Abstract
BACKGROUND
Gastric cancer (GC) is a highly prevalent gastrointestinal tract tumor. Several trials have demonstrated that the location of GC can affect patient prognosis. However, the factors determining tumor location remain unclear.
AIM
To investigate the tumor location of patients, we went on to study the influencing factors that lead to changes in the location of GC.
METHODS
A retrospective evaluation was carried out on 3287 patients who underwent gastrectomy for GC in Zhejiang Cancer Hospital. The patients were followed up post-diagnosis and post-gastrectomy. The clinicopathological variables and overall survival of the patients were recorded. By analyzing the location of GC, the tumor location was divided into four categories: “Upper”, “middle”, “lower”, and “total”. Statistical software was utilized to analyze the relationship of each variable with the location of GC.
RESULTS
A total of 3287 patients were included in this study. The clinicopathological indices of gender, age, serum levels of carcinoembryonic antigen (CEA), carbohydrate antigen (CA19-9) and CA72-4 levels, were significantly associated with tumor location in patients with GC. In addition, there was a strong correlation between GC location and the prognosis of postoperative patients. Specifically, patients with “lower” and “middle” GC demonstrated a better prognosis than those with tumors in other categories.
CONCLUSION
The five clinicopathological indices of gender, age, CEA, CA19-9 and CA72-4 levels exhibit varying degrees of influence on the tumor location. The tumor location correlates with patient prognosis following surgery.
Keywords: Gastric cancer, Clinicopathologic characteristics, Tumor marker, Tumor location, Overall survival
Core Tip: This study investigates how the location of gastric cancer (GC) impacts prognosis by analyzing data from 3287 patients who underwent gastrectomy. It reveals that clinicopathological factors, including gender, age, and serum levels of carcinoembryonic antigen, carbohydrate antigen (CA) 19-9, and CA72-4, significantly influence tumor location. Notably, GC located in the “lower” and “middle” regions correlates with a better overall survival compared to other locations. These findings underscore the importance of tumor location in predicting patient outcomes and guiding postoperative management strategies.
INTRODUCTION
Gastric cancer (GC) refers to a type of malignant tumor originating from the epithelial cells of the gastric mucosa. It is the fifth most common cancer worldwide and has the third highest mortality rate among all malignant tumors[1]. Adenocarcinoma is the most prevalent type of GC, accounting for more than 95% of cases, and is one of the most common malignant tumors in China. Symptoms of early GC are not obvious, leading to late-stage diagnosis and poor prognosis for many patients. The incidence and mortality rates of GC are high in East Asian countries, with approximately half of the world’s cases occurring in these countries[2]. Although the mortality rate of GC in China has decreased significantly with improvements in economic level and technology, especially in rural areas[3], GC is still an important cancer burden at present and one of the key issues in China’s cancer prevention and control strategies. According to various clinicopathological indices, the location of GC has been shown to impact patient prognosis, with different tumor locations correlated with prognostic survival. However, the factors influencing the different locations of GC remain controversial.
In this study, we retrospectively reported the clinicopathological characteristics and survival of 3287 GC patients over a 12-year period. We analyzed the effect of multiple variables on the tumor location and identified gender, age, carcinoembryonic antigen (CEA), CA19-9, and CA72-4 as independent risk factors for the location of GC. Moreover, our findings revealed that the main factors affecting the location of GC differed between male and female patients. Notably, further research is required to understand why survival rates vary according to the location of GC. These findings can aid in studying the factors influencing different GC locations, predicting the incidence, and assessing the survival prognosis of GC patients. This not only improves the detection rate of early GC during screening but also has far-reaching implications for the clinical treatment of the disease.
MATERIALS AND METHODS
Patients
A total of 3287 patients diagnosed with GC who underwent gastrectomy at Zhejiang Cancer Hospital from 2008 to 2019 were included in this study. We collected and analyzed the clinicopathological characteristics, tumor location, tumor marker levels, postoperative survival, surgical methods and extent of resection of GC patients for retrospective evaluation. The patients ranged in age from 25 to 95 years, and all underwent radical surgical treatment. Patients with postoperative residual GC, unsuccessful surgery or malignant metastases from other organs were excluded from this study. We followed these postoperative GC patients at regular intervals of 3-6 months, and data were collected accordingly. Prior to analyzing these patient data, personal information records, such as patient names and medical record numbers, were deidentified. All patients signed informed consent forms. The study was approved by the Ethics Committee of Zhejiang Cancer Hospital.
Clinicopathological characteristics
We collected data on the following variables: Age, gender, body mass index (BMI), pathological type, tumor location, surgical method, type of resection, pathological tumor-node-metastasis (pTNM, AJCC-TNM 8th), tumor differentiation grade, vascular tumor thrombus, nerve invasion, lymph node ratio, and preoperative tumor markers. Patients with abnormally elevated tumor markers were excluded from the analysis. Survival data were obtained by telephone follow-ups and medical records.
Division of gastric tumor location
In this study, the subsites of GC were classified as the cardia, gastric fundus, gastroesophageal junction, gastric body, greater curvature, lesser curvature, gastric antrum, pylorus, gastric horn, and distal stomach[4]. These sites were further categorized as being in the upper, middle, or lower third of the stomach. Tumors located primarily at the gastroesophageal junction, cardia or fundus were classified as being in the upper third of the stomach. Those located in the pylorus, sinus, horn of the stomach or distal stomach were classified as being in the lower third of the stomach. Tumors located in the middle of the stomach body, greater curvature, or lesser curvature were identified as being in the middle third of the stomach. Tumors involving two adjacent regions were classified according to their proximity: Those near the cardia were assigned to the upper third, while those near the pyloric antrum were assigned to the lower third. Tumors spanning non-adjacent regions or all three regions were categorized as “Total”. For statistical analysis and clarity, the classifications “Upper 1/3”, “Middle 1/3”, and “Lower 1/3” were simplified to “Upper”, “Middle”, and “Lower”, respectively, while “Total” remained unchanged.
Statistical analysis
The χ2 test was used to evaluate between-group differences in categorical variables. Bivariate correlations between study variables were calculated using Spearman’s rank correlation coefficient. The interaction between the dependent and independent variables was analyzed using logistic regression. P values < 0.05 were considered to indicate statistical significance. The study flowchart is shown in Figure 1. Statistical analyses were performed using IBM SPSS Statistics, version 25.0 (IBM Corp, Armonk, NY, United States) and GraphPad Prism 8, version 8.3.0 (GraphPad Software, San Diego, CA, United States). A random forest model was constructed using R version 4.3.1 (randomForestSRC package 3.2.3). The minimum depth method and variable importance (VIMP) method were used to filter important features, and the ggplot2 package 3.5.0 was used to visualize the images. Survival analysis was performed using the Kaplan-Meier survival curve method.
Figure 1.
Flow chart of the data selection and processing steps. AFP: Alpha-fetoprotein; CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; BMI: Body mass index; VIMP: Variable importance.
RESULTS
Location of GC affects postoperative survival
To assess the factors influencing the prognostic survival of GC patients, we constructed a random forest model to analyze the prognosis-related influencing factors. Patients surviving less than 12 months were excluded to refine the analysis. In this study, only patients who underwent laparoscopic gastrectomy or open gastrectomy were included, allowing us to exclude the effect of surgical modality on prognosis. Thus, we discovered a significant correlation between tumor location and patient prognosis (Figure 2).
Figure 2.
Forest plot of factors influencing survival in patients with gastric cancer. The forest map displays hazard ratios (HRs) and 95% confidence intervals (95%CIs) for various factors that influence tumor prognosis according to multiple Cox regression analyses. Each dot represents the HR for a specific factor, the horizontal line represents the 95%CI, and the vertical blank line represents the reference line (HR = 1.0), indicating no impact. Factors with good HR and CI on the right side of the line were significant predictors of poor prognosis. Values in parentheses are the highest normal levels for that tumor marker. CEA: Carcinoembryonic antigen; AFP: Alpha-fetoprotein; CA: Carbohydrate antigen; BMI: Body mass index; 95%CI: 95% confidence interval; HR: Hazard ratio; pTNM: Pathological tumor-node-metastasis.
To further examine how the location of GC impacts postoperative survival, 3287 patients were divided into four groups according to their stomach cancer location: “upper”, “middle”, “lower”, and “total”. Our clinical data showed the distribution of these cases as follows: Upper (52.4%), middle (30.6%), lower (15.4%), and total (1.6%) (Table 1). Survival prognosis was relatively better for patients with middle and lower GC compared to those with upper and total GC (Figure 3). Regarding the 5-year survival rate, no significant difference was noted between middle and lower GC patients. However, the overall survival (OS) rate disparity between patients with upper GC and those with total GC was more evident (Table 2). Our findings confirm that tumors situated nearer to the stomach’s proximal end correlate with shorter survival periods and poorer outcomes, which is consistent with previous studies[5-7]. Overall, the evidence suggests that the location of GC is a significant factor in determining patient prognosis.
Table 1.
Clinicopathological characteristics of gastric cancer patient samples, n = 3287, n (%)
|
Characteristics
|
Number of cases
|
| Gender | |
| Male | 2541 (77.3) |
| Female | 746 (22.7) |
| Age | |
| < 60 | 1191 (36.2) |
| ≥ 60 | 2096 (63.8) |
| BMI | |
| ≤ 18 | 296 (9.0) |
| 18-24 | 2090 (63.6) |
| ≥ 24 | 901 (27.4) |
| Family history | |
| No | 2102 (63.9) |
| Yes | 1185 (36.1) |
| Smoking history | |
| No | 1767 (53.8) |
| Yes | 1520 (46.2) |
| Drinking history | |
| No | 2261 (68.8) |
| Yes | 1026 (31.2) |
| Surgery methods | |
| Open | 2274 (84.4) |
| Laparoscopy | 513 (15.6) |
| Range of resection | |
| Proximal | 187 (5.7) |
| Distal | 1719 (52.3) |
| Total | 1381 (42.0) |
| Tumor location | |
| Middle | 1007 (30.6) |
| Lower | 507 (15.4) |
| Upper | 1721 (52.4) |
| Total | 52 (1.6) |
| Differentiation | |
| Well | 22 (0.7) |
| Well-moderate | 61 (1.9) |
| Moderate | 771 (23.5) |
| Moderate-poor | 1300 (39.5) |
| Poor | 1133 (34.5) |
| Pathological type | |
| Adenocarcinoma | 2283 (87.7) |
| PMGC | 206 (6.3) |
| MGC | 198 (6.0) |
| Vascular tumor thrombus | |
| No | 1720 (52.3) |
| Yes | 1567 (47.7) |
| Nerve invasion | |
| No | 1718 (52.3) |
| Yes | 1569 (47.7) |
| Maximum tumor diameter (cm) | |
| < 5 | 1982 (60.3) |
| ≥ 5 | 1305 (39.7) |
| Tumor depth | |
| T1 | 622 (18.9) |
| T2 | 447 (13.6) |
| T3 | 203 (6.2) |
| T4 | 2015 (61.3) |
| Lymph node metastasis | |
| N0 | 1142 (34.7) |
| N1 | 622 (18.9) |
| N2 | 703 (21.4) |
| N3 | 820 (24.9) |
| pTNM | |
| I | 788 (24.0) |
| II | 733 (22.3) |
| III | 1766 (53.7) |
| CEA | |
| < 5 | 2578 (78.4) |
| ≥ 5 | 709 (21.6) |
| CA19-9 | |
| < 37 | 2573 (78.3) |
| ≥ 37 | 714 (21.7) |
| CA125 | |
| < 35 | 3101 (94.3) |
| ≥ 35 | 186 (5.7) |
| AFP | |
| < 10 | 3110 (94.6) |
| ≥ 10 | 177 (5.4) |
| CA242 | |
| < 15 | 2735 (83.2) |
| ≥ 15 | 552 (16.8) |
| CA72-4 | |
| < 6.9 | 2661 (81.0) |
| ≥ 6.9 | 626 (19.0) |
AFP: Alpha-fetoprotein; CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; BMI: Body mass index; pTNM: Pathological tumor-node-metastasis; PMGC: Peritoneal metastases of gastric cancer; MGC: Mucinous gastric carcinoma.
Figure 3.
Kaplan-Meier survival curves for different locations of gastric cancer, where different colors represent different locations. Red: Lower 1/3; green: Middle 1/3; blue: Total; purple: Upper 1/3). The solid line represents the Kaplan-Meier estimates for each group. The shaded area around the survival curve represents the 95% confidence interval, representing the range of fluctuations in the true survival estimate. A P value less than 0.05 was considered to indicate statistical significance.
Table 2.
Five-year overall survival of patients with different tumor locations, %
|
Tumor location
|
OS
|
||||
|
1-year
|
2-year
|
3-year
|
4-year
|
5-year
|
|
| Upper | 89.7 | 73.3 | 52.3 | 40.6 | 32.2 |
| Middle | 93.5 | 78.1 | 53.6 | 41.8 | 30.4 |
| Lower | 91.2 | 77.9 | 57.8 | 44.4 | 33.5 |
| Total | 78.8 | 53.8 | 36.5 | 26.9 | 25.0 |
OS: Overall survival.
Location of GC is associated with multiple factors
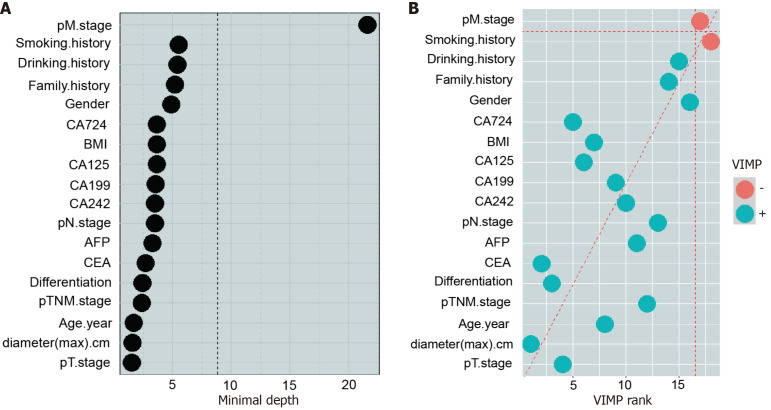
Subsequently, we examined the associations between various clinical variables and the anatomical location of GC. We identified the relevant factors contributing to the location of GC through the random forest model, the minimum depth method and the VIMP method (Figure 4 and Figure 5). This preliminary analysis paved the way for deeper exploration.
Figure 4.
Variable importance of the predictors for the predictive models for gastric cancer location. A: The minimum depth method was used to calculate the influencing factors related to the location of gastric cancer (GC). The vertical dashed line represents the minimum depth threshold; B: The variable importance method was used to calculate the influencing factors related to the location of GC. The green dots indicate strong correlations among impact factors, and the orange dots indicate weak correlations among impact factors. CEA: Carcinoembryonic antigen; AFP: Alpha-fetoprotein; CA: Carbohydrate antigen; BMI: Body mass index; pTNM: Pathological tumor-node-metastasis.
Figure 5.

Random forest plot of influencing factors related to gastric cancer location. According to the random forest model, the importance of each parameter to the degree of influence on the location of gastric cancer was calculated, and the histogram was drawn in order of importance. CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; BMI: Body mass index; pTNM: Pathological tumor-node-metastasis.
A series of one-way χ2 tests examining the association between patient variables and the location of GC indicated that gender, age, differentiation, extent of resection, vascular tumor thrombus, nerve invasion, pTNM stage, and serum levels of CEA, CA19-9, CA242, and CA72-4 were significant factors (P < 0.05) (Table 3). Furthermore, Spearman’s correlation analysis of GC location with relevant clinicopathological features revealed that gender (P = 0.001), age (P < 0.001), CEA (P < 0.001), CA19-9 (P < 0.001), CA242 (P < 0.001), and CA72-4 (P = 0.006) were significantly correlated with high confidence levels (Table 4). However, there was no significant correlation between GC location and BMI (P = 0.354), family history (P = 0.177), smoking history (P = 0.140), drinking history (P = 0.409), pathological type (P = 0.131), or alpha-fetoprotein (AFP) level (P = 0.197).
Table 3.
Correlations between tumor location and clinicopathologic characteristics of gastric cancer patients
| Characteristics |
Tumor location
|
||||
|
Middle
|
Lower
|
Upper
|
Total
|
P value
|
|
| Gender | |||||
| Male | 390 | 1285 | 826 | 40 | < 0.001 |
| Female | 117 | 436 | 181 | 12 | |
| Age | |||||
| < 60 | 228 | 692 | 248 | 23 | < 0.001 |
| ≥ 60 | 279 | 1029 | 759 | 29 | |
| BMI | |||||
| ≤ 18 | 40 | 161 | 89 | 6 | 0.354 |
| 18-24 | 306 | 1107 | 645 | 32 | |
| ≥ 24 | 161 | 453 | 273 | 14 | |
| Family history | |||||
| No | 320 | 1125 | 629 | 28 | 0.177 |
| Yes | 187 | 596 | 378 | 24 | |
| Smoking history | |||||
| No | 290 | 935 | 515 | 27 | 0.140 |
| Yes | 217 | 786 | 492 | 25 | |
| Drinking history | |||||
| No | 364 | 1178 | 682 | 37 | 0.409 |
| Yes | 143 | 543 | 325 | 15 | |
| Range of resection | |||||
| Proximal | 9 | 1 | 177 | 0 | < 0.001 |
| Distal | 197 | 1515 | 5 | 2 | |
| Total | 301 | 205 | 825 | 50 | |
| Differentiation | |||||
| Well | 2 | 15 | 5 | 0 | < 0.001 |
| Well-moderate | 4 | 37 | 19 | 1 | |
| Moderate | 101 | 363 | 300 | 7 | |
| Moderate-poor | 193 | 693 | 397 | 17 | |
| Poor | 207 | 613 | 286 | 27 | |
| Pathological type | |||||
| Adenocarcinoma | 460 | 1509 | 871 | 43 | 0.131 |
| PMGC | 20 | 111 | 72 | 3 | |
| MGC | 27 | 101 | 64 | 6 | |
| Vascular tumor thrombus | |||||
| No | 281 | 923 | 501 | 15 | 0.001 |
| Yes | 226 | 798 | 506 | 37 | |
| Nerve invasion | |||||
| No | 266 | 1023 | 421 | 8 | < 0.001 |
| Yes | 241 | 698 | 586 | 44 | |
| Maximum tumor diameter | |||||
| < 5 cm | 344 | 1129 | 505 | 4 | < 0.001 |
| ≥ 5 cm | 163 | 592 | 502 | 48 | |
| Tumor depth | |||||
| T1 | 103 | 433 | 86 | 0 | < 0.001 |
| T2 | 80 | 265 | 100 | 2 | |
| T3 | 40 | 96 | 64 | 3 | |
| T4 | 284 | 927 | 757 | 47 | |
| Lymph node metastasis | |||||
| N0 | 196 | 624 | 318 | 4 | < 0.001 |
| N1 | 101 | 331 | 186 | 4 | |
| N2 | 95 | 350 | 244 | 14 | |
| N3 | 115 | 416 | 259 | 30 | |
| pTNM | |||||
| I | 135 | 501 | 150 | 2 | < 0.001 |
| II | 136 | 377 | 218 | 2 | |
| III | 236 | 843 | 639 | 48 | |
| CEA | |||||
| < 5 | 424 | 1369 | 753 | 32 | < 0.001 |
| ≥ 5 | 83 | 352 | 254 | 20 | |
| CA19-9 | |||||
| < 37 | 423 | 1365 | 752 | 33 | < 0.001 |
| ≥ 37 | 84 | 356 | 255 | 19 | |
| CA125 | |||||
| < 35 | 474 | 1635 | 950 | 42 | < 0.001 |
| ≥ 35 | 33 | 86 | 57 | 10 | |
| AFP | |||||
| < 10 | 484 | 1626 | 954 | 46 | 0.197 |
| ≥ 10 | 23 | 95 | 53 | 6 | |
| CA242 | |||||
| < 15 | 439 | 1448 | 812 | 36 | 0.001 |
| ≥ 15 | 68 | 273 | 195 | 16 | |
| CA72-4 | |||||
| < 6.9 | 432 | 1391 | 802 | 36 | 0.008 |
| ≥ 6.9 | 75 | 330 | 205 | 16 | |
AFP: Alpha-fetoprotein; CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; BMI: Body mass index; pTNM: Pathological tumor-node-metastasis; PMGC: Peritoneal metastases of gastric cancer; MGC: Mucinous gastric carcinoma.
Table 4.
Spearman analysis of the correlation between tumor location and clinicopathological data
| Characteristics |
Tumor location
|
|
|
Spearman correlation
|
P value
|
|
| Gender | -0.056 | 0.001 |
| Age | 0.148 | < 0.001 |
| BMI | -0.022 | 0.197 |
| Family history | 0.019 | 0.279 |
| Smoking history | 0.040 | 0.020 |
| Drinking history | 0.022 | 0.202 |
| Range of resection | 0.256 | < 0.001 |
| Differentiation | -0.092 | < 0.001 |
| Pathological type | 0.041 | 0.019 |
| Vascular tumor thrombus | 0.054 | 0.002 |
| Nerve invasion | 0.128 | < 0.001 |
| Maximum tumor diameter | 0.169 | < 0.001 |
| Tumor depth | 0.190 | < 0.001 |
| Lymph node metastasis | 0.077 | < 0.001 |
| pTNM | 0.159 | < 0.001 |
| CEA | 0.083 | < 0.001 |
| CA19-9 | 0.080 | < 0.001 |
| CA125 | 0.013 | 0.452 |
| AFP | 0.014 | 0.410 |
| CA242 | 0.064 | < 0.001 |
| CA72-4 | 0.048 | 0.006 |
CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; BMI: Body mass index; pTNM: Pathological tumor-node-metastasis.
This comprehensive analysis underscores the multifactorial nature of GC localization and highlights key clinicopathological variables that are significantly associated with the disease.
The main factors affecting the location of GC
To explore the main factors affecting the location of GC, we conducted multiple logistic regression analysis. The analysis demonstrated a high degree of statistical significance (P < 0.001), indicating a strong agreement between the model’s predictions and the observed data. This confirms the robustness of our model. Importantly, the CA242 index did not achieve statistical significance (P > 0.05), indicating it does not independently affect the location of GC but may interact with other variables. Thus, the CA242 index was excluded from the final analysis of major influencing factors.
Several types of cancer, including those affecting the stomach, liver, and colon, are significantly more prevalent in men than in women[8-10]. Therefore, to more accurately understand the relationship between the location of GC and its influencing factors, we divided 3287 patients into two groups according to gender: Males (2541) and females (746) (Table 5). Multiple logistic regression analysis was then performed for both groups. In female patients, the main influencing factors for gastric body cancer were CA19-9 [P = 0.041, odds ratio (OR) = 2.399] and CA72-4 (P < 0.001, OR = 7.052). The probability of developing carcinoma of the gastric body was 2.399-fold and 7.052-fold higher in patients with high CA19-9 and CA72-4 expression, respectively. The main influencing factors for distal GC were age (P = 0.003, OR = 0.387), CEA level (P = 0.034, OR = 0.312), and CA72-4 level (P < 0.001, OR = 7.104). High CA72-4 expression increased the probability of developing distal gastric carcinoma by 7.104-fold compared to that of total GC. In addition, high CEA expression and age older than 60 years were associated with a higher probability of total stomach cancer than distal stomach cancer. For proximal GC, the main influencing factors were age (P < 0.001, OR = 0.150), CEA level (P = 0.010, OR = 0.241), and CA72-4 level (P < 0.001, OR = 8.152). High CA72-4 expression was associated with an 8.152-fold higher probability of proximal gastric carcinoma than total GC. Conversely, a high CEA level and an age over 60 years were linked to a higher probability of total GC than proximal GC (Table 6).
Table 5.
Tumor markers characteristics of gastric cancer patient samples (male and female, n = 3287), n (%)
|
Characteristics
|
Male
|
Female
|
| Age | ||
| < 60 | 852 (33.5) | 339 (45.4) |
| ≥ 60 | 1689 (66.5) | 407 (54.6) |
| CEA | ||
| < 5 | 1949 (76.7) | 629 (84.3) |
| ≥ 5 | 592 (23.3) | 117 (15.7) |
| CA19-9 | ||
| < 37 | 1988 (78.2) | 585 (78.4) |
| ≥ 37 | 553 (21.8) | 161 (21.6) |
| CA242 | ||
| < 15 | 2125 (83.6) | 610 (81.8) |
| ≥ 15 | 416 (16.4) | 136 (18.2) |
| CA72-4 | ||
| < 6.9 | 2051 (80.7) | 610 (81.8) |
| ≥ 6.9 | 490 (19.3) | 136 (18.2) |
CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen.
Table 6.
Logistic regression analysis of multiple clinicopathological characteristic variables related to the location of gastric cancer (female)
|
|
P value
|
OR
|
|
| Middle | CA19-9 | 0.041 | 2.339 |
| CA72-4 | < 0.001 | 7.052 | |
| Lower | Age | 0.003 | 0.387 |
| CEA | 0.034 | 0.312 | |
| CA72-4 | < 0.001 | 7.104 | |
| Upper | Age | < 0.001 | 0.150 |
| CEA | 0.010 | 0.241 | |
| CA72-4 | < 0.001 | 8.152 | |
CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; OR: Odd ratio.
In male patients, the primary influencing factors for gastric body cancer were CEA (P < 0.001, OR = 3.545) and CA19-9 (P < 0.001, OR = 2.246). Elevated levels of CEA and CA19-9, increased the probability of carcinoma in the gastric body by 3.545-fold and 2.246-fold, respectively, compared to total stomach cancer. For distal GC, the main influencing factors were CEA (P < 0.001, OR = 2.869) and CA19-9 (P < 0.001, OR = 1.932). High expression of CEA and CA19-9 increased the probabilities of distal gastric carcinoma by 2.869-fold and 1.932-fold, respectively. The primary influencing factors for proximal GC were age (P < 0.001, OR = 0.501), CEA level (P < 0.001, OR = 2.473), and CA19-9 level (P = 0.015, OR = 1.539). High expression of CEA and CA19-9 increased the probability of proximal gastric carcinoma by 2.473-fold and 1.539-fold, respectively, compared to that of total gastric carcinoma. Additionally, increasing age, especially above 60 years, was associated with a higher probability of total stomach cancer than proximal stomach cancer (Table 7).
Table 7.
Logistic regression analysis of multiple clinicopathological characteristic variables related to the location of gastric cancer (male)
|
|
P value
|
OR
|
|
| Middle | CEA | < 0.001 | 3.545 |
| CA19-9 | < 0.001 | 2.246 | |
| Lower | CEA | < 0.001 | 2.869 |
| CA19-9 | < 0.001 | 1.932 | |
| Upper | Age | < 0.001 | 0.501 |
| CEA | < 0.001 | 2.473 | |
| CA19-9 | 0.015 | 1.539 | |
CEA: Carcinoembryonic antigen; CA: Carbohydrate antigen; OR: Odd ratio.
Based on the foregoing analysis, CA72-4 has emerged as a pivotal tumor marker in female patients, particularly for assessing the incidence of GC subsites. In contrast, CA72-4 was not used to assess the incidence of GC sub-sites in male patients. Therefore, we believe that CA72-4 is an important tumor marker for studying gender difference in the location of GC. The CEA index is important for predicting total stomach cancer in females. In male patients, CEA and CA19-9 are prominent tumor markers for analyzing the incidence of GC.
DISCUSSION
In this study, we demonstrated that the location of GC was associated with patient survival: Patients with gastric body and distal GC exhibiting longer survival than those with proximal GC or total GC. Five variables [gender (P < 0.001), age (P < 0.001), CEA (P < 0.001), CA19-9 (P < 0.001), and CA72-4 (P = 0.006)] were found to influence the location of GC through univariate correlation analysis, Spearman correlation analysis, and logistic regression analysis. It was further inferred that age was an important factor in the development of total GC, CA72-4 was an important factor in the incidence of GC subsites in female patients, and CEA and CA19-9 were important factors in the incidence of GC subsites in male patients.
Differences in prognostic survival due to site of GC are associated with several factors. To further understand the causes of differences in postoperative patient survival based on GC location, we conducted a comprehensive search to summarize the physical, physiological, and genomic mechanisms affected by distal GC and proximal GC. This analysis was performed to elucidate the differences in survival prognosis attributable to tumor location. Some researchers have proposed that distal GC may have a better prognosis because of the early symptoms caused by pyloric obstruction, a complication that facilitates early diagnosis and treatment[11]. However, no conclusive evidence currently exists to support this hypothesis. A more reliable study demonstrated that gastric cardia cancer is highly invasive and more likely to involve blood vessels and lymph nodes than GC in other locations[12]. When GC invades large blood vessels, it may cause hemorrhage. Invasion of the esophagus can cause gastroesophageal stricture, resulting in dysphagia and subsequent malnutrition, both of which negatively impact survival. Moreover, vascular invasion and metastatic lymph node involvement have long been recognized as important factors in worsening the prognosis of patients with GC[13-15]. Reducing the incidence and mortality of GC remains a critical health objective. Our research proves that age, gender, and tumor markers influence the location of GC and patient survival. Previous studies have found that elevated serum levels of CEA and CA19-9 are closely related to tumor invasion, lymph node metastasis and TNM stage, while CA72-4 is more frequently observed in patients with vascular involvement[16-18]. Additionally, it has been postulated that elevated serum CEA levels may promote peritoneal tumor metastasis[19]. These results demonstrate that these tumor markers influence the prognostic survival of patients.
Contrary to our findings, some studies have shown that a smoking history is not only associated with an increased risk of GC overall[20], but specifically increases the risk of distal GC, without significantly affecting the risk of cardia or gastric body cancer[21]. In addition, an earlier case-control trial in Sweden identified BMI as an important factor in cardia cancer[22], with a higher prevalence of obesity among patients with this cancer type[23]. Furthermore, a Japanese study revealed that alcohol intake was associated with an increased risk of adenocarcinoma at the gastric cardia, possibly because alcohol promotes gastroesophageal reflux disease and thus increases the associated risk[24]. The variation in results may be attributed to difference in sample size. Previous studies on GC had sample sizes of fewer than 1000 patients, whereas our study included over 3000 patients. Therefore, our large sample size likely contributes to more accurate and objective findings. However, although factors such as family history[25] and AFP[26] have been reported to be associated with GC, there is limited conclusive evidence linking them to the tumor location. This is consistent with the results of our clinical data analysis.
This study has important clinical implications for the treatment of GC and addresses a gap in the existing literature. The identification of age, gender, and tumor markers (CEA, CA19-9, and CA72-4) as factors influencing GC location will contribute to the further development and standardization of clinical diagnostic methods with improved sensitivity and specificity. These findings may also enhance prognostic assessments for GC patients.
There are also some limitations in this study. First, the patient information collected was not comprehensive enough. Poor dietary habits are known to increase Helicobacter pylori infection, which causes persistent chronic inflammation of the gastric mucosa, and increases the risk of distal gastric adenocarcinoma[27,28]. Additionally, the incidence of GC varies greatly by geographic location, ethnicity, and socioeconomic status. Distal GC is more prevalent in developing countries, among Black populations, and in lower socioeconomic groups, whereas proximal tumors are more common in developed countries, among White populations, and in higher socioeconomic classes[29,30]. Unfortunately, these variables were not collected in our current study. Moreover, due to the vast geographic expanse of China, many post-surgical oncology patients return to local hospitals for follow-up treatment. We did not collect this data because of the lack of interoperability between medical record systems across hospitals in China, as well as patients’ limited ability to accurately report their treatment plans. Future research, if feasible, could benefit from sequencing tumor tissue specimens or conducting animal model experiments to further investigate the gene expression profiles of tumors at different gastric sites. Such studies could help explore the relationships between these differentially expressed genes and tumor markers and identify potential interconnected pathways that could guide subsequent evaluation of tumor location and prognosis.
CONCLUSION
In conclusion, this study illustrated that tumor location affects prognostic survival. In addition, we found that the five clinicopathological indices of gender, age, CEA, CA19-9, and CA72-4 levels exhibit varying degrees of influence on the location of GC. Therefore, early screening and early prevention are particularly important.
ACKNOWLEDGEMENTS
We thank all the laboratory members for insightful discussions.
Footnotes
Institutional review board statement: All study procedures, including animal care and handling, were approved by the Ethics Committee of Zhejiang Cancer Hospital (approval No. IRB-2024-604).
Informed consent statement: Informed consent was obtained from all participants involved in the study. Participants were provided with detailed information about the aims, methods, risks, benefits, and their rights to withdraw from the research at any time without penalty. All participants signed a consent form indicating their voluntary agreement to participate.
Conflict-of-interest statement: Author(s) certify that there is no conflict of interest related to the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C, Grade C
Novelty: Grade B, Grade B, Grade C, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C, Grade C
Scientific Significance: Grade B, Grade B, Grade B, Grade C
P-Reviewer: Jia JH; Sermet M; Song WC S-Editor: Chen YL L-Editor: A P-Editor: Guo X
Contributor Information
Yi-Xing Huang, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; School of Medicine, Zhejiang University, Hangzhou 310058, Zhejiang Province, China.
Han-Yi He, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Ken Chen, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Hai-Dong Liu, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Dan Zu, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Chen Liang, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Qi-Mei Bao, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Yang-Chan Hu, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Guo-Xia Liu, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Yu-Ke Zhong, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Chun-Kai Zhang, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Ming-Cong Deng, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China.
Yan-Hua He, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Ji Jing, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Yin Shi, School of Medicine, Zhejiang University, Hangzhou 310058, Zhejiang Province, China.
Sheng-Feng Xu, Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX 77030, United States.
Yao-Shu Teng, Department of Otorhinolaryngology, Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou 310006, Zhejiang Province, China.
Zu Ye, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China.
Xiang-Dong Cheng, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine, Chinese Academy of Sciences, Hangzhou 310022, Zhejiang Province, China; Key Laboratory of Prevention, Diagnosis and Therapy of Upper Gastrointestinal Cancer of Zhejiang Province, Hangzhou 310022, China; Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer, Zhejiang Cancer Hospital, Hangzhou 310022, China. yezuqscx@gmail.com.
Data sharing statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao K, Wu J. National trend of gastric cancer mortality in China (2003-2015): a population-based study. Cancer Commun (Lond) 2019;39:24. doi: 10.1186/s40880-019-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pithawa AK. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: pathophysiology, diagnosis, management. Med J Armed Forces India. 2011;63:205. [Google Scholar]
- 5.Salvon-Harman JC, Cady B, Nikulasson S, Khettry U, Stone MD, Lavin P. Shifting proportions of gastric adenocarcinomas. Arch Surg. 1994;129:381–8; discussion 388. doi: 10.1001/archsurg.1994.01420280053007. [DOI] [PubMed] [Google Scholar]
- 6.Shen Z, Ye Y, Xie Q, Liang B, Jiang K, Wang S. Effect of the number of lymph nodes harvested on the long-term survival of gastric cancer patients according to tumor stage and location: a 12-year study of 1,637 cases. Am J Surg. 2015;210:431–40.e3. doi: 10.1016/j.amjsurg.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Michelassi F, Takanishi DM Jr, Pantalone D, Hart J, Chappell R, Block GE. Analysis of clinicopathologic prognostic features in patients with gastric adenocarcinoma. Surgery. 1994;116:804–9; discussion 809. [PubMed] [Google Scholar]
- 8.Liu Y, Kaneko S, Sobue T. Trends in reported incidences of gastric cancer by tumour location, from 1975 to 1989 in Japan. Int J Epidemiol. 2004;33:808–815. doi: 10.1093/ije/dyh053. [DOI] [PubMed] [Google Scholar]
- 9.Hansson LE, Sparén P, Nyrén O. Increasing incidence of carcinoma of the gastric cardia in Sweden from 1970 to 1985. Br J Surg. 1993;80:374–377. doi: 10.1002/bjs.1800800338. [DOI] [PubMed] [Google Scholar]
- 10.Sons HU, Borchard F. Cancer of the distal esophagus and cardia. Incidence, tumorous infiltration, and metastatic spread. Ann Surg. 1986;203:188–195. doi: 10.1097/00000658-198602000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itano S. Early gastric cancer and its complications: bleeding, perforation and pyloric stenosis. Acta Med Okayama. 1983;37:431–440. doi: 10.18926/AMO/32420. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Okabayashi T, Nishimori I, Sugimoto T, Namikawa T, Dabanaka K, Tsujii S, Onishi S, Kobayashi M, Hanazaki K. Clinicopathologic features of adenocarcinoma at the gastric cardia: is it different from distal cancer of the stomach? J Am Coll Surg. 2008;206:306–310. doi: 10.1016/j.jamcollsurg.2007.06.306. [DOI] [PubMed] [Google Scholar]
- 13.del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, García-Muñiz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153–161. doi: 10.1007/s00432-007-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–567. doi: 10.1007/BF02573892. [DOI] [PubMed] [Google Scholar]
- 15.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 16.Wang JH, Mai C, Hong J, Zhang Q, Tang HS, Tang YQ, Cui SZ. [Predicting value of serum CEA and CA19-9 in neoadjuvant chemotherapy for advanced gastric carcinoma] Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:1273–1276. [PubMed] [Google Scholar]
- 17.Zhu YB, Ge SH, Zhang LH, Wang XH, Xing XF, DU H, Hu Y, Li YA, Jia YN, Lin Y, Fan B, Ji JF. [Clinical value of serum CEA, CA19-9, CA72-4 and CA242 in the diagnosis and prognosis of gastric cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:161–164. [PubMed] [Google Scholar]
- 18.Zhou J, Zhang L, Gu Y, Li K, Nie Y, Fan D, Feng Y. Dynamic expression of CEACAM7 in precursor lesions of gastric carcinoma and its prognostic value in combination with CEA. World J Surg Oncol. 2011;9:172. doi: 10.1186/1477-7819-9-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama M, Takashima I, Tanaka T, Yoshida K, Toge T, Nagata N, Iwamori S, Tamura Y. Carcinoembryonic antigen levels in the peritoneal cavity: useful guide to peritoneal recurrence and prognosis for gastric cancer. World J Surg. 1995;19:133–7; discussion 137. doi: 10.1007/BF00316997. [DOI] [PubMed] [Google Scholar]
- 20.Chao A, Thun MJ, Henley SJ, Jacobs EJ, McCullough ML, Calle EE. Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: The Cancer Prevention Study II. Int J Cancer. 2002;101:380–389. doi: 10.1002/ijc.10614. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi Y, Tsubono Y, Nakaya N, Kuriyama S, Shibuya D, Matsuoka H, Tsuji I. Cigarette smoking and the risk of gastric cancer: a pooled analysis of two prospective studies in Japan. Int J Cancer. 2004;112:1049–1055. doi: 10.1002/ijc.20518. [DOI] [PubMed] [Google Scholar]
- 22.Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 25.Bernini M, Barbi S, Roviello F, Scarpa A, Moore P, Pedrazzani C, Beghelli S, Marrelli D, de Manzoni G. Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric Cancer. 2006;9:9–13. doi: 10.1007/s10120-005-0350-7. [DOI] [PubMed] [Google Scholar]
- 26.Vatansever S, Özer MK, Erdoğan EI. Prognostic significance of α-fetoprotein in gastric adenocarcinoma. Prz Gastroenterol. 2022;17:35–40. doi: 10.5114/pg.2022.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: shifting the global burden. World J Gastroenterol. 2006;12:5458–5464. doi: 10.3748/wjg.v12.i34.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O’Brien DP, Romero-Gallo J, Peek RM Jr. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer. 1995;75:154–170. doi: 10.1002/1097-0142(19950101)75:1+<154::aid-cncr2820751305>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.