Abstract
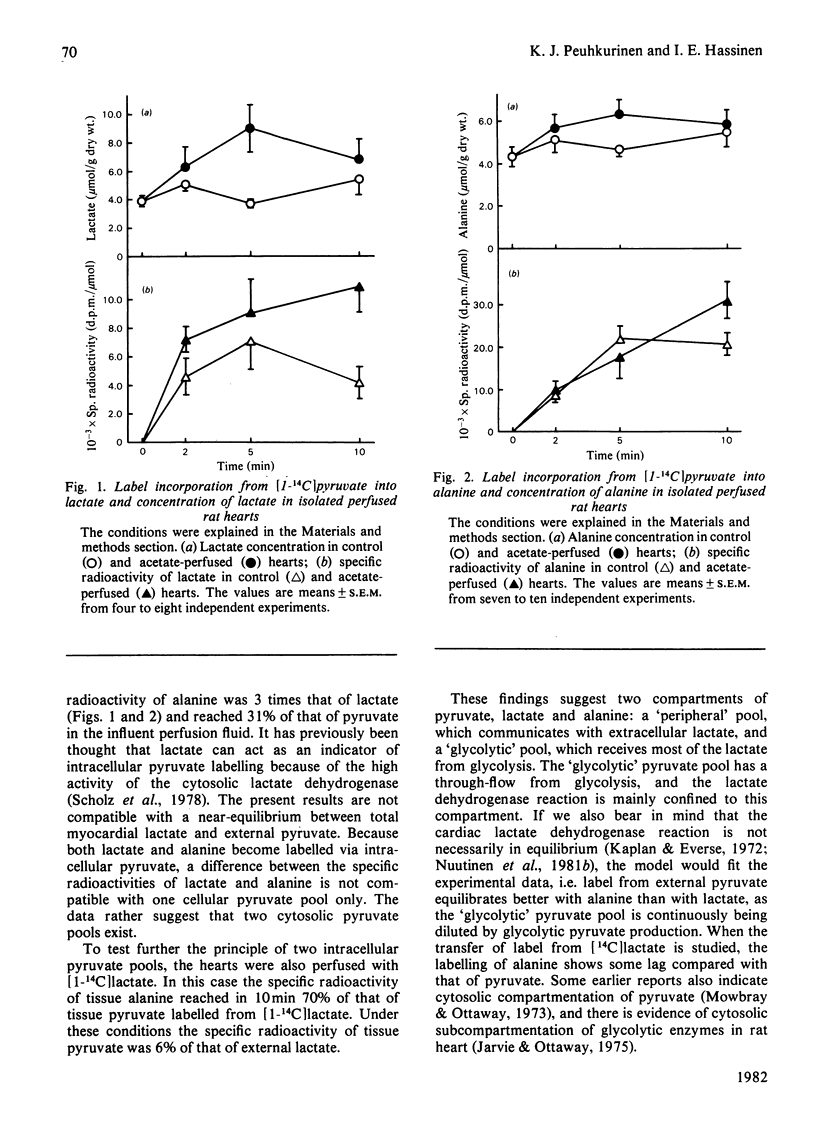
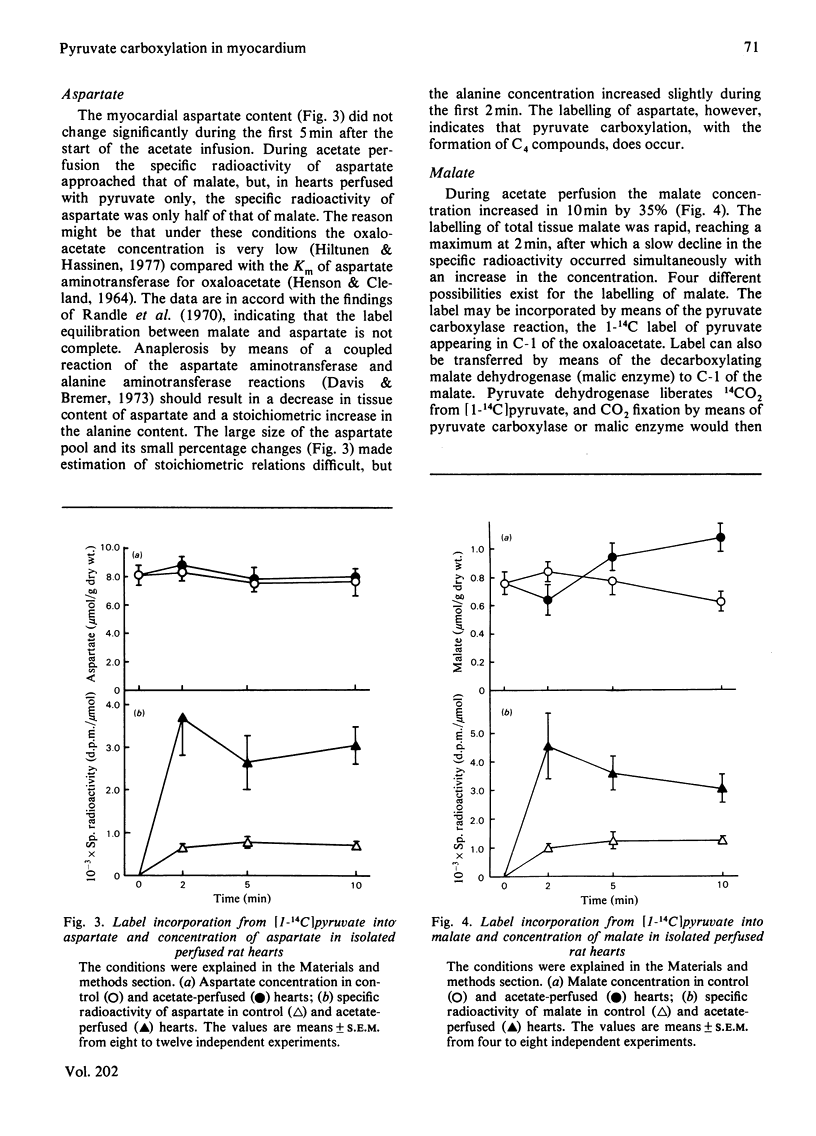
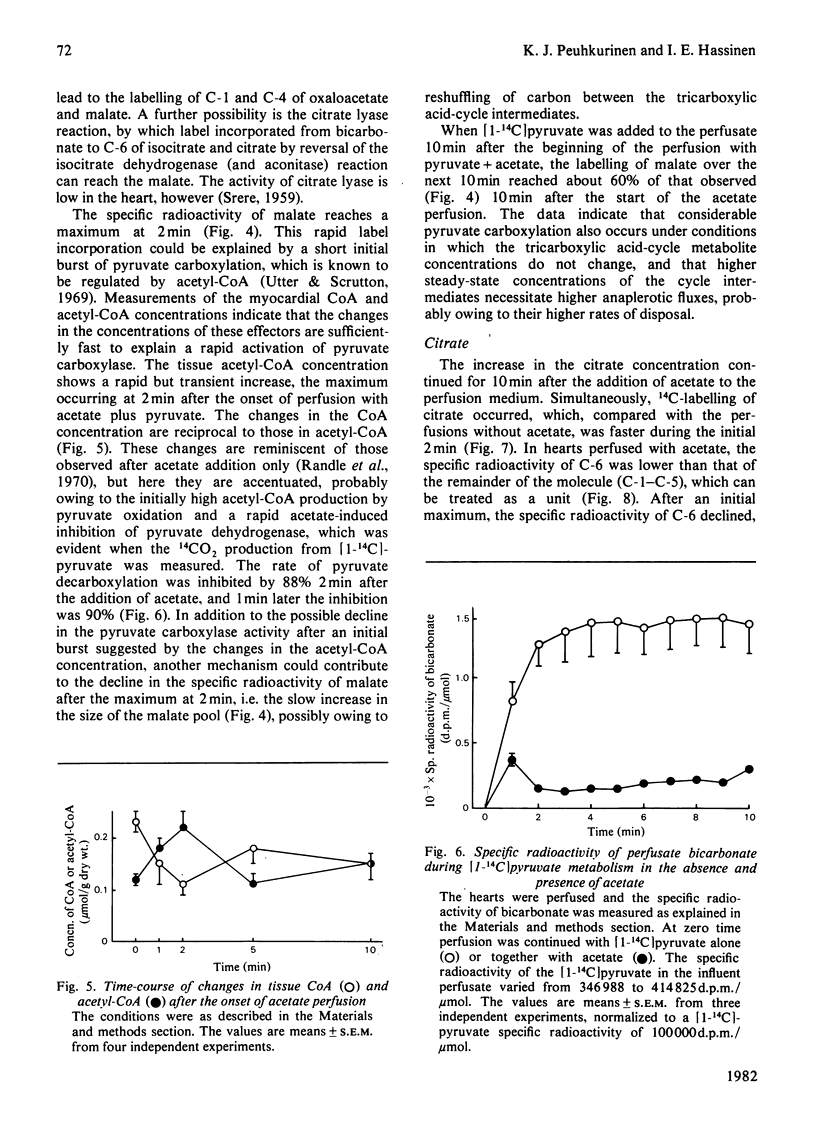
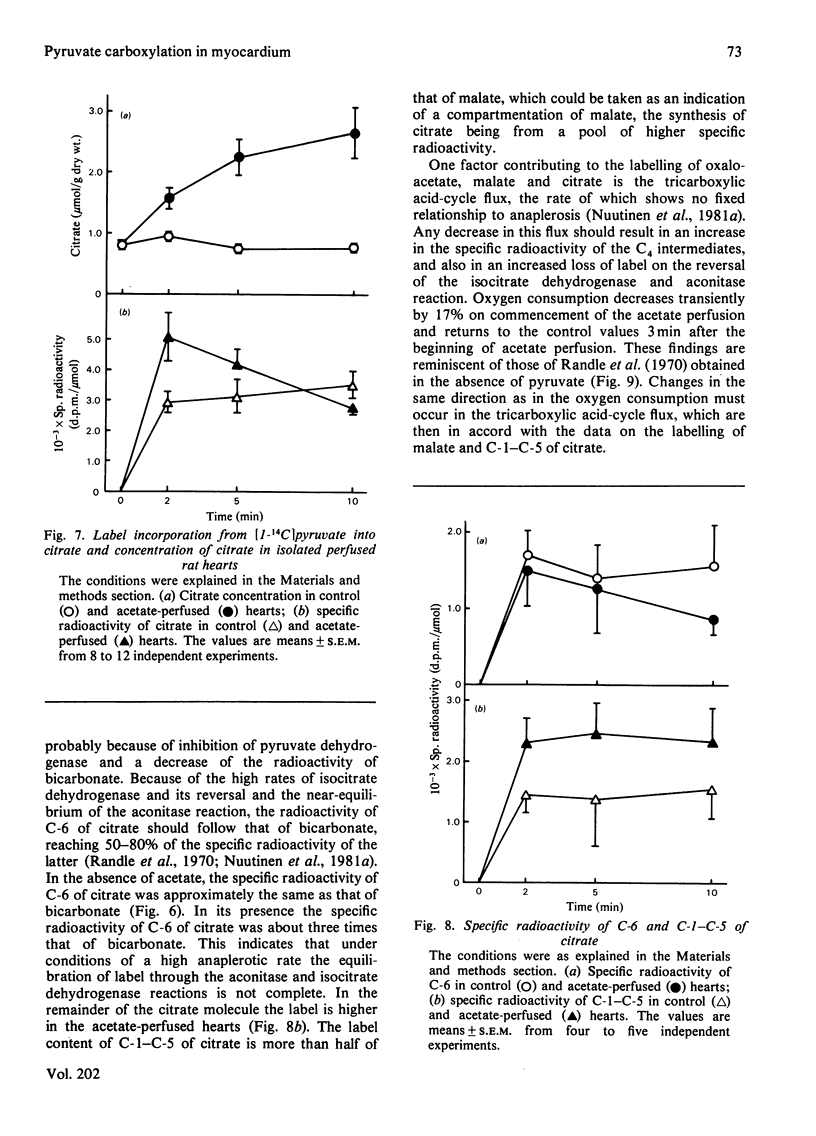
1. The role of pyruvate carboxylation in the net synthesis of tricarboxylic acid-cycle intermediates during acetate metabolism was studied in isolated rat hearts perfused with [1-14C]pyruvate. 2. The incorporation of the 14C label from [1-14C]pyruvate into the tricarboxylic acid-cycle intermediates points to a carbon input from pyruvate via enzymes in addition to pyruvate dehydrogenase and citrate synthase. 3. On addition of acetate, the specific radioactivity of citrate showed an initial maximum at 2 min, with a subsequent decline in labelling. The C-6 of citrate (which is removed in the isocitrate dehydrogenase reaction) and the remainder of the molecule showed differential labelling kinetics, the specific radioactivity of C-6 declining more rapidly. Since this carbon is lost in the isocitrate dehydrogenase reaction, the results are consistent with a rapid inactivation of pyruvate dehydrogenase after the addition of acetate, which was confirmed by measuring the 14CO2 production from [1-14C]pyruvate. 4. The results can be interpreted to show that carboxylation of pyruvate to the C4 compounds of the tricarboxylic acid cycle occurs under conditions necessitating anaplerosis in rat myocardium, although the results do not identify the enzyme involved. 5. The specific radioactivity of tissue lactate was too low to allow it to be used as an indicator of the specific radioactivity of the intracellular pyruvate pool. The specific radioactivity of alanine was three times that of lactate. When the hearts were perfused with [1-14C]lactate, the specific radioactivity of alanine was 70% of that of pyruvate. The results suggest that a subcompartmentation of lactate and pyruvate occurs in the cytosol.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta. 1971 Feb 2;231(1):32–47. doi: 10.1016/0005-2760(71)90253-0. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Bowman R. H. Effects of diabetes, fatty acids, and ketone bodies on tricarboxylic acid cycle metabolism in the perfused rat heart. J Biol Chem. 1966 Jul 10;241(13):3041–3048. [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- DAVIS E. J., QUASTEL J. H. THE EFFECTS OF SHORT-CHAIN FATTY ACIDS AND STARVATION ON THE METABOLISM OF GLUCOSE AND LACTATE BY THE PERFUSED GUINEA PIG HEART. Can J Biochem. 1964 Nov;42:1605–1621. doi: 10.1139/o64-172. [DOI] [PubMed] [Google Scholar]
- Davis E. J., Bremer J. Studies with isolated surviving rat hearts. Interdependence of free amino acids and citric-acid-cycle intermediates. Eur J Biochem. 1973 Sep 21;38(1):86–97. doi: 10.1111/j.1432-1033.1973.tb03037.x. [DOI] [PubMed] [Google Scholar]
- Davis E. J., Spydevold O., Bremer J. Pyruvate carboxylase and propionyl-CoA carboxylase as anaplerotic enzymes in skeletal muscle mitochondria. Eur J Biochem. 1980 Sep;110(1):255–262. doi: 10.1111/j.1432-1033.1980.tb04863.x. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., Ljunggren B., Norberg K., Siesjö B. K. Influence of complete ischemia on glycolytic metabolites, citric acid cycle intermediates, and associated amino acids in the rat cerebral cortex. Brain Res. 1974 Nov 15;80(2):265–279. doi: 10.1016/0006-8993(74)90690-8. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K. Metabolic effects of pent-4-enoate in isolated perfused rat heart. Biochem J. 1978 Feb 15;170(2):241–247. doi: 10.1042/bj1700241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvie D. R., Ottaway J. H. Localization in cardiac muscle of some enzymes related to glutamate metabolism. Histochem J. 1975 Mar;7(2):165–178. doi: 10.1007/BF01004560. [DOI] [PubMed] [Google Scholar]
- Kaplan N. O., Everse J. Regulatory characteristics of lactate dehydrogenases. Adv Enzyme Regul. 1972;10:323–336. doi: 10.1016/0065-2571(72)90021-0. [DOI] [PubMed] [Google Scholar]
- Kinnula V. L., Hassinen I. Effect of hypoxia on mitochondrial mass and cytochrome concentrations in rat heart and liver during postnatal development. Acta Physiol Scand. 1977 Apr;99(4):462–466. doi: 10.1111/j.1748-1716.1977.tb10399.x. [DOI] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Lin R. C., Davis E. J. Malic enzymes of rabbit heart mitochondria. Separation and comparison of some characteristics of a nicotinamide adenine dinucleotide-preferring and a nicotinamide adenine dinucleotide phosphate-specific enzyme. J Biol Chem. 1974 Jun 25;249(12):3867–3875. [PubMed] [Google Scholar]
- MORRISON J. F. The activation of aconitase by ferrous ions and reducing agents. Biochem J. 1954 Dec;58(4):685–692. doi: 10.1042/bj0580685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The effect of insulin and growth hormone on the flux of tracer from labelled lactate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):369–379. doi: 10.1111/j.1432-1033.1973.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Nagel W. O., Dauchy R. T., Sauer L. A. Mitochondrial malic enzymes. An association between NAD(P)+-dependent malic enzyme and cell renewal in Sprague-Dawley rat tissues. J Biol Chem. 1980 May 10;255(9):3849–3854. [PubMed] [Google Scholar]
- Opie L. H., Owen P. Effects of increased mechanical work by isolated perfused rat heart during production or uptake of ketone bodies. Assessment of mitochondrial oxidized to reduced free nicotinamide-adenine dinucleotide ratios and oxaloacetate concentrations. Biochem J. 1975 Jun;148(3):403–415. doi: 10.1042/bj1480403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Safer B., Williamson J. R. Mitochondrial-cytosolic interactions in perfused rat heart. Role of coupled transamination in repletion of citric acid cycle intermediates. J Biol Chem. 1973 Apr 10;248(7):2570–2579. [PubMed] [Google Scholar]
- Scholz R., Olson M. S., Schwab A. J., Schwabe U., Noell C., Braun W. The effect of fatty acids on the regulation of pyruvate dehydrogenase in perfused rat liver. Eur J Biochem. 1978 May 16;86(2):519–530. doi: 10.1111/j.1432-1033.1978.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Singer T. P., Kearney E. B., Kenney W. C. Succinate dehydrogenase. Adv Enzymol Relat Areas Mol Biol. 1973;37:189–272. doi: 10.1002/9780470122822.ch4. [DOI] [PubMed] [Google Scholar]
- Spydevold S., Davis E. J., Bremer J. Replenishment and depletion of citric acid cycle intermediates in skeletal muscle. Indication of pyruvate carboxylation. Eur J Biochem. 1976 Dec;71(1):155–165. doi: 10.1111/j.1432-1033.1976.tb11101.x. [DOI] [PubMed] [Google Scholar]
- Takala T. Protein synthesis in the isolated perfused rat heart. Effects of mechanical work load, diastolic ventricular pressure and coronary pressure on amino acid incorporation and its transmural distribution into left ventricular protein. Basic Res Cardiol. 1981 Jan-Feb;76(1):44–61. doi: 10.1007/BF01908162. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]



