Abstract
OBJECTIVES
To evaluate the feasibility, safety and quality of robotic-assisted mitral valve repair in complex versus non-complex cases during the early phase of a programme.
METHODS
Since the programme launch in September 2021 until February 2024, 100 patients underwent robotic-assisted mitral valve repair. Of them, 21 patients had complex repairs, while 79 had non-complex repairs. The median age was 58 years for complex cases and 61 years for non-complex cases (P = 0.36).
RESULTS
Bileaflet prolapse was significantly more prevalent in the complex group (52.4% vs 12.7%, P < 0.001). Neochord placement (61.9% vs 13.9%, P < 0.001) and commissuroplasty (28.6% vs 5.1%, P = 0.005) were more frequent in the complex group. The complex group had longer cardiopulmonary bypass times (161 vs 141 min, P < 0.001), aortic cross-clamp times (123 vs 102 min, P < 0.001) and leaflet repair times (43 vs 24 min, P < 0.001). Second pump runs were required more often for complex cases (23.8% vs 3.8%, P = 0.01). All patients left the operating room with residual mitral regurgitation of mild or less. Fewer complex patients were extubated in the operating room (42.9% vs 70.9%, P = 0.02), yet hospital stay was similar (4 vs 4 days, P = 0.56). There were no significant differences in postoperative adverse events. There were no differences in mitral regurgitation of mild or less 4 weeks post-surgery (95.2% vs 98.7%, P = 0.47).
CONCLUSIONS
Complex mitral valve repair can be safely and effectively performed with robotic assistance, even in the early phase of a programme. Despite longer operative and ventilation times in the complex group, hospital stay and postoperative adverse events remained similar.
Keywords: Mitral valve repair, Robotic cardiac surgery, Mitral valve prolapse
Robotic-assisted mitral valve repair (MVR) represents a significant advancement in heart surgery by offering precision and reducing invasiveness, potentially improving patient recovery and outcomes even compared to minimally invasive MVR through right thoracotomy [1–3].
Graphical Abstract
INTRODUCTION
Robotic-assisted mitral valve repair (MVR) represents a significant advancement in heart surgery by offering precision and reducing invasiveness, potentially improving patient recovery and outcomes even compared to minimally invasive MVR through right thoracotomy [1–3]. Despite these advances, the variability in anatomical complexity—from simple posterior leaflet pathology to more intricate anterior and bileaflet problems—raises questions regarding robotic interventions’ capabilities and overall effectiveness across different clinical scenarios [4–6]. Robotic cardiac surgeons have long advocated for the advantages of robotics in complex MVR. One recent paper has shown a significant increase in complex repair after switching from classic minimally invasive MVR to a robotic approach [1]. Another report demonstrated that robotic surgery can be performed across the whole spectrum of mitral valve (MV) pathology, and more than half of the cases in this series were complex repairs [4, 7].
Although complex robotic-assisted MVR has succeeded in established programmes, limited information on the early outcomes of newly introduced programmes is available [8, 9]. Our current study, therefore, aims to assess the feasibility, safety and quality of complex MVR using robotic assistance in the early programme phase, directly comparing it with non-complex MVR.
PATIENTS AND METHODS
Ethical statement
This study was approved by the University of Pittsburgh Institutional Review Board (IRB number 21080141). Written patients informed consent was obtained.
Study design
We conducted a retrospective database study by reviewing electronic medical records between September 2021 and February 2024. Robotic-assisted MVR was performed according to the methods previously described by us and others [10, 11].
Complexity of mitral regurgitation
MVR complexity was classified according to 2 categories: non-complex or complex. The non-complex group included cases of annuloplasty alone or repair of 1 leaflet segment (e.g. triangular excision–suture, placement of artificial chordae). The complex group consisted of cases involving the repair of more than 1 segment on the same leaflet (e.g. sliding plasty) or bileaflet repair. This classification was based on Loulmet et al. [4] with some modification, combining the complex and more complex as 1 group.
Statistical analysis
Statistical analysis was performed using the PRISM10 software package (GraphPad Software). Continuous variables are expressed as median and interquartile ranges, and categorical variables are expressed as the number of patients. We compared group characteristics using the Fisher exact or Mann–Whitney U-tests. All P values of <0.05 indicate a statistically significant difference.
RESULTS
Baseline characteristics
A total of 100 patients were included, with 21 in the complex group and 79 in the non-complex group. Table 1 shows patient demographics by group. The median age at the surgery was 58 and 61 years in the complex and non-complex groups, respectively (P = 0.36).
Table 1:
Patient characteristics
| Complex (n = 21) | Non-complex (n = 79) | P value | |||
|---|---|---|---|---|---|
| Age (year), median (IQR) | 58.0 | [50.0, 67.0] | 61.0 | [50.0, 69.0] | 0.36 |
| Female, n (%) | 2 | 9.5% | 30 | 38.0% | 0.02 |
| Body weight (kg), median (IQR) | 87.7 | [78.3, 98.6] | 77.0 | [66.4, 93.9] | 0.05 |
| BMI (m/kg2), median (IQR) | 27.7 | [25.4, 30.4] | 25.5 | [22.5, 29.1] | 0.07 |
| BSA (m2), median (IQR) | 2.1 | [2.0, 2.3] | 1.9 | [1.8, 2.2] | 0.05 |
| Diabetes mellitus, n (%) | 0 | 0.0% | 7 | 8.9% | 0.34 |
| Hypertension, n (%) | 13 | 61.9% | 52 | 65.8% | 0.80 |
| Smoking, n (%) | 7 | 33.3% | 73 | 92.4% | <0.001 |
| COPD, n (%) | 3 | 14.3% | 18 | 22.8% | 0.55 |
| Predicted DLCO (%), median (IQR) | 108.5 | [85.0, 119.5] | 86.0 | [77.0, 102.0] | 0.03 |
| Dialysis, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| CVD, n (%) | 0 | 0.0% | 1 | 1.3% | >0.99 |
| PVD, n (%) | 0 | 0.0% | 1 | 1.3% | >0.99 |
| Liver disease, n (%) | 2 | 9.5% | 1 | 1.3% | 0.11 |
| Liver cirrhosis, n (%) | 1 | 4.8% | 0 | 0.0% | 0.21 |
| Aspirin within 5 days, n (%) | 5 | 23.8% | 15 | 19.0% | 0.76 |
| Predicted risk permanent stroke (%), median (IQR) | 0.6 | [0.4, 0.9] | 0.7 | [0.4, 1.0] | 0.44 |
| Predicted risk renal failure (%), median (IQR) | 0.5 | [0.4, 0.8] | 0.5 | [0.3, 0.8] | 0.40 |
| STS risk of mortality (%), median (IQR) | 0.3 | [0.2, 0.6] | 0.4 | [0.3, 0.7] | 0.84 |
| Preoperative TEEa, n (%) | |||||
| MR | 0.12 | ||||
| None/trace | 0 | 0.0% | 0 | 0.0% | |
| Mild | 0 | 0.0% | 1 | 1.3% | |
| Moderate | 2 | 9.5% | 2 | 2.5% | |
| Moderate–severe | 1 | 4.8% | 0 | 0.0% | |
| Severe | 18 | 85.7% | 76 | 96.2% | |
| LVEF (%), median (IQR) | 55 | [55.0, 56.0] | 55 | [55.0, 58.0] | 0.7 |
| Location of prolapse, n (%) | <0.001 | ||||
| Anterior only | 1 | 4.8% | 3 | 3.80% | |
| Posterior only | 9 | 42.9% | 65 | 82.30% | |
| Bileaflet | 11 | 52.4% | 10 | 12.70% | |
| No prolapse | 0 | 0.0% | 1 | 1.30% | |
| Functional classification, n (%) | >0.99 | ||||
| Type I | 0 | 0.0% | 2 | 2.5% | |
| Type II | 21 | 100.0% | 77 | 97.5% | |
| Type IIIa | 0 | 0.0% | 0 | 0.0% | |
| Type IIIb | 0 | 0.0% | 0 | 0.0% | |
| Aetiology, n (%) | >0.99 | ||||
| Degenerative | 21 | 100.0% | 77 | 97.5% | |
| Barlow’s | 6 | 28.6% | 10 | 13.0% | |
| Functional | 0 | 0.0% | 1 | 1.3% | |
| Ischaemic | 0 | 0.0% | 0 | 0.0% | |
| Rheumatic | 0 | 0.0% | 0 | 0.0% | |
| Endocarditis | 0 | 0.0% | 1 | 1.3% | |
| Other | 0 | 0.0% | 0 | 0.0% | |
| SAM risk factors, median (IQR) | |||||
| Annular diameter (mm) | 38.4 | [34.0, 48.0] | 40.0 | [37.0, 45.0] | 0.32 |
| c-c diameter (mm) | 42.5 | [35.0, 46.6] | 43.0 | [38.0, 47.0] | 0.73 |
| AML length, mm | 27.5 | [25.0, 34.0] | 28.0 | [25.0, 32.0] | 0.75 |
| PML length (mm) | 20.0 | [17.0, 23.0] | 21.0 | [17.0, 24.0] | 0.56 |
| AML/PML ratio | 1.5 | [1.1, 1.9] | 1.4 | [1.2, 1.7] | 0.87 |
| Aorto-mitral angle degrees | 120.0 | [113.5, 125.0] | 120.0 | [111.5, 127.8] | 0.59 |
| C-sept distance (mm) | 28.0 | [26.0, 31.0] | 27.0 | [24.0, 31.0] | 0.36 |
| Basal septal diameter (mm) | 14.5 | [9.5, 21.0] | 13.0 | [10.0, 17.0] | 0.58 |
| LVEDD (mm) | 52.0 | [48.0, 60.0] | 53.0 | [47.0, 60.0] | 0.71 |
TEE is from the day of surgery.
Statistically significant P values are highlighted in bold.
AML: anterior mitral leaflet; BMI: body mass index; BSA: body surface area; c-c: commissure to commissure; COPD: chronic obstructive pulmonary disease; c-sept: commissure to septal; CVD: cerebral vascular disease; DLCO: diffusing capacity of the lungs for carbon monoxide; IQR: interquartile range; LVEDD: left ventricular endo diastolic diameter; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; PML: posterior mitral leaflet; PVD: peripheral vascular disease; SAM: systolic anterior motion; STS: Society of Thoracic Surgeons; TEE: transoesophageal echocardiogram.
The complex group had fewer females (9.5% vs 38.0%, P = 0.02) and higher body weight (87.7 kg vs 77.0 kg, P = 0.05) and body surface area (2.1 m2 vs 1.9 m2, P = 0.05). In addition, there were significant differences regarding smoking history, with only 33.3% being former or current smoker in the complex group, whereas 92.4% had a smoking history in the non-complex group (P < 0.001). The median predicted diffusion capacity of the lung for carbon monoxide was significantly higher in the complex group (108.5% vs 86.0%, P = 0.03). There were no differences between other past medical histories and preoperative risks as shown in Table 1.
Preoperative transoesophageal echocardiogram demonstrated no differences regarding the degree of mitral regurgitation (MR) and left ventricular ejection fraction.
There were significant differences in the location of prolapse between the 2 groups (P < 0.001). Bileaflet prolapse was more common in the complex group (52.4% vs 12.7%, P < 0.001), while isolated posterior prolapse was more frequent in the non-complex group (82.3%). All the patients in the complex group and 77 patients (97.5%) of the non-complex group were type II MR according to the Carpentier classification. Most of the patients’ MR was due to degenerative disease. Among patients with degenerative disease, there were 6 (28.6%) and 10 (13.0%) Barlow’s diseases in the complex group and in the non-complex group, respectively (P = 0.10). Systolic anterior motion risk factors were also measured with no significant differences among the groups and are shown in Table 1.
Intraoperative results
Table 2 shows the intraoperative data by group. The complex group had significantly longer total operative time, aortic cross-clamp time and cardiopulmonary bypass (CPB) time (all P < 0.001). When looking at the detailed procedural time, longer time was required for inspecting the native valve and repairing it in the complex group (P = 0.001 and P < 0.001, respectively). In addition, the complex group had more patients who required a 2nd pump run and MV replacement (P = 0.01 and 0.002, respectively). There were no differences in the number of conversions to sternotomy. Total incision length was longer in the complex group (P = 0.004), and this was due to longer mini-thoracotomy incision length in the complex group (P = 0.01).
Table 2:
Intraoperative data
| Complex (n = 21) | Non-complex (n = 79) | P value | |||
|---|---|---|---|---|---|
| Operative times (min), median (IQR) | |||||
| Total operative time | 360.0 | [304.0, 458.5] | 276.0 | [245.0, 306.0] | <0.001 |
| ACC time | 123.0 | [112.5, 194.5] | 102.0 | [81.0, 120.0] | <0.001 |
| CPB time | 161.0 | [144.0, 264.0] | 141.0 | [115.0, 159.0] | <0.001 |
| Port placement time | 17.0 | [11.0, 22.0] | 13.0 | [11.0, 15.8] | 0.06 |
| Robot docking time | 4.0 | [3.0, 5.8] | 3.5 | [3.0, 5.0] | 0.14 |
| Cannulation time | 41.0 | [28.5, 44.0] | 37.0 | [30.0, 44.5] | 0.88 |
| Valve inspection time | 7.0 | [3.0, 8.0] | 3.0 | [2.0, 4.0] | 0.001 |
| Leaflet repair time | 43.0 | [32.8, 78.0] | 24.0 | [16.0, 30.0] | <0.001 |
| Annuloplasty time | 25.0 | [20.5, 30.0] | 22.0 | [18.8, 27.0] | 0.06 |
| Left atrium closure time | 15.0 | [11.5, 21.0] | 14.5 | [12.0, 17.3] | 0.33 |
| Incisional lengths (cm), median (IQR) | |||||
| Mini-thoracotomy incision length | 8.0 | [6.0, 9.5] | 7.0 | [6.0, 8.0] | 0.01 |
| Cannulation incision length | 5.0 | [4.0, 5.0] | 5.0 | [4.0, 5.0] | 0.81 |
| Total incision length | 20.0 | [16.8, 23.0] | 17.0 | [15.5, 18.5] | 0.004 |
| Second pump run, n (%) | 5 | 23.8% | 3 | 3.8% | 0.01 |
| Conversion to sternotomy, n (%) | 2 | 9.5% | 2 | 2.5% | 0.19 |
| Reasons for conversion | |||||
| Bleeding control | 1 | 1 | |||
| Residual MR | 1 | 1 | |||
| Conversion to MV replacement, n (%) | 4 | 19.1% | 0 | 0.0% | 0.002 |
Statistically significant P values are highlighted in bold.
ACC: aortic cross-clamp; CPB: cardiopulmonary bypass; IQR: interquartile range; MR: mitral regurgitation; MV: mitral valve.
Type of repair
Table 3 demonstrates the detailed repair methods among the 2 groups. Resection was performed in majority of cases in both groups (81.0% in the complex group, 77.2% in the non-complex group, P > 0.99). On the other hand, significantly more patients required neochords (61.9% vs 13.9%, P < 0.001) and commissuroplasty (28.6% vs 5.1%, P = 0.005) in the complex group. More than 50% of patients had cleft closure in the complex group, whereas only 38% in the non-complex group; however, there was no significant difference (P = 0.14). There were no cases of Alfieri stitch in either group, and only 1 case of decalcification in the complex group (4.8%), showing no significant differences either (P > 0.99 and P = 0.21, respectively). An annuloplasty band was used in the majority of cases in either group, and a closed ring was used less frequently. Implant sizes were similar across both groups, with a median size of 30 mm. There were no differences in the number of concomitant surgeries in either group.
Table 3:
Surgical details
| Complex (n = 21) | Non-complex (n = 79) | P value | |||
|---|---|---|---|---|---|
| Type of repair, n (%) | |||||
| Resection | 17 | 81.0% | 61 | 77.2% | >0.99 |
| Neo chords | 13 | 61.9% | 11 | 13.9% | <0.001 |
| Commissuroplasty | 6 | 28.6% | 4 | 5.1% | 0.005 |
| Cleft closure | 12 | 57.1% | 30 | 38.0% | 0.14 |
| Alfieri stitch | 0 | 0.0% | 0 | 0.0% | >0.99 |
| Decalcification | 1 | 4.8% | 0 | 0.0% | 0.21 |
| Type of annuloplasty, n (%) | 0.68 | ||||
| Band | 20 | 95.2% | 71 | 89.9% | |
| Ring | 1 | 4.8% | 8 | 10.1% | |
| Implant size (mm), median (IQR) | 30.0 | [28.0, 31.0] | 30.0 | [28.0, 32.0] | 0.20 |
| Concomitant surgery, n (%) | |||||
| LAA closure | 6 | 28.6% | 11 | 13.9% | 0.19 |
| Tricuspid valve repair | 0 | 0.0% | 1 | 1.3% | >0.99 |
| PFO closure | 1 | 4.8% | 11 | 13.9% | 0.4514 |
| MAZE | 1 | 4.8% | 3 | 3.8% | >0.99 |
Statistically significant P values are highlighted in bold.
IQR: interquartile range; LAA: left atrial appendage; PFO: patent foramen ovale.
Postoperative results
Table 4 lists the postoperative data by group. Fewer patients were extubated in the operating room (OR) in the complex group compared to the non-complex group (42.9% vs 70.9%, P = 0.022), which was accompanied by longer ventilation time in the complex group (P = 0.002). Despite longer operation and ventilation time, there was no statistically significant difference in the length of intensive care unit (ICU) stay and total length of hospital stay between 2 groups. No systolic anterior motion was seen in the complex group. As shown in Table 4, there was no significant difference between groups concerning standard postoperative outcome parameters. No 30-day mortality was observed in either group.
Table 4:
Post-operative data
| Complex (n = 21) | Non-complex (n = 79) | P value | |||
|---|---|---|---|---|---|
| Postop total ventilation time (hour), median (IQR) | 2.3 | [0, 4.9] | 0 | [0, 2.5] | 0.002 |
| Extubation in the OR, n (%) | 9 | 42.9% | 56 | 70.9% | 0.022 |
| Postoperative length of ICU stay (hour), median (IQR) | 46.6 | [22.8, 74.6] | 48.4 | [26.3, 74.2] | 0.71 |
| Postoperative length of hospital stay (days), median (IQR) | 4 | [4.0, 6.0] | 4 | [3.0, 6.0] | 0.56 |
| SAM, n (%) | 0 | 0.0% | 3 | 3.8% | >0.99 |
| Transfusion, n (%) | 2.0 | 9.5% | 10.0 | 12.7% | >0.99 |
| Revision for bleeding, n (%) | 0 | 0.0% | 1 | 1.3% | >0.99 |
| Stroke, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| New post-operative AF, n (%) | 5 | 23.8% | 18 | 22.8% | >0.99 |
| Renal failure, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| Pneumonia, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| Leg ischaemia, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| Brachial plexus injury, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
| Wound infection, n (%) | 1 | 4.8% | 1 | 1.3% | 0.38 |
| 30-day mortality, n (%) | 0 | 0.0% | 0 | 0.0% | >0.99 |
Statistically significant P values are highlighted in bold.
AF: atrial fibrillation; ICU: intensive care unit; IQR: interquartile range; OR: operating room; SAM: systolic anterior motion.
Echocardiographic early outcome
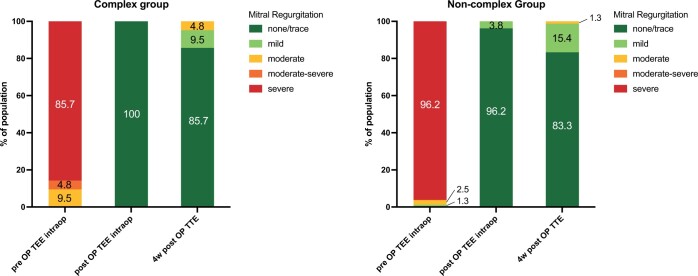
Table 5 demonstrates the postoperative echocardiographic data. All patients left OR with residual MR of mild or less. Mean MV gradient was 2.0 mmHg and left ventricular ejection fraction was 55.0% in both groups (P = 0.32 and 0.34, respectively). At 4 weeks post-surgery, there was 1 patient in each group with moderate MR (P = 0.73). Mean MV gradient increased to 3.0 mmHg for the complex group and 2.7 mmHg for the non-complex group (P = 0.23). Left ventricular ejection fraction was 55.0% in both groups (P = 0.94). Figure 1 shows the degree of pre- and postoperative MR.
Table 5:
Post-operative echocardiogram
| Complex | Non-complex | P value | |||
|---|---|---|---|---|---|
| Post-operative TEE in the OR | n = 21 | n = 79 | |||
| MR, n (%) | >0.99 | ||||
| None/trace | 21 | 100.0% | 76 | 96.2% | |
| Mild | 0 | 0.0% | 3 | 3.8% | |
| MV gradient (mmHg), median (IQR) | 2.0 | [1.5, 2.5] | 2.0 | [2.0, 3.0] | 0.32 |
| LVEF (%), median (IQR) | 55.0 | [55.0, 60.0] | 55.0 | [55.0, 55.0] | 0.34 |
| TTE 4 weeks post-surgery | n = 21 | n = 78 | |||
| MR, n (%) | 0.47 | ||||
| None/trace | 18 | 85.7% | 65 | 83.3% | |
| Mild | 2 | 9.5% | 12 | 15.4% | |
| Moderate | 1 | 4.8% | 1 | 1.3% | |
| Moderate-severe | 0 | 0% | 0 | 0% | |
| Severe | 0 | 0% | 0 | 0% | |
| MV gradient (mmHg), median (IQR) | 3 | [2.0, 4.5] | 3 | [2.0, 4.5] | 0.23 |
| LVEF (%), median (IQR) | 55 | [48.0, 60.0] | 55 | [48.0, 60.0] | 0.94 |
IQR: interquartile range; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MV: mitral valve; OR: operating room; TEE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram.
Figure 1:
Fraction of total of pre and postoperative mitral regurgitation among groups. All patients left the operating room with mild or less mitral regurgitation. There were no differences between the groups. OP: operative; TEE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram.
DISCUSSION
This is a retrospective study focusing on the feasibility and safety of complex MVR in the early phase of a robotic cardiac surgery programme, comparing outcomes between complex and non-complex cases. Loulmet et al. [4] showed that robotic-assisted MVR can be performed safely and effectively in a wide range of MV pathology. In addition, Suri et al. [6] demonstrated similar outcomes of robotic-assisted MVR in complex cases compared with non-complex cases. These studies, however, were performed in well-established programmes. Kakuta et al. [8] reported their first 100 robotic-assisted MVR cases with a very satisfactory result. Our study extends their study by explicitly comparing complex versus non-complex MVR outcomes within an early phase programme.
Percentage of complex repair
In our study, 21% of patients underwent complex MVR. Fujita et al. [1] reported 16% of complex repairs using a classic videoscopic approach through a mini-thoracotomy and 37% of that using a robotic approach. Suri et al. [6] performed complex robotic repair in 40.6% of their series, while Loulmet et al. [4] reported the highest rate at 52%, and the same group recently published that 74% of patients underwent concomitant complex subvalvular procedures [7]. Considering these series are from experienced centres, our lower rate of complex repair reflects a cautious approach during the implementation phase of a new programme.
Demographic profile
Notably, the complex group had more male patients and those with larger body surface area and heavier body weight. The result aligns with findings from Singh et al. [12], who reported that patients with mitral valve prolapse and severe MR who required valve surgery were more likely to be older men and more overweight. Furthermore, the same study observed that body mass index and hypertension were statistically independent predictors of the need for MV surgery in mitral valve prolapse patients with severe MR. On the other hand, the Mayo Clinic study found more females and younger patients in the complex repair group [6]. Overall, higher body mass index and body weight could be risk factors in the complexity of MV pathologies requiring intervention.
Mitral valve pathology and repair techniques
More than half of the complex group had had bileaflet prolapse, while most non-complex patients had isolated posterior leaflet prolapse. Notably, isolated P2 prolapse in patients with fibroelastic deficiency repair can be technically challenging due to additional manoeuvres like commissuroplasties or Alfieri stitches. In the non-complex group, all bileaflet prolapse cases were Barlow’s disease patients requiring only annuloplasty without leaflet manipulation, as described by Ben Zekry et al. [13]. Our findings align with Suri et al.’s [6] report, showing 91.3% isolated posterior leaflet pathology in the non-complex group and 82.3% bileaflet prolapse in the complex group. Their complex group also required significantly more neochord implantations and commissuroplasties.
Our concomitant procedure rates also match Suri et al.’s [6] findings, which also showed no significant difference between groups. Our rate of left atrial appendage closure, however, was higher. Our low rate of tricuspid valve repair can be explained by careful patient selection during the learning curve period of our programme, with more complex cases handled via sternotomy during the learning curve.
Intraoperative results
As expected, surgery-related times were longer in the complex group compared to the non-complex group. Total operative time increased by ∼33%, from four and a half hours in the non-complex group to 6 h in the complex group. Significant differences in myocardial ischaemic and CPB time were also noted, aligning with the largest series reporting on this topic [6]. While Loulmet et al. [4], with over 50% complex cases, reported slightly shorter operative times than our cohort, our CPB and aortic cross-clamp times for the non-complex group are within the limits reported in recent meta-analyses [14]. These times may not be much different from operative time reported for videoscopically assisted MVR through mini-thoracotomy; however, we believe major benefits in terms of visualization and ergonomics.
While there were no differences in conversion to sternotomy, more patients in the complex group required a 2nd pump run, and 4 needed conversion to MV replacement, compared to none in the non-complex group. This reflects the challenges associated with repairing complex prolapsed valves during the programme’s early phase. Our 4% conversion rate, though higher than other studies [1, 4–6, 9], aligns with the 3.6%–5.0% rate reported for videoscopically assisted MVR through mini-thoracotomy in the Society of Thoracic Surgeons (STS) database [3]. The lower conversion rate in robotic surgery was noted, but the learning curve was not analysed in that study.
Encouragingly, all patients in the complex group left the OR with none or trace residual MR. The same was true for 96.2% of the non-complex group, of whom only 3 showed mild regurgitation. This aligns with results from Gillinov et al. [9] with 99.7% of patients leaving the OR with none or mild regurgitation.
We noted 9.5% and 15.4% of mild MR in the complex group and the non-complex group, respectively, at the 4-week follow-up TTE, comparable to reports of others [6, 9], demonstrating that a satisfactory procedure could be performed in early phase programme. Our 2% rate of early moderate MR and post-repair MV gradients were also comparable to others [9].
Interestingly, the incision length was longer in the complex group. This may be due to a larger body surface area, and the need for enlargement in replacement cases. To our knowledge, no papers have reported on incision length in robotic MVR, making this an important new metric for evaluating surgical trauma.
Postoperative results
As expected, the extubation rate in the OR was lower, and ventilation time was ∼2 h longer in the complex group compared to the non-complex group. There was, however, no difference in the ICU length of stay or the total hospital length of stay. Our longer ICU length of stay is due to not having a dedicated intermediate care unit, and often, limited floor bed availability leads to holdups in the ICU. Some patients were discharged from the ICU directly. While Suri et al. [6] reported no difference in ICU stay between groups, Loulmet et al. [4] reported an extubation rate in the OR of 68% to 95%, which varied between quartiles in his series of 500 patients. We reached this level in our patients who underwent non-complex repair. Our overall 4-day length of stay for both groups was comparable to Loulmet et al.’s and slightly shorter than recent STS reports [3].
Our clinical results regarding perioperative end-points were satisfactory, with no permanent strokes, renal failure or mortality. In addition, the transfusion rates, revision for bleeding and atrial fibrillation were low and consistent with recent robotic MVR results in the STS database [3]. Despite the fact that the programme was a startup, complex robotic MVR did not increase perioperative morbidity or mortality.
Potential influence of learning curves
Our prolonged operative times and conversion to a replacement should be seen in the context of the surgeon and team’s learning curves in a new programme. Gillinov et al. [9] found that CPB and aortic cross-clamp times stabilize after 200–300 cases, emphasizing the importance of systematic training in high-volume centers. Similarly, Ramzy et al. [15] showed that technological updates and systematic training improve outcomes and reduce complications across 300 cases. These studies highlight the importance of continuous learning and adaptation in robotic-assisted procedures.
Limitations
Our study is from a single institution and retrospective study with a small sample size, specifically in the complex group. It may not fully capture the variability and potential confounders that could influence outcomes. Various surgeon and team learning curves were present. Additionally, the study has a short follow-up period, and long-term follow-up is warranted. The initiation of a multicentre prospective registry, involving several high-volume robotic centres, could provide more robust data.
CONCLUSION
In conclusion, our results indicate that complex MVR can be safely and effectively performed using a robotic minimally invasive approach, even within the early phase of a robotic heart surgery programme. While the complex group had longer operative times, more conversion to valve replacement, and longer ventilation time, there were no increases in ICU stay, hospital stay or postoperative adverse events. Early repair quality appears to meet current standards.
Glossary
ABBREVIATIONS
- CPB
Cardiopulmonary bypass
- ICU
Intensive care unit
- MR
Mitral regurgitation
- MV
Mitral valve
- MVR
Mitral valve repair
- OR
Operating room
- STS
Society of Thoracic Surgeons
Contributor Information
Kei Kobayashi, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Yizhan Guo, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Thomas E Rubino, Jr, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Luis E Ramirez, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Stephen D Waterford, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Ibrahim Sultan, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Victor D Morell, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
Johannes Bonatti, Division of Cardiac Surgery, Department of Cardiothoracic Surgery, University of Pittsburgh and UPMC Heart and Vascular Institute, Pittsburgh, PA, USA.
FUNDING
None declared.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Kei Kobayashi: Data curation; Formal analysis; Resources; Writing—original draft; Writing—review & editing. Yizhan Guo: Data curation. Thomas E. Rubino Jr.: Data curation. Luis E. Ramirez: Resources; Writing—review & editing. Stephen D. Waterford: Resources; Writing—review & editing. Ibrahim Sultan: Resources; Writing—review & editing. Victor D. Morell: Resources; Writing—review & editing. Johannes Bonatti: Conceptualization; Resources; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Jonas Pausch and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Fujita T, Kakuta T, Kawamoto N, Shimahara Y, Yajima S, Tadokoro N et al Benefits of robotically-assisted surgery for complex mitral valve repair. Interact CardioVasc Thorac Surg 2021;32:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mihaljevic T, Jarrett CM, Gillinov AM, Williams SJ, DeVilliers PA, Stewart WJ et al Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72–80.e4. [DOI] [PubMed] [Google Scholar]
- 3. Mori M, Parsons N, Krane M, Guy TS, Grossi EA, Dearani JA et al Robotic mitral valve repair for degenerative mitral regurgitation. Ann Thorac Surg 2024;117:96–104. [DOI] [PubMed] [Google Scholar]
- 4. Loulmet DF, Ranganath NK, Neuburger PJ, Nampiaparampil RG, Galloway AC, Grossi EA.. Can complex mitral valve repair be performed with robotics? An institution's experience utilizing a dedicated team approach in 500 patients. Eur J Cardiothorac Surg 2019;56:470–8. [DOI] [PubMed] [Google Scholar]
- 5. Roach A, Trento A, Emerson D, Gill G, Rowe G, Peiris A et al Durable robotic mitral repair of degenerative primary regurgitation with long-term follow-up. Ann Thorac Surg 2022;114:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suri RM, Taggarse A, Burkhart HM, Daly RC, Mauermann W, Nishimura RA et al Robotic mitral valve repair for simple and complex degenerative disease: midterm clinical and echocardiographic quality outcomes. Circulation 2015;132:1961–8. [DOI] [PubMed] [Google Scholar]
- 7. Dorsey M, James L, Shrivastava S, Loulmet D, Grossi EA.. Subvalvular techniques enhanced with endoscopic robotic mitral valve repair. JTCVS Tech 2023;22:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakuta T, Fukushima S, Shimahara Y, Yajima S, Tadokoro N, Minami K et al Early results of robotically assisted mitral valve repair in a single institution: report of the first 100 cases. Gen Thorac Cardiovasc Surg 2020;68:1079–85. [DOI] [PubMed] [Google Scholar]
- 9. Gillinov AM, Mihaljevic T, Javadikasgari H, Suri RM, Mick SL, Navia JL et al Early results of robotically assisted mitral valve surgery: analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82–91.e2. [DOI] [PubMed] [Google Scholar]
- 10. Gillinov M, Burns DJP, Wierup P.. The 10 commandments for mitral valve repair. Innovations (Phila) 2020;15:4–10. [DOI] [PubMed] [Google Scholar]
- 11. Ashraf F, Hasan I, Seese L, Deitz R, Yousef S, Sultan I et al Robotically assisted mitral valve repair—resectional techniques. J Vis Surg 2023;9:33. [Google Scholar]
- 12. Singh RG, Cappucci R, Kramer-Fox R, Roman MJ, Kligfield P, Borer JS et al Severe mitral regurgitation due to mitral valve prolapse: risk factors for development, progression, and need for mitral valve surgery. Am J Cardiol 2000;85:193–8. [DOI] [PubMed] [Google Scholar]
- 13. Ben Zekry S, Spiegelstein D, Sternik L, Lev I, Kogan A, Kuperstein R et al Simple repair approach for mitral regurgitation in Barlow disease. J Thorac Cardiovasc Surg 2015;150:1071–7.e1. [DOI] [PubMed] [Google Scholar]
- 14. Williams ML, Hwang B, Huang L, Wilson-Smith A, Brookes J, Eranki A et al Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. Ann Cardiothorac Surg 2022;11:490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramzy D, Trento A, Cheng W, De Robertis MA, Mirocha J, Ruzza A et al Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.