
BBB protective compounds in ischemic/stroke models
Oridonin, a natural diterpenoid compound extracted from Chinese herbs, has been proven to exert anti-oxidative stress effects in various disease models [291, 292]. Li et al. (2021) demonstrated that oridonin protects BBB integrity in an ischemic stroke (I/S) mouse model by upregulating TJ protein expression, inhibiting peripheral immune cell infiltration, and reducing neuroinflammation, ultimately lowering infarct volume [293]. Mechanistically, oridonin promotes NRF2 nuclear translocation through c-Jun N-terminal kinase (JNK) phosphorylation, allowing NRF2 to bind antioxidant response elements ARE and activate gene transcription [293, 294].
Rhein is the major ingredient of several traditional Chinese medicines (TCM) which has various pharmacological effects such as anti-inflammatory, antioxidant, and anti-cancer properties [295–297]. In the middle cerebral artery occlusion (MCAO) model in rats, Rhein improved neurological deficits, reduced infarct size, and preserved BBB integrity. Rhein’s neuroprotective effects are attributed to its NRF2-dependent activity, which reduces ROS, suppresses ferroptosis, and mitigates oxidative stress [298]. Rhein protected against Oxygen and Glucose Deprivation/Reoxygenation (OGD/R)-induced by regulating the NRF2 signaling pathway, and its effect was abolished upon NRF2 inhibition, suggesting that Rhein’s neuroprotective action is NRF2-dependent [298]. Although the molecular pathway behind NRF2 activation remains undescribed, however, molecular docking has been revealed to be capable of binding directly to the NRF2 protein [298].
Kinsenoside (KD), a major bioactive component in Anoectochilus roxburghii, has demonstrated efficacy in treating several disorders, such as diabetes, liver disease, osteoporosis, osteoarthritis, hyperlipidemia, endotoxin shock, and acute lung injury [299, 300]. Qiao et al. (2023) found that KD reduced infarct volume, neurological deficits, and brain edema while preserving BBB integrity by upregulating TJ proteins like Occludin, Claudin-5, and ZO-1 [301]. KD improved BBB structure and function, as evidenced by a lower 18 F-fluorodeoxyglucose pass rate of the BBB and upregulation of TJ proteins such as Occludin, Claudin-5, and ZO-1. It was proposed that the activation of NRF2 happened through the binding to ERK 1/2, which could promote NRF2 phosphorylation, causing its dissociation with KEAP1 and nuclear translocation, as well as, NRF2/HO-1 axis signaling protein stimulation [301].
Nomilin (NOM) is a triterpenoid, which exists in common edible citrus fruits. NOM has anticancer properties, immunomodulatory and antiproliferative activities, among others [302, 303]. Shi et al. (2019) investigated NOM’s neuroprotective effects in a MCAO stroke model and found it reduced infarct area, brain edema, and BBB disruption while stabilizing TJ proteins ZO-1 and Occludin [304]. Further results revealed that NOM treatment effectively mitigated oxidative stress and facilitated the expressions of NRF2 and NQO1, achieved by preventing the binding of KEAP1 to NRF2 [305], which might confirm that the loss of TJ proteins in the microvasculature was likely mediated by oxidative stress [304].
Procyanidin B2 (PB) composed of two molecules of the flavan-3-ol (−)-epicatechin is one of the most common procyanidins found in plants such as cocoa, apples, or grapes. PB is considered a bioactive component due to its benefits in health promotion as well as in the restoration and maintenance of homeostasis [306]. Wu et al. (2015) demonstrated PB’s neuroprotective effects in the MCAO stroke model, where it decreased infarct volume, reduced brain edema, and preserved BBB integrity by reducing Evans blue leakage and IgG levels as well as increasing TJ expression levels such as ZO-1 [307]. The activities of antioxidant enzymes were elevated, because PB reversed the suppression of NRF2 nuclear translocation, and increased the protein expression of HO-1, Glutathione S-transferase-α (GSTα), and NQO1 in the ipsilateral ischemic area of the brain [307]. PB’s effect on NRF2 increased activated is mediated through the deacetylase sirtuin-1 (SIRT1), which induces NRF2 transcription [308]. SIRT1 has been linked to increased NRF2 transcription and improved DNA binding of NRF2 to its target genes by promoting the deacetylation of NRF2, stabilizing the transcription factor and enhancing its activity [309]. This interaction improves NRF2’s ability to bind to antioxidant response elements (ARE) in the DNA, leading to the activation of numerous genes involved in the antioxidant defense mechanism [308].
Dimethyl fumarate (DMF) is known for its anti-inflammatory and anti-oxidative activity in a variety of tissues and cell types. DMF is a well-characterized activator of NRF2, acting as an electrophile which reacts to KEAP1 redox-sensitive cysteines, promoting a conformation change which is unable to recruit the ubiquitination machinery, allowing for the free NRF2 to travel to the nucleus and promote its transcription [263]. In vitro, DMF and its primary metabolite monomethyl fumarate (MMF) increased the survival rate of astrocytes and neurons exposed to oxidative stress conditions [310–312]. Clinically used for treating psoriasis [313] and, more recently, for multiple sclerosis [314, 315], Kunze et al. (2015) validated the DMF effect in it stabilized the BBB by preventing disruption of endothelial TJs and gap formation, and decreased matrix metalloproteinase activity in the brain stroke model [316], as well as inhibited inflammatory cytokine expression and attenuated leukocyte transmigration. DMF activated the NRF2 pathway as shown by the upregulation of several target genes in the brain in vivo, as well as in cerebral endothelial cells and astrocytes in vitro, where DMF also increased the protein abundance of nuclear NRF2 [316].
BBB protective compounds in traumatic brain injury models
Sulforaphane (SFN) is an isothiocyanate abundant in cruciferous vegetables (e.g., broccoli), and was identified as being one of the most potent inducers of NRF2 [317, 318]. SFN acts as an electrophile, inhibiting the KEAP1 degradation pathway [290], and showcases antioxidant, antiproliferative, and anticarcinogenic properties [319]. Zhao et al. (2007) analyzed the effect of the NRF2 activation by SFN in traumatic brain injury (TBI) models using pneumatic piston-induced injury [152]. Postinjury administration of SFN reduced the loss of endothelial cell markers and TJ proteins and preserved BBB function. This effect was NRF2-dependent, as benefits were not observed in Nrf2-deficient mice or those pretreated with NRF2-decoy oligonucleotide [152].
Allyl isothiocyanate (AITC) is a member of a group of naturally occurring compounds called isothiocyanates and is found in radish, mustard, and wasabi [320]. These compounds have been shown to exhibit antioxidant, anti-inflammatory, anticancer, and antimicrobial properties by inducing NRF2 activation [321–324]. In a cryogenic TBI model in mice, Caglayan et al. (2019) analyzed its effects on NRF2 and NF-κB signaling pathways [325]. AITC which was administered immediately after the injury significantly decreased infarct volume and BBB permeability. Protein levels of pro-inflammatory cytokines IL-1β and IL6, glial fibrillary acidic protein (GFAP), and NF-κB were decreased, while NRF2 and neural cell adhesion molecule levels were increased with AITC when compared with vehicle control [325].
Fisetin is a flavonoid present in fruits and vegetables like apples, strawberries, grapes, cucumbers, and persimmon, known for its anti-carcinogenic, anti-inflammatory, and antioxidant effects [326, 327]. In addition, fisetin was also a potent scavenger of reactive oxygen species (ROS). It has been used to prevent oxidative stress-induced pathologies, such as Alzheimer’s and Parkinson’s diseases, skin damage, liver injury, and diabetic neuropathy [328–331]. Zhang et al. (2018) demonstrated that fisetin improved neurological function, reduced brain edema, and preserved BBB integrity in TBI [332]. Administration of fisetin suppressed neuron cell death and apoptosis, increased the expression of B-cell lymphoma 2 (BCL-2), and decreased the expression of Bcl-2-associated X protein (BAX) and caspase-3 after TBI. In addition, fisetin activated the NRF2/ARE pathway following TBI. However, fisetin only failed to suppress oxidative stress in Nrf2-deficient mice [332]. Activation of NRF2 by fisetin has been described to occur by activating the PI3K/AKT pathway, which, in turn, prevents NRF2 degradation by the β-TrCP1 pathway through GSK3 inhibitory phosphorylation [333].
BBB protective compounds in cigarette smoking toxicity models
Metformin (MF) widely used in diabetes management, also has possible renoprotective properties as well as neuroprotective effects on BBB integrity [334–337]. Prasad et al. (2017) found that MF, through NRF2 activation, counteracted cigarette smoke (CS) toxicity by preserving TJ protein expression, reducing inflammation and oxidative stress, and normalizing GLUT-1 and thrombomodulin levels [87] which drastically reduces CS toxicity at the cerebrovascular level. Although the mechanism by which can activate NRF2 remains to be fully elucidated, it has been known to inhibit AMPK. AMPK addresses phosphorylation to NRF2 and prevents its activation by degradation through β-TrCP2. Thus, MF can lead to the induction of a selected group of NRF2-target genes [336, 338].
Rosiglitazone (RSG), is a thiazolidinedione compound that is well known to improve insulin resistance through regulating adiponectin gene expression and is used for the treatment of type-2 diabetes mellitus [339]. It is considered to act as a transcription factor peroxisome proliferator-activated receptor (PPARγ) agonist [340, 341]. Since chronic smoking and diabetes carry similar risks for cerebrovascular diseases and stroke, it is plausible that RSG can prevent/reduce BBB impairment promoted by chronic TS and recent e-cigarette vaping exposure. Although the exact mechanism of RSG is not fully understood, previous studies have revealed that RSG can promote counteractive protective mechanisms primarily associated with the enhancement of NRF2 activity through activation of the peroxisome proliferator-activated receptor gamma [342]. as well as through the PI3K/AKT pathway [343]. Sivandzade et al. (2019) reported that RSG increased PPARγ expression and NRF2 activity, which protected the BBB in models of tobacco smoke and e-cigarette exposure [279].
BBB protective compounds in sepsis-associated encephalopathy models
Liensinine, an alkaloid extracted from lotus plumule [344], has multiple biological activities, such as anti-oxidative stress [345, 346], anti-inflammation [347], and anti-hypertension [348, 349]. Wang et al. (2023) found that liensinine preserved BBB integrity in a sepsis-associated encephalopathy (SAE) model by upregulating TJ proteins and decreasing oxidative stress through NRF2 activation [350]. Mice were pretreated with LPS, which triggered brain necrosis and disrupted the integrity and permeability of the BBB. While liensinine restored cerebrum structure and improved BBB integrity with upregulated TJs proteins, decreased Evans-Blue leakage and fibrinogen expression with decreased MMP-2/9 in serum, thereby reducing BBB permeability. Moreover, LPS triggered cerebrum oxidative stress and inflammation, whereas liensinine enhanced antioxidant enzyme activities and weakened malondialdehyde through the NRF2 pathway [350], which could be by the inhibition of KEAP1 binding to NRF2 [351].
Astragaloside IV (ASIV) is one of the active components present in Astragalus membranaceous, a plant used in traditional Chinese medicine. Many studies have shown that the administration of ASIV may facilitate the alleviation of CNS diseases, such as multiple sclerosis, traumatic brain injury, and cerebral ischemia or ischemia/reperfusion [352–358], through antioxidant, anti-inflammation, or anti-apoptosis. Li et al. (2017) demonstrated ASIV’s efficacy in preventing BBB disruption by increasing TJ protein expression and reducing VCAM-1 in LPS-stimulated mice [132]. ASIV was found to prevent the leakage of BBB in LPS-induced mice, which was accompanied by increased ZO-1 and Occludin but reduced VCAM-1 in brain microvessels. Moreover, in bEnd.3 cells line, ASIV mitigated the increased permeability induced by LPS. ASIV also enhanced the expression of TJ proteins such as ZO-1, Occludin, and Claudin-5 in LPS-stimulated bEnd.3 cells. Meanwhile, it inhibited inflammatory responses and prevented the monocyte adhesion onto bEnd.3 cells upon LPS stimulation. Further study disclosed that ASIV could alleviate ROS levels and activate the NRF2 antioxidant pathway in bEnd.3 cells. When Nrf2 was silenced, the protective effect of ASIV was abolished [132]. It has been proposed to activate NRF2 through different pathways, such as SIRT1 and PI3K/AKT pathway [359–361].
Baicalin is one of the main bioactive components in the extract of Scutellaria baicalensis. It has anti-inflammatory, anti-cancer, anti-diabetic, anti-thrombotic, cardioprotective, liver protection, and neuroprotective pharmacological properties [362]. Thus, Wang, X. et al. 2021 demonstrated that treatment with baicalin can inhibit the production of pro-inflammatory cytokines induced by LPS in mice and bEnd.3 cells, including IL-1β and TNF-α [99]. At the same time, LPS caused a decrease in TJ proteins in the BBB, but baicalin can alleviate the damage by up-regulating Claudin-5 and ZO-1 protein expression. In addition, the results showed that baicalin reduced the production of ROS and oxidative parameter malonaldehyde (MDA) in bEnd.3 cells and promoted the production of SOD, and up-regulated the expression of NRF2, HO-1, and NQO1 [99]. The mechanism of this change was mediated by activating the NRF2 signaling pathway through the KEAP1 pathway [363].
GYY4137, a new synthetic compound of hydrogen sulfide (H2S), can release lower concentrations of H2S over a longer period in the form of an aqueous solution, and it has extensive biological benefits. Currently, GYY4137 is widely used in studies focusing on the protective effects of H2S [364, 365]. In septic mice, Cui et al. (2021) found that GYY4137 inhibited TJ degradation and oxidative stress by activating NRF2 through KEAP1 modification [112]. These results suggested that administrated GYY4137, in combination with LPS, significantly alleviated the clinical symptoms, improving the pathological abnormalities of septic mice. Moreover, the degradation of TJs in the BBB was considerably inhibited by GYY4137, and significantly attenuated inflammation and apoptosis in the brain.
Fenretinide (FEN), the synthetic retinoid 4-hydroxy(phenyl) retinamide, is a KEAP1-NRF2 protein-protein interaction inhibitor. It has been widely studied in cancer therapeutic because of its favorable toxicological profile and is presently undergoing phase II clinical trials for the suppression of insulin resistance in obese humans with hepatic steatosis [366–368].
Previous studies have shown that FEN reduced the expression of pro-inflammatory genes and the levels of oxidative stress markers after spinal cord injury (SCI) [369]. Li et al. (2020) observed that the FEN treatment markedly improved NRF2 expression and nuclear translocation in bEnd.3 cells, and promoted NRF2/ARE transcription activity, and its downstream signals, which were NRF2-dependent [48], possibly acting as an NRF2-KEAP1 Protein-Protein Interaction-inhibitor. FEN also exhibited a cytoprotective role in LPS-stimulated bEnd.3 cells through improving antioxidant capacity and inhibiting inflammation by blocking NF-κB signaling. In a mouse model with brain injury induced by LPS, FEN administration markedly attenuated the behavior impairments, BBB, and histological changes in hippocampus samples [48].
Even though other NRF2 inducers have been tested for BBB protection in various neurological conditions, studies exploring NRF2 compounds in diabetes, obesity, hypertension, and certain neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s, Huntington’s) remain limited, indicating a significant area for future research.
Conclusion remarks
There is a growing list of CNS pathologies involving BBB dysfunction, including multiple sclerosis [370–372]; hypoxia and ischemia [373–375]; edema [375, 376]; Parkinson’s disease [377–380], Alzheimer’s disease [378, 380–383]. Other well-known diseases in which a disturbed Blood-Brain Barrier (BBB) plays a recognized role include epilepsy [384, 385]; tumors [386, 387] lysosomal storage diseases [388, 389] and glaucoma [390, 391] in blood-retinal barrier (BRB) injury. The barrier dysfunction can range from mild and transient TJs opening to chronic barrier breakdown [392–394], and changes in transport systems and enzymes can also occur. While the causal relationship between BBB disturbance and disease initiation is not always clear, its contribution to disease progression is well documented [395, 396].
Research has identified multiple mechanisms contributing to neurological disorders during secondary injury, including inflammatory responses, oxidative stress, mitochondrial dysfunction, and Blood-Brain Barrier (BBB) disruption. NRF2 plays a crucial role in this context, as its activation helps mitigate oxidative stress and inflammation. Furthermore, NRF2 has been shown to regulate tight junctions and matrix metalloproteinases, which are vital for maintaining BBB integrity under neurophysiological conditions.
Numerous compounds, both phytochemicals derived from fruits, vegetables, and medicinal herbs, as well as synthetic agents that inhibit the KEAP1-NRF2 interaction, exhibit significant neuroprotective effects. These effects include enhancing BBB integrity, preserving neuronal viability, and curbing excessive inflammation through NRF2-mediated oxidative stress responses. Despite extensive evidence of the neuroprotective properties of various phytochemicals in vitro and in vivo, there remains a notable gap in effective clinical applications. In spite of promising preclinical data, translating these findings into effective clinical treatments remains a challenge. To date, only a few compounds, like sulforaphane and dimethyl fumarate (DMF), have progressed to clinical trials targeting CNS diseases and may offer protection against BBB damage [397, 398]. Repurposing existing drugs, such as metformin and rosiglitazone (currently used in diabetes treatment), for CNS diseases is an attractive option due to their known mechanisms involving similar pathways.
To optimize the therapeutic potential of NRF2 activators for BBB protection, careful consideration must be given to patient selection, drug dosage, delivery methods, timing of administration, and administration protocols to maximize benefits while minimizing side effects. Additionally, the safety and pharmacokinetics of these phytochemicals are still poorly understood, highlighting the need for further research to facilitate their clinical introduction.
Acknowledgements
This article is based upon work from COST Action CA20121, supported by COST (European Cooperation in Science and Technology) (https://www.cost.eu) (https://benbedphar.org/about-benbedphar/).
Abbreviations
- Aβ
Amyloid-β
- AD
Alzheimer´s Disease
- AGEs
Advanced Glycation End products
- AITC
Allyl Isothiocyanate
- AJs
Adherens Junctions
- AKT
Protein kinase B (PKB)
- ALM
Lipoamide
- ALS
Amyotrophic Lateral Sclerosis
- ANG-II
Angiotensin II
- ARE
Antioxidant Response Elements
- ASIV
Astragaloside IV
- α-SYN
α-synuclein
- BAX
BCL-2-Associated X protein
- BACE1
Beta-site Amyloid precursor protein Cleaving Enzyme 1
- BACH1
BTB Domain And CNC Homolog 1
- BBB
Blood-Brain Barrier
- BCL-2
B-Cell Lymphoma 2
- BEC
Brain Endothelial Cells
- BRB
Blood-Retinal Barrier
- β-TrCP
β-Transducin repeat-Containing Protein
- CAT
Catalase
- CAP
Capsaicin
- CBF
Cerebral Blood Flow
- CLP
Cecal Ligation and Puncture
- CNS
Central Nervous System
- CO
Carbon monoxide
- COX-2
Cyclooxygenase-2
- CS
Cigarette Smoking
- DMF
Dimethyl Fumarate
- EC
Endothelial Cells
- ECM
Extra Cellular Matrix
- EpRE
Electrophile Response Elements
- ERK
Extracellular signal-Regulated Kinases
- FEN
Fenretinide
- FPN
Ferroportin
- FSP1
Ferroptosis Suppressor Protein-1
- FTH1
Ferritin heavy chain
- GCLC
Catalytic subunits of γ-glutamyl synthase
- GCLM
Modulatory subunits of γ-glutamyl synthase
- GFAP
Glial Fibrillary Acidic Protein
- GLUT1
Glucose Transporter 1
- GPX4
Glutathione Peroxidase-4
- GPX
Glutathione peroxidase
- GSH
Glutathione
- GSK-3
Glycogen synthase kinase
- GSTα
Glutathione S-Transferase-α
- H2S
Hydrogen Sulfide
- HAND
HIV-Associated Neurocognitive Disorder
- HIF-1α
Hypoxia-inducible factor 1-alpha
- HNE
4-hydroxynonenal
- HO-1
Heme Oxygenase 1
- HSP
Heat Shoot Proteins
- IFN-γ
Interferon-γ
- IgG
Immunoglobulin G
- IKKβ
Inhibitor of nuclear factor Kappa-B Kinase subunit β
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- iNOS
Inducible Nitric Oxide Synthase
- I/R
Ischemia/Reperfusion injury
- I/S
Ischemic/Stroke
- JNK
C-Jun N-terminal kinase
- KD
Kinsenoside
- KEAP1
Kelch-like-ECH-Associated Protein 1
- LB
Lewy Body
- LPS
Lipopolysaccharide
- MAF
Musculoaponeurotic Fibrosarcoma
- MCAO
Middle Cerebral Artery Occlusion
- MDA
Malonaldehyde
- MF
Metformin
- MMF
Monomethyl Fumarate
- MMPs
Matrix Metalloproteinases
- MS
Multiple Sclerosis
- NF-κB
Nuclear Factor kappa-light-chain-enhancer of activated B
- NFT
Neuro Fibrillary Tangle
- NO
Nitrogen monoxide
- NOM
Nomilin
- NOS
Nitrogen monoxide Species
- NOX2
NADPH Oxidase 2
- NOX4
NADPH Oxidase 4
- NQO1
NAD(P)H Quinone Oxidoreductase-1
- NRF2
Nuclear factor erythroid-Related Factor 2
- NVU
NeuroVascular Unit
- OGD/R
Oxygen and Glucose Deprivation/Reoxygenation
- ox-LDL
Oxidized Low-density Lipoprotein
- PB
Procyanidin B2
- PD
Parkinson´s Disease
- PECAM1
Platelet EC Adhesion Molecules 1
- PI3K
Phosphoinositide 3-Kinases
- PKC
Protein Kinase C
- PPARγ
Peroxisome Proliferator-Activated Receptor-γ
- PVN
Hypothalamic paraVentricular Nucleus
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- RSG
Rosiglitazone
- SAE
Sepsis-Associated Encephalopathy
- SCI
Spinal Cord Injury
- SFN
Sulforaphane
- SHRs
Spontaneously Hypertensive Rats
- SIRT1
Deacetylase Sirtuin-1
- SNpc
Substantia Nigra pars compacta
- SOD
Superoxide Dismutase
- SP
Senile Plaque
- SVID
Small Vessel Ischemic Disease
- TAMP
Tight-junction Associated MARVEL Protein
- TBI
Traumatic Brain Injury
- TCM
Traditional Chinese Medicine
- TGF-β
Transforming Growth Factor-β
- TJs
Tight Junctions
- TNF-α
Tumor Necrosis Factor-α
- TrkB
Tropomyosin-related Kinase Receptor type B
- TS
Tobacco Smoke
- VCAM-1
Vascular Cell Adhesion Molecule-1
- VCID
Vascular Cognitive Impairment and Dementia
- VE-Cadherin
Vascular Endothelial-Cadherin
- VEGF
Vascular Endothelial Growth Factor
- VEGFR2
Vascular Endothelial Growth Factor Receptor 2
- ZO-1
Zonula Occludens-1
Author contributions
EC and AJGY contributed to the conceptualization and overall structure of the manuscript. EC and AJGY did the literature search, drafted the content of the manuscript, devised all the figures, and edited the manuscript. EC, AC and AJGY substantially revised and edited the manuscript, ensuring its quality and coherence. All authors have read and approved the final version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy and Competitiveness (MINECO) (grands PDC2021-121421-I00, PDC2022-1337665-I00, PID2022-141786OB-I00 and PID2019-110061RB-I00). The Autonomous Community of Madrid (grands S2017BMD-3827 and P2022_BMD-7230). EC is holder of an FPU contract of MIU (Ministry of Universities FPU2021, FPU21/02505).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eduardo Cazalla, Antonio Cuadrado and Ángel Juan García-Yagüe have contributed equally to this work.
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. [DOI] [PubMed] [Google Scholar]
- 2.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song L, Yan Y, Marzano M, Li Y. Studying Heterotypic Cell(-)Cell interactions in the human brain using pluripotent stem cell models for Neurodegeneration. Cells. 2019;8(4). [DOI] [PMC free article] [PubMed]
- 4.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in Health and Disease. Neuron. 2017;96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a Systems Medicine Approach. Pharmacol Rev. 2018;70(2):348–83. [DOI] [PubMed] [Google Scholar]
- 9.Lastra D, Escoll M, Cuadrado A. Transcription factor NRF2 participates in cell cycle progression at the level of G1/S and mitotic checkpoints. Antioxid (Basel). 2022;11(5). [DOI] [PMC free article] [PubMed]
- 10.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. [DOI] [PubMed] [Google Scholar]
- 11.Hayes JD, Chowdhry S, Dinkova-Kostova AT, Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of beta-TrCP and GSK-3. Biochem Soc Trans. 2015;43(4):611–20. [DOI] [PubMed] [Google Scholar]
- 12.Hozzein WN, Badr G, Badr BM, Allam A, Ghamdi AA, Al-Wadaan MA, et al. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol Immunol. 2018;103:322–35. [DOI] [PubMed] [Google Scholar]
- 13.He F, Ru X, Wen T. NRF2, a transcription factor for stress response and Beyond. Int J Mol Sci. 2020;21(13). [DOI] [PMC free article] [PubMed]
- 14.Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res. 2021;34:43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. [DOI] [PubMed] [Google Scholar]
- 17.Mao L, Yang T, Li X, Lei X, Sun Y, Zhao Y, et al. Protective effects of sulforaphane in experimental vascular cognitive impairment: contribution of the Nrf2 pathway. J Cereb Blood Flow Metab. 2019;39(2):352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sajja RK, Green KN, Cucullo L. Altered Nrf2 signaling mediates hypoglycemia-induced blood-brain barrier endothelial dysfunction in vitro. PLoS ONE. 2015;10(3):e0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, et al. Nrf2 Deficiency exacerbates obesity-Induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, Neuroinflammation, Amyloidogenic Gene expression, and Cognitive decline in mice, mimicking the aging phenotype. J Gerontol Biol Sci Med Sci. 2018;73(7):853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfieri A, Srivastava S, Siow RCM, Cash D, Modo M, Duchen MR, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–22. [DOI] [PubMed] [Google Scholar]
- 21.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE. 2010;5(10):e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam SJ, Richmond DL, Kaminker JS, Modrusan Z, Martin-McNulty B, Cao TC, et al. Death receptors DR6 and TROY regulate brain vascular development. Dev Cell. 2012;22(2):403–17. [DOI] [PubMed] [Google Scholar]
- 23.Zolotoff C, Voirin AC, Puech C, Roche F, Perek N. Intermittent hypoxia and its impact on Nrf2/HIF-1alpha expression and ABC transporters: an in Vitro Human Blood-Brain Barrier Model Study. Cell Physiol Biochem. 2020;54(6):1231–48. [DOI] [PubMed] [Google Scholar]
- 24.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322(5905):1247–50. [DOI] [PubMed] [Google Scholar]
- 25.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 2017;219(1):22–96. [DOI] [PubMed] [Google Scholar]
- 28.Dithmer S, Blasig IE, Fraser PA, Qin Z, Haseloff RF. The Basic requirement of tight Junction proteins in blood-brain barrier function and their role in pathologies. Int J Mol Sci. 2024;25(11). [DOI] [PMC free article] [PubMed]
- 29.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121(Pt 3):298–305. [DOI] [PubMed] [Google Scholar]
- 30.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2(1):a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321(1):89–96. [DOI] [PubMed] [Google Scholar]
- 32.Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273(6):C1859–67. [DOI] [PubMed] [Google Scholar]
- 33.Saito AC, Higashi T, Fukazawa Y, Otani T, Tauchi M, Higashi AY, et al. Occludin and Tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol Biol Cell. 2021;32(8):722–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell. 2008;19(5):1862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, et al. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208(6):821–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampugnani MG, Dejana E. The control of endothelial cell functions by adherens junctions. Novartis Found Symp. 2007;283:4–13. discussion – 7, 238 – 41. [DOI] [PubMed] [Google Scholar]
- 37.Miller G. Drug targeting. Breaking down barriers. Science. 2002;297(5584):1116–8. [DOI] [PubMed] [Google Scholar]
- 38.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589(17):4125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23(23):8786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajares M, Cuadrado A, Rojo AI. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017;11:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pajares M, Jimenez-Moreno N, Dias IHK, Debelec B, Vucetic M, Fladmark KE, et al. Redox control of protein degradation. Redox Biol. 2015;6:409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pajares M, Jimenez-Moreno N, Garcia-Yague AJ, Escoll M, de Ceballos ML, Van Leuven F, et al. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12(10):1902–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181(1):680–9. [DOI] [PubMed] [Google Scholar]
- 48.Li T, Zheng LN, Han XH. Fenretinide attenuates lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed Pharmacother. 2020;125:109680. [DOI] [PubMed] [Google Scholar]
- 49.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–22. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami Y, Kinoshita M, Mori Y, Okochi S, Hirano S, Shimoda I, et al. The Y54(L)W mutation of anti-leukotriene C4 single-chain antibody increases affinity to leukotriene E4. J Biochem. 2017;161(1):79–86. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava R, Fernandez-Gines R, Encinar JA, Cuadrado A, Wells G. The current status and future prospects for therapeutic targeting of KEAP1-NRF2 and beta-TrCP-NRF2 interactions in cancer chemoresistance. Free Radic Biol Med. 2022;192:246–60. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32(32):3765–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojo AI, Rada P, Egea J, Rosa AO, Lopez MG, Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci. 2008;39(1):125–32. [DOI] [PubMed] [Google Scholar]
- 54.Baird L, Yamamoto M. The Molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 2020;40(13). [DOI] [PMC free article] [PubMed]
- 55.Yusoff FM, Maruhashi T, Kawano KI, Nakashima A, Chayama K, Tashiro S, et al. Bach1 plays an important role in angiogenesis through regulation of oxidative stress. Microvasc Res. 2021;134:104126. [DOI] [PubMed] [Google Scholar]
- 56.Aliev G, Obrenovich ME, Reddy VP, Shenk JC, Moreira PI, Nunomura A, et al. Antioxidant therapy in Alzheimer’s disease: theory and practice. Mini Rev Med Chem. 2008;8(13):1395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280(4):C719–41. [DOI] [PubMed] [Google Scholar]
- 58.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 2012;1822(5):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. [DOI] [PubMed] [Google Scholar]
- 60.Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999;31(4):261–72. [DOI] [PubMed] [Google Scholar]
- 61.Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011;17(33):3729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng ZV, Lyu H, Lam SYE, Lam PK, Poon WS, Wong GKC. The dynamics of Microglial polarization reveal the Resident neuroinflammatory responses after subarachnoid hemorrhage. Transl Stroke Res. 2020;11(3):433–49. [DOI] [PubMed] [Google Scholar]
- 64.Che J, Sun Y, Deng Y, Zhang J. Blood-brain barrier disruption: a culprit of cognitive decline? Fluids Barriers CNS. 2024;21(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Chen F, Zhang Y, Zheng X, Liu S, Tang M, et al. Biphasic effect of sulforaphane on angiogenesis in hypoxia via modulation of both Nrf2 and mitochondrial dynamics. Food Funct. 2022;13(5):2884–98. [DOI] [PubMed] [Google Scholar]
- 66.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng D, Zhou J, Liu H, Wu X, Li F, Zhao J, et al. Astrocytic NDRG2-PPM1A interaction exacerbates blood-brain barrier disruption after subarachnoid hemorrhage. Sci Adv. 2022;8(39):eabq2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wenzel P, Kossmann S, Munzel T, Daiber A. Redox regulation of cardiovascular inflammation - immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol Med. 2017;109:48–60. [DOI] [PubMed] [Google Scholar]
- 72.Nair S, Doh ST, Chan JY, Kong AN, Cai L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer. 2008;99(12):2070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rushworth SA, Zaitseva L, Murray MY, Shah NM, Bowles KM, MacEwan DJ. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-kappaB and underlies its chemo-resistance. Blood. 2012;120(26):5188–98. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58(5):588–98. [DOI] [PubMed] [Google Scholar]
- 76.Patel M. Targeting oxidative stress in Central Nervous System disorders. Trends Pharmacol Sci. 2016;37(9):768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salim S. Oxidative stress and the Central Nervous System. J Pharmacol Exp Ther. 2017;360(1):201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yaribeygi H, Panahi Y, Javadi B, Sahebkar A. The underlying role of oxidative stress in neurodegeneration: a mechanistic review. CNS Neurol Disord Drug Targets. 2018;17(3):207–15. [DOI] [PubMed] [Google Scholar]
- 79.Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4(+)T cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Z, Mao X, Liu A, Gao X, Chen X, Ye M, et al. Osthole, a natural coumarin improves cognitive impairments and BBB dysfunction after transient global brain ischemia in C57 BL/6J mice: involvement of Nrf2 pathway. Neurochem Res. 2015;40(1):186–94. [DOI] [PubMed] [Google Scholar]
- 81.Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117–27. [DOI] [PubMed] [Google Scholar]
- 82.Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5(3):575–85. [DOI] [PubMed] [Google Scholar]
- 83.Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76(8):967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Wang L, Du N, Yin X, Shao H, Yang L. Ramelteon ameliorates LPS-Induced hyperpermeability of the blood-brain barrier (BBB) by activating Nrf2. Inflammation. 2021;44(5):1750–61. [DOI] [PubMed] [Google Scholar]
- 85.Prasad S, Sajja RK, Park JH, Naik P, Kaisar MA, Cucullo L. Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers CNS. 2015;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sajja RK, Prasad S, Tang S, Kaisar MA, Cucullo L. Blood-brain barrier disruption in diabetic mice is linked to Nrf2 signaling deficits: role of ABCB10? Neurosci Lett. 2017;653:152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prasad S, Sajja RK, Kaisar MA, Park JH, Villalba H, Liles T, et al. Role of Nrf2 and protective effects of metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naik P, Sajja RK, Prasad S, Cucullo L. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC Neurosci. 2015;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sajja RK, Kaisar MA, Vijay V, Desai VG, Prasad S, Cucullo L. In Vitro Modulation of Redox and Metabolism Interplay at the brain vascular endothelium: genomic and proteomic profiles of sulforaphane activity. Sci Rep. 2018;8(1):12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang T, Sun Y, Mao L, Zhang M, Li Q, Zhang L, et al. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018;17:323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. [DOI] [PubMed] [Google Scholar]
- 92.Hilliard A, Mendonca P, Russell TD, Soliman KFA. The Protective effects of flavonoids in Cataract formation through the activation of Nrf2 and the inhibition of MMP-9. Nutrients. 2020;12(12). [DOI] [PMC free article] [PubMed]
- 93.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21(1):1–14. [DOI] [PubMed] [Google Scholar]
- 94.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):673–87. [PubMed] [Google Scholar]
- 96.Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappab activity, tumor necrosis factor-alpha, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010;2010:238321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gu Y, Zheng G, Xu M, Li Y, Chen X, Zhu W, et al. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem. 2012;120(1):147–56. [DOI] [PubMed] [Google Scholar]
- 98.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Yu JY, Sun Y, Wang H, Shan H, Wang S. Baicalin protects LPS-induced blood-brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int Immunopharmacol. 2021;96:107725. [DOI] [PubMed] [Google Scholar]
- 100.Tamiya S, Wormstone IM, Marcantonio JM, Gavrilovic J, Duncan G. Induction of matrix metalloproteinases 2 and 9 following stress to the lens. Exp Eye Res. 2000;71(6):591–7. [DOI] [PubMed] [Google Scholar]
- 101.Lee SH, Sohn DH, Jin XY, Kim SW, Choi SC, Seo GS. 2’,4’,6’-tris(methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-alpha-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem Pharmacol. 2007;74(6):870–80. [DOI] [PubMed] [Google Scholar]
- 102.Kim BC, Jeon WK, Hong HY, Jeon KB, Hahn JH, Kim YM et al. The anti-inflammatory activity of Phellinus linteus (Berk. & M.A. Curt.) is mediated through the PKCdelta/Nrf2/ARE signaling to up-regulation of heme oxygenase-1. J Ethnopharmacol. 2007;113(2):240-7. [DOI] [PubMed]
- 103.Saw CL, Yang AY, Huang MT, Liu Y, Lee JH, Khor TO, et al. Nrf2 null enhances UVB-induced skin inflammation and extracellular matrix damages. Cell Biosci. 2014;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. [DOI] [PubMed] [Google Scholar]
- 105.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. [DOI] [PubMed] [Google Scholar]
- 106.Palmiotti CA, Prasad S, Naik P, Abul KM, Sajja RK, Achyuta AH, et al. In vitro cerebrovascular modeling in the 21st century: current and prospective technologies. Pharm Res. 2014;31(12):3229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol Neurodegener. 2011;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polotsky VY, Savransky V, Bevans-Fonti S, Reinke C, Li J, Grigoryev DN, et al. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol Genomics. 2010;41(3):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heiss EH, Schachner D, Zimmermann K, Dirsch VM. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 2013;1(1):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol. 2007;33(1):86–98. [DOI] [PubMed] [Google Scholar]
- 111.Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of blood-brain barrier in Alzheimer’s Disease. J Alzheimers Dis. 2018;63(4):1223–34. [DOI] [PubMed] [Google Scholar]
- 112.Cui W, Chen J, Yu F, Liu W, He M. GYY4137 protected the integrity of the blood-brain barrier via activation of the Nrf2/ARE pathway in mice with sepsis. FASEB J. 2021;35(7):e21710. [DOI] [PubMed] [Google Scholar]
- 113.Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. 2019;39(8):1542–9. [DOI] [PubMed] [Google Scholar]
- 114.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–81. [DOI] [PubMed] [Google Scholar]
- 115.Jurcau A, Ardelean AI. Oxidative stress in Ischemia/Reperfusion injuries following Acute ischemic stroke. Biomedicines. 2022;10(3). [DOI] [PMC free article] [PubMed]
- 116.Kamal FZ, Lefter R, Jaber H, Balmus IM, Ciobica A, Iordache AC. The Role of Potential Oxidative Biomarkers in the Prognosis of Acute Ischemic Stroke and the Exploration of Antioxidants as Possible Preventive and Treatment Options. Int J Mol Sci. 2023;24(7). [DOI] [PMC free article] [PubMed]
- 117.Yang Z, Zhao TZ, Zou YJ, Zhang JH, Feng H. Hypoxia induces autophagic cell death through hypoxia-inducible factor 1alpha in microglia. PLoS ONE. 2014;9(5):e96509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci (Landmark Ed). 2011;16(5):1733–45. [DOI] [PubMed] [Google Scholar]
- 119.Li M, Ke J, Deng Y, Chen C, Huang Y, Bian Y, et al. The Protective Effect of Liquiritin in Hypoxia/Reoxygenation-Induced disruption on blood brain barrier. Front Pharmacol. 2021;12:671783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41(2–3):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hahn KR, Kwon HJ, Yoon YS, Kim DW, Hwang IK. Phosphoglycerate kinase 1 protects against ischemic damage in the gerbil hippocampus. Aging. 2022;14(22):8886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amin N, Abbasi IN, Wu F, Shi Z, Sundus J, Badry A, et al. The Janus face of HIF-1alpha in ischemic stroke and the possible associated pathways. Neurochem Int. 2024;177:105747. [DOI] [PubMed] [Google Scholar]
- 124.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–7. [DOI] [PubMed] [Google Scholar]
- 125.Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. 2016;25(5):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cyran AM, Zhitkovich A. HIF1, HSF1, and NRF2: oxidant-responsive Trio raising Cellular defenses and engaging Immune System. Chem Res Toxicol. 2022;35(10):1690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bae T, Hallis SP, Kwak MK. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med. 2024;56(3):501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63(1):70–80. [DOI] [PubMed] [Google Scholar]
- 129.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–19. [DOI] [PubMed] [Google Scholar]
- 130.McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, et al. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110(1):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li H, Wang P, Huang F, Jin J, Wu H, Zhang B, et al. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol Appl Pharmacol. 2018;340:58–66. [DOI] [PubMed] [Google Scholar]
- 133.Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, et al. Tight junction proteins at the blood-brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76(10):1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Winkler L, Blasig R, Breitkreuz-Korff O, Berndt P, Dithmer S, Helms HC, et al. Tight junctions in the blood-brain barrier promote edema formation and infarct size in stroke - ambivalent effects of sealing proteins. J Cereb Blood Flow Metab. 2021;41(1):132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tavakkoli A, Iranshahi M, Hasheminezhad SH, Hayes AW, Karimi G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother Res. 2019;33(9):2256–73. [DOI] [PubMed] [Google Scholar]
- 136.Huynh LM, Burns MP, Taub DD, Blackman MR, Zhou J. Chronic neurobehavioral impairments and decreased hippocampal expression of genes important for brain glucose utilization in a mouse model of mild TBI. Front Endocrinol (Lausanne). 2020;11:556380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, et al. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med. 2013;60:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bhowmick S, D’Mello V, Ponery N, Abdul-Muneer PM. Neurodegeneration and sensorimotor deficits in the mouse model of traumatic brain Injury. Brain Sci. 2018;8(1). [DOI] [PMC free article] [PubMed]
- 139.Chen Q, Wu B, Shi Z, Wang Y, Yuan Y, Chen X, et al. LncRNA H19 knockdown promotes neuropathologic and functional recovery via the Nrf2/HO-1 axis after traumatic brain injury. CNS Neurosci Ther. 2024;30(7):e14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 141.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakano-Kobayashi A, Canela A, Yoshihara T, Hagiwara M. Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proc Natl Acad Sci U S A. 2023;120(33):e2303809120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C, Zhao XM, Wang Q, Du TT, Zhang MX, Wang HZ, et al. Astrocyte-derived SerpinA3N promotes neuroinflammation and epileptic seizures by activating the NF-kappaB signaling pathway in mice with temporal lobe epilepsy. J Neuroinflammation. 2023;20(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chandran R, Kim T, Mehta SL, Udho E, Chanana V, Cengiz P, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab. 2018;38(10):1818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng H, Wang P, Wang N, Dong W, Chen Z, Wu M et al. Neuroprotection of NRF2 against Ferroptosis after traumatic brain Injury in mice. Antioxid (Basel). 2023;12(3). [DOI] [PMC free article] [PubMed]
- 147.Pietsch EC, Chan JY, Torti FM, Torti SV. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J Biol Chem. 2003;278(4):2361–9. [DOI] [PubMed] [Google Scholar]
- 148.Li C, Wu Z, Xue H, Gao Q, Zhang Y, Wang C, et al. Ferroptosis contributes to hypoxic-ischemic brain injury in neonatal rats: role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci Ther. 2022;28(12):2268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen T, Xu YP, Chen Y, Sun S, Yan ZZ, Wang YH. Arc regulates brain damage and neuroinflammation via Sirt1 signaling following subarachnoid hemorrhage. Brain Res Bull. 2023;203:110780. [DOI] [PubMed] [Google Scholar]
- 150.Xu Y, Jia B, Li J, Li Q, Luo C. The interplay between Ferroptosis and neuroinflammation in Central Neurological disorders. Antioxid (Basel). 2024;13(4). [DOI] [PMC free article] [PubMed]
- 151.Cheng Y, Gao Y, Li J, Rui T, Li Q, Chen H, et al. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic Biol Med. 2023;194:184–98. [DOI] [PubMed] [Google Scholar]
- 152.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27(38):10240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Munoz Maniega S, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, Heye AK, et al. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37(2):644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304(12):H1598–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cheon EJ. Hypertension and cognitive dysfunction: a narrative review. J Yeungnam Med Sci. 2023;40(3):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5(3):255–61. [DOI] [PubMed] [Google Scholar]
- 158.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–93. [DOI] [PubMed] [Google Scholar]
- 159.Bai J, Yu XJ, Liu KL, Wang FF, Jing GX, Li HB, et al. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol Appl Pharmacol. 2017;333:100–9. [DOI] [PubMed] [Google Scholar]
- 160.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-kappabin the paraventricular nucleus. Hypertension. 2012;59(1):113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gabor A, Leenen FH. Central neuromodulatory pathways regulating sympathetic activity in hypertension. J Appl Physiol (1985). 2012;113(8):1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oliveira-Sales EB, Nishi EE, Carillo BA, Boim MA, Dolnikoff MS, Bergamaschi CT, et al. Oxidative stress in the sympathetic premotor neurons contributes to sympathetic activation in renovascular hypertension. Am J Hypertens. 2009;22(5):484–92. [DOI] [PubMed] [Google Scholar]
- 163.Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol. 2014;276(2):115–20. [DOI] [PubMed] [Google Scholar]
- 164.Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation. 2015;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, et al. Central Renin-Angiotensin System activation and inflammation Induced by High-Fat Diet sensitize angiotensin II-Elicited hypertension. Hypertension. 2016;67(1):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen Y, Yuan T, Zhang H, Yan Y, Wang D, Fang L, et al. Activation of Nrf2 attenuates pulmonary vascular remodeling via inhibiting endothelial-to-mesenchymal transition: an insight from a plant Polyphenol. Int J Biol Sci. 2017;13(8):1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jia XY, Yang Y, Jia XT, Jiang DL, Fu LY, Tian H, et al. Capsaicin pretreatment attenuates salt-sensitive hypertension by alleviating AMPK/Akt/Nrf2 pathway in hypothalamic paraventricular nucleus. Front Neurosci. 2024;18:1416522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu KLH, Wu CW, Chao YM, Hung CY, Chan JYH. Impaired Nrf2 regulation of mitochondrial biogenesis in rostral ventrolateral medulla on hypertension induced by systemic inflammation. Free Radic Biol Med. 2016;97:58–74. [DOI] [PubMed] [Google Scholar]
- 169.Gao L, Zimmerman MC, Biswal S, Zucker IH. Selective Nrf2 gene deletion in the Rostral Ventrolateral Medulla evokes hypertension and Sympathoexcitation in mice. Hypertension. 2017;69(6):1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brand-Miller JC. Glycemic load and chronic disease. Nutr Rev. 2003;61(5 Pt 2):S49–55. [DOI] [PubMed] [Google Scholar]
- 171.Huh S. Adherence of the Annals of Pediatric Endocrinology & Metabolism to the principles of transparency and best practice in Scholarly Publishing. Ann Pediatr Endocrinol Metab. 2018;23(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74(1):70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shimizu F, Sano Y, Tominaga O, Maeda T, Abe MA, Kanda T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-beta by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol Aging. 2013;34(7):1902–12. [DOI] [PubMed] [Google Scholar]
- 174.Banks WA. The blood-brain barrier interface in diabetes Mellitus: dysfunctions, mechanisms and approaches to treatment. Curr Pharm Des. 2020;26(13):1438–47. [DOI] [PubMed] [Google Scholar]
- 175.Shah GN, Morofuji Y, Banks WA, Price TO. High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: implications for cerebral microvascular disease in diabetes. Biochem Biophys Res Commun. 2013;440(2):354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sajja RK, Prasad S, Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS. 2014;11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salameh TS, Mortell WG, Logsdon AF, Butterfield DA, Banks WA. Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS. 2019;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yan Z, Wang C, Meng Z, Gan L, Guo R, Liu J et al. C1q/TNF-Related protein 3 prevents Diabetic Retinopathy via AMPK-Dependent stabilization of blood-retinal barrier tight junctions. Cells. 2022;11(5). [DOI] [PMC free article] [PubMed]
- 179.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. [DOI] [PubMed] [Google Scholar]
- 180.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–45. [DOI] [PubMed] [Google Scholar]
- 181.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. [DOI] [PubMed] [Google Scholar]
- 182.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3(1):46–56. [DOI] [PubMed] [Google Scholar]
- 183.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39(2):227–36. [DOI] [PubMed] [Google Scholar]
- 184.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94(5):609–16. [DOI] [PubMed] [Google Scholar]
- 185.Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med. 2004;37(11):1694–702. [DOI] [PubMed] [Google Scholar]
- 186.Miyata T, Sugiyama S, Suzuki D, Inagi R, Kurokawa K. Increased carbonyl modification by lipids and carbohydrates in diabetic nephropathy. Kidney Int Suppl. 1999;71:S54–6. [DOI] [PubMed] [Google Scholar]
- 187.Churchman AT, Anwar AA, Li FYL, Sato H, Ishii T, Mann GE, et al. Transforming growth factor-beta1 elicits Nrf2-mediated antioxidant responses in aortic smooth muscle cells. J Cell Mol Med. 2009;13(8B):2282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis. 2011;21(4):277–85. [DOI] [PubMed] [Google Scholar]
- 189.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57(10):2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10(8):923–34. [DOI] [PubMed] [Google Scholar]
- 191.Toth RK, Warfel NA. Strange bedfellows: nuclear factor, erythroid 2-Like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in Tumor Hypoxia. Antioxid (Basel). 2017;6(2). [DOI] [PMC free article] [PubMed]
- 192.Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020;25(24). [DOI] [PMC free article] [PubMed]
- 193.Kocahan S, Dogan Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: the brain, neural Pathology, N-methyl-D-aspartate receptors, tau protein and other Risk factors. Clin Psychopharmacol Neurosci. 2017;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Anwar MM, Ozkan E, Shomalizadeh N, Sapanci S, Ozler C, Kesibi J, et al. Assessing the role of primary healthy microglia and gap junction blocker in hindering Alzheimer’s disease neuroinflammatory type: early approaches for therapeutic intervention. Front Neurosci. 2022;16:1041461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qu L, Ji L, Wang C, Luo H, Li S, Peng W, et al. Synthesis and evaluation of multi-target-directed ligands with BACE-1 inhibitory and Nrf2 agonist activities as potential agents against Alzheimer’s disease. Eur J Med Chem. 2021;219:113441. [DOI] [PubMed] [Google Scholar]
- 196.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68(21):1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862(5):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28(7):977–86. [DOI] [PubMed] [Google Scholar]
- 200.Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, et al. Brain clearance of Alzheimer’s amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10(4):359–68. [DOI] [PubMed] [Google Scholar]
- 201.Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis. 2001;3(1):23–30. [DOI] [PubMed] [Google Scholar]
- 202.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115(11):3285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pan W, Solomon B, Maness LM, Kastin AJ. Antibodies to beta-amyloid decrease the blood-to-brain transfer of beta-amyloid peptide. Exp Biol Med (Maywood). 2002;227(8):609–15. [DOI] [PubMed] [Google Scholar]
- 205.Keaney J, Walsh DM, O’Malley T, Hudson N, Crosbie DE, Loftus T, et al. Autoregulated paracellular clearance of amyloid-beta across the blood-brain barrier. Sci Adv. 2015;1(8):e1500472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS ONE. 2011;6(8):e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Asghari K, Niknam Z, Mohammadpour-Asl S, Chodari L. Cellular junction dynamics and Alzheimer’s disease: a comprehensive review. Mol Biol Rep. 2024;51(1):273. [DOI] [PubMed] [Google Scholar]
- 208.Govindpani K, McNamara LG, Smith NR, Vinnakota C, Waldvogel HJ, Faull RL et al. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J Clin Med. 2019;8(5). [DOI] [PMC free article] [PubMed]
- 209.Ivanidze J, Mackay M, Hoang A, Chi JM, Cheng K, Aranow C, et al. Dynamic contrast-enhanced MRI reveals unique blood-brain barrier permeability characteristics in the Hippocampus in the normal brain. AJNR Am J Neuroradiol. 2019;40(3):408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martins T, Baptista S, Goncalves J, Leal E, Milhazes N, Borges F, et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011;1411:28–40. [DOI] [PubMed] [Google Scholar]
- 211.Dubey S, Heinen S, Krantic S, McLaurin J, Branch DR, Hynynen K, et al. Clinically approved IVIg delivered to the hippocampus with focused ultrasound promotes neurogenesis in a model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2020;117(51):32691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ghiso J, Tomidokoro Y, Revesz T, Frangione B, Rostagno A. Cerebral amyloid angiopathy and Alzheimer’s Disease. Hirosaki Igaku. 2010;61(Suppl):S111–24. [PMC free article] [PubMed] [Google Scholar]
- 213.Fan W, Chen H, Li M, Fan X, Jiang F, Xu C, et al. NRF2 activation ameliorates blood-brain barrier injury after cerebral ischemic stroke by regulating ferroptosis and inflammation. Sci Rep. 2024;14(1):5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Branca C, Ferreira E, Nguyen TV, Doyle K, Caccamo A, Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2017;26(24):4823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Joshi G, Gan KA, Johnson DA, Johnson JA. Increased Alzheimer’s disease-like pathology in the APP/ PS1DeltaE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol Aging. 2015;36(2):664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bahn G, Park JS, Yun UJ, Lee YJ, Choi Y, Park JS, et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc Natl Acad Sci U S A. 2019;116(25):12516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yuan M, Wang Y, Huang Z, Jing F, Qiao P, Zou Q, et al. Impaired autophagy in amyloid-beta pathology: a traditional review of recent Alzheimer’s research. J Biomed Res. 2022;37(1):30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016;131(5):645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35(11 Suppl 1):2623–7. [DOI] [PubMed] [Google Scholar]
- 220.Price JM, Sutton ET, Hellermann A, Thomas T. beta-amyloid induces cerebrovascular endothelial dysfunction in the rat brain. Neurol Res. 1997;19(5):534–8. [DOI] [PubMed] [Google Scholar]
- 221.Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, Singh AK, et al. The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J Neurochem. 2012;123(Suppl 2):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ng AYH, Li Z, Jones MM, Yang S, Li C, Fu C et al. Regulator of G protein signaling 12 enhances osteoclastogenesis by suppressing Nrf2-dependent antioxidant proteins to promote the generation of reactive oxygen species. Elife. 2019;8. [DOI] [PMC free article] [PubMed]
- 223.Castillo-Carranza DL, Nilson AN, Van Skike CE, Jahrling JB, Patel K, Garach P, et al. Cerebral Microvascular Accumulation of Tau Oligomers in Alzheimer’s Disease and related tauopathies. Aging Dis. 2017;8(3):257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Reuck JL, Deramecourt V, Cordonnier C, Leys D, Pasquier F, Maurage CA. Cerebrovascular lesions in patients with frontotemporal lobar degeneration: a neuropathological study. Neurodegener Dis. 2012;9(4):170–5. [DOI] [PubMed] [Google Scholar]
- 225.Majerova P, Garruto RM, Kovac A. Cerebrovascular inflammation is associated with tau pathology in Guam parkinsonism dementia. J Neural Transm (Vienna). 2018;125(7):1013–25. [DOI] [PubMed] [Google Scholar]
- 226.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21(8):880–6. [DOI] [PubMed] [Google Scholar]
- 227.Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23(3):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Costea L, Meszaros A, Bauer H, Bauer HC, Traweger A, Wilhelm I et al. The blood-brain barrier and its intercellular junctions in Age-related Brain disorders. Int J Mol Sci. 2019;20(21). [DOI] [PMC free article] [PubMed]
- 229.Milenkovic I, Petrov T, Kovacs GG. Patterns of hippocampal tau pathology differentiate neurodegenerative dementias. Dement Geriatr Cogn Disord. 2014;38(5–6):375–88. [DOI] [PubMed] [Google Scholar]
- 230.Kapasi A, Yu L, Petyuk V, Arfanakis K, Bennett DA, Schneider JA. Association of small vessel disease with tau pathology. Acta Neuropathol. 2022;143(3):349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Majerova P, Michalicova A, Cente M, Hanes J, Vegh J, Kittel A, et al. Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS ONE. 2019;14(5):e0217216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen Y, He Y, Han J, Wei W, Chen F. Blood-brain barrier dysfunction and Alzheimer’s disease: associations, pathogenic mechanisms, and therapeutic potential. Front Aging Neurosci. 2023;15:1258640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zilka N, Stozicka Z, Kovac A, Pilipcinec E, Bugos O, Novak M. Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy. J Neuroimmunol. 2009;209(1–2):16–25. [DOI] [PubMed] [Google Scholar]
- 234.Lee DC, Rizer J, Selenica ML, Reid P, Kraft C, Johnson A, et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. 2010;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang JY, Liu SJ, Li HL, Wang JZ. Microtubule-associated protein tau is a substrate of ATP/Mg(2+)-dependent proteasome protease system. J Neural Transm (Vienna). 2005;112(4):547–55. [DOI] [PubMed] [Google Scholar]
- 236.Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117(3):648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83(1):176–85. [DOI] [PubMed] [Google Scholar]
- 238.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, et al. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27(5):1119–30. [DOI] [PubMed] [Google Scholar]
- 239.Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009;18(21):4153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E, et al. Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. 2010;6(7):e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014;5:3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18(4):251–9. [DOI] [PubMed] [Google Scholar]
- 243.Al-Bachari S, Naish JH, Parker GJM, Emsley HCA, Parkes LM. Blood-brain barrier leakage is increased in Parkinson’s Disease. Front Physiol. 2020;11:593026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35(5):747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pienaar IS, Lee CH, Elson JL, McGuinness L, Gentleman SM, Kalaria RN, et al. Deep-brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson’s disease. Neurobiol Dis. 2015;74:392–405. [DOI] [PubMed] [Google Scholar]
- 246.Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem. 1995;65(2):710–24. [DOI] [PubMed] [Google Scholar]
- 247.Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, et al. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23(2):154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Heithoff BP, George KK, Phares AN, Zuidhoek IA, Munoz-Ballester C, Robel S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia. 2021;69(2):436–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pivoriunas A, Verkhratsky A. Astrocyte-Endotheliocyte Axis in the regulation of the blood-brain barrier. Neurochem Res. 2021;46(10):2538–50. [DOI] [PubMed] [Google Scholar]
- 250.Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89(2):464–73. [DOI] [PubMed] [Google Scholar]
- 251.Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet. 1999;353(9157):981–2. [DOI] [PubMed] [Google Scholar]
- 252.Calkins MJ, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010;115(2):557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang J, Sun H, Zhu L, Du L, Ma Y, Ma Y, et al. Micro Ribonucleic Acid 27a aggravates ferroptosis during early ischemic stroke of rats through nuclear factor erythroid-2-Related factor 2. Neuroscience. 2022;504:10–20. [DOI] [PubMed] [Google Scholar]
- 254.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning protects astrocytes against Oxygen glucose Deprivation Via the Nuclear erythroid 2-Related factor 2 pathway. Transl Stroke Res. 2018;9(2):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ding Y, Chen M, Wang M, Li Y, Wen A. Posttreatment with 11-Keto-beta-boswellic acid ameliorates cerebral ischemia-reperfusion Injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol. 2015;52(3):1430–9. [DOI] [PubMed] [Google Scholar]
- 256.Bogale TA, Faustini G, Longhena F, Mitola S, Pizzi M, Bellucci A. Alpha-Synuclein in the regulation of Brain endothelial and perivascular cells: gaps and future perspectives. Front Immunol. 2021;12:611761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim KS, Park JY, Jou I, Park SM. Regulation of Weibel-Palade body exocytosis by alpha-synuclein in endothelial cells. J Biol Chem. 2010;285(28):21416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kuan WL, Bennett N, He X, Skepper JN, Martynyuk N, Wijeyekoon R, et al. Alpha-synuclein pre-formed fibrils impair tight junction protein expression without affecting cerebral endothelial cell function. Exp Neurol. 2016;285(Pt A):72–81. [DOI] [PubMed] [Google Scholar]
- 259.Song W, Patel A, Qureshi HY, Han D, Schipper HM, Paudel HK. The Parkinson disease-associated A30P mutation stabilizes alpha-synuclein against proteasomal degradation triggered by heme oxygenase-1 over-expression in human neuroblastoma cells. J Neurochem. 2009;110(2):719–33. [DOI] [PubMed] [Google Scholar]
- 260.Zukor H, Song W, Liberman A, Mui J, Vali H, Fillebeen C, et al. HO-1-mediated macroautophagy: a mechanism for unregulated iron deposition in aging and degenerating neural tissues. J Neurochem. 2009;109(3):776–91. [DOI] [PubMed] [Google Scholar]
- 261.Riedel M, Goldbaum O, Schwarz L, Schmitt S, Richter-Landsberg C. 17-AAG induces cytoplasmic alpha-synuclein aggregate clearance by induction of autophagy. PLoS ONE. 2010;5(1):e8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, et al. TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem. 2002;83(4):846–54. [DOI] [PubMed] [Google Scholar]
- 263.Lastres-Becker I, Garcia-Yague AJ, Scannevin RH, Casarejos MJ, Kugler S, Rabano A, et al. Repurposing the NRF2 activator Dimethyl Fumarate as Therapy against Synucleinopathy in Parkinson’s Disease. Antioxid Redox Signal. 2016;25(2):61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lianos EA, Detsika MG. Immune-related functions of Heme Oxygenase-1. Antioxid (Basel). 2023;12(7). [DOI] [PMC free article] [PubMed]
- 265.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280(27):25350–60. [DOI] [PubMed] [Google Scholar]
- 266.Mancuso C, Bonsignore A, Di Stasio E, Mordente A, Motterlini R. Bilirubin and S-nitrosothiols interaction: evidence for a possible role of bilirubin as a scavenger of nitric oxide. Biochem Pharmacol. 2003;66(12):2355–63. [DOI] [PubMed] [Google Scholar]
- 267.Horvath S, Langfelder P, Kwak S, Aaronson J, Rosinski J, Vogt TF, et al. Huntington’s disease accelerates epigenetic aging of human brain and disrupts DNA methylation levels. Aging. 2016;8(7):1485–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagace M, Kuan WL, Saint-Pierre M, et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann Neurol. 2015;78(2):160–77. [DOI] [PubMed] [Google Scholar]
- 269.Savage JC, St-Pierre MK, Carrier M, El Hajj H, Novak SW, Sanchez MG, et al. Microglial physiological properties and interactions with synapses are altered at presymptomatic stages in a mouse model of Huntington’s disease pathology. J Neuroinflammation. 2020;17(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019;94:112–20. [DOI] [PubMed] [Google Scholar]
- 271.O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20(6):252–8. [DOI] [PubMed] [Google Scholar]
- 272.Huang Z, Ji H, Shi J, Zhu X, Zhi Z. Engeletin attenuates Abeta1-42-Induced oxidative stress and neuroinflammation by Keap1/Nrf2 pathway. Inflammation. 2020;43(5):1759–71. [DOI] [PubMed] [Google Scholar]
- 273.Jin YN, Yu YV, Gundemir S, Jo C, Cui M, Tieu K, et al. Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant huntingtin. PLoS ONE. 2013;8(3):e57932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Naik P, Cucullo L. Pathobiology of tobacco smoking and neurovascular disorders: untied strings and alternative products. Fluids Barriers CNS. 2015;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Naik P, Fofaria N, Prasad S, Sajja RK, Weksler B, Couraud PO, et al. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91(12):2525–38. [DOI] [PubMed] [Google Scholar]
- 277.Hutamekalin P, Farkas AE, Orbok A, Wilhelm I, Nagyoszi P, Veszelka S, et al. Effect of nicotine and polyaromtic hydrocarbons on cerebral endothelial cells. Cell Biol Int. 2008;32(2):198–209. [DOI] [PubMed] [Google Scholar]
- 278.Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, et al. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am J Physiol Lung Cell Mol Physiol. 2014;307(3):L205–18. [DOI] [PubMed] [Google Scholar]
- 279.Sivandzade F, Cucullo L. Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci. 2019;20(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing New Clinical Criteria for septic shock: for the Third International Consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ren C, Yao RQ, Zhang H, Feng YW, Yao YM. Sepsis-associated encephalopathy: a vicious cycle of immunosuppression. J Neuroinflammation. 2020;17(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017;43(8):1075–84. [DOI] [PubMed] [Google Scholar]
- 283.Wu XX, Huang XL, Chen RR, Li T, Ye HJ, Xie W, et al. Paeoniflorin prevents intestinal barrier disruption and inhibits lipopolysaccharide (LPS)-Induced inflammation in Caco-2 cell monolayers. Inflammation. 2019;42(6):2215–25. [DOI] [PubMed] [Google Scholar]
- 284.Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia ZY. Lipopolysaccharide (LPS) aggravates high glucose- and Hypoxia/Reoxygenation-Induced Injury through activating ROS-Dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:8151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Fan Y, Yang X, Tao Y, Lan L, Zheng L, Sun J. Tight junction disruption of blood-brain barrier in white matter lesions in chronic hypertensive rats. NeuroReport. 2015;26(17):1039–43. [DOI] [PubMed] [Google Scholar]
- 286.Tsoyi K, Jang HJ, Nizamutdinova IT, Park K, Kim YM, Kim HJ, et al. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3beta/GATA-6 signaling pathways in TNF-alpha-activated human endothelial cells. Atherosclerosis. 2010;213(1):115–21. [DOI] [PubMed] [Google Scholar]
- 287.Yu Y, Feng J, Lian N, Yang M, Xie K, Wang G, et al. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. Int Immunopharmacol. 2020;85:106585. [DOI] [PubMed] [Google Scholar]
- 288.Haas de Mello A, Liu T, Garofalo RP, Casola A. Hydrogen Sulfide Donor GYY4137 Rescues NRF2 Activation in Respiratory Syncytial Virus Infection. Antioxidants (Basel). 2022;11(7). [DOI] [PMC free article] [PubMed]
- 289.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–38. [DOI] [PubMed] [Google Scholar]
- 290.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14(12):2347–60. [DOI] [PubMed] [Google Scholar]
- 291.Seo EJ, Fischer N, Efferth T. Phytochemicals as inhibitors of NF-kappaB for treatment of Alzheimer’s disease. Pharmacol Res. 2018;129:262–73. [DOI] [PubMed] [Google Scholar]
- 292.Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45(5):466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li L, Cheng SQ, Guo W, Cai ZY, Sun YQ, Huang XX, et al. Oridonin prevents oxidative stress-induced endothelial injury via promoting Nrf-2 pathway in ischaemic stroke. J Cell Mol Med. 2021;25(20):9753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ye S, Hu X, Sun S, Su B, Cai J, Jiang J. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1. Eur J Pharmacol. 2024;974:176620. [DOI] [PubMed] [Google Scholar]
- 295.Cho JH, Chae JI, Shim JH. Rhein exhibits antitumorigenic effects by interfering with the interaction between prolyl isomerase Pin1 and c-Jun. Oncol Rep. 2017;37(3):1865–72. [DOI] [PubMed] [Google Scholar]
- 296.Ge H, Tang H, Liang Y, Wu J, Yang Q, Zeng L, et al. Rhein attenuates inflammation through inhibition of NF-kappaB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang QW, Su Y, Sheng JT, Gu LM, Zhao Y, Chen XX, et al. Anti-influenza a virus activity of rhein through regulating oxidative stress, TLR4, akt, MAPK, and NF-kappaB signal pathways. PLoS ONE. 2018;13(1):e0191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Liu H, Zhang TA, Zhang WY, Huang SR, Hu Y, Sun J. Rhein attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through NRF2/SLC7A11/GPX4 pathway. Exp Neurol. 2023;369:114541. [DOI] [PubMed] [Google Scholar]
- 299.Lu L, Xiong Y, Lin Z, Chu X, Panayi AC, Hu Y, et al. Advances in the therapeutic application and pharmacological properties of kinsenoside against inflammation and oxidative stress-induced disorders. Front Pharmacol. 2022;13:1009550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Qi CX, Zhou Q, Yuan Z, Luo ZW, Dai C, Zhu HC, et al. Kinsenoside: a promising bioactive compound from Anoectochilus Species. Curr Med Sci. 2018;38(1):11–8. [DOI] [PubMed] [Google Scholar]
- 301.Qiao N, An Z, Fu Z, Chen X, Tong Q, Zhang Y, et al. Kinsenoside alleviates oxidative stress-induced blood-brain barrier dysfunction via promoting Nrf2/HO-1 pathway in ischemic stroke. Eur J Pharmacol. 2023;949:175717. [DOI] [PubMed] [Google Scholar]
- 302.Raphael TJ, Kuttan G. Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine. 2003;10(6–7):483–9. [DOI] [PubMed] [Google Scholar]
- 303.Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr Cancer. 2001;40(2):180–4. [DOI] [PubMed] [Google Scholar]
- 304.Shi YS, Zhang Y, Liu B, Li CB, Wu J, Li Y. Nomilin protects against cerebral ischemia-reperfusion induced neurological deficits and blood-brain barrier disruption via the Nrf2 pathway. Food Funct. 2019;10(9):5323–32. [DOI] [PubMed] [Google Scholar]
- 305.Xue XH, Xue JX, Hu W, Shi FL, Yang Y. Nomilin targets the Keap1-Nrf2 signalling and ameliorates the development of osteoarthritis. J Cell Mol Med. 2020;24(15):8579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Martinez-Micaelo N, Gonzalez-Abuin N, Pinent M, Ardevol A, Blay M. Procyanidin B2 inhibits inflammasome-mediated IL-1beta production in lipopolysaccharide-stimulated macrophages. Mol Nutr Food Res. 2015;59(2):262–9. [DOI] [PubMed] [Google Scholar]
- 307.Wu S, Yue Y, Li J, Li Z, Li X, Niu Y, et al. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol Nutr Food Res. 2015;59(10):1930–41. [DOI] [PubMed] [Google Scholar]
- 308.Li Y, Zhu Y, Hu F, Liu L, Shen G, Tu Q. Procyanidin B2 regulates the Sirt1/Nrf2 signaling pathway to improve random-pattern skin flap survival. Phytother Res. 2023;37(9):3913–25. [DOI] [PubMed] [Google Scholar]
- 309.Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Albrecht P, Bouchachia I, Goebels N, Henke N, Hofstetter HH, Issberner A, et al. Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflammation. 2012;9:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–92. [DOI] [PubMed] [Google Scholar]
- 312.Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther. 2012;341(1):274–84. [DOI] [PubMed] [Google Scholar]
- 313.Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. German Multicentre Study. Br J Dermatol. 1998;138(3):456–60. [DOI] [PubMed] [Google Scholar]
- 314.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97. [DOI] [PubMed] [Google Scholar]
- 315.Havrdova E, Hutchinson M, Kurukulasuriya NC, Raghupathi K, Sweetser MT, Dawson KT, Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of:, Gold R, Kappos L, Arnold D, Miller RJ, Phillips DH et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med JT, Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97. Expert Opin Pharmacother. 2013;14(15):2145-56. [DOI] [PubMed]
- 316.Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M, et al. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol. 2015;266:99–111. [DOI] [PubMed] [Google Scholar]
- 317.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem. 2013;329:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89(6):2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yang L, Palliyaguru DL, Kensler TW. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol. 2016;43(1):146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3(3):233–55. [DOI] [PubMed] [Google Scholar]
- 321.Wang Q, Bao Y. Nanodelivery of natural isothiocyanates as a cancer therapeutic. Free Radic Biol Med. 2021;167:125–40. [DOI] [PubMed] [Google Scholar]
- 322.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, et al. Allyl Isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–7. [DOI] [PubMed] [Google Scholar]
- 323.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134(8):2004–10. [DOI] [PubMed] [Google Scholar]
- 324.Smith T, Musk SR, Johnson IT. Allyl Isothiocyanate selectively kills undifferentiated HT29 cells in vitro and suppresses aberrant crypt foci in the colonic mucosa of rats. Biochem Soc Trans. 1996;24(3):381S. [DOI] [PubMed] [Google Scholar]
- 325.Caglayan B, Kilic E, Dalay A, Altunay S, Tuzcu M, Erten F, et al. Allyl Isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-kappaB pathways in traumatic brain injury in mice. Mol Biol Rep. 2019;46(1):241–50. [DOI] [PubMed] [Google Scholar]
- 326.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130(9):2243–50. [DOI] [PubMed] [Google Scholar]
- 327.Zhou CH, Wang CX, Xie GB, Wu LY, Wei YX, Wang Q, et al. Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR 4/NF-kappaB signaling pathway. Brain Res. 2015;1629:250–9. [DOI] [PubMed] [Google Scholar]
- 328.Nabavi SF, Braidy N, Habtemariam S, Sureda A, Manayi A, Nabavi SM. Neuroprotective effects of Fisetin in Alzheimer’s and Parkinson’s diseases: from Chemistry to Medicine. Curr Top Med Chem. 2016;16(17):1910–5. [DOI] [PubMed] [Google Scholar]
- 329.Sandireddy R, Yerra VG, Komirishetti P, Areti A, Kumar A. Fisetin imparts Neuroprotection in Experimental Diabetic Neuropathy by modulating Nrf2 and NF-kappaB pathways. Cell Mol Neurobiol. 2016;36(6):883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sun Q, Zhang W, Zhong W, Sun X, Zhou Z. Dietary fisetin supplementation protects against Alcohol-Induced Liver Injury in mice. Alcohol Clin Exp Res. 2016;40(10):2076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wu PY, Lyu JL, Liu YJ, Chien TY, Hsu HC, Wen KC et al. Fisetin regulates Nrf2 expression and the inflammation-related signaling pathway to prevent UVB-Induced skin damage in hairless mice. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed]
- 332.Zhang L, Wang H, Zhou Y, Zhu Y, Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018;118:304–13. [DOI] [PubMed] [Google Scholar]
- 333.Moustafa PE, Abo El Nasr NME, Shabana ME, Saleh DO. Fisetin mitigates letrozole-induced polycystic ovarian syndrome in rats: crosstalk of AMPK/PI3K/AKT-mediated-Nrf2 antioxidant defense mechanism and the inflammasome NLRP3/NF-kappaB P65/IL-1beta signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2024;397(10):8077–8088. [DOI] [PubMed]
- 334.Jaikumkao K, Thongnak L, Htun KT, Pengrattanachot N, Phengpol N, Sutthasupha P, et al. Dapagliflozin and metformin in combination ameliorates diabetic nephropathy by suppressing oxidative stress, inflammation, and apoptosis and activating autophagy in diabetic rats. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166912. [DOI] [PubMed] [Google Scholar]
- 335.Ekperikpe US, Mandal S, Holt SJ, Daniels JK, Johnson TD, Cooper JS, et al. Metformin reduces insulin resistance and attenuates progressive renal injury in prepubertal obese Dahl salt-sensitive rats. Am J Physiol Ren Physiol. 2023;325(3):F363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747–54. [DOI] [PubMed] [Google Scholar]
- 337.Liu Y, Tang G, Li Y, Wang Y, Chen X, Gu X, et al. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J Neuroinflammation. 2014;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Matzinger M, Fischhuber K, Poloske D, Mechtler K, Heiss EH. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020;29:101393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Malinowski JM, Bolesta S. Rosiglitazone in the treatment of type 2 diabetes mellitus: a critical review. Clin Ther. 2000;22(10):1151–68. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 340.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, et al. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(12):2627–33. [DOI] [PubMed] [Google Scholar]
- 341.Wang X, Wang Z, Liu JZ, Hu JX, Chen HL, Li WL, et al. Double antioxidant activities of rosiglitazone against high glucose-induced oxidative stress in hepatocyte. Toxicol Vitro. 2011;25(4):839–47. [DOI] [PubMed] [Google Scholar]
- 342.Markowicz-Piasecka M, Sikora J, Szydlowska A, Skupien A, Mikiciuk-Olasik E, Huttunen KM. Metformin - a future therapy for neurodegenerative diseases: theme: Drug Discovery, Development and Delivery in Alzheimer’s Disease Guest Editor: Davide Brambilla. Pharm Res. 2017;34(12):2614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ye Y, Zhou W, Ren Y, Lu J, Chen A, Jin R, et al. The ameliorating effects of Guizhi Fuling Wan combined with rosiglitazone in a rat ovarian model of polycystic ovary syndrome by the PI3K/AKT/NF-kappaB and Nrf2/HO-1 pathways. Gynecol Endocrinol. 2023;39(1):2254848. [DOI] [PubMed] [Google Scholar]
- 344.Sharma BR, Gautam LN, Adhikari D, Karki R. A Comprehensive Review on Chemical profiling of Nelumbo Nucifera: potential for Drug Development. Phytother Res. 2017;31(1):3–26. [DOI] [PubMed] [Google Scholar]
- 345.Liang L, Ye S, Jiang R, Zhou X, Zhou J, Meng S. Liensinine alleviates high fat diet (HFD)-induced non-alcoholic fatty liver disease (NAFLD) through suppressing oxidative stress and inflammation via regulating TAK1/AMPK signaling. Int Immunopharmacol. 2022;104:108306. [DOI] [PubMed] [Google Scholar]
- 346.Xie Y, Zhang Y, Zhang LT, Zeng SX, Guo ZB, Zheng BD. Protective effects of alkaloid compounds from Nelumbinis Plumula on tert-butyl hydroperoxide-induced oxidative stress. Molecules. 2013;18(9):10285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zhou M, Jiang M, Ying X, Cui Q, Han Y, Hou Y, et al. Identification and comparison of anti-inflammatory ingredients from different organs of Lotus nelumbo by UPLC/Q-TOF and PCA coupled with a NF-kappaB reporter gene assay. PLoS ONE. 2013;8(11):e81971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Dong ZX, Zhao X, Gu DF, Shi YQ, Zhang J, Hu XX, et al. Comparative effects of liensinine and neferine on the human ether-a-go-go-related gene potassium channel and pharmacological activity analysis. Cell Physiol Biochem. 2012;29(3–4):431–42. [DOI] [PubMed] [Google Scholar]
- 349.Liang X, Wang S, Wang L, Ceylan AF, Ren J, Zhang Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission. Pharmacol Res. 2020;157:104846. [DOI] [PubMed] [Google Scholar]
- 350.Wang G, Sun Y, Yang Q, Dai D, Zhang L, Fan H, et al. Liensinine, a alkaloid from lotus Plumule, mitigates lipopolysaccharide-induced sepsis-associated encephalopathy through modulation of nuclear factor erythroid 2-related factor-mediated inflammatory biomarkers and mitochondria apoptosis. Food Chem Toxicol. 2023;177:113813. [DOI] [PubMed] [Google Scholar]
- 351.Zhang X, Yuan S, Fan H, Zhang W, Zhang H. Liensinine alleviates sepsis-induced acute liver injury by inhibiting the NF-kappaB and MAPK pathways in an Nrf2-dependent manner. Chem Biol Interact. 2024;396:111030. [DOI] [PubMed] [Google Scholar]
- 352.Liu C, Vojnovic D, Kochevar IE, Jurkunas UV. UV-A irradiation activates Nrf2-Regulated antioxidant defense and induces p53/Caspase3-Dependent apoptosis in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2016;57(4):2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Liu HS, Shi HL, Huang F, Peterson KE, Wu H, Lan YY, et al. Astragaloside IV inhibits microglia activation via glucocorticoid receptor mediated signaling pathway. Sci Rep. 2016;6:19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yang ST, Lin JW, Chiu BY, Hsu YC, Chang CP, Chang CK. Astragaloside improves outcomes of traumatic brain injury in rats by reducing microglia activation. Am J Chin Med. 2014;42(6):1357–70. [DOI] [PubMed] [Google Scholar]
- 355.Liu G, Song J, Guo Y, Wang T, Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct. 2013;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu JP, et al. Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur J Pharmacol. 2009;606(1–3):137–41. [DOI] [PubMed] [Google Scholar]
- 357.Wang HL, Zhou QH, Xu MB, Zhou XL, Zheng GQ. Astragaloside IV for experimental focal cerebral ischemia: preclinical evidence and possible mechanisms. Oxid Med Cell Longev. 2017;2017:8424326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Gu DM, Lu PH, Zhang K, Wang X, Sun M, Chen GQ, et al. EGFR mediates astragaloside IV-induced Nrf2 activation to protect cortical neurons against in vitro ischemia/reperfusion damages. Biochem Biophys Res Commun. 2015;457(3):391–7. [DOI] [PubMed] [Google Scholar]
- 359.Wan D, Zhu Z, Zhou J, Deng Z, Lei P, Liu Q, et al. Astragaloside IV protects LO2 cells from oxidative damage caused by radiation-induced bystander effect through Akt/Nrf2 pathway. Toxicol Res (Camb). 2023;12(4):635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ma J, Chen T, Wang R. Astragaloside IV ameliorates cognitive impairment and protects oligodendrocytes from antioxidative stress via regulation of the SIRT1/Nrf2 signaling pathway. Neurochem Int. 2023;167:105535. [DOI] [PubMed] [Google Scholar]
- 361.Su X, Guo H, Zhou Y, Cao A, Shen Q, Zhu B, et al. Astragaloside IV attenuates high glucose-induced NF-kappaB-mediated inflammation through activation of PI3K/AKT-ERK-dependent Nrf2/ARE signaling pathway in glomerular mesangial cells. Phytother Res. 2023;37(9):4133–48. [DOI] [PubMed] [Google Scholar]
- 362.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of Baicalin: a review. Brain Sci. 2018;8(6). [DOI] [PMC free article] [PubMed]
- 363.Chen Y, Bao S, Wang Z, Fang Z, Tang H. Baicalin promotes the sensitivity of NSCLC to cisplatin by regulating ferritinophagy and macrophage immunity through the KEAP1-NRF2/HO-1 pathway. Eur J Med Res. 2024;29(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Rose P, Dymock BW, Moore PK. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015;554:143–67. [DOI] [PubMed] [Google Scholar]
- 365.Grambow E, Mueller-Graf F, Delyagina E, Frank M, Kuhla A, Vollmar B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets. 2014;25(3):166–74. [DOI] [PubMed] [Google Scholar]
- 366.Pavone ME, Malpani SS, Dyson M, Kim JJ, Bulun SE. Fenretinide: a potential treatment for endometriosis. Reprod Sci. 2016;23(9):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Dong R, Gong Y, Meng W, Yuan M, Zhu H, Ying M, et al. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. 2017;388:43–53. [DOI] [PubMed] [Google Scholar]
- 368.Morrice N, McIlroy GD, Tammireddy SR, Reekie J, Shearer KD, Doherty MK, et al. Elevated fibroblast growth factor 21 (FGF21) in obese, insulin resistant states is normalised by the synthetic retinoid Fenretinide in mice. Sci Rep. 2017;7:43782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lopez-Vales R, Redensek A, Skinner TA, Rathore KI, Ghasemlou N, Wojewodka G, et al. Fenretinide promotes functional recovery and tissue protection after spinal cord contusion injury in mice. J Neurosci. 2010;30(9):3220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Correale J, Villa A. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity. 2007;40(2):148–60. [DOI] [PubMed] [Google Scholar]
- 371.Zierfuss B, Larochelle C, Prat A. Blood-brain barrier dysfunction in multiple sclerosis: causes, consequences, and potential effects of therapies. Lancet Neurol. 2024;23(1):95–109. [DOI] [PubMed] [Google Scholar]
- 372.Rani A, Ergun S, Karnati S, Jha HC. Understanding the link between neurotropic viruses, BBB permeability, and MS pathogenesis. J Neurovirol. 2024;30(1):22–38. [DOI] [PubMed] [Google Scholar]
- 373.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5(1):71–81. [DOI] [PubMed] [Google Scholar]
- 374.Liu S, Agalliu D, Yu C, Fisher M. The role of pericytes in blood-brain barrier function and stroke. Curr Pharm Des. 2012;18(25):3653–62. [DOI] [PubMed] [Google Scholar]
- 375.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22(5):E4. [DOI] [PubMed] [Google Scholar]
- 377.Khor SLQ, Ng KY, Koh RY, Chye SM. Blood-brain barrier and neurovascular unit dysfunction in Parkinson’s Disease: from clinical insights to pathogenic mechanisms and Novel Therapeutic approaches. CNS Neurol Disord Drug Targets. 2024;23(3):315–30. [DOI] [PubMed] [Google Scholar]
- 378.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transpl. 2007;16(3):285–99. [DOI] [PubMed] [Google Scholar]
- 379.Wong YY, Wu CY, Yu D, Kim E, Wong M, Elez R, et al. Biofluid markers of blood-brain barrier disruption and neurodegeneration in Lewy body spectrum diseases: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2022;101:119–28. [DOI] [PubMed] [Google Scholar]
- 380.Wu YC, Bogale TA, Koistinaho J, Pizzi M, Rolova T, Bellucci A. The contribution of beta-amyloid, tau and alpha-synuclein to blood-brain barrier damage in neurodegenerative disorders. Acta Neuropathol. 2024;147(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Acharya NK, Grossman HC, Clifford PM, Levin EC, Light KR, Choi H, et al. A chronic increase in blood-brain barrier permeability facilitates Intraneuronal deposition of exogenous bloodborne Amyloid-Beta1-42 peptide in the brain and leads to Alzheimer’s disease-relevant cognitive changes in a mouse model. J Alzheimers Dis. 2024;98(1):163–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kumar Nelson V, Jha NK, Nuli MV, Gupta S, Kanna S, Gahtani RM, et al. Unveiling the impact of aging on BBB and Alzheimer’s disease: factors and therapeutic implications. Ageing Res Rev. 2024;98:102224. [DOI] [PubMed] [Google Scholar]
- 383.Liu R, Collier JM, Abdul-Rahman NH, Capuk O, Zhang Z, Begum G. Dysregulation of Ion Channels and transporters and blood-brain barrier dysfunction in Alzheimer’s Disease and Vascular Dementia. Aging Dis. 2024;15(4):1748–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129(Pt 1):18–35. [DOI] [PubMed] [Google Scholar]
- 385.Reiss Y, Bauer S, David B, Devraj K, Fidan E, Hattingen E, et al. The neurovasculature as a target in temporal lobe epilepsy. Brain Pathol. 2023;33(2):e13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65(24):11419–28. [DOI] [PubMed] [Google Scholar]
- 387.Ahmed MH, Canney M, Carpentier A, Idbaih A. Overcoming the blood brain barrier in glioblastoma: Status and future perspective. Rev Neurol (Paris). 2023;179(5):430–6. [DOI] [PubMed] [Google Scholar]
- 388.Begley DJ, Pontikis CC, Scarpa M. Lysosomal storage diseases and the blood-brain barrier. Curr Pharm Des. 2008;14(16):1566–80. [DOI] [PubMed] [Google Scholar]
- 389.Urayama A. [The blood-brain barrier and neurodegenerative lysosomal storage diseases]. Brain Nerve. 2013;65(2):153–63. [PubMed] [Google Scholar]
- 390.Grieshaber MC, Flammer J. Does the blood-brain barrier play a role in Glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S115–21. [DOI] [PubMed] [Google Scholar]
- 391.Alarcon-Martinez L, Shiga Y, Villafranca-Baughman D, Cueva Vargas JL, Vidal Paredes IA, Quintero H, et al. Neurovascular dysfunction in glaucoma. Prog Retin Eye Res. 2023;97:101217. [DOI] [PubMed] [Google Scholar]
- 392.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130(1):55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Schiera G, Di Liegro CM, Schiro G, Sorbello G, Di Liegro I. Involvement of astrocytes in the formation, maintenance, and function of the blood-brain barrier. Cells. 2024;13(2). [DOI] [PMC free article] [PubMed]
- 394.Stamp MEM, Halwes M, Nisbet D, Collins DJ. Breaking barriers: exploring mechanisms behind opening the blood-brain barrier. Fluids Barriers CNS. 2023;20(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–36. [DOI] [PubMed] [Google Scholar]
- 396.Fong H, Zhou B, Feng H, Luo C, Bai B, Zhang J et al. Recapitulation of structure-function-regulation of blood-brain barrier under (patho)physiological conditions. Cells. 2024;13(3). [DOI] [PMC free article] [PubMed]
- 397.DiNicolantonio JJ, McCarty MF, Assanga SI, Lujan LL, O’Keefe JH. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart. 2022;9(1). [DOI] [PMC free article] [PubMed]
- 398.Zimmerman AW, Singh K, Connors SL, Liu H, Panjwani AA, Lee LC, et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with Autism Spectrum Disorder. Mol Autism. 2021;12(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.