Abstract
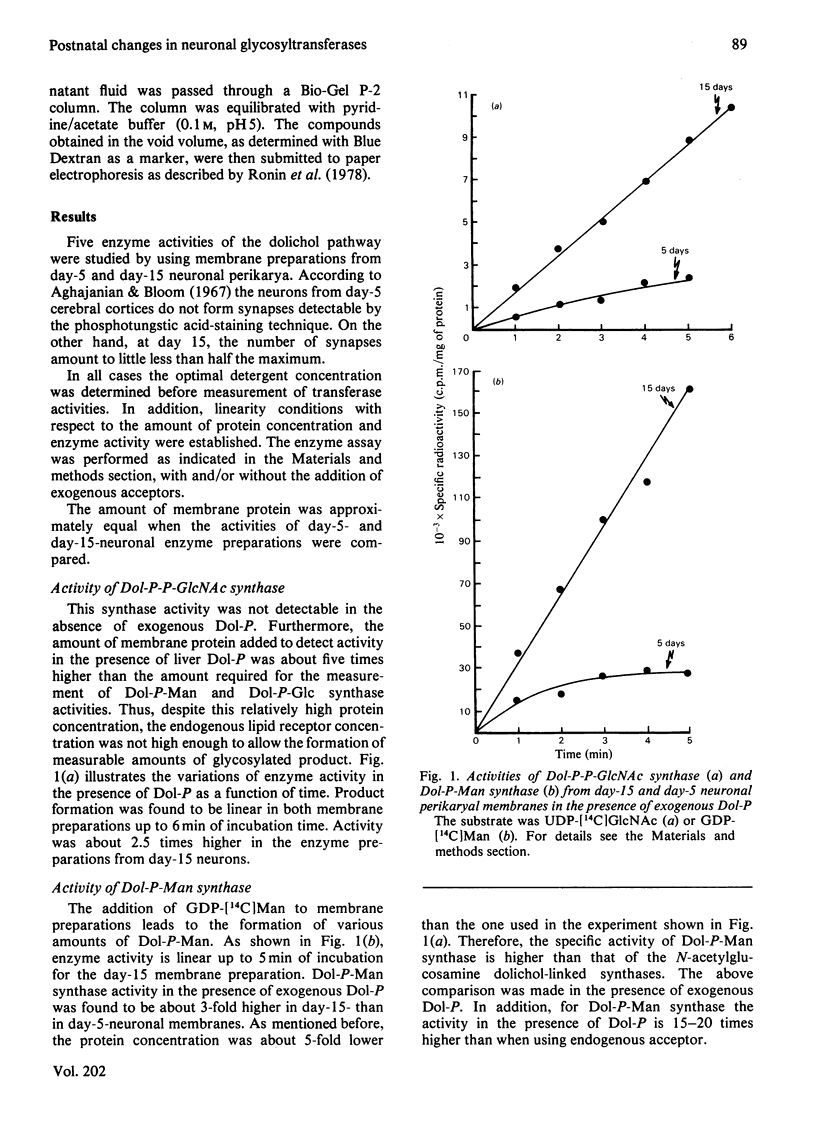
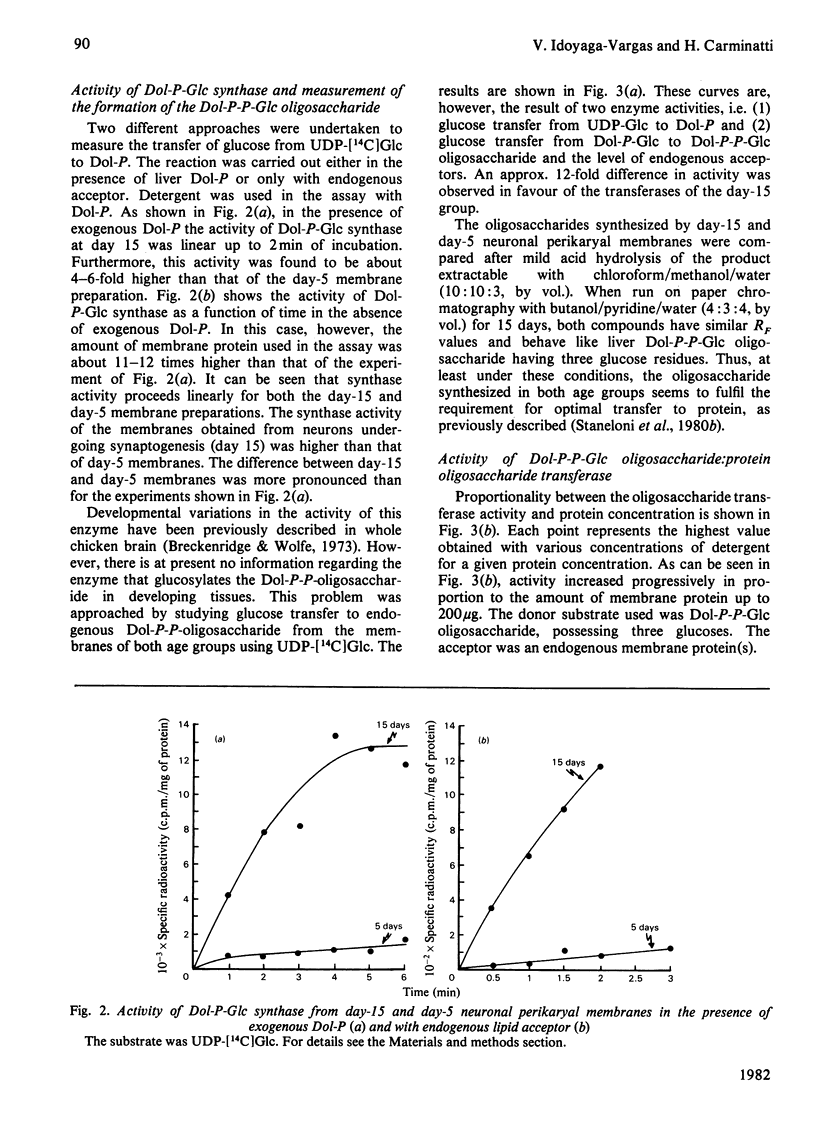
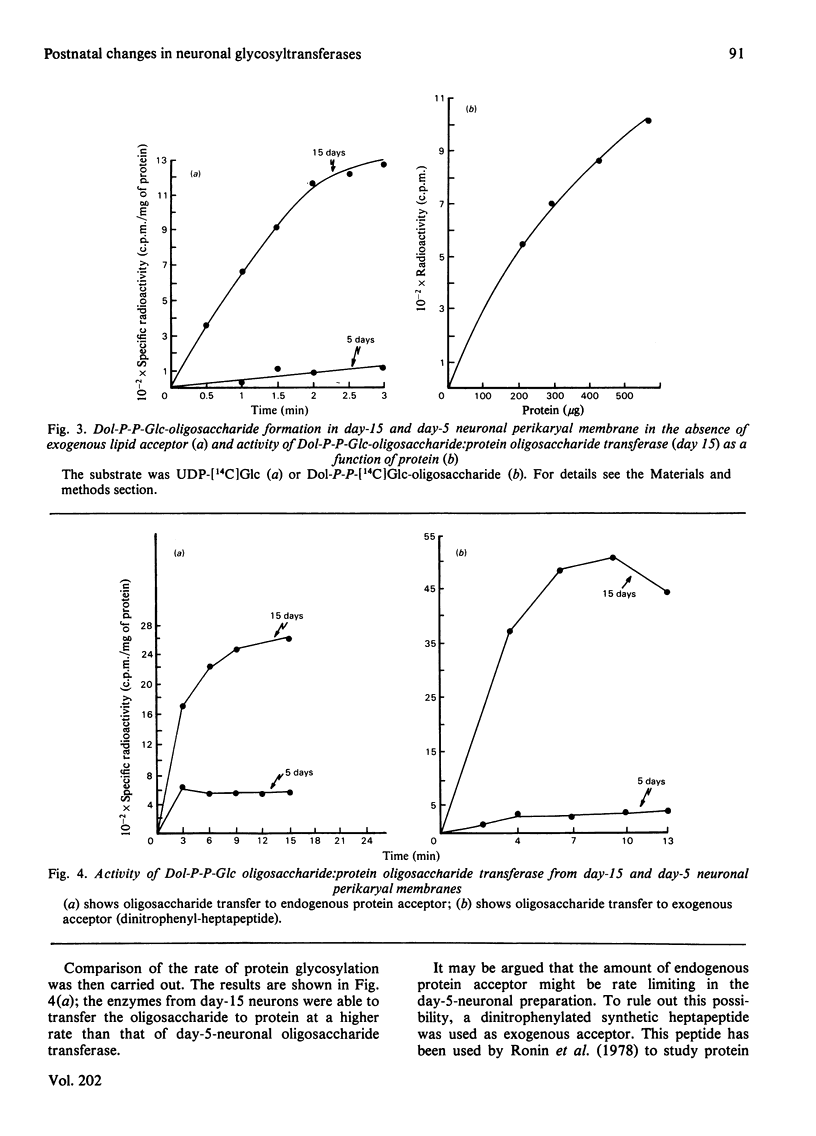
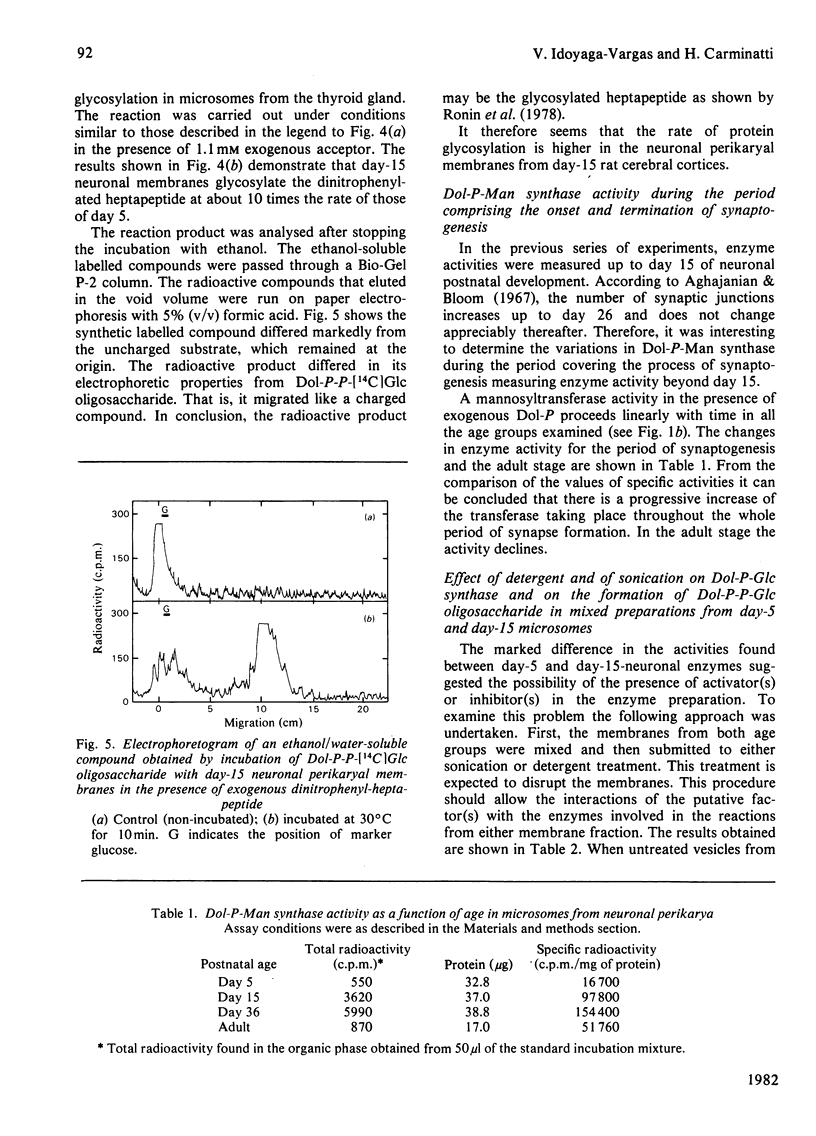
Neuronal perikarya were isolated from rat cerebral cortex at different stages of postnatal development. Membranes sedimenting at 100000 g were obtained from these neurons to study several glycosyltransferases of the dolichol pathway. Enzyme activities from stages before and during synapse formation were compared (days 5 and 15 respectively). Dolichyl diphosphate (Dol-P-P) N-acetylglucosamine, dolichyl phosphate mannose and dolichyl phosphate glucose synthases and the enzymes catalysing Dol-P-P-GlcNAc2Man9Glc3 formation were higher at day 15 of postnatal development. The glycosyl transfer of the latter compound to endogenous protein(s) as well as to a dinitrophenyl-heptapeptide was also measured. The activity was higher at day 15. Furthermore, the activity of dolichyl phosphate mannose synthase was also measured during the time when the number of synapses ceased to increase (day 36) and in the adult stage. The activity of dolichyl phosphate mannose synthase was higher at day 36 than at day 15, and declined in the adult stage. From these results it may be concluded that there is an increase in the glycosylation of asparagine-type glycoproteins during synapse formation in the neurons of the cerebral cortex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967 Dec;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Behrens N. H., Carminatti H., Staneloni R. J., Leloir L. F., Cantarella A. I. Formation of lipid-bound oligosaccharides containing mannose. Their role in glycoprotein synthesis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3390–3394. doi: 10.1073/pnas.70.12.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge W. C., Wolfe L. S. The effect of dolichol phosphate on the synthesis of lipid bound sugars in embryonic chick brain. FEBS Lett. 1973 Jan 1;29(1):66–68. doi: 10.1016/0014-5793(73)80017-1. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. An electron microscopic study of neurons during postnatal development of the rat cerebral cortex. J Comp Neurol. 1968 May;133(1):17–44. doi: 10.1002/cne.901330103. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto K., Kornfeld S. The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980 May 25;255(10):4441–4446. [PubMed] [Google Scholar]
- Chapman A., Trowbridge I. S., Hyman R., Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979 Jul;17(3):509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- Harford J. B., Waechter C. J. A developmental change in dolichyl phosphate mannose synthase activity in pig brain. Biochem J. 1980 May 15;188(2):481–490. doi: 10.1042/bj1880481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford J. B., Waechter C. J., Saul R., DeVries G. H. Evidence for the biosynthesis of mannosylphosphoryldolichol and N-acetylglucosaminylpyrophosphoryldolichol by an axolemma-enriched membrane preparation from bovine white matter. J Neurochem. 1979 Jan;32(1):91–98. doi: 10.1111/j.1471-4159.1979.tb04514.x. [DOI] [PubMed] [Google Scholar]
- Hemming F. W. Dolichol phosphate, a coenzyme in the glycosylation of animal membrane-bound glycoproteins. Biochem Soc Trans. 1977;5(4):1223–1231. doi: 10.1042/bst0051223. [DOI] [PubMed] [Google Scholar]
- Idoyaga Vargas V., Carminatti H. Glycosylation of endogenous protein(s) of the rough and smooth microsomes by a lipid sugar intermediate. Mol Cell Biochem. 1977 Jul 5;16(2):171–176. doi: 10.1007/BF01732058. [DOI] [PubMed] [Google Scholar]
- James M. J., Kandutsch A. A. Regulation of hepatic dolichol synthesis by beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1980 Sep 25;255(18):8618–8622. [PubMed] [Google Scholar]
- Kean E. L. Stimulation by GDP-mannose of the biosynthesis of N-acetylglucosaminylpyrophosphoryl polyprenols by the retina. J Biol Chem. 1980 Mar 10;255(5):1921–1927. [PubMed] [Google Scholar]
- Krusius T., Finne J., Margolis R. U., Margolis R. K. Structural features of microsomal, synaptosomal, mitochondrial, and soluble glycoproteins of brain. Biochemistry. 1978 Sep 5;17(18):3849–3854. doi: 10.1021/bi00611a026. [DOI] [PubMed] [Google Scholar]
- Margolis R. K., Preti C., Lai D., Margolis R. U. Developmental changes in brain glycoproteins. Brain Res. 1976 Aug 13;112(2):363–369. doi: 10.1016/0006-8993(76)90290-0. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Ronin C., Granier C., van Rietschoten J., Bouchilloux S. Enzymatic transfer of oligosaccharide from oligosaccharide-lipids to an Asn-Ala-Thr containing heptapeptide. Biochem Biophys Res Commun. 1978 Apr 14;81(3):772–778. doi: 10.1016/0006-291x(78)91418-3. [DOI] [PubMed] [Google Scholar]
- Sellinger O. Z., Azcurra J. M., Johnson D. E., Ohlsson W. G., Lodin Z. Independence of protein synthesis and drug uptake in nerve cell bodies and glial cells isolated by a new technique. Nat New Biol. 1971 Apr 21;230(16):253–256. doi: 10.1038/newbio230253a0. [DOI] [PubMed] [Google Scholar]
- Sellinger O. Z., Johnson D. E., Santiago J. C., Idoyaga-Vargas V. A study of the biochemical differentiation of neurons and glia in the rat cerebral cortex. Prog Brain Res. 1973;40(0):331–347. doi: 10.1016/S0079-6123(08)60698-6. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Ugalde R. A., Leloir L. F. Addition of glucose to dolichyl diphosphate oligosaccharide and transfer to protein. Eur J Biochem. 1980 Apr;105(2):275–278. doi: 10.1111/j.1432-1033.1980.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Harford J. B. Evidence for the enzymatic transfer of N-acetylglucosamine from UDP--N-acetylglucosamine into dolichol derivative and glycoproteins by calf brain membranes. Arch Biochem Biophys. 1977 May;181(1):185–198. doi: 10.1016/0003-9861(77)90497-0. [DOI] [PubMed] [Google Scholar]



