Abstract
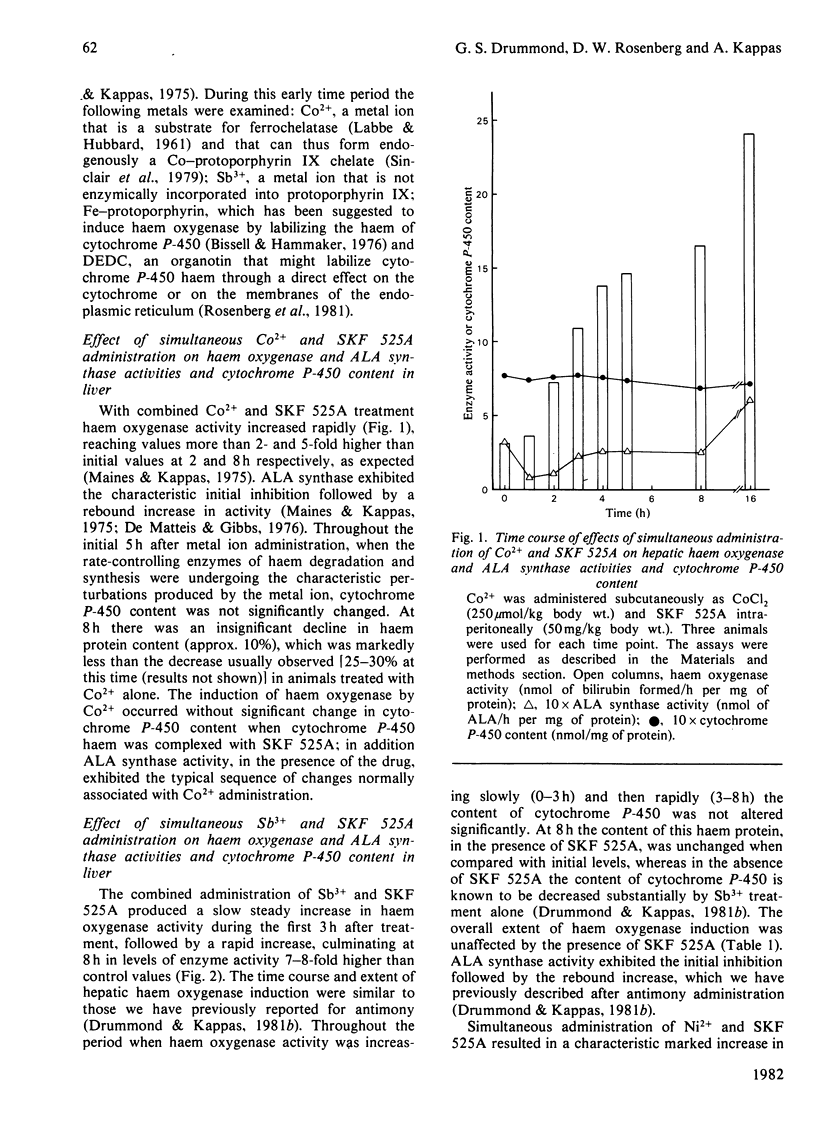
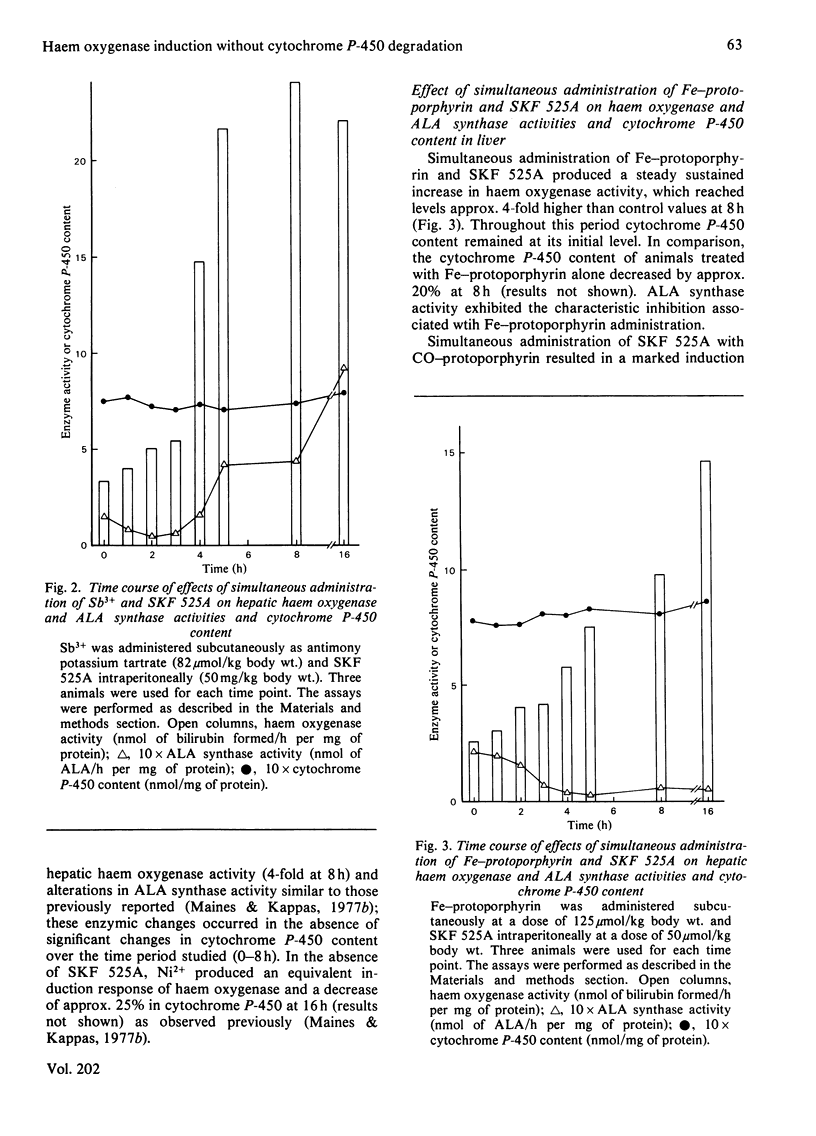
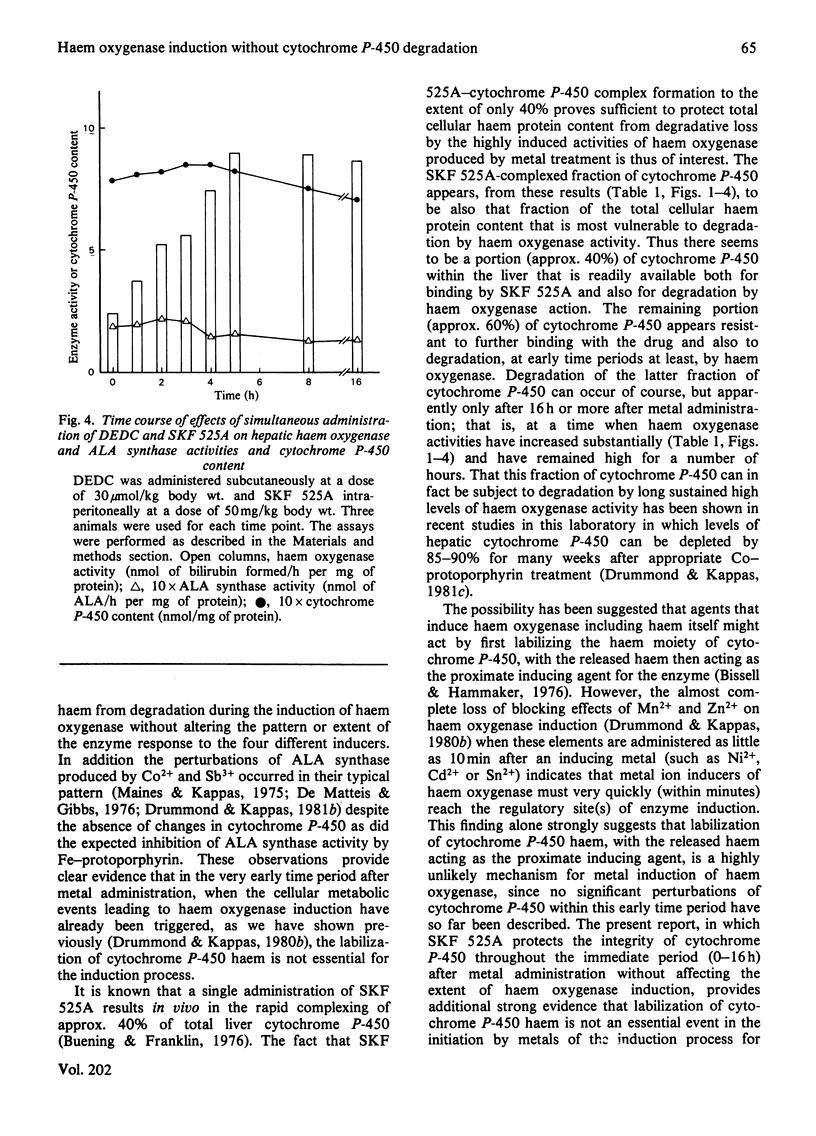
The induction of hepatic haem oxygenase (EC 1.14.99.3) by a series of metals, organometals and metalloporphyrins was examined in vivo in the presence of compound SKF 525A, which is known to complex with the prosthetic group of cytochrome P-450. Concurrent administration of SKF 525A and an inducing metal did not affect the extent and time course of haem oxygenase induction. The decrease in cytochrome P-450 content normally associated with metal administration was, however, prevented, indicating that haem oxygenase induction by metals can proceed without the significant labilization of the haem moiety of cytochrome P-450. In addition, the integrity of this haem protein can be maintained by chemical means in the presence of sustained high activities of haem oxygenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell D. M., Hammaker L. E. Cytochrome P-450 heme and the regulation of hepatic heme oxygenase activity. Arch Biochem Biophys. 1976 Sep;176(1):91–102. doi: 10.1016/0003-9861(76)90144-2. [DOI] [PubMed] [Google Scholar]
- Buening M. K., Franklin M. R. SKF 525-A inhibition, induction, and 452-nm complex formation. Drug Metab Dispos. 1976 May-Jun;4(3):244–255. [PubMed] [Google Scholar]
- De Matteis F., Unseld A. Increased liver haem degradation caused by foreign chemicals: a comparison of the effects of 2-allyl-2-isopropylacetamide and cobaltous chloride. Biochem Soc Trans. 1976;4(2):205–209. doi: 10.1042/bst0040205. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Metal ion interactions in the control of haem oxygenase induction in liver and kidney. Biochem J. 1980 Nov 15;192(2):637–648. doi: 10.1042/bj1920637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Potent heme-degrading action of antimony and antimony-containing parasiticidal agents. J Exp Med. 1981 Feb 1;153(2):245–256. doi: 10.1084/jem.153.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Metal specificity of the ironprotoporphyrin chelating enzyme from rat liver. Biochim Biophys Acta. 1961 Sep 2;52:130–135. doi: 10.1016/0006-3002(61)90910-6. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: blockade of metal effects by thiols. Proc Natl Acad Sci U S A. 1977 May;74(5):1875–1878. doi: 10.1073/pnas.74.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Rosenberg D. W., Drummond G. S., Cornish H. C., Kappas A. Prolonged induction of hepatic haem oxygenase and decreases in cytochrome P-450 content by organotin compounds. Biochem J. 1980 Aug 15;190(2):465–468. doi: 10.1042/bj1900465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Kappas A., Bernstein S. E., Alvares A. P. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J Biol Chem. 1979 Feb 10;254(3):729–735. [PubMed] [Google Scholar]
- Schenkman J. B., Wilson B. J., Cinti D. L. Dimethylaminoethyl 2,2-diphenylvalerate HCl (SKF 525-A)--in vivo and in vitro effects of metabolism by rat liver microsomes--formation of an oxygenated complex. Biochem Pharmacol. 1972 Sep 1;21(17):2373–2383. doi: 10.1016/0006-2952(72)90389-9. [DOI] [PubMed] [Google Scholar]
- Sinclair P., Gibbs A. H., Sinclair J. F., de Matteis F. Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem J. 1979 Mar 15;178(3):529–538. doi: 10.1042/bj1780529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]



