Abstract
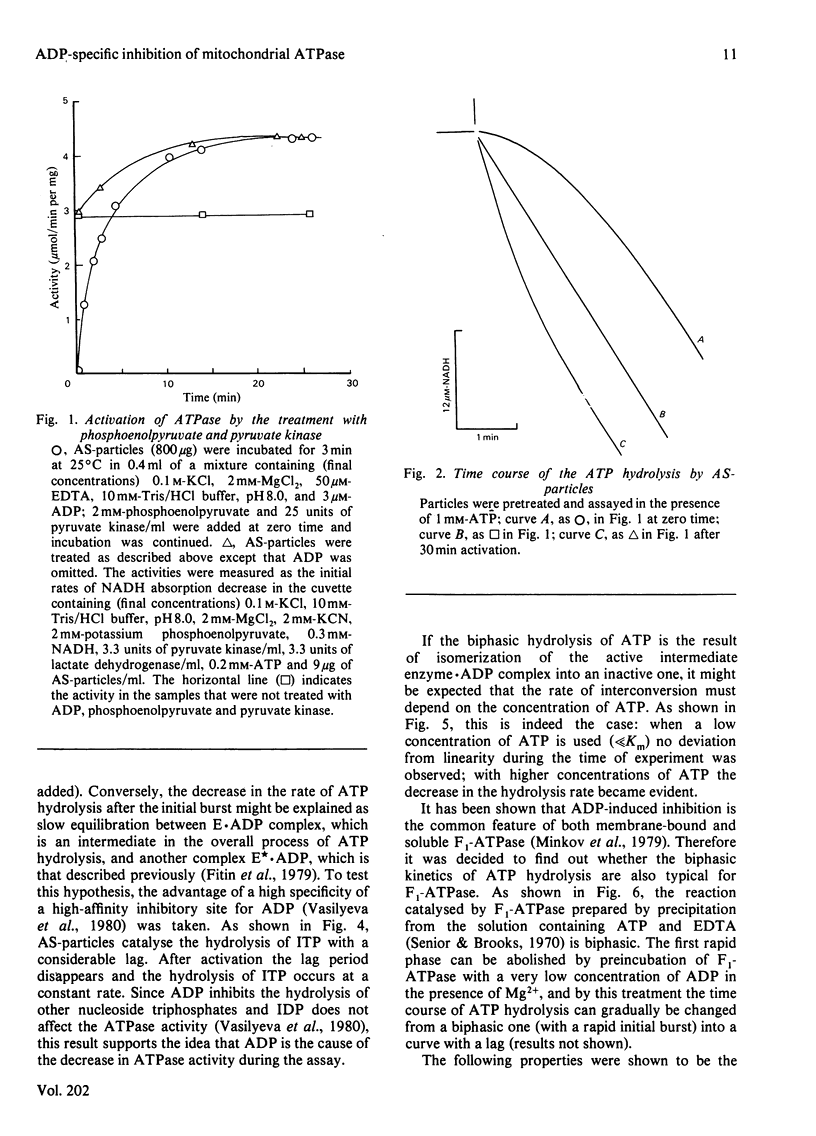
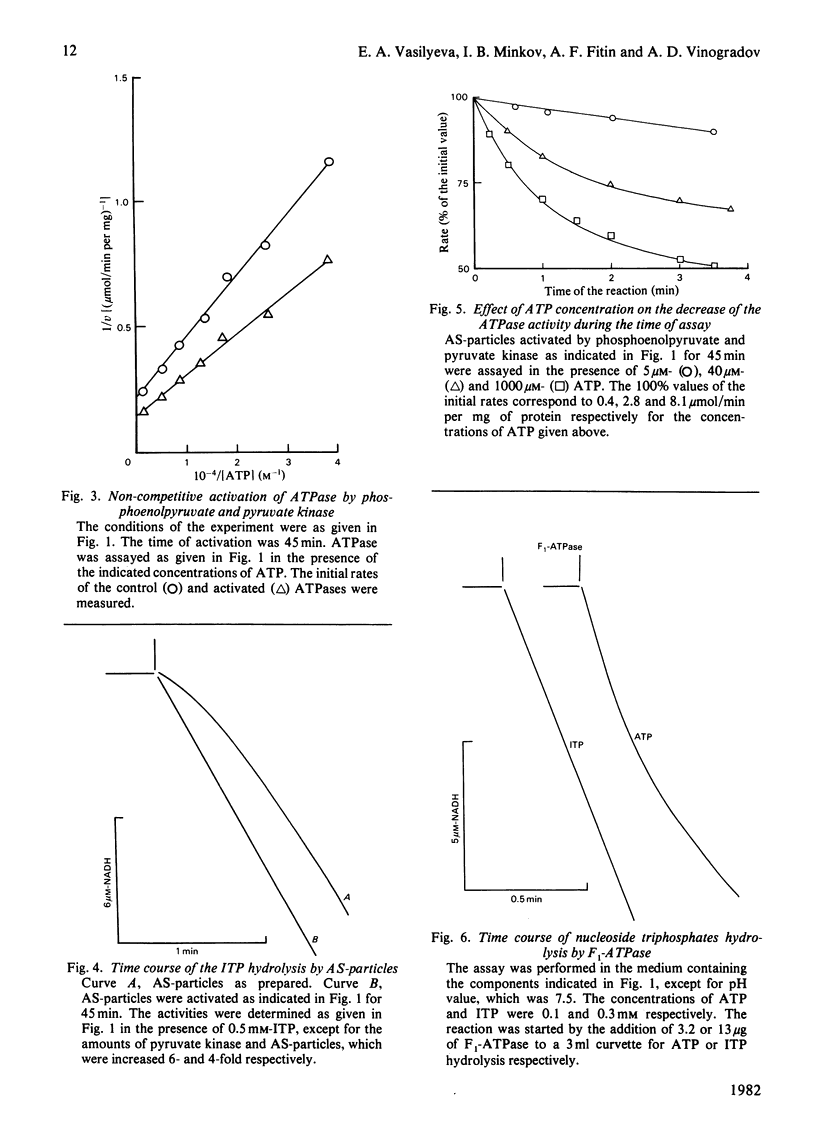
1. A substantial increase of the initial rate of ATP hydrolysis was observed after preincubation of bovine heart submitochondrial particles with phosphoenolpyruvate and pyruvate kinase. 2. The activation was accompanied by an increase of Vmax, without change of Km for ATP. 3. The activated particles catalysed the biphasic hydrolysis of ATP in the presence of an ATP-regenerating system; the initial rapid phase was followed by a second, slower, phase in a time-dependent fashion. 4. The higher the ATP concentration used as a substrate, the higher is the rate of transition between these two phases. 5. The particles catalysed the hydrolysis of ITP with a lag phase; after preincubation with phosphoenolpyruvate and pyruvate kinase, ITP was hydrolysed at a constant rate. 6. Qualitatively the same phenomena were observed when soluble mitochondrial ATPase (F1-ATPase) prepared by the conventional method in the presence of ATP was used as nucleotide triphosphatase. 7. A kinetic scheme is proposed, in which the intermediate active enzyme-product complex (E.ADP) formed during ATP hydrolysis is in slow equilibrium with the inactive E*.ADP complex forming as a result of dislocation of ADP from the active site of ATPase to the other site, which is not in rapid equilibrium with the surrounding medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsen R., Moudrianakis E. N. Roles for metal ions in the hydrolysis of adenosine triphosphate by the 13S coupling factors of bacterial and mitochondrial oxidative phosphorylation. Biochemistry. 1973 Jul 17;12(15):2926–2933. doi: 10.1021/bi00739a024. [DOI] [PubMed] [Google Scholar]
- Akimenko V. K., Minkov I. B., Bakeeva L. E., Vinogradov A. D. Vydelenie i svoistva rastvorimoi ATFazy mitokhondrii serdtsa byka. Biokhimiia. 1972 Mar-Apr;37(2):348–359. [PubMed] [Google Scholar]
- Fitin A. F., Vasilyeva E. A., Vinogradov A. D. An inhibitory high affinity binding site for ADP in the oligomycin-sensitive ATPase of beef heart submitochondrial particles. Biochem Biophys Res Commun. 1979 Jan 30;86(2):434–439. doi: 10.1016/0006-291x(79)90884-2. [DOI] [PubMed] [Google Scholar]
- Garrett N. E., Penefsky H. S. Interaction of adenine nucleotides with multiple binding sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1975 Sep 10;250(17):6640–6647. [PubMed] [Google Scholar]
- HOMMES F. A. THE SUCCINATE-LINKED NICOTINAMIDE-ADENINE DINUCLEOTIDE REDUCTION IN SUBMITOCHONDRIAL PARTICLES. I. KINETIC STUDIES OF THE REACTION. Biochim Biophys Acta. 1963 Oct 1;77:173–182. doi: 10.1016/0006-3002(63)90490-6. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Interaction of Mg+2 with beef heart mitochondrial ATPase (F1). Biochem Biophys Res Commun. 1979 Nov 14;91(1):233–238. doi: 10.1016/0006-291x(79)90608-9. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Radda G. K., Slater E. C. Tightly bound nucleotides of the energy-transducing ATPase, and their role in oxidative phosphorylation. II. The beef heart mitochondrial system. Biochim Biophys Acta. 1977 Mar 11;459(3):560–572. doi: 10.1016/0005-2728(77)90054-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Mitchell R. A. Arsenate and phosphate as modifiers of adenosine triphosphate driven energy-linked reduction. Kinetic study of the effects of modifiers on inhibition by adenosine diphosphate. Biochemistry. 1972 Jun 6;11(12):2278–2283. doi: 10.1021/bi00762a011. [DOI] [PubMed] [Google Scholar]
- Höckel M., Hulla F. W., Risi S., Dose K. Kinetic studies on bacterial plasma membrane ATPase (F1). Nucleotide-induced long term inactivation of ATP hydrolyzing activity is linked to the formation of multiple "tight" enzyme nucleotide complexes. J Biol Chem. 1978 Jun 25;253(12):4292–4296. [PubMed] [Google Scholar]
- Kayalar C., Rosing J., Boyer P. D. 2,4-Dinitrophenol causes a marked increase in the apparent Km of Pi and of ADP for oxidative phosphorylation. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1153–1159. doi: 10.1016/s0006-291x(76)80252-5. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Skulachev V. P. H+-Adenosine triphosphatase and membrane energy coupling. Biochim Biophys Acta. 1977 Jun 21;463(1):29–89. doi: 10.1016/0304-4173(77)90003-9. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- Minkov I. B., Fitin A. F., Vasilyeva E. A., Vinogradov A. D. Mg2+-induced ADP-dependent inhibition of the ATPase activity of beef heart mitochondrial coupling factor F1. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1300–1306. doi: 10.1016/0006-291x(79)92150-8. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Active/inactive state transitions of mitochondrial ATPase molecules influenced by Mg2+, anions and aurovertin. FEBS Lett. 1975 Aug 1;56(1):55–61. doi: 10.1016/0014-5793(75)80110-4. [DOI] [PubMed] [Google Scholar]
- PENEFSKY H. S., PULLMAN M. E., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. II. Participation of a soluble adenosine tolphosphatase in oxidative phosphorylation. J Biol Chem. 1960 Nov;235:3330–3336. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Pedersen P. L. ATP-dependent reactions catalyzed by inner membrane vesicles of rat liver mitochondria. Kinetics, substrate specificity, and bicarbonate sensitivity. J Biol Chem. 1976 Feb 25;251(4):934–940. [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- Roveri O. A., Muller J. L., Wilms J., Slater E. C. The pre-steady state and steady-state kinetics of the ATPase activity of mitochondrial F1. Biochim Biophys Acta. 1980 Feb 8;589(2):241–255. doi: 10.1016/0005-2728(80)90041-9. [DOI] [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial ATPase. Evidence for nucleotide binding at separate regulatory and catalytic sites. J Biol Chem. 1975 Oct 10;250(19):7848–7853. [PubMed] [Google Scholar]
- Schuster S. M., Reinhart G. D., Lardy H. A. Studies on the kinetic mechanism of oxidative phosphorylation. J Biol Chem. 1977 Jan 25;252(2):427–432. [PubMed] [Google Scholar]
- Selwyn M. J. Preparation and general properties of a soluble adenosine triphosphatase from mitochondria. Biochem J. 1967 Oct;105(1):279–288. doi: 10.1042/bj1050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Vasilyeva E. A., Fitin A. F., Minkov I. B., Vinogradov A. D. Kinetics of interaction of adenosine diphosphate and adenosine triphosphate with adenosine triphosphatase of bovine heart submitochondrial particles. Biochem J. 1980 Jun 15;188(3):807–815. doi: 10.1042/bj1880807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyeva E. A., Minkov I. B., Fitin A. F., Vinogradov A. D. Kinetic mechanism of mitochondrial adenosine triphosphatase. Inhibition by azide and activation by sulphite. Biochem J. 1982 Jan 15;202(1):15–23. doi: 10.1042/bj2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J. Proton-driven phosphorylation reactions in mitochondrial and chloroplast membranes. FEBS Lett. 1975 May 1;53(2):123–125. doi: 10.1016/0014-5793(75)80001-9. [DOI] [PubMed] [Google Scholar]


