Abstract
OBJECTIVES
To assess the incremental prognostic value of right ventricular free wall longitudinal strain over conventional risk scores in predicting the peri-operative mortality in patients with severe tricuspid regurgitation (TR) undergoing isolated tricuspid valve (TV) surgery.
METHODS
We retrospectively enrolled 110 consecutive patients with severe TR who underwent isolated TV surgery between November 2016 and July 2022 at San Raffaele Hospital, Milan, Italy. Exclusion criteria were previous TV surgery, urgent surgery, complex congenital heart disease, active endocarditis and inadequate acoustic window. Baseline clinical data were included, as well as laboratory tests and clinical risk score, as TRI-SCORE and MELD-XI. The clinical outcome was peri-operative mortality, defined as all-cause mortality within 30 days.
RESULTS
The final cohort included 79 patients. The end-point occurred in 7 patients (9%), who died within 30 days after isolated TV surgery. Receiver operator characteristic curves analysis showed that, among parameters of right ventricular function, right ventricular free wall longitudinal strain was the best parameter to predict peri-operative mortality (AUC: 0.854, 95% CI 0.74–0.96, P = 0.005, sensitivity 68%, specificity 100%). At univariable analysis, left ventricular ejection fraction, diabetes mellitus, creatinine, estimated glomerular filtration rate, serum sodium, MELD-XI, TRI-SCORE, right ventricular areas, right ventricular global longitudinal strain, right ventricular free wall longitudinal strain, fractional area change and the ratio between right ventricular free wall longitudinal strain/pulmonary arterial systolic pressure were significantly associated with the end-point. The combination of TRI-SCORE and right ventricular Strain, evaluating right ventricular systolic function with speckle-tracking echocardiography, outperformed classic TRI-SCORE in outcome prediction (AUC 0.874 vs 0.787, P = 0.05).
CONCLUSIONS
Right ventricular free wall longitudinal strain has an incremental prognostic value over conventional parameters and significantly improves the ability of clinical scores to predict peri-operative mortality in patients undergoing isolated TV surgery.
Keywords: Tricuspid regurgitation, Isolated tricuspid valve surgery, Right ventricular free wall longitudinal strain
Severe tricuspid regurgitation (TR) is associated with significant mortality and morbidity [1–3], leading to progressive right ventricular (RV) dilatation and dysfunction, right heart failure and ultimately end-organ damage [4].
Graphical Abstract
INTRODUCTION
Severe tricuspid regurgitation (TR) is associated with significant mortality and morbidity [1–3], leading to progressive right ventricular (RV) dilatation and dysfunction, right heart failure and ultimately end-organ damage [4].
According to current guidelines [5], surgery remains the gold standard treatment for TR. Isolated TV surgery (ITVS) is encumbered by a mortality ranging from as low as 2% to >30% [6–8].
Recently, a validated surgical risk score (TRI-SCORE) [9] was able to stratify patient mortality in the perioperative period. The score is formed by eight parameters, and the novel and valuable feature is the inclusion of liver and RV function [10]. TRI-SCORE incorporates RV function evaluated through Tricuspid Annular Plane Systolic Excursion (TAPSE) and systolic peak at Tissue Doppler Imaging (S-TDI), carrying the intrinsic limitations of these parameters, especially in the setting of severe TR.
In fact, conventional echocardiographic parameters, such as TAPSE (Tricuspid Annular Plane Systolic Excursion), S’ TDI (peak Systolic wave at Tissue Doppler Imaging) or fractional area change (FAC), are load and angle dependent, without exploring all RV walls or considering flow direction. Strain analysis, and in particular RV free wall longitudinal strain (RVFWLS), reflects RV contractility more accurately [11], and has recently emerged as a prognostic indicator in patients with TR [12–14].
The aim of this study was to explore the independent and incremental prognostic value of RVFWLS isolated or embedded in a clinical risk score, such as the TRI-SCORE, in predicting short-term outcomes of patients with severe TR undergoing ITVS.
MATERIALS AND METHODS
Study population
We retrospectively enrolled 110 consecutive patients with severe TR with complete echocardiographic evaluation who underwent ITVS between November 2016 and July 2022 at San Raffaele Hospital, Milan, Italy. Exclusion criteria were previous TV surgery, urgent surgery, complex congenital heart disease, active endocarditis and inadequate acoustic window.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board (LOCOMOTRI study protocol). For retrospective studies, the ethical committee waived informed consent.
Data collection
The following clinical data were collected: age, sex, BMI (body mass index), cardiovascular risk factors, chronic kidney, pulmonary, liver, cerebrovascular and coronary artery disease, prior pacemaker implantation and surgical/transcatheter heart interventions, congenital heart disease, history of intravenous drug abuse and acute decompensated congestive heart failure. Signs and symptoms of fluid overload were recorded as well as information regarding diuretic therapy and functional capacity, as evaluated by New York Heart Association (NYHA) class.
Laboratory tests were measured at baseline, specifically: haemoglobin, creatinine, estimated glomerular filtration rate according to the Cockroft-Gault formula, bilirubin, plasmatic sodium, NT-proBNP and TnT (Troponin T).
Transthoracic echocardiography
Complete transthoracic echocardiograms were performed at rest with commercially available ultrasound systems (Vivid E9 and Vivid E95, General Electric Healthcare, Milwaukee, WI, USA). Median time from echocardiography to intervention was 9 days.
Data were acquired as per current recommendations for cardiac chambers quantification and the severity of TR was assessed by multiparametric approach using jet size, VC, PISA radius, EROA PISA, 3D VC area (when available) and hepatic vein flow [15–17] and graded as severe, massive or torrential [18].
In particular, RV global longitudinal strain (RVGLS) was calculated as the average of the 6 RV segments (basal, mid and apical segments of both the free wall and the septum), while RVFWLS as the average of the 3 segments of RV free wall (basal, mid and apical segments). RVFWLS and RVGLS were used as absolute values, instead of negative, to avoid confusion. RV dysfunction was defined according to guidelines [19]. Right ventricle-pulmonary artery (RV-PA) coupling was evaluated non-invasively using the ratio between TAPSE and SPAP and between RVFWLS and SPAP [20, 21].
Data were analysed off-line on a dedicated workstation (ECHOPAC BT 11; GE, Milwaukee, WI, USA).
Clinical risk score
The TRI-SCORE was calculated to stratify patients in low (≤3), moderate (4–5), or high (≥6) surgical risk, giving a value of 1 to the following variables (maximum score 12): age ≥ 70 years, NYHA functional classes III–IV, right-sided heart failure signs (severe jugular venous distention, ascites and/or marked peripheral oedema), daily dose of furosemide ≥ 125 mg, glomerular filtration rate <30 ml/min, elevated total bilirubin (above our laboratory threshold of 1.2 mg/dl), left ventricular ejection fraction (LVEF) <60% and moderate/severe RV dysfunction (semi-quantitatively assessed using a multi-parametric approach integrating TAPSE < 17 mm and/or Doppler tissue imaging peak systolic annular velocity S’ < 9.5 cm/s and/or and according to visual assessment) [9]. In addition, we built a modified version of the classic TRI-SCORE, a combination of TRI-SCORE and longitudinal Strain, using RVFWLS for assessing RV function instead of TAPSE and S’ TDI (assigning 2 points in case of RVFWLS lower than the cut-off defined by ROC analysis).
Follow up and end-point
The clinical outcome was all-cause 30-day mortality; if hospital discharge occurred before 30 days, patients were followed-up by phone interview, analysis of hospital database and/or ambulatory visit.
Statistical analysis
The normal distribution of continuous variables was tested with Kolmogorov–Smirnov test. Continuous variables are reported as mean ± standard deviation or median (interquartile range, IQR) and were compared using the Student’s t-test or Mann–Whitney U-test, respectively. Categorical variables are reported as counts and percentages: the Chi-square test was used when the expected cell counts were 5 or greater; in cases where any cell in the contingency table had an expected count below 5, Fisher’s exact test was applied.
Receiver operator characteristic (ROC) curves and the areas under the curves were calculated for echocardiographic parameters of RV function and used to find a cut off discriminating the occurrence of the end-point at follow-up. In addition, ROC analysis was performed to compare the ability to predict the primary end-point of TRI-SCORE and the combination of TRI-SCORE with Strain. The comparison between the AUCs was performed using the DeLong’s test.
The difference between Chi-square (χ2) was assessed by the likelihood ratio test, while Cox regression was used to identify independent predictors of 30-day mortality.
Survival rates were estimated with the Kaplan–Meier method and differences between groups compared by the log-rank test. Follow-up was censored at the time of last follow-up or when the primary end-point occurred.
The intra- and interobserver reproducibility of strain analysis was assessed by 2 independent readers in a set of 10 random cases and tested by calculating the intra-class correlation coefficient for RVFWLS.
A 2-sided P < 0.05 was considered statistically significant. Statistical analysis was performed by SPSS 24.0 software (SPSS, Inc., Chicago, IL, USA).
RESULTS
Clinical and echocardiographic characteristics of the population
During the study period, 110 patients underwent isolated TV surgery: as 25 patients were excluded due to technical reasons leading to inadequate strain analysis and 6 because of previous TV surgery, the final population included 79 patients. Twenty-two patients underwent TV repair with annuloplasty, while 57 TV replacement (54 received a bioprosthetic valve and 3 received a mechanical valve).
Baseline clinical characteristics are shown in Table 1: patients had a median age of 66 (IQR: 57–75) years, were predominantly female, with a high prevalence of atrial fibrillation. Almost half of patients had a history of previous cardiac surgery and 37% of the total population was very symptomatic for heart failure (NYHA classes III/IV). According to the TRI-SCORE, the majority of patients (n = 53, 67%) had a low surgical risk, while 10% of patients had a high surgical risk with a TRI-SCORE >6 points.
Table 1:
Baseline clinical and echocardiographic characteristics
| Clinical characteristics | n = 79 |
|---|---|
| Age (years) | 66 (IQR 57-75) |
| Sex: female | 54 (68%) |
| Hypertension | 63 (63%) |
| Diabetes mellitus | 8 (10%) |
| Smoking history | 25 (32%) |
| Hypercholesterolemia | 24 (30%) |
| COPD | 8 (10%) |
| Atrial fibrillation | 36 (46%) |
| Pacemaker/ICD | 16 (20%) |
| CAD | 11 (14 %) |
| BMI (kg/m2) | 24.6 + 5.4 |
| Haemoglobin (g/dl) | 12.6 + 1.9 |
| Creatinine (mg/dl) | 1 + 0.4 |
| eGFR (ml/min) | 73.9 + 27.9 |
| Total bilirubin (mg/dl) | 0.88 + 0.6 |
| Sodium (mEq/l) | 139 + 3.7 |
| Furosemide dose (mg) | 82 mg + 127 mg |
|
|
| TRI-SCORE (points) | 3 (IQR 1–4) |
| Surgical risk according to TRI-SCORE | |
|
53 (67%) |
|
18 (22%) |
|
8 (10%) |
| Surgical risk according to TRI-SCORE and RV Strain | |
|
47 (59%) |
|
20. (25%) |
|
12 (15%) |
| Charlson comorbidity index | 4 (IQR 0 - 2) |
| Functional class | |
| NYHA classes I–II | 50 (63%) |
| NYHA classes III–IV | 29 (37%) |
| Signs of right heart failure | |
| Ascites | 1 (1%) |
| Hepatomegaly | 7 (9%) |
| Peripheral oedema | 18 (23%) |
| Fatigue | 42 (53%) |
| Dyspepsia | 3 (4%) |
| Jugular venous distension | 3 (4%) |
| Previous cardiac surgery | 35 (44%) |
|
32 |
|
2 |
|
1 |
| Type of TV surgery | |
|
22 (28%) |
|
54 (68%) |
|
3 (4%) |
|
28 (35%) |
| Echocardiographic characteristics | |
| TR aetiology | |
| Organic TR | 28 (35%) |
|
3 (4%) |
|
5 (6%) |
|
7 (9%) |
|
1 (1%) |
| Functional TR | 51 (65%) |
|
31 (39%) |
|
20 (25%) |
| Grade of TR | |
|
58 (73%) |
|
14 (18%) |
|
7 (9%) |
| VC (mm) | 10.6 ± 4.13 |
| EROA PISA (cm2) | 0.7 ± 0.49 |
| RA area (cm2) | 30.6 ± 12.1 |
| TA (SL diameter, mm) | 45.8 ± 7.7 |
| TA dilatation (TA diameter ≥ 21 mm/m2) | 72 (91%) |
| RV basal end diastolic diameter (mm) | 47.9 ± 8.8 |
| RV mid end diastolic diameter (mm) | 37.4 ± 6.9 |
| RV end diastolic area (cm2) | 23.8 ± 9.3 |
| RV end systolic area (cm2) | 13.5 ± 6.5 |
| TAPSE (mm) | 20.6 ± 4.9 |
| S’ TDI (cm/s) | 11.9 ± 3.4 |
| FAC (%) | 43.7 ± 8 |
| RVFWLS (absolute value, %) | 21.6 ± 6.3 |
| RVGLS (absolute value, %) | 19.3 ± 6.2 |
| SPAP (mmHg) | 39.1 ± 9.1 |
| RV-PA coupling | |
|
0.56 ± 0.02 |
|
0.54 ± 0.2 |
| Estimated RAP (mmHg) | 9.8 ± 5.2 |
| LV EF (%) | 58.9 ± 6.7 |
| LV EF > 50% | 72 (91%) |
| LV end diastolic volume/BSA (ml/m2) | 51.1 ± 13.2 |
| Average E/E’ | 7.2 ± 3.4 |
| LA/BSA (ml/m2) | 60.4 ± 52.6 |
| Aortic regurgitation | |
|
46 (58%) |
|
28 (35%) |
|
5 (7%) |
|
0 (0%) |
| Aortic stenosis | |
|
78 (99%) |
|
0 (0%) |
|
1 (1%) |
|
0 (0%) |
| Mitral regurgitation | |
|
33 (42%) |
|
28 (35%) |
|
18 (23%) |
|
0 (0%) |
| Mitral stenosis | |
|
75 (95%) |
|
4 (5%) |
|
0 (0%) |
|
0 (0%) |
| Tricuspid stenosis | |
|
73 (91%) |
|
2 (3%) |
|
2 (3%) |
|
2 (3%) |
| Pulmonary regurgitation | |
|
77 (97%) |
|
2 (3%) |
|
0 (0%) |
|
0 (0%) |
| Pulmonary stenosis | 0 (0%) |
Abbreviations: BMI: body mass index; COPD: chronic obstructive pulmonary disease; CAD coronary artery disease; CABG: coronary artery bypass graft; eGFR: estimated glomerular filtration rate; ICD: implantable cardioverter defibrillator; NYHA: New York Heart Association; BSA: body surface area; LV EF: left ventricular ejection fraction; EROA: effective regurgitant orifice area; RA: right atrial; RV, right ventricle; LA: left atrial; PASP: pulmonary artery systolic pressure; RAP: right atrial pressure; VC: vena contracta; TA: tricuspid annulus.
Baseline echocardiographic characteristics of patients are presented in Table 1.
Approximately 90% of the population presented with preserved left ventricular ejection fraction (LVEF > 50%). The most frequent aetiology of TR was functional (65%). TR was severe, massive and torrential in 73%, 18% and 9% of patients, respectively.
The prevalence of RV systolic dysfunction, according to recommended cut offs [22], was 19%, 29% and 11% based on TAPSE, S’ TDI and FAC, respectively (Fig. 1); the prevalence of RV dysfunction significantly increased according to RVFWLS (43%): 25–40% of patients with normal RV function according to each conventional echocardiographic parameters had impaired RV systolic function according to RVFWLS (Fig. 1). The intraclass correlation coefficient for RVFWLS was very good (0.83), as assessed in a random set of 10 patients.
Figure 1:
Prevalence of RV dysfunction according to different echocardiographic indices (upper panel) and reclassification of RV function according to RVFWLS (lower panel).
End-point and prognostic role of RV function
The primary end-point of peri-operative mortality occurred in 7 patients (9%) with a median time from intervention of 15 (IQR 5–20) days. Data on ‘residual TR’ were available for 76 out of 79 patients. The vast majority of patients (65 patients, 85%) had none or trace residual TR, 7 patients (9%) had mild residual TR, and 4 patients (5%) had moderate residual TR. In univariable analysis, the presence of moderate residual TR was not associated 30-day mortality (HR 0.5, 0.1–777, P = 0.38), nor did the presence of more than mild residual TR (HR 0.39, 0.1–337, P = 0.14). At univariable analysis, LVEF, diabetes mellitus, creatinine, eGFR, serum sodium, MELD XI, TRI-SCORE and the combination of TRI-SCORE and Strain, RV end-systolic and end-diastolic area, RVGLS, RVFWLS and FAC were predictors of peri-operative mortality (Table 2).
Table 2:
Univariable analysis for predictors of 30-day mortality
| Univariable analysis | ||
|---|---|---|
| HR (95% CI) | P-value | |
| Age | 1.08 (0.98–1.18) | 0.113 |
| Sex: male | 0.919 (0.18–4.74) | 0.92 |
| Hypertension | 2.22 (0.41–12.12) | 0.36 |
| Diabetes mellitus | 10.66 (2.14–53.07) | 0.004 |
| Hypercholesterolemia | 1.14 (0.209–6.22) | 0.88 |
| Smoking history | 2.1 (0.42–10.38) | 0.37 |
| COPD | 0.42 (0.01–4016.7) | 0.59 |
| Atrial fibrillation | 1.75 (0.9–3.42) | 0.101 |
| CAD | 0.039 (0–717.43) | 0.517 |
| BMI | 1.04 (0.93–1.17) | 0.454 |
| Haemoglobin (g/dl) | 0.76 (0.52–1.1) | 0.136 |
| Creatinine (mg/dl) | 19.37 (4.33–86.57) | <0.001 |
| eGFR (ml/min) | 0.93 (0.94–0.99) | 0.002 |
| Na+ (mEq/l) | 0.806 (0.696–0.932) | 0.004 |
| Total bilirubin (mg/dl) | 1.84 (0.79–4.3) | 0.157 |
| NYHA class III or IV | 3.51 (0.64–19.19) | 0.147 |
| Previous CV surgery | 3.13 (0.61–16.15) | 0.172 |
| Moderate MR | 1.38 (0.27–7.11) | 0.8 |
| Beating heart surgery | 0.957 (CI 0.59–1.55) | 0.85 |
| TRI-SCORE | 1.54 (1.08–2.2) | 0.02 |
| TRI-SCORE and RV Strain | 1.705 (1.20–2.42) | 0.003 |
| Charlson comorbidity index | 0.46 (0.09–2.34) | 0.358 |
| MELD XI | 1.298 (1.12–1.51) | 0.001 |
| RV end diastolic area (cm2) | 1.10 (1.01–1.2) | 0.02 |
| RV end systolic area (cm2) | 1.13 (1.02–1.25) | 0.02 |
| RA volume (ml) | 1.01 (0.1–1.02) | 0.158 |
| TA diameter (mm) | 1.02 (0.93–1.12) | 0.64 |
| TAPSE | 0.9 (0.78–1.05) | 0.172 |
| S’ TDI | 0.71 (0.51–0.97) | 0.03 |
| FAC | 0.91 (0.84–0.98) | 0.013 |
| RVFWLS | 0.83 (0.73–0.94) | 0.003 |
| RVGLS | 0.84 (0.74–0.96) | 0.008 |
| PASP | 1.03 (0.95–1.11) | 0.54 |
| LVEF | 0.88 (0.79–0.98) | 0.017 |
| E/E’ | 1.05 (0.7–1.59) | 0.81 |
| TAPSE/PASP | 0.02 (0.01–2.27) | 0.1 |
| RVFWLS/PASP | 0.001 (0.01–0.26) | 0.016 |
| Organic vs functional TR | 42.49 (0.07–25035.8) | 0.249 |
| Residual TR after surgery ≥ 1+ | 0.39 (0.1–337) | 0.14 |
Bold values denote statistical significance at the p < 0.05 level.
Among parameters of RV function, ROC curve analysis showed that RVFWLS cutoff of 20% was the best parameter of RV function to predict peri-operative mortality (AUC: 0.833, IC 0.72–0.95, P = 0.005, sensitivity 68%, specificity 100%) (Fig. 2). Patients that died at 30 days had significantly lower RVFWLS compared with those who survived (16.7 + 5.6 vs 22.3 + 6.1; P = 0.017).
Figure 2:
ROC curve analysis with echocardiographic parameters of RV function.
Patients with RV dysfunction defined by RVFWLS < 20% had lower both 30-day and overall survival compared with patients with preserved RV function (80 ± 0.7% vs 100%, log-rank P 0.001) (Fig. 3; Supplementary Material, Fig. S1).
Figure 3:
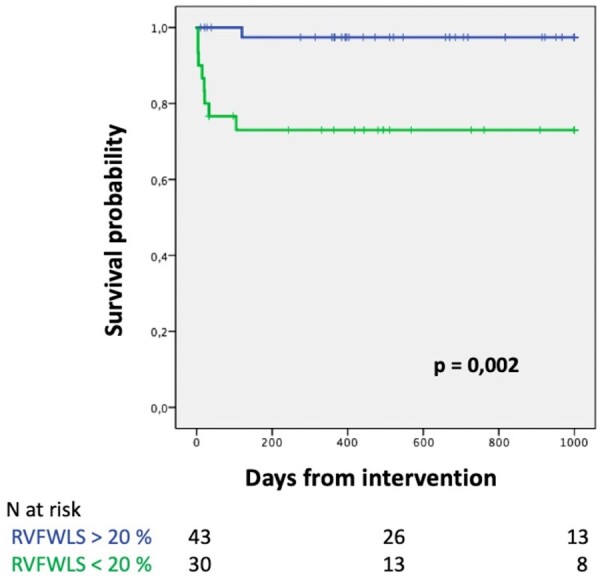
Kaplan–Meier curves according to RV dysfunction defined by RVFWLS.
In ROC curve analysis, the combination of TRI-SCORE and RV Strain, assessing RV dysfunction by speckle-tracking echocardiography, outperformed the TRI-SCORE in predicting outcome (AUC 0.874 vs 0.787, P = 0.05, Fig. 4).
Figure 4:
ROC curve analysis including different TRI-SCORE models.
In addition, the incremental value of RVFWLS to predict survival over TRI-SCORE was assessed by sequential Cox analysis: adding TAPSE to TRI-SCORE did not significantly increase the global χ2 values; however, adding RVFWLS significantly improved the global χ2 values (P = 0.281 vs P = 0.008) (Fig. 5).
Figure 5:
Incremental value of the RVFWLS and TAPSE over TRI-SCORE for predicting the primary end-point by global χ2 changes in sequential Cox analysis.
DISCUSSION
The main results of the present study are:
in all-comers surgical candidates undergoing ITVS in a high-volume tertiary centre, the peri-operative mortality was not negligible despite the majority of patients presented normal RV function, as assessed by conventional parameters;
a high proportion of patients undergoing surgery had an advanced stage of the disease, as shown by more than severe TR grade and a reduction of the RV function assessed by longitudinal strain;
TRI-SCORE showed a good predictability of peri-operative mortality, further increasing when RV function was evaluated by RVFWLS.
In our surgical cohort, TV intervention was associated with a mortality of 9%, which is consistent with previous data [23–25]. High mortality rates following TV surgery could be explained by the late referral of patients with severe TR, with advanced right heart failure [25–27].
Among baseline clinical characteristics, diabetes mellitus, serum sodium levels, TRI-SCORE and hepatorenal dysfunction as assessed by MELD XI were predictors of all-cause death at univariable analysis, consistent with previous evidence [9, 28, 29], emphasizing the loss of benefit when TR is treated in the advanced stage of the disease and in the presence of multiorgan failure. It is interesting that in the univariable analysis a number of the parameters of TRI-SCORE were not significant in our cohort: age, NYHA class, bilirubin levels as opposed to the TRI-SCORE itself, whereas in the paper by Dreyfus et al. [9] these were highly significant. This could be related to the relatively small study population, and the characteristics of the cohorts: compared with the paper by Dreyfus et al. [9], our population was older (mean age 66 vs 60 years), without elevated total bilirubin, and with lower functional NYHA class (NYHA classes III–IV 37% vs 47%).
Current guidelines acknowledge the importance of early treatment in order to avoid poor clinical outcomes, but clear indications of the timing of intervention based on thresholds of RV function have not been defined yet [5]. This does not necessarily imply that RV failure is the main cause of death after surgery, and missing echocardiographic data after surgery hampered this analysis in our work. Evaluation of RV function is particularly challenging in the context of severe, or more than severe, TR due to the backward stroke volume, not taken into account by conventional parameters, having the potential to overestimate RV function [30]. In our experience, TAPSE was not predictive of mortality. Some authors have previously shown its association with long-term mortality [31–33]. However, these studies differed for the inclusion of moderate TR, the low rates of intervention and the higher percentage of patients with decreased TAPSE. Conversely, in our population RV dysfunction when assessed by conventional methods was relatively low, whereas longitudinal strain was able to identify a higher rate of systolic impairment, as already known in the literature [13]. The present preliminary study, despite the small sample size, adds to the previous literature that RVFWLS provided incremental prognostic value to predict peri-operative mortality at 30 days in respect to conventional parameters such as FAC and S’ TDI.
In addition, TAPSE/PASP ratio, a surrogate of RV-PA coupling, in the univariate analysis did not correlate with outcome (Table 2), maybe because also PASP seems not to be associated with prognosis, maybe because in the context of severe TR, such as in our population, echocardiographic estimation of PASP loses accuracy, and also TAPSE could overestimates RV systolic function. Patients undergoing TV surgery have a narrower range of PASP, due to possible selection bias, thus maybe hampering the prognostic role of this ratio in this specific population.
Finally, the combination of TRI-SCORE-and RV Strain, that is the TRI-SCORE in which RV function is evaluated by RVFWLS, had a higher prognostic power to predict peri-operative mortality after ITVS, as compared with the classic TRI-SCORE that is based on TAPSE (Chi-square 12.024 vs 8.145; P = 0.008).
Early detection of RV dysfunction is crucial in patients with TR. Our findings may be a proof of concept to apply to patients with severe TR in order to break the vicious circle of volume overload and RV remodelling before irreversible damage occurs.
Limitations
This is a retrospective, single centre study with a relatively small sample size and focusing on 30-day mortality; due to the retrospective nature, pre-operative management could have differed among patients. Measurements by 3D echocardiography, CMR and cardiac catheterization, and the assessment of invasive pulmonary pressures were not available for the majority of patients. Missing adequate echocardiographic views for strain analysis in some cases could have hampered our statistical analysis; lack of echocardiographic data at follow-up, and in particular data on residual TR, prevented us from analysing pre- and post-surgical changes as well as the impact of post-operative parameters on outcomes.
CONCLUSIONS
Severe TR is associated with significant morbidity and mortality, and advanced stages of RV dysfunction carry the worst clinical outcomes. RVFWLS could detect earlier RV dysfunction as compared with conventional echocardiographic parameters, especially in the setting of severe TR. Although a definitive conclusion cannot be drawn, the addition of RVFWLS to the TRI-SCORE, a well-known clinical risk score, improves the ability to predict the risk of in-hospital mortality. Our data represent a proof of concept that need to be confirmed in large clinical trial.
Supplementary Material
Glossary
ABBREVIATIONS
- ITVS
Isolated TV surgery
- RV
Right ventricle
- RVFWLS
Right ventricular free wall longitudinal strain
- TR
Tricuspid regurgitation
- TV
Tricuspid valve
Contributor Information
Francesco Ancona, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Matteo Bellettini, Division of Cardiology, Cardiovascular and Thoracic Department, Città della Salute e della Scienza Hospital, Turin, Italy; Department of Medical Sciences, University of Turin, Turin, Italy.
Giovanni Polizzi, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Gabriele Paci, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Davide Margonato, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Giacomo Ingallina, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Stefano Stella, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Giorgio Fiore, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Annamaria Tavernese, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Martina Belli, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Federico Biondi, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Alessandro Castiglioni, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Paolo Denti, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Heart Valve Center, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Nicola Buzzatti, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Heart Valve Center, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Gaetano Maria De Ferrari, Division of Cardiology, Cardiovascular and Thoracic Department, Città della Salute e della Scienza Hospital, Turin, Italy; Department of Medical Sciences, University of Turin, Turin, Italy.
Ottavio Alfieri, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Elisabetta Lapenna, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Michele De Bonis, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Francesco Maisano, Department of Cardiac Surgery, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy; Heart Valve Center, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Eustachio Agricola, Cardiovascular Imaging Unit, Cardio-Thoracic-Vascular Department, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
No funding was received.
Conflict of interest: Michele De Bonis is a co-founder, CMO and Stock shareholder of StarTric srl; other authors reported no disclosures related to the present manuscript. Paolo Denti served as Consultant for InnovHeart, Picardia, HVR, Approxima and received Speaker Honoraria from Abbott and Edwards. Francesco Maisano received Grant and/or Research Institutional Support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, Venus and Consulting fees, Honoraria personal and Institutional from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Mtex, Venus, Squadra, Valgen Royalty Income/IP Rights Edwards Lifesciences and is shareholder (including share options) of Magenta, Transseptalsolutions, 4Tech.
DATA AVAILABILITY
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Author contributions
Francesco Ancona, MD: Conceptualization; Writing—original draft. Matteo Bellettini: Conceptualization; Formal analysis; Writing—original draft. Giovanni Polizzi: Data curation; Writing—original draft. Gabriele Paci: Data curation. Davide Margonato: Data curation; Supervision; Writing—review & editing. Giacomo Ingallina: Formal analysis; Methodology; Supervision; Writing—review & editing. Stefano Stella: Supervision. Giorgio Fiore: Supervision. Annamaria Tavernese: Supervision. Martina Belli: Supervision. Federico Biondi: Supervision. Alessandro Castiglioni: Visualization. Paolo Denti: Supervision. Nicola Buzzatti: Visualization. Gaetano Maria De Ferrari: Supervision. Ottavio Alfieri: Supervision. Elisabetta Lapenna: Supervision. Michele De Bonis: Supervision. Francesco Maisano: Supervision. Eustachio Agricola: Supervision; Writing—review & editing
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Amedeo Anselmi, Mateo Marin-Cuartas, George Gradinariu and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Nath J, Foster E, Heidenreich PA.. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43:405–9. doi: 10.1016/j.jacc.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 2. Wang TKM, Mentias A, Akyuz K, Kirincich J, Crane A, Popovic Z. et al. Effect of tricuspid valve repair or replacement on survival in patients with isolated severe tricuspid regurgitation. Am J Cardiol 2022;162:163–9. doi: 10.1016/j.amjcard.2021.08.069 [DOI] [PubMed] [Google Scholar]
- 3. Prihadi EA, van der Bijl P, Gursoy E, Abou R, Mara Vollema E, Hahn R et al Development of significant tricuspid regurgitation over time and prognostic implications: new insights into natural history. Eur Heart J 2018;39:3574–81. doi: 10.1093/eurheartj/ehy352 [DOI] [PubMed] [Google Scholar]
- 4. Taramasso M, Gavazzoni M, Pozzoli A, Dreyfus G, Bolling S, George I et al Tricuspid regurgitation. JACC Cardiovasc Imaging 2019;12:605–21. doi: 10.1016/j.jcmg.2018.11.034 [DOI] [PubMed] [Google Scholar]
- 5. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 6. Dreyfus J, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E. et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304–17. doi: 10.1093/eurheartj/ehaa643 [DOI] [PubMed] [Google Scholar]
- 7. Kim JB, Jung S-H, Choo SJ, Chung CH, Lee JW.. Surgical outcomes of severe tricuspid regurgitation: predictors of adverse clinical outcomes. Heart 2013;99:181–7. doi: 10.1136/heartjnl-2012-302856 [DOI] [PubMed] [Google Scholar]
- 8. Kim Y-J, Kwon D-A, Kim H-K, Park J, Hahn S, Kim K. et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 2009;120:1672–8. doi: 10.1161/CIRCULATIONAHA.109.849448 [DOI] [PubMed] [Google Scholar]
- 9. Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie-Badie Y, Bouchery M. et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J 2022;43:654–62. doi: 10.1093/eurheartj/ehab679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombo A, Maisano F.. A new tool for the forgotten valve: a score to predict the risk of surgery. Eur Heart J 2022;43:663–5. doi: 10.1093/eurheartj/ehab799 [DOI] [PubMed] [Google Scholar]
- 11. Badano LP, Kolias TJ, Muraru D, Abraham T, Aurigemma G, Edvardsen T. et al. ; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2018;19:591–600. doi: 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 12. Prihadi EA, van der Bijl P, Dietz M, Abou R, Vollema E, Marsan N. et al. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging 2019;12:e008666. doi: 10.1161/CIRCIMAGING.118.008666 [DOI] [PubMed] [Google Scholar]
- 13. Ancona F, Melillo F, Calvo F, Attalla El Halabieh N, Stella S, Capogrosso C. et al. Right ventricular systolic function in severe tricuspid regurgitation: prognostic relevance of longitudinal strain. Eur Heart J Cardiovasc Imaging 2021;22:868–75. doi: 10.1093/ehjci/jeab030 [DOI] [PubMed] [Google Scholar]
- 14. Romano S, Dell'atti D, Judd RM, Kim RJ, Weinsaft JW, Kim J. et al. Prognostic value of feature-tracking right ventricular longitudinal strain in severe functional tricuspid regurgitation. JACC Cardiovasc Imaging 2021;14:1561–8. doi: 10.1016/j.jcmg.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn P. et al. Recommendations for noninvasive evaluation of native valvular regurgitation. J Am Soc Echocardiogr 2017;30:303–71. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 17. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R et al; Scientific Document Committee of the European Association of Cardiovascular Imaging. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging 2022;23:e171–232. doi: 10.1093/ehjci/jeab253 [DOI] [PubMed] [Google Scholar]
- 18. Hahn RT, Zamorano JL.. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging 2017;18:1342–3. doi: 10.1093/ehjci/jex139 [DOI] [PubMed] [Google Scholar]
- 19. Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P. et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ Cardiovasc Imaging 2016;9:e003866. doi: 10.1161/CIRCIMAGING.115.003866 [DOI] [PubMed] [Google Scholar]
- 20. Fortuni F, Butcher SC, Dietz MF, van der Bijl P, Prihadi E, De Ferrari G. et al. Right ventricular–pulmonary arterial coupling in secondary tricuspid regurgitation. Am J Cardiol 2021;148:138–45. doi: 10.1016/j.amjcard.2021.02.037 [DOI] [PubMed] [Google Scholar]
- 21. Ünlü S, Bézy S, Cvijic M, Duchenne J, Delcroix M, Voigt J-U.. Right ventricular strain related to pulmonary artery pressure predicts clinical outcome in patients with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 2023;24:635–42. doi: 10.1093/ehjci/jeac136 [DOI] [PubMed] [Google Scholar]
- 22. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher M, Chandrasekaran K. et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786–8. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Färber G, Marx J, Scherag A, Saqer I, Diab M, Sponholz C. et al. Risk stratification for isolated tricuspid valve surgery assisted using the Model for End-Stage Liver Disease score. J Thorac Cardiovasc Surg 2023;166:1433–41.e1. doi: 10.1016/j.jtcvs.2021.11.102 [DOI] [PubMed] [Google Scholar]
- 24. Russo M, Di Mauro M, Saitto G, Lio A, Berretta P, Taramasso M. et al. Outcome of patients undergoing isolated tricuspid repair or replacement surgery. Eur J Cardiothorac Surg 2022;62(3):ezac230. doi: 10.1093/ejcts/ezac230 [DOI] [PubMed] [Google Scholar]
- 25. Kundi H, Popma JJ, Cohen DJ, Liu D, Laham R, Pinto D. et al. Prevalence and outcomes of isolated tricuspid valve surgery among Medicare beneficiaries. Am J Cardiol 2019;123:132–8. doi: 10.1016/j.amjcard.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 26. Carrascal Y, Segura B, Sánchez C, Velasco E.. Delay of surgical treatment of severe tricuspid regurgitation and outcomes in patients with left-sided heart valve disease. Rev Esp Cardiol (Engl Ed) 2023;76:453–9. doi: 10.1016/j.rec.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 27. Sala A, Lorusso R, Bargagna M, Ascione G, Ruggeri S, Meneghin R. et al. Isolated tricuspid valve surgery: first outcomes report according to a novel clinical and functional staging of tricuspid regurgitation. Eur J Cardiothorac Surg 2021;60:1124–30. doi: 10.1093/ejcts/ezab228 [DOI] [PubMed] [Google Scholar]
- 28. Nishiura N, Kitai T, Okada T, Sano M, Miyawaki N, Kim K. et al. Long-term clinical outcomes in patients with severe tricuspid regurgitation. J Am Heart Assoc 2023;12:e025751. doi: 10.1161/JAHA.122.025751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Y, Liu Y, Seto W, Wu M, Yu Y, Lam Y. et al. Prognostic value of hepatorenal function by modified model for end-stage liver disease (MELD) score in patients undergoing tricuspid annuloplasty. J Am Heart Assoc 2018;7(14):e009020. doi: 10.1161/JAHA.118.009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M. et al. Two-dimensional strain–a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 31. Galloo X, Stassen J, Butcher SC, Meucci M, Dietz M, Mertens B. et al. Staging right heart failure in patients with tricuspid regurgitation undergoing tricuspid surgery. Eur J Cardiothorac Surg 2022;62(2):ezac290. doi: 10.1093/ejcts/ezac290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subbotina I, Girdauskas E, Bernhardt A, Sinning C, Reichenspurner H, Sill B.. Comparison of outcomes of tricuspid valve surgery in patients with reduced and normal right ventricular function. Thorac Cardiovasc Surg 2017;65:617–25. doi: 10.1055/s-0037-1604450 [DOI] [PubMed] [Google Scholar]
- 33. Dietz MF, Prihadi EA, van der Bijl P, Goedemans L, Mertens B, Gursoy E et al Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation 2019;140:836–45. doi: 10.1161/CIRCULATIONAHA.119.039630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author on reasonable request.







