Abstract
Background
Local infiltration analgesia (LIA) is a crucial component of multimodal analgesia that enhances recovery after total hip arthroplasty (THA) and knee arthroplasty (TKA). However, LIA can cause fatal local anesthetic systemic toxicity (LAST). The incidences of LIA-induced LAST in different surgeries and anesthetic agents have not been well investigated.
Methods
This observational study enrolled 1,267 adult patients who received LIA with bupivacaine for primary THA or TKA under general anesthesia at a single medical center between January 2020 and October 2021. LAST incidence was graded by severity: severe (refractory seizure or arrhythmia requiring lipid rescue), major (seizure or arrhythmia requiring prompt medication), and minor (all other) events. Patient demographics, surgical and anesthesia details, recovery profiles, and LAST incidences were recorded and analyzed.
Results
The incidence of severe LAST events was 2.41 per 1000 in unilateral THA, 0 in TKA, and 3.16 per 1000 in the entire cohort. The odds ratio of developing major LAST events was 4.35 in patients undergoing unilateral THA compared with those undergoing unilateral TKA in a matched comparison. Patients who underwent bilateral THA had the highest risk of developing LIA-induced LAST. Additionally, patients using propofol infusion for anesthesia maintenance had a lower risk of seizures and tremors than those using sevoflurane inhalation. The odds ratio of major LAST events was 0.47 in matched comparisons.
Conclusions
LIA was associated with a significantly higher risk of LAST in the THA group than in the TKA group. Propofol maintenance reduces the likelihood of seizures and tremors compared with sevoflurane inhalation. Exploring strategies to reduce the incidence of LIA-induced LAST is essential to improve perioperative patient safety.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-024-02816-y.
Keywords: Lipid emulsion, Local anesthetic systemic toxicity, Local infiltration analgesia, Total hip arthroplasty, Total knee arthroplasty
Background
As the population ages and the obesity epidemic continues, the requirement for joint replacement surgery owing to joint degeneration is increasing. Data from the National Inpatient Samples of the United States from 2000 to 2014 projects that the annual volume of primary total knee arthroplasty (TKA) will surge by 71% (to 1.26 million surgeries) by 2030, while the volume of primary total hip arthroplasty (THA) is expected to increase by 85% (to 0.635 million surgeries) by 2030 [1]. Proper management of postoperative pain after total joint arthroplasty is the key to enhancing recovery after surgery, often involving multimodal analgesia that combines local infiltration analgesia (LIA), peripheral nerve blocks, and two or more analgesics with different mechanisms [2]. Surgeon-performed LIA is motor-sparing and offers tremendous advantages over traditional opioids, including enhanced early mobilization, improved joint range of motion, reduced total analgesic consumption, less postoperative vomiting, and facilitated rehabilitation [3, 4].
LIA with bupivacaine for THA and TKA has become routine practice at our institute to facilitate patient recovery, because it significantly reduces postoperative pain and meperidine use [5]. However, the incidence of postoperative nausea and vomiting is higher in patients receiving LIA because of the systemic effects of local anesthetics [5]. Despite this, most patients experience rapid recovery after surgery under the fast-track THA and TKA protocols and can be discharged on the first postoperative day with satisfactory mobility. Nevertheless, LIA with high-dose bupivacaine carries the potential risk of local anesthetic systemic toxicity (LAST). An American single-center observational cohort study found that the incidence of LIA-induced LAST requiring rescue lipid emulsion was 0.7 per 1000 and 2.0 per 1000 following unilateral TKA and THA, respectively [6]. Clinical manifestations of LAST include seizures, new-onset arrhythmia, unconsciousness, and respiratory suppression, etc. These LAST events were frequently managed in the post-anesthesia recovery unit (PACU) with interventions such as nasal/oral airway insertion for airway support and medications including midazolam, atropine, and antiarrhythmics. For severe LAST events defined as refractory seizures or arrhythmias that persisted despite the use of midazolam or antiarrhythmics, a rescue infusion of lipid emulsion was administered [7].
Fortunately, most of these LAST events were self-limiting and resolved within two hours after the LIA procedures without impeding early ambulation and rehabilitation. However, it is unclear whether these LAST events delay patient recovery after THA or TKA, and the incidences of LIA-induced LAST among various surgeries and anesthetic agents remain unclear. Therefore, this retrospective cohort study aimed to evaluate the incidence of LAST events and the recovery profiles of patients who underwent LIA for fast-track THA and TKA at our institute. We classified LAST events as severe (refractory seizure or arrhythmia requiring rescue lipid emulsion), major (seizure, bradycardia, new-onset arrhythmia), or minor (other LAST symptoms) according to their clinical manifestations. Subsequently, we made propensity-matched comparison of the incidence of LAST events in patients undergoing THA versus TKA and in those maintained with propofol versus sevoflurane.
Methods
Study population
This retrospective single-center study aimed to analyze the incidences of LAST after LIA for THA and TKA, as well as the influence of sevoflurane inhalation or propofol infusion on LAST occurrences. This cohort study enrolled patients who underwent LIA for primary THA or TKA at the Linkou Chang Gung Memorial Hospital between January 2020 and October 2021. The study was approved by the Institutional Ethics Committee of the Chang Gung Medical Foundation (approval number: 202200080B0). The need for informed consent was waived because of the retrospective design of the study and the use of anonymized personal information.
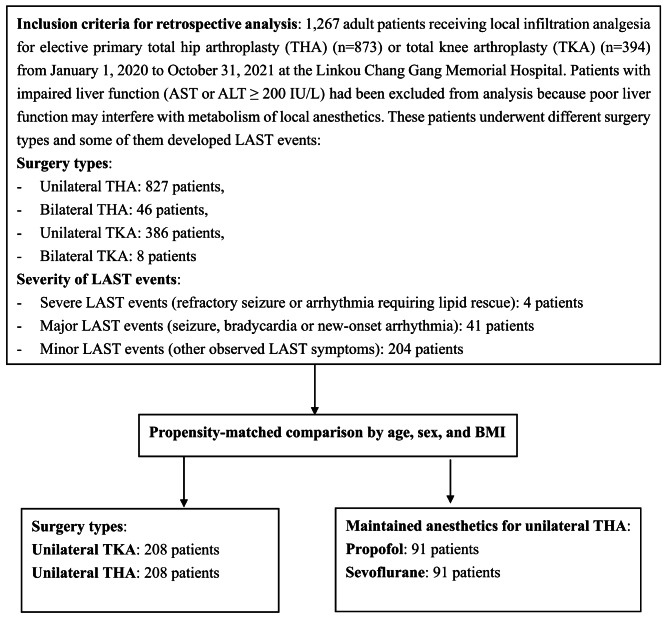
The study design and group allocations are shown in Fig. 1. This study enrolled 1,267 adult patients who underwent LIA for primary THA or TKA using a preset fast-track protocol. Patients with impaired liver function (AST or ALT ≥ 200 IU/L) were excluded from this study because poor liver function may interfere with LA metabolism and the production of albumin and α1-acid glycoprotein, which binds with unbound LA and reduces its plasma concentration [8]. Among these patients, 873 underwent THA (827 unilateral and 46 bilateral), and 394 underwent TKA (386 unilateral and 8 bilateral). Subsequently, we conducted a propensity score-matched comparison for subgroup analysis by matching age, sex, and body mass index (BMI). The subgroup analysis included comparisons of LAST incidence between the unilateral THA (n = 208) and unilateral TKA (n = 208) groups and between the propofol (n = 91) and sevoflurane (n = 91) groups in patients undergoing unilateral THA. All surgeries were performed by the same orthopedic team, with THA solely by P-H Hsieh and TKA by P-H Hsieh and his colleagues. The fast-track protocol for THA and TKA was designed to provide optimal patient care, enabling rapid recovery after surgery with satisfactory mobility. All enrolled patients underwent preoperative optimization, intraoperative stress minimization, and early postoperative mobilization. Preoperative optimization includes smoking cessation for more than four weeks and medication adjustments such as holding anticoagulants (e.g. warfarin). Intraoperative stress minimization includes active air warming, controlled hypotension to reduce blood loss, antimicrobial prophylaxis, prevention of postoperative nausea and vomiting, and multimodal analgesia. Postoperative early mobilization includes early oral intake and aggressive physical therapy. The multimodal analgesia protocol includes parenteral non-steroidal anti-inflammatory drugs, acetaminophen, parecoxib, LIA, ice packing, and intramuscular opioid rescue to promote early rehabilitation and ensure smooth next-day discharge for most patients. Patient baseline demographics, surgical and anesthesia details (including anesthetic usage, blood loss, LA dose, and anesthesia duration), and postoperative recovery profiles (including numeric rating scale [NRS], LAST events, and duration of hospitalization) were obtained from hospital records for subsequent comparison.
Fig. 1.
Flow chart of the study design and group allocation
General anesthesia, joint replacement surgery, and prescriptions for LIA and lipid emulsion
All patients recruited in this cohort underwent joint replacement surgeries under general anesthesia. General anesthesia was induced with fentanyl (0.5–1.5 mcg/kg), 2% xylocaine (20 mg), propofol (1.5–2.0 mg/kg), and cisatracurium (0.1–0.2 mg/kg) or rocuronium (0.5–1.0 mg/kg), and then maintained with either sevoflurane (Ultane, Abbott, UK) or propofol (Fresofol, Fresenius Kabi, Austria) after tracheal intubation. In all TKA surgeries, a tourniquet was used to reduce intraoperative blood loss.
In our fast-track THA and TKA protocol, the prescription for LIA involved administering 2–3 mg/kg of bupivacaine (maximal 300 mg for unilateral LIA) (0.5%; AstraZeneca, France) mixed with normal saline to create a 60 ml LIA mixture. Half of the dose was infiltrated beneath the tensor fascia lata before arthrotomy (or the posterior capsule in TKA), while the other half was infiltrated into the gluteus medius muscle (or the anterior capsule in TKA) and subcutaneous tissue after implantation and reduction [9]. Previous pharmacokinetic studies on nerve block or epidural anesthesia have indicated that the median time to peak plasma concentration of bupivacaine is 20–40 min, but the bupivacaine concentration curve after LIA has not been investigated [10]. To monitor for possible LAST symptoms, patients receiving LIA were observed in the PACU for 1–2 h.
Management of LAST events
Upon wound closure, all patients were expected to undergo smooth extubation within 10 min and were then transferred to the PACU to make the operating room available for the next surgery. However, if the extubation criteria were not met or the patient experienced delayed emergence, delayed extubation in the PACU was performed at the discretion of the duty anesthesiologists. Patients receiving high-dose LA for LIA have an increased probability of developing LAST. The major systemic toxicities of LAST involve the central nervous system (CNS), cardiovascular, and respiratory systems. CNS-related manifestations included seizures (generalized convulsions), agitation (irritability or restlessness), tremors (focal muscle twitching), and delayed emergence (defined as a Glasgow Coma Scale (GCS) score < 8 upon PACU arrival). Cardiovascular-related toxicities included ventricular arrhythmia (ventricular tachycardia, or ventricular fibrillation), severe bradycardia (heart rate ≤ 45 bpm), and new-onset arrhythmia (ventricular premature contractions, widened QRS complex, prolonged QT Interval, atrioventricular block, other complex arrhythmias) [11]. Respiratory suppression was indicated by dyspnea or apnea, delayed extubation, oral/nasal airway insertion for airway support, and oxygen desaturation (SpO2 < 95% in breathing room air) during postoperative care. A rescue dose of lipid emulsion was infused in patients who developed severe LAST events with refractory seizures or ventricular arrhythmia despite repeated administration of midazolam or antiarrhythmics. After observation in the PACU for 1–2 h and achieving a stable condition (Modified Aldrete Score of ≥ 8 and a NRS for pain of ≤ 3), these patients were transferred to the medical ward for further care. However, the residual systemic effects of LA, such as dizziness or postoperative nausea and vomiting, might persist even though ondansetron (8 mg; Supren, Taiwan Biotech Co.) was routinely administered to every patient before the end of surgery. Each LAST event in our study was diagnosed by a duty anesthesiologist and an attending physician in the PACU and recorded using a comprehensive quality assurance system. We utilized electronic anesthesia records and nursing records in the PACU and wards to reconfirm the association of these relevant events with LAST. This thorough examination included intraoperative and postoperative occurrences along with objective measurements to ensure consistent and accurate documentation of LAST events. The definitions and sources of the LAST events are summarized in Supplementary Table 1.
Comparison of LAST incidence rates in different surgical types and maintained anesthetics
In clinical practice, varying incidence rates of LAST events have been observed with different surgical types and maintained anesthetics. We observed a higher incidence of LAST in patients who underwent THA than in those who underwent TKA, even when the same LIA dosages of bupivacaine was administered. Consequently, we conducted a propensity-matched comparison of the incidence of LAST events in patients undergoing unilateral THA versus TKA and in those maintained with propofol versus sevoflurane. For maintenance of anesthesia during THA, either intravenous propofol or inhaled sevoflurane was used at the discretion of the duty anesthesiologist, whereas during TKA, anesthesia was mostly maintained with sevoflurane. For intravenous propofol maintenance, an effect-site target-controlled infusion (TCI) pump (using Marsh model) was employed, with the effect-site concentration (Ce) titrated between 3 and 4 µg/mL or a bispectral index (BIS) of 40–60 if used. The maintained dosage of sevoflurane was set at 2–3% or a BIS of 40–60 if used. Theoretically, propofol is a lipid-based emulsion that might reduce the plasma concentration of LA and the occurrence of LAST. However, we encountered a limited availability of TCI machines during the COVID-19 pandemic; therefore, propofol infusion could only be used when TCI was available for THA patients. Therefore, we compared the incidences of LAST between patients who received propofol infusion and those who received sevoflurane inhalation for THA.
Statistical analysis
The primary outcome of this observational study was the comparison of LAST incidence between the THA and TKA groups and between the propofol infusion and sevoflurane inhalation groups. To simplify the analysis, we classified observed LAST events as severe (refractory seizure or arrhythmia requiring rescue lipid emulsion), major (seizure, bradycardia, and new-onset arrhythmia), and minor (other symptoms). Owing to substantial differences in the baseline characteristics of the patients among the compared groups (THA vs. TKA and propofol vs. sevoflurane), propensity score-matched comparisons were performed. Propensity scores were obtained using multivariate logistic regression and matched according to age, sex, and BMI. Each patient who received propofol was matched with a counterpart who received sevoflurane, and each patient who underwent THA was matched with a counterpart who underwent TKA. Continuous variables (e.g., age) were analyzed using independent sample t-tests, whereas categorical variables (e.g., sex) were evaluated using Fisher’s exact tests. Association analyses for binary outcomes (such as LAST events) were conducted using a univariate logistic regression model, and continuous outcomes (such as pain scores) were analyzed using linear regression. Within-pair outcome dependency was dealt with using the robust standard error known as the generalized estimating equation. The relationship between bupivacaine dose and the incidence of composite LAST events (as well as its components) in patients undergoing unilateral THA was explored using univariate logistic regression, which was treated with a restricted cubic spline variable with three knots. All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc. NC, USA), and a two-sided p value of < 0.05 was considered statistically significant.
Results
Demographics of patients receiving THA and TKA and their incidence of LAST events
The general characteristics of patients who underwent LIA for THA and TKA and the incidences of severe, major, and minor LAST events are presented in Table 1. Patients selected for bilateral THA or TKA generally had fewer chronic diseases but experienced longer durations of anesthesia and PACU stays, greater blood loss, and longer hospitalizations (usually discharged on the second postoperative day) than those who underwent unilateral THA or TKA. As higher doses of bupivacaine were administered in the LIA for bilateral surgeries, these patients tended to have more LAST events than those undergoing unilateral surgeries. LIA for unilateral TKA resulted in substantially fewer LAST events than unilateral THA. Similarly, patients undergoing bilateral TKA experienced a lower incidence of major LAST events (12.50%) compared to those undergoing bilateral THA (21.73%).
Table 1.
Patient demographics in THA and TKA groups in the entire cohort
| Variables | THA group (n = 873) | TKA group (n = 394) | ||
|---|---|---|---|---|
| Surgery | Unilateral (n = 827) | Bilateral (n = 46) | Unilateral (n = 386) | Bilateral (n = 8) |
| Age (years) | 60.06 ± 13.92 | 51.82 ± 13.44 | 72.31 ± 7.82 | 64.12 ± 6.43 |
| Male, n (%) | 291 (35.18) | 21 (45.65) | 92 (23.82) | 2 (25.00) |
| BMI (kg/M2) | 25.62 ± 4.24 | 25.47 ± 4.37 | 28.62 ± 6.99 | 28.32 ± 4.79 |
| Maintained anesthetics | ||||
| Propofol | 167 (20.19) | 8 (17.39) | 19 (4.92) | 0 (0) |
| Sevoflurane | 660 (79.81) | 38(82.61) | 367 (95.08) | 8 (100) |
| Chronic diseases | ||||
| Hypertension | 335 (40.51) | 9 (19.56) | 214 (55.44) | 5 (62.50) |
| Diabetes | 87 (10.52) | 1 (2.17) | 98 (25.38) | 0 (0) |
| Liver cirrhosis | 4 (0.48) | 0 (0) | 0 (0) | 0 (0) |
| Arrhythmia | 30 (3.62) | 2 (4.34) | 4 (1.04) | 1 (12.50) |
| CNS disease | 23 (2.78) | 0 (0) | 9 (2.33) | 0 (0) |
| End stage renal disease | 6 (0.73) | 0 (0) | 3 (0.77) | 0 (0) |
| Duration of anesthesia (min) | 71.24 ± 8.36 | 131.61 ± 15.58 | 80.16 ± 12.82 | 173.62 ± 21.82 |
| Duration of PACU stay (min) | 65.28 ± 13.74 | 98.22 ± 22.43 | 62.46 ± 10.52 | 79.50 ± 24.75 |
| Intraoperative blood loss (ml) | 205.67 ± 116.76 | 348.91 ± 177.79 | 118.19 ± 44.82 | 256.25 ± 105.01 |
| Hospitalization (day) | 3.02 ± 0.14 | 3.76 ± 0.56 | 3.03 ± 0.17 | 3.50 ± 0.53 |
| Pain score at 24 h (NRS) | 2.28 ± 0.82 | 2.36 ± 0.68 | 1.91 ± 0.62 | 2.25 ± 0.46 |
| Bupivacaine dose | 277.42 ± 30.56 | 372.78 ± 91.03 | 278.46 ± 41.42 | 400.00 ± 169.03 |
| Severe LAST events | 2 (0.24) | 2 (4.35) | 0 (0) | 0 (0) |
| Major LAST events | 47 (5.82) | 10 (21.73) | 5 (1.27) | 1 (12.50) |
| Seizure | 18 (2.17) | 9 (19.57) | 0 (0) | 1 (12.50) |
| Bradycardia or arrhythmia | 30 (3.62) | 4 (8.69) | 5 (1.29) | 1 (12.50) |
| Minor LAST events | ||||
| Tremor | 30 (3.62) | 11 (23.91) | 1 (0.32) | 1 (12.50) |
| Agitation | 24 (2.90) | 9 (19.56) | 1 (0.32) | 1 (12.50) |
| Delayed emergence | 125 (15.11) | 19 (41.30) | 9 (2.33) | 1 (12.50) |
| Delayed extubation | 63 (7.62) | 8 (17.39) | 7 (1.81) | 0 (0) |
| Airway insertion | 144 (17.41) | 22 (47.83) | 2 (0.63) | 1 (12.50) |
| Oxygen requirement | 76 (9.19) | 5 (10.87) | 4 (1.26) | 1 (12.50) |
| Post-operative vomiting | 42 (5.08) | 4 (8.69) | 23 (5.95) | 1 (12.50) |
Central nervous system (CNS) disease includes history of stroke, Parkinson’s disease, and brain tumor. Data are presented as number (%) or mean ± standard deviation. *p < 0.05 represented a significant difference between two groups. Abbreviation: BMI, body mass index; CNS, central nervous system; LAST, local anesthetic systemic toxicity; NRS, numeric rating scale; PACU, post-anesthesia care unit; THA, total hip arthroplasty; TKA, total knee arthroplasty
The characteristics of the patients who developed severe and major LAST events are shown in Table 2. Among the enrolled 1,267 patients, four developed severe LAST events (incidence: 4/1,267 = 3.16 per 1000) requiring rescue lipid infusion to alleviate refractory seizures, defined as persistent seizures despite repeated midazolam administration. Fortunately, ventricular arrhythmia or cardiac arrest was not observed in patients with severe LAST events. Among these four events, two occurred in bilateral THA and two occurred in unilateral THA. Additionally, 41 patients developed major LAST events (incidence: 41/1,267 = 32.36 per 1000) such as seizures, bradycardia, and new-onset arrhythmias requiring prompt medications such as midazolam, atropine, or antiarrhythmics. These severe or major LAST events were associated with longer PACU stays but did not impede early ambulation, as most patients could still ambulate like other patients by the evening of the operation day.
Table 2.
Characteristics of patients developing severe and major LAST events after LIA
| Outcomes | Severe LAST event (n = 4) | Major LAST event (n = 41) |
|---|---|---|
| incidence (in the entire cohort) (per 1000) | 3.16 | 32.36 |
| Rescue lipid infusion | 4 (100) | 0 |
| Seizure | 4 (100) | 11 (26.83) |
| Ventricular arrhythmia | 0 | 0 |
| Bradycardia or new-onset arrhythmia | 3 (75) | 32 (78.05) |
| Other symptoms | ||
| Delayed emergence | 4 (100) | 17 (41.46) |
| Delayed extubation | 2 (50) | 9 (21.95) |
| Post-operative vomiting | 1 (25) | 6 (14.63) |
| Surgery | ||
| Unilateral THA | 2 (50) | 33 (80.49) |
| Bilateral THA | 2 (50) | 3 (7.32) |
| Unilateral TKA | 0 (0) | 3 (7.32) |
| Bilateral TKA | 0 (0) | 2 (4.88) |
| Maintained anesthetics | ||
| Sevoflurane | 4 (100) | 33 (80.49) |
| Propofol | 0 (0) | 8 (19.51) |
| Age (years old) | 52.33 ± 5.51 | 65.14 ± 12.72 |
| Male, n (%) | 3 (75) | 9 (21.95) |
| BMI (kg/M2) | 23.71 ± 5.02 | 25.31 ± 3.57 |
| Chronic diseases | ||
| Arrhythmia | 1 (25) | 1 (2.44) |
| Liver cirrhosis | 0 (0) | 0 (0) |
| End stage renal disease | 0 (0) | 1 (2.44) |
| CNS disease | 0 (0) | 0 (0) |
| Intraoperative blood loss (ml) | 300 ± 100 | 240.24 ± 119.48 |
| Duration of anesthesia (min) | 111.33 ± 36.23 | 76.21 ± 21.45 |
| Duration of PACU stay (min) | 112.61 ± 24.42 | 85.17 ± 21.68 |
| Bupivacaine dose (mg) | 365.67 ± 86.75 | 291.09 ± 60.11 |
| Hospitalization (days) | 4.0 ± 1.0 | 3.73 ± 0.26 |
| Pain score at 24 h (NRS) | 1.67 ± 0.57 | 1.92 ± 0.65 |
Data were presented as number (%) or mean ± standard deviation. *p < 0.05 represented a significant difference. Abbreviations: BMI, body mass index; CNS, central nervous system; LAST, local anesthetic systemic toxicity; LIA, local infiltration analgesia; NRS, numeric rating scale; PACU, post-anesthesia care unit; THA, total hip arthroplasty; TKA, total knee arthroplasty
Patients undergoing LIA for THA had a higher incidence of LAST than patients undergoing TKA
We conducted a propensity-matched comparison based on age, sex, and BMI to compare the incidence of LAST between patients undergoing unilateral THA and those undergoing TKA. The demographic characteristics of the matched patients are shown in Table 3. No significant differences were found in chronic diseases between the THA and TKA groups; however, inherent differences were observed in surgery type, such as longer anesthesia duration in TKA and higher blood loss in THA (a tourniquet was applied in all TKA patients in this cohort). A matched comparison of the incidence of LAST in the THA and TKA groups is presented in Table 4. The odds ratio (OR) of developing major LAST events (seizures, bradycardia, and new-onset arrhythmia) in patients undergoing THA compared to those undergoing TKA was 4.35 (p = 0.031). In the THA group, LIA increased the incidence of CNS adverse events, including tremors (p = 0.023) and delayed emergence (p < 0.001), and respiratory adverse events, such as delayed extubation (p < 0.001) and airway requirement (p < 0.001), compared with the TKA group (Table 4). LIA also increased the incidence of CV adverse event as bradycardia or meaningful arrhythmias in the THA group compared with the TKA group (OR = 3.45, p = 0.047).
Table 3.
Patient demographics in matched unilateral THA and TKA groups
| Variables | TKA group (n = 208) | THA group (n = 208) | p value |
|---|---|---|---|
| Age (years) | 70.74 ± 7.96 | 70.61 ± 8.17 | 0.977 |
| Male, n (%) | 40 (19.23) | 44 (21.15) | 0.625 |
| BMI (kg/M2) | 27.08 ± 4.01 | 27.29 ± 4.03 | 0.605 |
| Chronic diseases | |||
| Hypertension | 131 (62.98) | 130 (62.50) | 0.919 |
| Diabetes | 44 (21.15) | 41 (19.71) | 0.715 |
| Coronary artery disease | 4 (1.92) | 6 (2.88) | 0.522 |
| Arrhythmia | 4 (1.92) | 9 (4.33) | 0.159 |
| CNS disease | 5 (2.40) | 8 (3.85) | 0.398 |
| Asthma/COPD | 11 (5.29) | 7 (3.37) | 0.335 |
| Malignancy | 11 (5.29) | 8 (3.85) | 0.367 |
| Duration of surgery (min) | 57.56 ± 11.80 | 42.34 ± 8.72 | < 0.001* |
| Duration of anesthesia (min) | 84.70 ± 13.20 | 70.34 ± 9.19 | < 0.001* |
| Duration of PACU stay (min) | 68.46 ± 14.44 | 78.62 ± 18.64 | < 0.001* |
| Intraoperative blood loss (ml) | 118.11 ± 49.25 | 214.42 ± 94.33 | < 0.001* |
| Hospitalization (day) | 3.03 ± 0.17 | 3.01 ± 0.12 | 0.314 |
| Bupivacaine dose | 277.42 ± 30.56 | 272.78 ± 31.03 | 0.765 |
| Pain score at 24 h (NRS) | 2.07 ± 0.55 | 2.28 ± 0.81 | 0.631 |
Central nervous system (CNS) disease includes history of stroke, Parkinson’s disease, and brain tumor. Data are presented as number (%) or mean ± standard deviation. Continuous variables were compared using independent sample T-test, while categorical variables were analyzed using Fisher’s exact tests. *p < 0.05 represented a significant difference between two groups. Abbreviation: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CNS, central nervous system; NRS, numeric rating scale; PACU, post-anesthesia care unit; THA, total hip arthroplasty; TKA, total knee arthroplasty
Table 4.
Incidence rates of LAST events in matched unilateral THA and TKA groups
| Outcomes | TKA (n = 208) | THA (n = 208) | OR (95% CI) | p value |
|---|---|---|---|---|
| Major LAST events | 3 (1.44) | 13 (6.25) | 4.35 (1.41–16.67) | 0.031* |
| Seizure | 0 (0) | 5 (2.40) | 11.11 (0.61–97.68) | 0.103 |
| Bradycardia or new-onset arrhythmia | 3 (1.44) | 11 (5.29) | 3.45 (1.01–11.11) | 0.047* |
| Minor LAST events | ||||
| Tremor | 3 (1.44) | 13 (6.25) | 4.55 (1.49–12.54) | 0.023* |
| Agitation | 1 (0.48) | 4 (1.92) | 3.03 (0.47–19.88) | 0.241 |
| Delayed emergence | 7 (3.37) | 41 (19.71) | 6.67 (2.94–14.28) | < 0.001* |
| Delayed extubation | 5 (2.40) | 28 (13.59) | 5.88 (2.32–14.28) | < 0.001* |
| Airway insertion | 7 (3.37) | 45 (21.63) | 7.69 (3.33–16.66) | < 0.001* |
| Oxygen requirement | 25 (12.02) | 37 (17.96) | 1.58 (0.93–2.78) | 0.095 |
| Post-operative vomiting | 15 (7.21) | 13 (6.25) | 0.86 (0.40–1.85) | 0.701 |
Data are presented as number (%). Odds ratio and p value were calculated by using univariate logistic regression. *p < 0.05 represented a significant difference between two groups. Abbreviation: CI, confidence interval; LAST, local anesthetic systemic toxicity; OR, odds ratio; THA, total hip arthroplasty; TKA, total knee arthroplasty
Propofol infusion reduced seizures and tremors in THA patients
A propensity-matched comparison by age, sex, and BMI in patients receiving unilateral THA further divided the patients into propofol (n = 91) and sevoflurane (n = 91) groups according to the anesthetics used for anesthesia maintenance. Demographic comparisons revealed no significant differences between the propofol and sevoflurane groups (Table 5). A comparison of LAST incidence between patients maintained with sevoflurane and those maintained with propofol is shown in Table 6. The odds ratio of developing major LAST events in patients maintained with sevoflurane inhalation compared to those maintained with propofol infusion was 2.12 (p = 0.042). Interestingly, propofol infusion significantly reduced the incidence of seizures (p = 0.016) and tremors (p = 0.024) compared with sevoflurane inhalation, though no significant differences were found in other LAST events. However, as this comparison involved fewer cases, these results might be interpreted with caution.
Table 5.
Demographics of the sevoflurane and propofol groups in patients who underwent unilateral THA
| Variables | Propofol (n = 91) | Sevoflurane (n = 91) | p value |
|---|---|---|---|
| Age (years) | 60.18 ± 13.30 | 60.02 ± 13.13 | 0.959 |
| Male, n (%) | 28 (30.77) | 30 (32.97) | 0.663 |
| BMI (kg/M2) | 26.59 ± 4.44 | 25.45 ± 4.28 | 0.074 |
| Chronic diseases | |||
| Hypertension | 30 (32.97) | 38 (41.76) | 0.273 |
| Diabetes | 9 (9.89) | 10 (10.98) | 0.710 |
| Coronary artery disease | 2 (2.20) | 2 (2.20) | 1.000 |
| Arrhythmia | 1 (1.10) | 1 (1.10) | 1.000 |
| CNS disease | 1 (1.10) | 4 (4.39) | 0.391 |
| Asthma/COPD | 0 (0) | 0 (0) | NA |
| Malignancy | 1 (1.10) | 2 (2.20) | 0.687 |
| Duration of surgery (min) | 42.88 ± 7.39 | 43.73 ± 9.91 | 0.543 |
| Duration of anesthesia (min) | 70.73 ± 8.40 | 73.51 ± 10.79 | 0.078 |
| Duration of PACU stay (min) | 79.26 ± 19.82 | 76.82 ± 16.24 | 0.056 |
| Intraoperative blood loss (mL) | 254.48 ± 120.36 | 254.20 ± 133.26 | 0.620 |
| Hospitalization (day) | 3.01 ± 0.10 | 3.03 ± 0.16 | 0.460 |
| Bupivacaine dose | 274.21 ± 29.56 | 275.450 ± 23.32 | 0.732 |
| Pain score at 24 h (NRS) | 2.18 ± 0.76 | 2.08 ± 0.31 | 0.769 |
Central nervous system (CNS) disease includes history of stroke, Parkinson’s disease, and brain tumor. Data are presented as number (%) or mean ± standard deviation. Continuous variables were compared using independent sample T-test, while categorical variables were analyzed using Fisher’s exact tests. *p < 0.05 represented a significant difference between two groups. Abbreviation: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CNS, central nervous system; NRS, numeric rating scale; THA, total hip arthroplasty; PACU, post-anesthesia care unit
Table 6.
Incidence rates of LAST events in patients receiving sevoflurane or propofol maintenance for unilateral THA
| Outcomes | Propofol (n = 91) | Sevoflurane (n = 91) | OR (95% CI) | p value |
|---|---|---|---|---|
| Major LAST events | 3 (3.30) | 6 (6.59) | 2.12 (1.08–4.65) | 0.042* |
| Seizure | 1 (1.09) | 4 (4.39) | 3.14 (1.68–6.85) | 0.016* |
| Bradycardia or new-onset arrhythmia | 3 (3.30) | 5 (5.49) | 1.45 (0.38–5.51) | 0.587 |
| Minor LAST events | ||||
| Tremor | 1(1.10) | 6 (6.59) | 4.18 (2.24–8.68) | 0.024* |
| Agitation | 1 (1.10) | 2 (2.19) | 1.28 (0.17-10.00) | 0.812 |
| Delayed emergence | 18 (19.78) | 20 (21.97) | 1.12 (0.41–1.65) | 0.575 |
| Delayed extubation | 14 (15.38) | 16 (17.58) | 0.91 (0.43–1.95) | 0.813 |
| Airway insertion | 16 (17.58) | 17 (18.68) | 0.94 (0.46–1.93) | 0.872 |
| Oxygen requirement | 10 (10.99) | 11 (12.08) | 1.02 (0.34-2.00) | 0.667 |
| Post-operative vomiting | 5 (5.49) | 6 (6.59) | 0.75 (0.24–2.33) | 0.623 |
Data are presented as number (%). Odds ratio and p value were calculated by using univariate logistic regression. *p < 0.05 represented a significant difference between two groups. Abbreviation: CI, confidence interval; LAST, local anesthetic systemic toxicity; OR, odds ratio; THA, total hip arthroplasty
Dose-response relationship in patients receiving unilateral THA
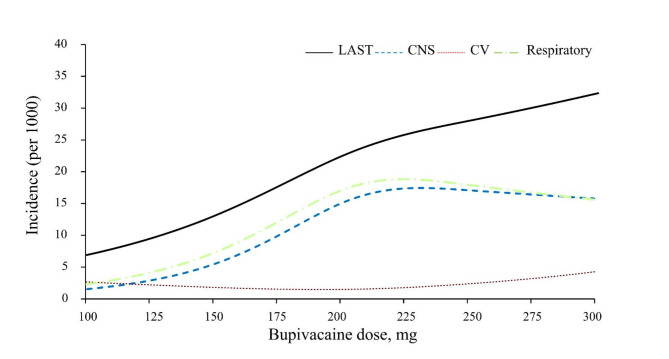
Using a restricted cubic spline model, the available dose-response relationship between bupivacaine dose and overall LAST incidence in patients undergoing unilateral THA is illustrated in Fig. 2. Considering the limited number of cases and available bupivacaine doses, it is difficult to establish a correlation equation between bupivacaine dose and LAST incidence. However, the overall incidence of LAST events was positively correlated with the bupivacaine dose.
Fig. 2.
The relationship between bupivacaine dose and the incidence of overall LAST events (including central nervous system (CNS), cardiovascular (CV), and respiratory events) in patients receiving unilateral total hip arthroplasty. CNS events include seizures, tremors, agitation, and delayed emergence. Respiratory events include delayed extubation, airway insertion, and oxygen requirements
Discussion
This retrospective single-center cohort study aimed to identify the incidence rates of LAST in patients receiving LIA for fast-track THA and TKA, and to investigate the efficacy of propofol maintenance infusion in reducing the incidence of LAST. Our results, which were derived from a large sample size, present detailed LAST events and recovery profiles, thus providing valuable evidence for clinical practice. First, our data revealed that the incidence of LIA-induced LAST was higher in the unilateral THA than in the unilateral TKA group (OR: 4.35, p = 0.031). LIA-induced LAST events after TKA were rare even after bilateral TKA. Second, propofol infusion for anesthesia maintenance decreased the incidence of seizures compared with sevoflurane inhalation (OR: 0.47, p = 0.042). Patients who developed severe or major LAST events had a prolonged PACU stay but seemingly experienced less postoperative pain, and these LAST events did not delay postoperative ambulation and recovery. Further pharmacokinetic studies are required to construct a plasma bupivacaine concentration curve after LIA and its association with LAST symptoms to unravel the causal relationship underlying our findings. Although this study can only demonstrate an association instead of a causal relationship, it highlights that LIA-induced LAST can be particularly severe in patients undergoing bilateral THA, a phenomenon that has not been previously reported in the literature. This finding suggests that the current LIA dosage might be inappropriate for patients undergoing bilateral THA, highlighting the importance of developing safer LIA strategies to mitigate potential LAST risks while preserving effective analgesia.
LIA provides effective opioid-free analgesia when combined with multimodal analgesia after total joint arthroplasty, accelerating functional recovery and reducing complications, and is a pivotal component of the ERAS program, particularly for TKA [4, 12–14]. Although clinical evidence supporting the use of LIA for THA is limited, a meta-analysis found that LIA resulted in improved pain relief and decreased opioid consumption in patients undergoing THA [15]. Leveraging the advantages of LIA in THA and TKA and addressing potential LAST events, our results showed that LIA effectively enhanced patient recovery after THA and TKA. Patients undergoing LIA for bilateral THA or TKA were discharged with fair ambulation within two days after joint replacement surgery. Despite infrequent reports of LIA-induced LAST, our single-center cohort study found that the incidence of severe LAST was 2.41 per 1000 in unilateral THA and 3.16 in the entire cohort (possibly because of the inclusion of bilateral surgeries), which was slightly higher than the reported incidence of LAST associated with peripheral nerve blocks (1.8 per 1000 in the United States Inpatient Database from 2006 to 2014) [16]. The incidence of severe LAST after LIA reported in a previous cohort was 2.0 per 1000 for unilateral THA, which is similar to our results [6]. Fortunately, most of these LAST events resolved within two hours of conservative treatment in the PACU, except for a few patients who experienced symptoms such as dizziness, nausea, or vomiting the day after surgery [5].
In our retrospective single-center cohort, patients who underwent THA had a higher incidence of LAST than those who underwent TKA (OR, 4.35; p = 0.031), which was also reported in a previous cohort [6]. The heightened vascularity, larger vessel size surrounding the hip acetabulum, and increased blood loss in THA compared with TKA might contribute to accelerated LA absorption and higher LAST incidence following LIA for THA. Several large database analyses have found higher blood transfusion rates in primary THA than in primary TKA [17]. Since tourniquets were applied in all TKA surgeries and were released after LIA, tourniquets could significantly delay the increase in plasma LA concentration in TKA patients compared with THA patients. However, clinical LAST events were not reported in previous small-scale population pharmacokinetic analyses of LIA for THA and TKA, and their reported free plasma ropivacaine concentrations were all below the CNS toxic threshold (0.600 mcg/ml) [18–21]. Affas et al. reported their study of LIA for THA using 200 mg ropivacaine and found the maximal unbound plasma ropivacaine concentration to be 0.032 mcg/ml within 30 h after LIA [22]. Gromov et al. reported their study on 28 patients with LIA using 400 mg ropivacaine injected periarticularly in each knee and found the peak plasma concentrations of free ropivacaine to be 0.095 mcg/ml in bilateral TKA [23]. Despite this, our cohort evaluated LAST incidence in an Asian population, who might be more susceptible to LAST events owing to naturally lower α1-acid glycoprotein levels compared with Western populations [24]. In addition, we administered bupivacaine instead of ropivacaine for LIA, which is more cardiotoxic than ropivacaine [7]. These factors might account for these previously unreported LAST events observed in our retrospective cohort study.
We also investigated the influence of different maintained anesthetics on LIA-induced LAST. Although a previous EEG study found that sevoflurane might be epileptogenic at 1.5 and 2 minimum alveolar concentrations (MAC), a meta-analysis of clinical trials showed that sevoflurane- and propofol-maintained anesthesia had a comparable incidence of postoperative seizures [25, 26]. Our study revealed that patients receiving propofol infusion had a decreased incidence of seizures and tremors compared with those receiving sevoflurane inhalation. Although propofol is dissolved in a 10% MCT/LCT triglyceride solution, it cannot substitute for a 20% lipid emulsion for LAST resuscitation because of its strong cardio-depressive effect [7]. An experimental piglet model demonstrated that propofol did not increase the time to cardiac arrest after bupivacaine infusion compared with sevoflurane [27]. Combining our results with those previous studies, propofol infusion appears to merely mitigate LIA-induced CNS toxicity, but has no effect on CV toxicity compared to sevoflurane. This CNS-specific benefit of propofol might be attributed to its residual anticonvulsant effects during anesthesia emergence rather than a reduction in plasma LA concentrations [28].
The development of LAST is associated with the vascularity of the injection site, drug properties, LA dosage, volume, the presence of vasoconstrictors, the level of binding proteins such as albumin and α1-acid glycoprotein, and the patient’s comorbidities [29]. Populations identified as susceptible to LAST events include patients with extreme ages (neonates and elderly); pregnant women; individuals with renal diseases, cardiac diseases, and hepatic dysfunction; and those with lower levels of α1-acid glycoprotein [21, 30]. However, these documented risk factors have not been observed in our patients who developed severe or major LAST events. A possible explanation might be that awareness of these documented risk factors for LAST resulted in reduced bupivacaine doses, and thus fewer LAST events in these populations. Further pharmacokinetic studies to determine the plasma albumin and α1-acid glycoprotein concentrations and to stratify susceptible populations for LIA-induced LAST might be required to develop safer LIA strategy.
Our observational study showed that the LIA protocol for fast-track THA induces a nonnegligible incidence of LAST events. Although these events are often self-limiting without serious consequences, several alternatives may help to resolve this dilemma. These recommendations include reducing the LA dosage in LIA, using ropivacaine as an alternative, adding additives to LIA mixtures, and using liposomal bupivacaine. Reducing the LA dose for LIA without diminishing its analgesic effects may be the most straightforward method for attenuating LIA-induced LAST. The bupivacaine dose used in our fast-track protocol (2–3 mg/kg bupivacaine, maximum 300 mg, for unilateral LIA) was based on a previous trial at our institute, in which LIA using 300 mg bupivacaine for unilateral THA produced relatively safe results [5]. Although LIA using a bupivacaine dose of up to 370 mg for unilateral THA was utilized in the latest American cohort [6], which is higher than our dose, we should keep in mind that 150–175 mg of bupivacaine was recommended as the maximum dose in the 2004 guideline [31]. Although the analgesic efficacy might be reduced if a lower LA dose is utilized, the analgesic effects could be compensated for by parenteral acetaminophen injection, additives, or motor-sparing blocks, such as the PENG block for THA or iPACK block for TKA [32–34]. Using ropivacaine as an alternative choice of LA might be attractive for reducing the cardiotoxicity of bupivacaine. Previous pharmacokinetic studies using ropivacaine in LIA for THA showed a safe pharmacokinetic profile [22]. The addition of dexamethasone or dexmedetomidine to the LIA mixture or through intravenous administration has been proven to prolong analgesia and reduce the required LA dosage, thus reducing the risks of LAST [35, 36]. Liposomal bupivacaine, a lipid-encapsulated bupivacaine designed for slow release over 72 h, gained Food and Drug Administration approval in 2011 and has demonstrated safety and efficacy in LIA injections for TKA [37, 38]. Although liposomal bupivacaine is not currently available in Taiwan, its potential use holds promise in providing an improved safety profile with an extended duration of analgesia.
This study evaluated the full spectra of LIA-induced LAST events in THA and TKA; these major and minor LAST events after LIA are rarely discussed in the literature. Our results showed that patients who underwent bilateral THA had the highest risk of developing LAST events compared to those who underwent other surgeries. We also compared the clinical incidence of LIA-induced LAST in patients who underwent THA versus TKA and in patients who were maintained with propofol infusion versus sevoflurane inhalation. Such comparisons have not yet been reported in the literature. The sample size of our cohort was sufficiently large to represent the general incidence of LIA-induced LAST. Although our defined major and minor LAST events might have other causes such as general anesthetics or patient factors, severe LAST requiring lipid rescue infusion is highly specific in reflecting LIA-induced LAST. Our retrospective analysis serves as a reminder that LAST is an ever-present concern that cannot be overlooked when using high-dose LA for LIA or nerve blocks, especially in an Asian population that has a naturally lower level of α1-acid glycoprotein compared with Western populations. Adequate preparations for managing LAST should always be undertaken.
The present study had several limitations. First, as an observational cohort study, inherent demographic differences existed between the THA and TKA groups and other comparisons; however, these differences could not be completely eliminated by propensity score matching. Second, LAST might not fully account for our observed major and minor LAST events, such as agitation, delayed emergence, delayed extubation, airway insertion, and postoperative vomiting, which might be caused by other anesthetics, medical histories, or surgical factors. Thus, our results might overestimate these LAST events. Unfortunately, because LIA has been routinely administered to all patients undergoing THA and TKA patients in our hospital for years, we were unable to compare patients with and without LIA to determine the exact proportion of these incidences attributable to LIA. However, since all patients received the same care from the same medical team, and we derived our results through group comparisons, LIA-induced LAST might still be the most possible explanation for the excessively higher incidences of LAST in the THA and sevoflurane groups. Third, non-recorded LAST events, such as subclinical tremors or agitation, QT prolongation, or short-term arrhythmia, were not accounted for in this cohort, potentially resulting in an underestimation. Fourth, the plasma levels of albumin, α1-acid glycoprotein, and bupivacaine concentration after LIA were not measured in this observational study. Therefore, our retrospective observational study in a nonrandomized sample could only establish an association instead of causality between LIA dosage and LAST events. Further pharmacokinetic studies are required to establish a correlation between plasma bupivacaine concentrations and the observed LAST events to elucidate our findings.
Conclusions
The present study revealed that patients who received LIA with bupivacaine as motor-sparing analgesia during unilateral THA were associated with a higher incidence of LIA-induced LAST events, including tremors and arrhythmias, than those who underwent unilateral TKA. Patients who received propofol maintenance infusion were associated with a lower incidence of LIA-induced seizures and tremors than those who received sevoflurane inhalation. Finding strategies to recalibrate the balance between the LIA regimen and the risk of LAST will enhance patient safety, particularly in those undergoing bilateral THA. Further prospective studies are required to establish the association between plasma bupivacaine concentration and the observed LAST events to validate our findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Raising Statistics Consultant Inc. for statistical assistance. We would like to thank Editage for English editing.
Abbreviations
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- BIS
Bispectral index
- BMI
Body mass index
- CI
Confidence interval
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- CV
Cardiovascular
- EEG
Electroencephalography
- ERAS
Enhanced recovery after surgery
- GCS
Glasgow coma scale
- LA
Local anesthetics
- LAST
Local anesthetic systemic toxicity
- iPACK block
Infiltration of local anesthetic between the Popliteal Artery and Capsule of the Knee block
- LCT
Long chain triglyceride
- LIA
Local infiltration analgesia
- MAC
Minimum alveolar concentration
- MCT
Medium chain triglyceride
- NRS
Numeric rating scale
- OR
Odds ratio
- PACU
Post-anesthesia care unit
- PENG block
PEricapsular Nerve Group block
- TCI
Target-controlled infusion
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
Author contributions
All authors contributed to the study conception and design of this study. Material preparation and data collection were performed by H-T Lin, P-H Hsieh, J-T Liou, Y-T Chung, Y-C Lin, and Y-F Tsai. Analysis was performed by H-T Lin and Y-F Tsai. The first draft of the manuscript was written by H-T Lin and Y-F Tsai, and all the authors commented on the previous versions of the manuscript. All authors have reviewed and approved the final manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 111-2320-B-182 A-008-MY3), and Chang Gung Memorial Hospital, Taiwan (CMRPG3K1051-3 and CMRPG3N0591). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Data availability
The data is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of the Chang Gung Medical Foundation (approval number: 202200080B0). The need for informed consent was waived because of the retrospective design of the study and the use of anonymized personal information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yung-Fong Tsai and Yen-Chu Lin contributed equally to this work.
References
- 1.Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455–60. [DOI] [PubMed] [Google Scholar]
- 2.Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62–72. [DOI] [PubMed] [Google Scholar]
- 3.Qi BC, Yu J, Qiao WS. Comparison of intrathecal morphine versus local infiltration analgesia for pain control in total knee and hip arthroplasty: a meta-analysis. Med (Baltim). 2020;99(36):e21971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen LO, Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014;113(3):360–74. [DOI] [PubMed] [Google Scholar]
- 5.Chen DW, Hu CC, Chang YH, Lee MS, Chang CJ, Hsieh PH. Intra-articular bupivacaine reduces postoperative pain and meperidine use after total hip arthroplasty: a randomized, double-blind study. J Arthroplasty. 2014;29(12):2457–61. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell K, Cai E, Miller B, Jenkins K, McAllister RK, Fettiplace M, et al. Local anesthetic systemic toxicity from local infiltration anesthesia in total joint arthroplasty: a single center retrospective study. Reg Anesth Pain Med. 2023. 10.1136/rapm-2023-104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal JM, Barrington MJ, Fettiplace MR, Gitman M, Memtsoudis SG, Morwald EE, et al. The third American Society of Regional Anesthesia and Pain Medicine Practice Advisory on local anesthetic systemic toxicity: executive Summary 2017. Reg Anesth Pain Med. 2018;43(2):113–23. [DOI] [PubMed] [Google Scholar]
- 8.Burjorjee J, Phelan R, Hopman WM, Ho AM, Nanji S, Jalink D, Mizubuti GB. Plasma bupivacaine levels (total and free/unbound) during epidural infusion in liver resection patients: a prospective, observational study. Reg Anesth Pain Med. 2022. 10.1136/rapm-2022-103683. [DOI] [PubMed] [Google Scholar]
- 9.Kurosaka K, Tsukada S, Ogawa H, Nishino M, Yoshiya S, Hirasawa N. Comparison of early-stage and late-stage Periarticular Injection for Pain Relief after total hip arthroplasty: a double-blind randomized controlled trial. J Arthroplasty. 2020;35(5):1275–80. [DOI] [PubMed] [Google Scholar]
- 10.Heppolette CAA, Brunnen D, Bampoe S, Odor PM. Clinical pharmacokinetics and pharmacodynamics of Levobupivacaine. Clin Pharmacokinet. 2020;59(6):715–45. [DOI] [PubMed] [Google Scholar]
- 11.Gitman M, Fettiplace MR, Weinberg GL, Neal JM, Barrington MJ. Local anesthetic systemic toxicity: a narrative literature review and clinical update on Prevention, diagnosis, and management. Plast Reconstr Surg. 2019;144(3):783–95. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright TW, Gill M, McDonald DA, Middleton RG, Reed M, Sahota O, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: enhanced recovery after surgery (ERAS((R))) Society recommendations. Acta Orthop. 2020;91(1):3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Changjun C, Xin Z, Yue L, Liyile C, Pengde K. Key elements of enhanced recovery after total joint arthroplasty: a reanalysis of the enhanced recovery after surgery guidelines. Orthop Surg. 2023;15(3):671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Changjun C, Jingkun L, Yun Y, Yingguang W, Yanjun R, Debo Z, et al. Enhanced recovery after total joint arthroplasty (TJA): a contemporary systematic review of clinical outcomes and usage of key elements. Orthop Surg. 2023;15(5):1228–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma HH, Chou TA, Tsai SW, Chen CF, Wu PK, Chen WM. The efficacy of intraoperative periarticular injection in total hip arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morwald EE, Zubizarreta N, Cozowicz C, Poeran J, Memtsoudis SG. Incidence of local anesthetic systemic toxicity in Orthopedic patients receiving peripheral nerve blocks. Reg Anesth Pain Med. 2017;42(4):442–5. [DOI] [PubMed] [Google Scholar]
- 17.Bedard NA, Pugely AJ, Lux NR, Liu SS, Gao Y, Callaghan JJ. Recent trends in blood utilization after primary hip and knee arthroplasty. J Arthroplasty. 2017;32(3):724–7. [DOI] [PubMed] [Google Scholar]
- 18.Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997;78(5):507–14. [DOI] [PubMed] [Google Scholar]
- 19.Miller RJ, Cameron AJ, Dimech J, Orec RJ, Lightfoot NJ. Plasma ropivacaine concentrations following local infiltration analgesia in total knee arthroplasty: a pharmacokinetic study to Determine Safety following fixed-dose administration. Reg Anesth Pain Med. 2018;43(4):347–51. [DOI] [PubMed] [Google Scholar]
- 20.Bakker SMK, Fenten MGE, Touw DJ, van den Bemt BJF, Heesterbeek PJC, Scheffer GJ, Stienstra R. Pharmacokinetics of 400 mg locally infiltrated Ropivacaine after total knee arthroplasty without Perioperative Tourniquet Use. Reg Anesth Pain Med. 2018;43(7):699–704. [DOI] [PubMed] [Google Scholar]
- 21.Kazune S, Nurka I, Zolmanis M, Paulausks A, Bandere D. Systemic ropivacaine concentrations following local infiltration analgesia and femoral nerve block in older patients undergoing total knee arthroplasty. Local Reg Anesth. 2023;16:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Affas F, Eksborg S, Wretenberg P, Olofsson C, Stiller CO. Ropivacaine pharmacokinetics after local infiltration analgesia in hip arthroplasty. Anesth Analg. 2014;119(4):996–9. [DOI] [PubMed] [Google Scholar]
- 23.Gromov K, Grassin-Delyle S, Foss NB, Pedersen LM, Nielsen CS, Lamy E, et al. Population pharmacokinetics of ropivacaine used for local infiltration anaesthesia during primary total unilateral and simultaneous bilateral knee arthroplasty. Br J Anaesth. 2021;126(4):872–80. [DOI] [PubMed] [Google Scholar]
- 24.Feely J, Grimm T. A comparison of drug protein binding and alpha 1-acid glycoprotein concentration in Chinese and caucasians. Br J Clin Pharmacol. 1991;31(5):551–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaaskelainen SK, Kaisti K, Suni L, Hinkka S, Scheinin H. Sevoflurane is epileptogenic in healthy subjects at surgical levels of anesthesia. Neurology. 2003;61(8):1073–8. [DOI] [PubMed] [Google Scholar]
- 26.Chui J, Mariappan R, Mehta J, Manninen P, Venkatraghavan L. Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: systematic review and meta-analysis. Can J Anaesth. 2014;61(4):347–56. [DOI] [PubMed] [Google Scholar]
- 27.Mauch J, Kutter AP, Martin Jurado O, Spielmann N, Frotzler A, Bettschart-Wolfensberger R, Weiss M. Bupivacaine toxicity and propofol anesthesia: animal study on intravascular bupivacaine injection. Anaesthesist. 2011;60(9):814–8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Yu Y, Lu Y, Yue H. Systematic review and meta-analysis of propofol versus barbiturates for controlling refractory status epilepticus. BMC Neurol. 2019;19(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo I, Akpa BS. Validity of the lipid Sink as a mechanism for the reversal of local anesthetic systemic toxicity a physiologically based pharmacokinetic model study. Anesthesiology. 2013;118(6):1350–61. [DOI] [PubMed] [Google Scholar]
- 30.El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29(6):564–75. discussion 524. [DOI] [PubMed] [Google Scholar]
- 32.Memtsoudis SG, Cozowicz C, Bekeris J, Bekere D, Liu J, Soffin EM, et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary hip and knee arthroplasty: recommendations from the International Consensus on Anesthesia-related outcomes after surgery (ICAROS) group based on a systematic review and meta-analysis of current literature. Reg Anesth Pain Med. 2021;46(11):971–85. [DOI] [PubMed] [Google Scholar]
- 33.Sogbein OA, Sondekoppam RV, Bryant D, Johnston DF, Vasarhelyi EM, MacDonald S, et al. Ultrasound-guided motor-sparing knee blocks for postoperative Analgesia following total knee arthroplasty: a Randomized Blinded Study. J Bone Joint Surg Am. 2017;99(15):1274–81. [DOI] [PubMed] [Google Scholar]
- 34.Yeoh SR, Chou Y, Chan SM, Hou JD, Lin JA. Pericapsular Nerve Group Block and Iliopsoas Plane Block: a scoping review of quadriceps weakness after two Proclaimed Motor-Sparing hip blocks. Healthc (Basel). 2022;10(8). [DOI] [PMC free article] [PubMed]
- 35.Wang XF, Luo XL, Liu WC, Hou BC, Huang J, Zhan YP, Chen SB. Effect of dexmedetomidine priming on convulsion reaction induced by lidocaine. Med (Baltim). 2016;95(43):e4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong B, Oh C, Jo Y, Chung W, Park E, Park H, Yoon S. The Effect of Intravenous Dexamethasone and Dexmedetomidine on Analgesia Duration of Supraclavicular Brachial Plexus Block: a Randomized, Four-Arm, Triple-Blinded, placebo-controlled trial. J Pers Med. 2021;11(12). [DOI] [PMC free article] [PubMed]
- 37.Springer BD, Mason JB, Odum SM. Systemic safety of Liposomal Bupivacaine in simultaneous bilateral total knee arthroplasty. J Arthroplasty. 2018;33(1):97–101. [DOI] [PubMed] [Google Scholar]
- 38.Marino J, Scuderi G, Dowling O, Farquhar R, Freycinet B, Overdyk F. Periarticular knee injection with liposomal bupivacaine and continuous femoral nerve Block for Postoperative Pain Management after total knee arthroplasty: a Randomized Controlled Trial. J Arthroplasty. 2019;34(3):495–500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available from the corresponding author on reasonable request.