Abstract
Merkel cell–neurite complexes (MNCs) are enriched in touch-sensitive areas, including whisker hair follicles and the glabrous skin of the rodent's paws, where tactile stimulation elicits slowly adapting type 1 (SA1) tactile impulses to encode for the sense of touch. Recently, we have shown with rodent whisker hair follicles that SA1 impulses are generated through fast excitatory synaptic transmission at MNCs and driven by acid-sensing ion channels (ASICs). However, it is currently unknown whether, besides whisker hair follicles, ASICs also play an essential role in generating SA1 impulses from MNCs of other body parts in mammals. In the present study, we attempted to address this question by using the skin–nerve preparations made from the hindpaw glabrous skin and tibial nerves of both male and female rodents and applying the pressure-clamped single–fiber recordings. We showed that SA1 impulses elicited by tactile stimulation to the rat hindpaw glabrous skin were largely diminished in the presence of amiloride and diminazene, two ASIC channel blockers. Furthermore, using the hindpaw glabrous skin and tibial nerve preparations made from the mice genetically deleted of ASIC3 channels (ASIC3−/−), we showed that the frequency of SA1 impulses was significantly lower in ASIC3−/− mice than in littermate wild-type ASIC3+/+ mice, a result consistent with the pharmacological experiments with ASIC channel blockers. Our findings suggest that ASIC channels are essential for generating SA1 impulses to underlie the sense of touch in the glabrous skin of rodent hindpaws.
Keywords: acid-sensing ion channel, low threshold mechanoreceptor, Merkel cell–neurite complex, Piezo2 channel, sense of touch, slowly adapting type 1 response
Significance Statement
Merkel cell–neurite complexes (MNCs) are enriched in touch-sensitive areas, including whisker hair follicles and the glabrous skin of the rodent's paws, where tactile stimulation elicits slowly adapting type 1 (SA1) tactile impulses to encode for the sense of touch. Here, using the skin–nerve preparations made from the hindpaw glabrous skin and tibial nerves of rodents and applying the pressure-clamped single–fiber recordings, we have demonstrated that ASIC channels are essential for generating SA1 impulses at MNCs in the glabrous skin of rodent hindpaws. Thus, ASIC channels at MNCs may play a key role in the sense of touch to the skin of mammals.
Introduction
The sense of touch is essential for sensory tasks in daily life, including environmental exploration, social interaction, and tactile discrimination. These sensory tasks are performed mainly by tactile end organs, such as Merkel discs (Merkel, 1875; Iggo and Muir, 1969; Halata et al., 2003), Meissner's corpuscles, Ruffini corpuscles, and Pacinian corpuscles in the skin (Johnson, 2001; Handler and Ginty, 2021). Merkel discs, also known as Merkel cell–neurite complexes (MNCs; Merkel, 1875; Iggo and Muir, 1969; Johnson, 2001; Halata et al., 2003; Handler and Ginty, 2021), consist of Merkel cells and their associated Aβ-afferent neurites (Iggo and Muir, 1969; Halata et al., 2003; Handler and Ginty, 2021). They are located throughout the skin layers of the body and are most abundant in whisker hair follicles of nonprimates and fingertips of primates (Iggo and Muir, 1969; Carvell and Simons, 1990; Ebara et al., 2002; Prigg et al., 2002; Zimmerman et al., 2014). Tactile stimuli to MNCs, such as skin indentation, pressure, and whisker hair movement, elicit slowly adapting type 1 (SA1) impulses of action potentials (APs) on Aβ-afferent fibers. SA1 impulses are characteristic tactile responses (Iggo and Muir, 1969; Blake et al., 1997; Johnson, 2001; Bensmaia et al., 2006) essential for the sense of touch and sensory tasks such as tactile discrimination (Carvell and Simons, 1990; Blake et al., 1997; Johnson, 2001). The ability to generate SA1 impulses in MNCs can be compromised under clinical conditions such as chemotherapy and diabetes (Chang and Gu, 2019; Garcia-Mesa et al., 2021), which may be an underlying mechanism for losing touch sensation or numbness in the fingers and toes in patients with these clinical conditions.
The molecular mechanism by which tactile stimuli generate SA1 impulses in Aβ-afferent fibers is not fully understood. Previous studies have identified Piezo2 channels (Coste et al., 2010) as mechanical transducers at MNCs (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014), and the activation of Piezo2 channels by tactile stimuli elicits Ca2+-APs on Merkel cells (Ikeda et al., 2014). It has been proposed that excitation on Merkel cells by tactile stimuli may drive the generation of SA1 impulses on Aβ-afferent fibers through excitatory synaptic transmission. Glutamate, serotonin, or norepinephrine has previously been suggested as the transmitter mediating the synaptic transmission at MNCs (Halata et al., 2003; Chang et al., 2017; Hoffman et al., 2018). Utilizing AP impulses recorded from Aβ-afferent fibers in an ex vivo skin–nerve preparation, a previous study provided evidence of norepinephrine's necessity and sufficiency, acting at β2-adrenergic receptors, for SA1 impulse generation at MNCs (Hoffman et al., 2018). Interestingly, we have not observed evidence of the role of norepinephrine and β2-adrenergic receptors in our recent study on synaptic transmission at the MNCs in the ex vivo whisker hair follicle preparations (Yamada et al., 2024). This discrepancy was thought to reflect the different anatomical origins of the preparations (skin touch domes vs whisker hair follicles), differential consequences of ex vivo tissue preparations, or recording conditions (EPSCs vs AP impulses; Hoffman et al., 2018; Jeon and Caterina, 2024; Yamada et al., 2024). Using the rodent whisker hair follicle preparations and applying patch-clamp recordings directly to Aβ-fiber axon terminals at MNCs, our recent study has demonstrated that tactile signals at MNCs are conveyed from Merkel cells to Aβ-afferent fibers through fast excitatory synaptic transmission, which generates SA1 impulses on Aβ-afferent fibers to encode for the sense of touch. We further have shown that the excitatory synaptic transmission at MNCs in whisker hair follicles is mediated by acid-sensing ion channels (ASICs); the protons released from Merkel cells are synaptic transmitters at the MNC synapses (Yamada et al., 2024).
ASICs are a subclass of the degenerin/epithelial Na+ channel family gated by protons (Geffeney and Goodman, 2012; Krishtal, 2015). These Na+-selective channels can be activated by extracellular protons in an acidic pH ranging from 7.0 to 4.5 (Kellenberger and Schild, 2015). Currently, four ASIC subunits have been identified in mammalian afferent neurons (Deval and Lingueglia, 2015), and functional ASICs are formed by ASIC1, ASIC2, and ASIC3 as homomers or heteromers in afferent neurons (Benson et al., 2002). Initially, ASICs were considered mechanical transducers of mammals, similar to their homolog degenerin Mec-4/Mec-10 in Caenorhabditis elegans (Waldmann et al., 1997; Geffeney and Goodman, 2012; Omerbasic et al., 2015; Cheng et al., 2018). However, studies have failed to establish that ASICs are mechanical transducers in mammals over the past several decades (Price et al., 2000, 2001; Drew et al., 2004; Kang et al., 2012; Omerbasic et al., 2015). Nevertheless, our recent study with rodent whisker hair follicles has indicated that ASICs are involved in tactile functions at MNCs by mediating fast excitatory synaptic transmission to generate SA1 impulses on Aβ-afferent fibers (Yamada et al., 2024). In that study, we have shown that pharmacological inhibition and genetic deletion of ASIC3 channels diminished fast excitatory synaptic transmission at the MNCs and abolished SA1 impulses on Aβ-afferent fibers in the whisker hair follicles of rodents (Yamada et al., 2024). One question that remains to be answered is whether ASIC channels also mediate the generation of SA1 impulses in the MNCs of the skin. To address this question, in the present study, we used the skin–nerve preparations made from rodents' hindpaw glabrous skin and tibial nerves; we recorded SA1 impulses elicited by mechanical stimulation and investigated the effects on SA1 impulses by pharmacological block and genetic deletion of ASIC channels.
Materials and Methods
Animals
Sprague Dawley rats (Envigo) aged 3–4 weeks (both males and females) were used in this study. ASIC3 gene knock-out (KO) mice (ASIC3−/−, both males and females) and their littermate wild-type (WT) mice (ASIC3+/+) were generated from heterozygous ASIC3 mouse breeders (ASIC3+/−, Jackson Laboratory). C57BL/6J mice were also used in experiments (WT, Jackson Laboratory). Nav1.8-ChR2-EYFP mice, including males and females, were generated by crossing Nav1.8-Cre mice with Ai32 (RCL-ChR2 (H134R)/EYFP) mice. The Nav1.8-Cre mice were generously provided by Dr. John Wood at the University College London and transferred to our laboratory from Dr. Stephen Waxman's lab at Yale University. Ai32 mice were purchased from Jackson Laboratory. We performed a crossbreeding of Nav1.8-Cre mice with Ai32 (RCL-ChR2(H134R)/EYFP) mice to establish the Nav1.8-Cre+;ChR2-EYFP loxP/+ mouse line, referred to as Nav1.8-ChR2-EYFP hereafter. The mice used in this study were 11–17 weeks old. All animals were housed in a temperature-controlled room at 23°C and maintained on a 12 h light/dark cycle. Animal care and use adhere to the guidelines for the care and use of experimental animals set forth by the National Institutes of Health. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Ex vivo skin–nerve preparations
Animals (rats and mice) were anesthetized with 5% isoflurane and then killed by decapitation. The glabrous skin in the plantar and toe regions of the hindpaw and the medial plantar and tibial nerves were dissected using a pair of dissection scissors under a dissection microscope. The skin–nerve preparation was placed in a 60 mm recording chamber with a Sylgard Silicone-coated bottom. Fat, muscle, and connective tissues on the nerves and the skin were carefully removed using forceps. The skin was affixed to the bottom of the chamber with tissue pins, with the inside facing up for the rat skin and facing down for the mouse skin, while the nerve bundle was affixed with a tissue anchor within the same recording chamber. The cutting end of the nerve bundle was briefly exposed to a mixture of 0.05% Dispase II (Roche, catalog #11534200) plus 0.05% collagenase (Sigma-Aldrich, catalog #C0130) for 30–60 s, after which the enzyme was washed off using the normal Krebs’ solution (see below). This gentle enzyme treatment aimed to facilitate the separation of individual afferent fibers at the cutting end of the nerve bundle, allowing for the aspiration of a single fiber into the recording electrode for pressure-clamped single–fiber recordings (see below). Subsequently, the recording chamber was mounted on the stage of an Olympus BX51WI upright microscope. The skin–nerve preparation was continuously superfused with a normal Krebs bath solution that contained the following (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. The pH of the solution was adjusted to 7.3, and the osmolarity was adjusted to 325 mOsm. Krebs’ bath solution was saturated with 95% O2 and 5% CO2. Throughout the experiments, Krebs’ bath solution in the recording chamber was maintained at 28–32°C.
Pressure-clamped single–fiber recordings
The pressure-clamped single–fiber recording was conducted similarly as described in our previous studies (Sonekatsu et al., 2020) for detecting AP impulses elicited by mechanical stimulation. Briefly, the recording electrodes for pressure-clamped single–fiber recordings were made with thin-walled borosilicate glass tubing without filament (inner diameter, 1.12 mm; outer diameter, 1.5 mm; World Precision Instruments). These electrodes were fabricated using a P-97 Flaming/Brown Micropipette Puller (Sutter Instrument), and the tip of each electrode was fire-polished to a final size of 4–10 µm in diameter using a microforge (MF-900, Narishige). The recording electrode, filled with Krebs’ bath solution, was mounted onto an electrode holder connected to a high-speed pressure–clamp device (ALA Scientific Instruments) to allow fine controls of intraelectrode pressures. Under a 40× objective, the end of the individual afferent nerve was visualized and isolated by applying a low positive pressure (∼10 mmHg or 0.19 Psi) to the recording electrode. Subsequently, the end of a single nerve fiber was aspirated into the recording electrode under a negative pressure of ∼10 mmHg. Once the nerve fiber's end entered the recording electrode at ∼10 µm, the electrode pressure was readjusted to −3 ± 2 mmHg and maintained at this pressure throughout the experiment. Nerve impulses in the single afferent fiber were recorded under the I0 configuration and amplified using a Multiclamp 700B amplifier (Molecular Devices) in the AC recording mode (100 times AC membrane potential, 5 times gain, 0.1 Hz AC filter, 3 kHz Bessel filter). The analog signals were digitized by the Digidata 1550B (Molecular Devices) and sampled at a rate of 25 kHz with the Axon Clampex 11 software (Molecular Devices). All experiments were carried out at 30 ± 2°C.
To determine the conduction velocity (CV) of the recorded afferent fibers, AP impulses were initiated by electrical stimulation using a bipolar stimulation electrode placed on the tibial nerve bundle. The distance between the site of electrical stimulation and the recording site was ∼10–14 mm. Electrical stimuli consisted of monophasic square pulses generated by an electronic stimulator (Master-9, A.M.P.I) with a stimulation isolator (ISO-Flex, A.M.P.I) and delivered to the stimulation electrode. The duration of each stimulation pulse was 200 µs for Aβ-fibers.
Mechanical stimulation
For a recorded afferent fiber, its mechanosensitive site (receptive field) in the hindpaw glabrous skin was first located using a glass rod. Poking the mechanosensitive receptive field of the recorded afferent fiber with the glass rod would produce APs that the recording electrode could detect. In the present study, all data were collected from mechanosensitive receptive sites, i.e., mechanoreceptors, in the glabrous skin of the hindpaw. Once a mechanoreceptor was identified, the von Frey thresholds were determined using the following steps. The glabrous skin of the hindpaws was poked by von Frey filaments, starting with lower force to higher force; each von Frey filament was applied five times. Von Frey threshold was determined when a filament with a specific force elicited AP impulses, whereas a lower force of von Frey filament did not induce any AP impulses. Mechanical stimulation was applied to the same receptive field using a force-calibrated mechanical indenter (300C-I, Aurora Scientific) to determine mechanical responses. The tip of the indenter had a diameter of 0.8 mm. The indenter was connected to a Digidata 1550B Digitizer to enable the generation of ramp-and-hold mechanical stimulation commands by the pClamp 11 software. Before applying mechanical stimulation, the indenter tip was gently lowered to the surface of the receptive field with a 10 mN force. Subsequently, the 10 mN force was canceled to 0, ensuring that the tip of the indenter contacted the surface of the receptive field without applying force. Under the force control module, ramp-and-hold mechanical stimuli were applied to the mechanoreceptor of the glabrous skin. The step force commanders were calibrated by applying indenter at toe tips, paw pads, and other areas of the plantar skin. The actual forces applied to these skin areas were measured and used. Unless otherwise indicated, the ramp-and-hold force steps were at 0, 5, 15, 30, and 80 mN. Unless otherwise specified, the ramp duration (dynamic phase) was 10 ms, and the holding duration (static phase) was 0.98 s.
Afferent impulses evoked by mechanical stimulation were recorded using pressure-clamped single–fiber recordings. The signals were amplified using the Multiclamp 700B amplifier in the AC recording mode (100 times AC membrane potential, 5 times gain, 0.1 Hz AC filter, 3 kHz Bessel filter) and sampled at a rate of 25 kHz with the Axon Clampex 11 software (Molecular Devices).
Patch-clamp recordings from Aβ-afferent neurons in ex vivo whole-mount DRGs
Nav1.8-ChR2-EYFP mice were either directly used or used 5 d after injecting the fluorescent dye DiD into the foot pads of their right hindpaws. DiD (1 mM in ethanol) was injected at two different sites in each hindpaw at a volume of 2.5 µl each injection site. The injections of DiD were to retrogradely label the DRG neurons that innervated the foot pads so that these neurons could be recorded. The Nav1.8-ChR2-EYFP mice were deeply anesthetized with isoflurane and decapitated. DRGs at the level of L4 and L5 with their attached peripheral afferent bundles (6–13 mm) were rapidly dissected and placed in ice-cold Krebs’ solution. Connective tissues on the surface of the DRGs were carefully removed with fine forceps. DRGs with their peripheral afferent bundles were then affixed in a recording chamber by a tissue anchor and submerged in Krebs’ solution that contained the following (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. Krebs’ solution was saturated with 95% O2 and 5% CO2 and had pH of 7.35 and osmolarity of 325 mOsm and at room temperature of 24°C. DRGs were exposed to 0.06% Dispase II and 0.06% collagenase in Krebs’ solution for 6 min, and then DRGs were continuously perfused at 2 ml/min with Krebs’ solution. Under the fluorescent and DIC-IR microscope, whole-cell patch–clamp recordings were performed on the large-sized Nav1.8-ChR2-EYFP–negative DRG neurons with diameters ≥26 µm. The reason for making recordings from these DRG neurons was because they were mainly Aβ-afferents with SA1-type LTMRs based on our recent study (Yamada et al., 2023). In a different set of experiments with DiD-injected animals, the large-sized DiD-labeled Nav1.8-ChR2-EYFP–negative DRG neurons were selected for recordings. After filling the recording electrode internal solution, the electrode resistance ranged from 5 to 6 MΩ. The recording electrode internal solution contained the following (in mM): 105 K-gluconate, 35 KCl, 0.5 CaCl2, 2.4 MgCl2, 5 EGTA, 10 HEPES, 5 Na2ATP, and 0.33 GTP-TRIS salt; the pH of the solution was adjusted to 7.35 with KOH. The junction potential of the recording condition was 12 mV, as calculated based on ionic concentrations of internal and bath solutions using the pCLAMP 11 software (Molecular Devices). After establishing whole-cell access, recordings were performed under the current-clamp configuration to classify the types of large-sized Nav1.8-ChR2-EYFP–negative DRG neurons based on the CV of their afferent fibers. APs were evoked at the peripheral end of afferent nerve bundles using a suction stimulation electrode. The suction stimulation electrode was fire-polished and had a tip size of ∼1 mm in diameter. The peripheral end of the afferent nerve was aspirated into the suction stimulation electrode under negative pressure. APs were evoked by monophasic square wave pulses generated by pClamp11 software and delivered via a stimulation isolator and the suction stimulation electrode to the peripheral ends of the afferent nerves. The duration of the stimulation pulse was 50 µs. CV was calculated based on the latency of APs and the length of axons. The latency of APs was measured from the time of stimulation that was marked by a stimulation artifact to the time when AP was initiated at the recorded DRG neuron. The length of the axon was the distance between the stimulation site and the recording site. The afferents with CV >10 m/s were considered Aβ-afferents (Yamada et al., 2023). AP width was measured as the duration from 50% of AP upstroke to 50% of AP repolarization. Proton-evoked currents were recorded under the voltage-clamp configuration with neurons held at −72 mV (voltage command of −60 mV). Unless otherwise indicated, membrane voltages mentioned in the texts for this set of experiments have been corrected for the calculated junction potentials of 12 mV. To record proton-evoked current, a Krebs’ solution with a pH of 5 (acidified by citric acid) was applied for 1 s to the recorded neurons. Signals of current-clamp recordings were low-pass filtered at 2 kHz and sampled at 50 kHz. Signals of voltage-clamp experiments were amplified using an Axopatch 200B amplifier, filtered at 2 kHz, and sampled at 10 kHz using pCLAMP 11 software (Molecular Devices).
Pharmacology
Pharmacological agents were made in Krebs’ solution and applied to the skin–nerve preparations through bath applications. Unless otherwise indicated, each compound was perfused to the skin–nerve preparations for 10 min. The testing compounds were delivered through a tube with an internal diameter of 1 mm, and the tube outlet was positioned 0.5 cm away from the recording site. The tube was connected to a peristaltic pump (Miniplus 3 Peristaltic Pumps, Gilson), and testing compounds were bath applied by the drug delivery pump at a rate of 2 ml/min. To examine the effects of ASIC channel blockers on mechanically evoked impulses, mechanically evoked AP impulses were determined before (control) and following the bath application of amiloride (200 µM) or diminazene (100 µM) for 10 min. To examine the effects of ASIC channel blockers on caffeine-induced impulses, caffeine was bath applied to the receptive field in the absence (control) and presence of amiloride (200 µM) or diminazene (100 µM). To examine the effects of protons on DRG neurons, protons were applied to DRG neurons through an acidified Krebs’ solution, pH 5 (acidified by citric acid).
Immunohistochemical staining in mouse DRG sections
L4 and L5 DRGs were harvested from WT (C57) and ASIC3−/− mice at 3–4 months old for immunostaining to determine ASIC3 expression. In brief, animals were anesthetized with isoflurane, transcardially exsanguinated with 0.1 M phosphate-buffered (PB) solution, and perfused with 4% paraformaldehyde (PFA) in 0.1 M PB solution. L4 and L5 DRGs were removed and placed in 4% PFA in 0.1 M PB solution for 20–60 min and then placed in 20% sucrose for cryoprotection overnight at 4°C. The DRGs were then embedded in optimal cutting temperature (OCT) compound (Baxter Scientific) and cut on a cryostat (Leica Biosystems) into 12 µm sections. The sections of DRGs were thaw-mounted onto slides and allowed to air-dry for 16 min. DRG sections were then encircled with hydrophobic resin (PAP Pen, The Binding Site). The slide-mounted sections were rinsed three times with the BupH Modified Dulbecco's phosphate buffer solution (PBS; Thermo Fisher Scientific) that contained 0.1% triton (Millipore Sigma; PBST) and then sequentially incubated at room temperature with ethanol solutions at the concentrations of 50% for 10 min, 70% for 10 min, and 50% for 10 min. The slides were rinsed three times with the PBST solution, and sections were incubated with 10% normal goat serum in PBS for 30 min at room temperature. The sections were incubated with a polyclonal rabbit anti-ASIC3 antibody (1:400 diluted with 5% normal goat serum in PBST; Alomone Labs) at 4°C overnights. In some experiments, the sections were incubated with a polyclonal chicken anti-NF200 antibody (EMD Millipore). Following three rinses with PBST solution, the sections were incubated with a secondary antibody for 1.5 h at room temperature. The secondary antibody (1:500 in 5% normal goat serum in PBST) was a goat anti-rabbit IgG conjugated with Alexa Fluor 568 (Thermo Fisher Scientific) and a goat anti-chicken IgG conjugated with Alexa Fluor 488 (Thermo Fisher Scientific). The sections were rinsed three times with PBS solution and coverslipped with the ProLong Diamond Antifade Mountant medium (Thermo Fisher Scientific). Slices were viewed, and images were taken using an FV3000 confocal microscope (Olympus). Image processing and scale bar addition were done using the cellSens imaging software (Olympus).
Immunohistochemistry of glabrous skin cryosections
Mice at Postnatal Day (P)14 were anesthetized using ketamine/xylazine/acepromazine cocktail and transcardially perfused with 4% PFA in PBS. Hindpaw glabrous skin tissues containing foot pads were dissected and postfixed for 2 h in 4% PFA in PBS at 4°C, followed by three rinses in PBS. Tissues were cryoprotected in 30% sucrose in PBS overnight at 4°C and embedded in OCT. Sections were cut at 40 µm using a cryostat (RWD Minux FS800). After sorting, sections were collected in multiwell plates and further transferred in 1.5 ml Eppendorf tubes. Sections were washed in PBS containing 0.3% Triton X-100 (three times for 10 min) and then blocked in PBS containing 5% lamb serum and 0.3% Triton X-100 (PBT) for 1 h at room temperature. Primary antibodies were diluted in the same blocking buffer, incubated overnight at 4°C, and then washed in PBT (three times for 10 min). Secondary antibodies were incubated in a blocking buffer at 1:500 dilution for 1 h at room temperature. Sections were then washed in PBT (three times for 10 min), mounted with Fluoromount-G (Southern Biotech) and coverslip application, and sealed using clear nail polish (Thermo Fisher Scientific). Primary antibodies used include chicken anti-NF200 Heavy (Aves Labs) 1:800, rabbit anti-Asic3 (Alomone Labs) 1:500, and rat anti-TROMA-1 (Developmental Studies Hybridoma Bank, DSHB) 1:100. Secondary antibodies (all 1:500) used are Alexa Fluor 488-conjugated goat anti-chicken antibody, Alexa Fluor 594-conjugated goat anti-rat antibody, and Alexa Fluor 647-conjugated goat anti-rabbit antibody. Secondary antibodies were purchased from either Invitrogen or the Jackson ImmunoResearch Laboratories. Images were taken using a Leica SP5II confocal microscope (fluorescent). Image processing and scale bar addition were done in Fiji ImageJ.
Whole-mount immunostaining of hairy skin
Whole-mount immunostaining of hairy skin was performed as previously described (Rutlin et al., 2014; Gautam et al., 2024). After deep anesthesia, mice of P14 age were transcardially perfused using 4% PFA. An electric trimmer and Nair cream were used to remove hair from the back. Tape-striping using lab tape and Kimwipes was performed until the skin glistered. The back skin tissue was dissected and rinsed in PBS 2–3 times for 5 min. The big skin piece was further cut into several small pieces (∼5 mm2). At least six pieces per mouse were collected. The skin samples were fixed for another 2 h in 4% PFA/PBS at 4°C, and any excess fat and hair were removed, followed by PBS rinses thrice at room temperature. At room temperature, washing was done every 30 min for ∼4 h with PBST (0.5% Triton X-100). Primary antibodies [chicken anti-NF200 Heavy (Aves Labs) 1:500, rabbit anti-Asic3 (Alomone Labs) 1:400, rat anti-TROMA-1 (DSHB) 1:50] in chilled blocking solution (5% heat-inactivated goat serum, 75% PBST, 20% DMSO) were applied and incubated on a rocker at room temperature for 4 d. Tissues were rinsed in PBST three times, followed by a wash every 30 min for 6 h with PBST. Tissues were then incubated in secondary antibodies in blocking solution at RT for 2 d (Alexa Fluor 488-conjugated goat anti-chicken antibody, Alexa Fluor 594-conjugated goat anti-rat antibody, and Alexa Fluor 635-conjugated goat anti-rabbit antibody, all 1:500). PBST was used to rinse the tissues for three times followed by a wash every 30 min for at least 6 h with PBST. Tissues were dehydrated (2 h for each) in serial MeOH (methanol)/PBS dilutions (25, 50, 80, 100) rocking at room temperature. The tissue was kept in 1:1 MeOH:BABB (one part benzyl alcohol:two parts benzyl benzoate) solution for 2 h rocking at room temperature. The tissue was cleared in 100% BABB and mounted on a slide (with little BABB). Four drops of Dow Corning High Vacuum Grease were applied at four corners around the tissue, and a cover glass was applied over it with gentle pressure so that the coverslip would stick with the mounting tissue and grease. Images were taken using a Leica SP5II confocal microscope (fluorescent). Image processing and scale bar addition were done in Fiji ImageJ.
Data analysis
Electrophysiological data were collected from the skin–nerve preparations and DRGs of both male and female rats and male and female mice. We observed no signs of differences in electrophysiological results between the male and female animals. Thus, their data were combined for analysis. Electrophysiological data were analyzed using the CLAMPFIT 11 software. Statistical analyses were performed using GraphPad Prism (version 10). Unless otherwise indicated, data were reported as individual values and mean ± SEM of n independent observations. Data normality was analyzed using the Shapiro–Wilk test. Statistical significance was evaluated using unpaired and paired Student's t tests, one-way ANOVA with Tukey's post hoc test, and two-way ANOVA with Bonferroni’s post hoc test. Differences were significant with *p < 0.05, **p < 0.01, and ***p < 0.001, and not significant (ns) with p ≥ 0.05.
Results
We used the skin–nerve preparations made from the rat hindpaw glabrous skin and tibial nerves and applied the pressure-clamped single–fiber recording to record SA1 impulses following mechanical indentation (Fig. 1A). In this set of experiments, afferent fiber CV was determined following electrical stimulation of the tibial nerve to evoke an AP impulse, and the latency of the AP impulse was used to calculate the CV (Fig. 1A,B). The afferent fibers with a CV over 10 m/s were considered Aβ-fibers (Fig. 1C), and these Aβ-fibers were further investigated for their mechanical responses. The afferent fibers whose CV were <10 m/s were not included in the present study. We tested the mechanical threshold of the Aβ-fibers innervating the glabrous skin of the hindpaws (Fig. 1D), and those with a mechanical threshold of 4 mN or lower were considered Aβ-fiber low threshold mechanoreceptors (LTMRs; Fig. 1D). Many Aβ-fiber LTMRs exhibited SA1 impulses in response to mechanical indentation (Fig. 1E), with AP impulses slowly adapted and the impulse firing irregularly (Fig. 1F,G). The coefficient of variance for these Aβ-fiber LTMRs was >0.5, suggesting that these were Aβ-fiber SA1 LTMRs (SA1; Wellnitz et al., 2010). Additionally, we also encountered Aβ-fiber LTMRs that exhibited rapidly adapting (RA) type and slowly adapting type 2 (SA2) impulses in the recordings from the rat hindpaw glabrous skin (data not shown); these were Aβ-fiber RA LTMRs (RA) and Aβ-fiber SA2 LTMRs (SA2), respectively.
Figure 1.
SA1 impulses recorded from the skin–nerve preparation made from the glabrous skin and tibial nerves of rat hindpaws. A, Diagram illustrates the pressure-clamped single–fiber recordings of SA1 impulses following mechanical stimulation applied to the glabrous skin of rat hindpaws. SA1 impulses were evoked by a mechanical probe. An electrical stimulator was used to directly evoke AP impulses to determine AP CV. B, Sample trace shows an AP impulse (arrowhead indicated) elicited by electrical stimulation of the tibial nerve for determining CV. The arrow indicates an electrical stimulation artifact. C, Summary data of the CV of SA1 LTMRs (n = 15). D, Mechanical thresholds of SA1 LTMRs (n = 15). E, Sample trace shows AP impulses evoked from an SA1 LTMR by a mechanical indentation at a 15 mN force. The duration (1 s) of the mechanical indentation is indicated below the AP impulses. F, The plot of instantaneous frequency of the SA1 impulses in E. G, Coefficient of variance of SA1 impulses from SA1 LTMRs (n = 15). Data represent individual observations and mean ± SEM.
We investigated whether mechanical indentation-elicited SA1 impulses in the rat hindpaw glabrous skin could be suppressed by ASIC channel blockers. In this set of experiments, SA1 impulses were evoked by mechanical indentation at 15 mN in the absence and presence of the ASIC channel blocker amiloride. As shown in Figure 2, A and C, the application of the mechanical indentation-elicited robust SA1 impulses in the absence of amiloride with the impulse frequency of 25.5 ± 7.0 Hz (n = 7), and the frequency of SA1 impulses was significantly decreased to 6.6 ± 1.8 Hz in the presence of 200 µM amiloride (n = 7; Fig. 2C). The mechanical indentation-evoked SA1 impulses largely recovered after the wash-off of amiloride (Fig. 2A,C). We further examined the effects of diminazene, an ASIC channel blocker structurally distinct from amiloride (Lingueglia and Lazdunski 2013), on the SA1 impulses evoked by mechanical indentation at 15 mN (Fig. 2B,D). In this set of experiments, the mechanical indentation-elicited robust SA1 impulses in the absence of diminazene with the impulse frequency of 25.4 ± 5.0 Hz (n = 7), and the frequency of SA1 impulses was significantly decreased to 2.2 ± 0.9 Hz in the presence of 100 µM diminazene (n = 7; Fig. 2D). The mechanical indentation-evoked SA1 impulses largely recovered after the wash-off of diminazene (Fig. 2A,D).
Figure 2.
SA1 impulses elicited by mechanical indentation are suppressed pharmacologically by ASIC blockers. A, Sample traces show SA1 impulses before (control, left), following the application of 200 µM amiloride (middle), and after the wash-off of amiloride (right). B, Similar to A, except diminazene (100 µM) was tested. In both A and B, SA1 impulses were elicited by mechanical indentation at 15 mN. C, Pooled data of the experiments exemplified in A, showing the suppression of SA1 impulses by 200 µM amiloride (n = 7). D, Pooled data of the experiments exemplified in B, showing the suppression of SA1 impulses by 100 µM diminazene (n = 7). Data represent individual observations and mean ± SEM; *p < 0.05; **p < 0.01; ns, not significantly different; one-way ANOVA with Tukey's post hoc test.
We have previously found that caffeine increases AP impulses in SA1 LTMRs in whisker hair follicles (Yamada et al., 2024). Similarly, we found that caffeine significantly increased AP impulses in SA1 LTMRs in the skin–nerve preparations made from the glabrous skin and tibial nerves of rat hindpaws (Fig. 3). AP impulses were 298 ± 222 impulses/5 min (n = 5) in the absence of caffeine and significantly increased to 1,642 ± 327 impulses/5 min (n = 5) in the presence of 10 mM caffeine (Fig. 3B). However, caffeine-induced increases of AP impulses in SA1 LTMRs were significantly abolished in the presence of 200 µM amiloride (468 ± 145 impulses/5 min; n = 5; Fig. 3B).
Figure 3.
Impulses of Aβ-fiber SA1 LTMRs induced by caffeine are suppressed pharmacologically by ASIC blockers. A, Sample traces show impulses of an SA1 LTMR before (control, top), following the application of 10 mM caffeine (middle) and the application of 10 mM caffeine plus 200 µM amiloride (bottom). B, Pooled data of the experiments exemplified in A, showing the suppression of caffeine-induced impulses by 200 µM amiloride (n = 5). Data represent individual observations and mean ± SEM; *p < 0.05; **p < 0.01; one-way ANOVA with Tukey's post hoc test.
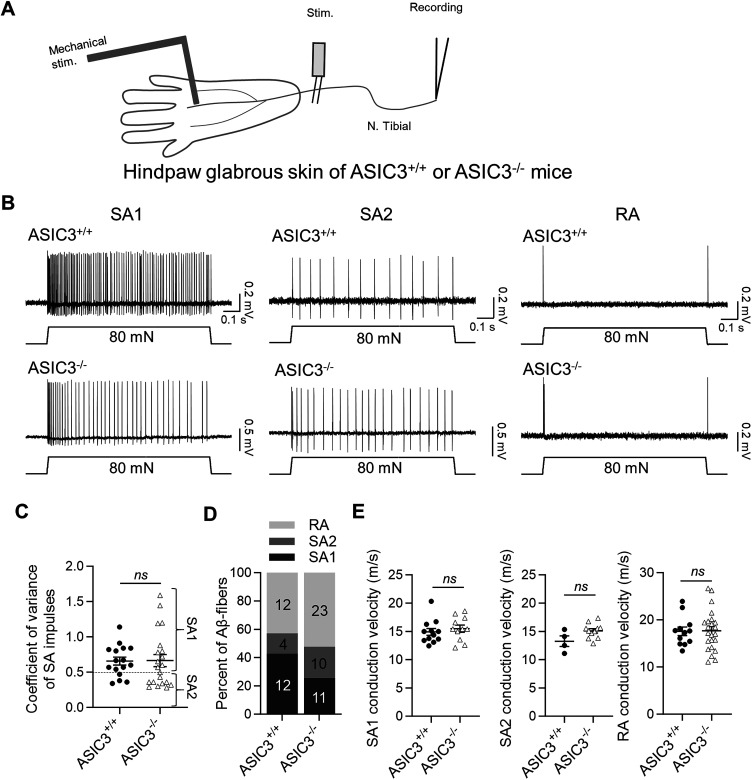
Our recent study has shown that ASIC3-containing ASIC channels are essential for generating SA1 impulses in MNCs of the whisker hair follicles of mice (Yamada et al., 2024). In the present study, we further investigated whether ASIC3 is essential to generate SA1 impulses in the MNCs of the hindpaw glabrous skin of mice. To test this idea, we used the skin–nerve preparation made from the hindpaw glabrous skin and tibial nerves (Fig. 4A) of the WT mice (ASIC3+/+) and the mice whose ASIC3 was genetically deleted (ASIC3−/−). As shown in Figure 4B, three types of Aβ-fiber LTMRs were identified in the hindpaw glabrous skin, including SA1, SA2, and RA LTMRs, in both ASIC3+/+ and ASIC3−/− mice. The RA LTMRs displayed AP impulses only in the dynamic phase of the mechanical indentation step, whereas SA1 and SA2 LTMRs displayed AP impulses during both dynamic and static phases of the mechanical indentation step (Fig. 4B). In the present study, we differentiated SA1 and SA2 responses by the coefficient of variance of their impulses (Fig. 4C), with the coefficient of variance larger than 0.5 being SA1 and equal to or smaller than 0.5 being SA2 (Wellnitz et al., 2010). Previous studies have shown that SA1 impulses (responses) are generated from MNCs, i.e., SA1 LTMRs (Iggo and Muir, 1969; Halata et al., 2003; Handler and Ginty, 2021). In ASIC3+/+ mice, SA1, RA, and SA2 proportions were 43%, 43%, and 14%, respectively (Fig. 4D). However, in ASIC3−/− mice, SA1, RA, and SA2 proportions were 26%, 52%, and 22%, respectively, which shows a tendency to reduce SA1 LTMRs and increase RA and SA2 LTMRs. There is no significant difference in SA1, SA2, and RA CV between the ASIC3+/+ and ASIC3−/− mice (Fig. 4E).
Figure 4.
Mechanical indentation-elicited impulses of Aβ-fiber LTMRs in hindpaw glabrous skin of ASIC3+/+ and ASIC3−/− mice. A, Diagram illustrates the pressure-clamped single–fiber recordings of mechanical indentation-elicited impulses of Aβ-fiber LTMRs in the hindpaw glabrous skin of mice. B, Three sets of sample traces show AP impulses evoked by mechanical indentation from an SA1 LTMR (left), SA2 LTMR, and RA LTMR. Mechanical indentation was applied at 80 mN force with a duration of 1 s. C, Coefficient of variance of SA LTMR (SA1 and SA2 LTMRs) recorded from the hindpaw glabrous skin of ASIC3+/+ and ASIC3−/− mice. The dashed line indicates the coefficient of variance value of 0.5, the level that is used to classify SA LTMRs into SA1 LTMRs (coefficient of variance ≥0.5) and SA2 LTMRs (coefficient of variance <0.5). D, The percentage of Aβ-fiber LTMRs being SA1 (black), SA2 (dark gray), and RA (light gray), as recorded from the hindpaw glabrous skin of ASIC3+/+ and ASIC3−/− mice. E, CV of SA1 LTMRs (left panel), SA2 LTMRs (middle panel), and RA LTMRs (right panel), as recorded from the hindpaw glabrous skin of ASIC3+/+ and ASIC3−/− mice. Data represent individual observations and mean ± SEM; ns, not significantly significant; Student's t test.
We investigated the mechanical sensitivity of the three types of Aβ-fiber LTMRs in the glabrous skin of ASIC3+/+ and ASIC3−/− mice. This was achieved by determining the von Frey thresholds and the impulse frequencies elicited by different forces of mechanical indentations (Fig. 5). The von Frey thresholds for the SA1 LTMRs were 0.80 ± 0.12 mN (n = 11) in ASIC3−/− mice, not significantly different from those of ASIC3+/+ mice (0.76 ± 0.14 mN; n = 13). However, the frequencies of SA1 impulses evoked by different indentation forces were significantly lower in ASIC3−/− mice than in ASIC3+/+ mice (Fig. 5B). For example, with the indentation forces of 30 mN, the frequencies of SA1 impulses were 60.1 ± 10.3 (n = 10) Hz in ASIC3+/+ mice and significantly decreased to 31.3 ± 6.6 (n = 11) Hz in ASIC3−/− mice. For the SA2 LTMRs, the von Frey thresholds were 0.59 ± 0.13 mN (n = 10) in ASIC3−/− mice, not significantly different from those of ASIC3+/+ mice (0.97 ± 0.36 mN; n = 4; Fig. 5C). The frequencies of SA2 impulses evoked by different indentation forces were also not significantly different between ASIC3−/− mice and ASIC3+/+ mice (Fig. 5D). For the RA LTMRs, the von Frey thresholds were 0.61 ± 0.15 mN (n = 12) in ASIC3−/− mice, not significantly different from those of ASIC3−/− mice (0.62 ± 0.09 mN; n = 23; Fig. 5E). Interestingly, the frequencies of RA impulses evoked by the indentation force of 80 mN were significantly higher in ASIC3−/− mice (2.89 ± 0.41 Hz; n = 19) than in ASIC3+/+ mice (1.83 ± 0.27; n = 12; Fig. 5F).
Figure 5.
Comparison of mechanical responses of Aβ-fiber LTMRs in the hindpaw glabrous skin between ASIC3+/+ and ASIC3−/− mice. A, Von Frey threshold of SA1 LTMRs recorded from the hindpaw glabrous skin of ASIC3+/+ (solid circles) and ASIC3−/− mice (open triangles). B, Frequency of SA1 impulses in response to mechanical indentation at the forces of 0.5, 30, and 80 mN applied to the hindpaw glabrous skin of ASIC3+/+ (solid circles; n = 10) and ASIC3−/− mice (open triangles; n = 11). C, Similar to A, except SA2 LTMRs were examined in the hindpaw glabrous skin of ASIC3+/+ (solid circles) and ASIC3−/− mice (open triangles). D, Similar to B, except SA2 LTMRs were examined in the hindpaw glabrous skin of ASIC3+/+ (solid circles; n = 4) and ASIC3−/− mice (open triangles; n = 10). E, Similar to A, except RA LTMRs were examined in the hindpaw glabrous skin of ASIC3+/+ (solid circles) and ASIC3−/− mice (open triangles). F, Similar to B, except RA LTMRs were tested in the hindpaw glabrous skin of ASIC3+/+ (solid circles; n = 12) and ASIC3−/− mice (open triangles; n = 19). A–F, Mechanical indentation was applied for 1 s, and frequencies of the impulses were the averaged values in 1 s. Data represent individual observations and mean ± SEM; #p < 0.05; ###p < 0.001; *p < 0.05; **p < 0.01; ns, not significantly significant; two-way ANOVA with Bonferroni’s post hoc test or Student's t test.
Since SA1 impulses recorded in the glabrous skin–nerve preparation are conducted by Aβ-afferent fibers whose soma are located in L4/L5 DRGs, we examined whether ASIC3 channels were expressed on L4/L5 DRG neurons that likely gave rise to Aβ-afferent fibers. Strong ASIC3-immunoreactivity (ASIC3-ir) was observed in many small- to large-sized DRG neurons (Fig. 6A,B). The ASIC3-ir specifically represented the expression of ASIC3 channels on these DRG neurons since no ASIC3-ir could be observed in the DRG sections obtained from ASIC3−/− mice (Fig. 6C). The large-sized DRG neurons with ASIC3-ir were also NF200-immunoreactivity positive, suggesting they were Aβ-afferent fibers (Fig. 6D,F).
Figure 6.
ASIC3 expression on the neurons of lumbar DRGs of mice. A, Sample image shows ASIC3-ir on an L5 DRG section of a WT mouse. B, Histogram shows the cell diameter distribution of ASIC3-ir–positive neurons in L4 and L5 DRG sections. The ASIC3-ir–positive neurons were pooled from 24 DRG sections (L4 and L5) of five WT mice. C, Sample image shows the lack of ASIC3-ir in an L5 DRG section of an ASIC3−/− mouse. D–F, Sample images show double immunostaining for ASIC3 (ASIC3-ir, D) and NF200 (NF200-ir, E) in an L5 DRG section of a WT mouse. F is the overlay image of D and E to show the coimmunostaining of ASIC3 and NF200 in some large-sized DRG neurons.
We performed immunohistochemical staining on the cryosections of the glabrous skin of the hindpaws. In this set of experiments, we used an antibody against NF200 to identify afferent nerves, an antibody against Troma1 to identify Merkel cells, and an antibody against ASIC3 to identify ASIC3 expression. As shown in Figure 7A–D, some afferent fibers stained with NF200-immunoreactivity were near Merkel cells that showed Troma1-immunoreactivity. ASIC3-ir was observed on some structures that closely contacted Merkel cells (Fig. 7C,D), suggesting these ASIC3-ir structures may be the postsynaptic sites of MNCs. Interestingly, ASIC3-ir was not colocalized with NF200-immunoreactivity on most parts of afferent fibers, raising the possibility that ASIC3 expression is restricted to the postsynaptic sites. A similar expression pattern of ASIC3-ir was observed in the back hairy skin in whole-mount tissue preparation (Fig. 7E–H).
Figure 7.
Immunohistochemical staining of Troma1, NF200, and ASIC3 in mouse glabrous and hairy skin. A–D, Images show immunoreactivity of NF200 (A), Troma1 (B), and ASIC3 (C) in the cryosections of the plantar glabrous skin of a mouse hindpaw. D is the overlay image from A, B, and C, showing the innervation of Merkel cells (Troma1-staining) by primary afferent nerves (NF200-staining) and the close contact to the Merkel cells by some ASIC3-ir–positive structures in the glabrous skin. Arrows in D indicate two sites where the ASIC3-ir–positive structures contact Merkel cells. E–H, Similar to A–D, except that the immunohistochemical staining was performed on the back hairy skin of the whole-mount tissue preparations of a mouse. The overlay image in H demonstrates the innervation of Merkel cells (Troma1-staining) by primary afferent nerves (NF200-staining) and the close contact to the Merkel cells by the ASIC3-ir–positive structures in the hairy skin as well. Arrows in H indicate two sites where the ASIC3-ir–positive structures contact Merkel cells.
We have previously shown that Nav1.8-ChR2-EYFP–negative Aβ-afferents innervating the glabrous skin of mouse hindpaws were primarily SA1 LTMRs (Yamada et al., 2023). The present study examined whether Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4/L5 DRGs expressed functional ASIC channels to mediate inward currents (Fig. 8A–D). In this set of experiments, Nav1.8-ChR2-EYFP mice were used, and recordings were performed on large-sized Nav1.8-ChR2-EYFP–negative neurons in L4/L5 DRGs obtained from Nav1.8-ChR2-EYFP mice (Fig. 8A–D). The CV for all the neurons included in this study was >10 m/s (16.3 ± 1.4 m/s; n = 11; Fig. 8E,F). The soma diameters ranged from 27 to 40 µm (32.6 ± 1.4 µm; n = 11). The AP width was under 0.8 ms (0.63 ± 0.03 ms; n = 11). These features were consistent with the properties of Aβ-LTMRs shown in our previous studies (Yamada et al., 2023). When an acidified Krebs’ solution, pH 5, was applied to these large-sized Nav1.8-ChR2-EYFP–negative DRG neurons, most responded to protons with inward currents (Fig. 8I,J). Of 16 proton-sensitive Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons, the average amplitudes of the proton-evoked currents were 394.5 ± 86.6 pA (n = 16; Fig. 8K). We further determined whether the afferent neurons that give rise to SA1 LTMRs in the foot pads of mice may express functional ASIC channels. We injected the fluorescent dye DiD to label these neurons in L4 DRGs retrogradely. The large-sized DiD–labeled afferent neurons that were Nav1.8-ChR2-EYFP-negative (Fig. 9A–E) were then recorded for their response to protons (Fig. 9F–H). As shown in Figure 9F–H, applying an acidified Krebs’ solution, pH 5, evoked inward currents in most (9/11) of these neurons. The proton-evoked currents were 92.3 ± 12.0 pA (n = 9; Fig. 9H).
Figure 8.
Proton-evoked inward currents in Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4 DRGs of mice. A, Experimental setting for recordings of proton-evoked currents in Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4 DRGs. An L4 DRG with an attached peripheral afferent bundle was anchored in a recording chamber. Whole-cell patch–clamp recordings were made from DRG neurons. Electrical stimulation was applied to the peripheral afferent bundle with a suction electrode to evoke APs. As illustrated in A, an acidified Krebs’ solution, pH 5, was applied to the recorded neuron. B, C, Image shows L4 DRG neurons under 40× bright-field (B) and fluorescent microscopy (C). An asterisk in each image indicates a large-sized Nav1.8-ChR2-EYFP–negative Aβ-afferent neuron. The shadow of a recording electrode can be seen on the right side of the asterisk. D, Overlay image of B and C to show the Nav1.8-ChR2-EYFP–negative (asterisk indicated) and Nav1.8-ChR2-EYFP–positive (green color) Aβ-afferent neurons. E, Sample trace shows an AP evoked by electrical stimulation to an afferent nerve bundle at its peripheral site, and the AP was recorded from a Nav1.8-ChR2-EYFP–negative Aβ-afferent neuron. F–H, Pooled data show CV (F), soma diameters (G), and AP widths (H) of Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons. I, Sample trace shows an inward current evoked by protons, pH 5 (Krebs’ solution), in a Nav1.8-ChR2-EYFP–negative Aβ-afferent neuron. J, The percentage of Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons being proton-responsive (n = 16) and not proton-responsive (n = 9). K, Pooled data of proton-evoked inward currents in Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4 DRGs (n = 16). Data represent individual observations and mean ± SEM.
Figure 9.
Proton-evoked inward currents in Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons that innervate the foot pads of mouse hindpaws. A–D, Images show L4 DRG neurons under 40× bright-field (A) and fluorescent microscopy (B–D). The L4 DRG was obtained from a Nav1.8-ChR2-EYFP mouse whose hindpaw foot pads were injected with the fluorescent dye DiD. DiD-labeled neurons and Nav1.8-ChR2-EYFP–positive neurons were shown in B (red) and C (green), respectively. D is the overlay image of B and C to show DiD-labeled and Nav1.8-ChR2-EYFP–negative DRG neurons (asterisk indicated) that were selected for patch-clamp recordings. E, Pooled data shows the soma diameters of the DiD-labeled Nav1.8-ChR2-EYFP–negative DRG neurons being recorded. F, Sample trace shows an inward current evoked by protons, pH 5 (Krebs’ solution), in a DiD-labeled Nav1.8-ChR2-EYFP–negative Aβ-afferent neuron. G, The percentage of DiD-labeled Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons being proton-responsive (n = 9) and not proton-responsive (n = 2). H, Pooled data of proton-evoked inward currents in DiD-labeled Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4 DRGs (n = 9). Data represent individual observations and mean ± SEM.
Earlier experiments (Fig. 4, 5) showing the effects on SA1 impulses by ASIC3 KO were performed with mechanical indentations applied at a relatively short duration of 1 s, which showed significantly lower SA1 impulse frequencies in ASIC3−/− mice than in ASIC3+/+ mice (Fig. 5B). In the final set of experiments, we examined whether the differences in SA1 impulses between ASIC3−/− mice and ASIC3+/+ mice could extend beyond 1 s. In this set of experiments, SA1 impulses were elicited by an indentation force of 80 mN applied to the receptive field for 2.5 s (Fig. 10A). The SA1 impulse frequencies were also significantly higher in ASIC3−/− mice than in ASIC3+/+ mice in the late phase of mechanical indentation, from the indentation time of 1 to 2.5 s (Fig. 10B). For example, at the time point of 2.5 s, the SA1 impulse frequency was 10.6 ± 2.8 Hz (n = 11) in ASIC3−/− mice, significantly lower than that in ASIC3+/+ (36.0 ± 5.0 Hz; n = 10). Overall, for the whole indentation period of 2.5 s, the SA1 frequencies were also significantly lower in ASIC3−/− mice (23.4 ± 5.0 Hz; n = 11) than in ASIC3+/+ mice (54.4 ± 6.5 Hz; n = 10; Fig. 10C).
Figure 10.
Mechanical responses of Aβ-fiber SA1 LTMRs in the hindpaw glabrous skin of ASIC3+/+ and ASIC3−/− mice with prolonged mechanical indentation. A, Sample traces of SA1 impulses elicited by mechanical indentation at 80 mN. Mechanical indentation was applied to the glabrous skin of the hindpaw of an ASIC3+/+ (top panel) and an ASIC3−/− mouse (bottom panel) for a duration of 2.5 s. B, Time course of the frequency of SA1 impulses evoked by 80 mN mechanical indentation. The indentation was applied for 2.5 s onto the hindpaw glabrous skin of an ASIC3+/+ (solid circle) and an ASIC3−/− mice (open triangles). The gray color-shaded area indicates the late-phase SA1 impulses. C, Averaged frequency of SA1 impulses during 2.5 s mechanical indentation at 80 mN. The mechanical indentations were applied to the hindpaw glabrous skin of ASIC3+/+ (circles) and ASIC3−/− mice (triangles). Data represent individual observations and mean ± SEM; ###p < 0.001; *p < 0.05; **p < 0.01; two-way ANOVA with Bonferroni’s post hoc test (late-phase SA1 impulses in B) or Student's t test (C).
Discussion
In the present study, with the glabrous skin–tibial nerve preparations, we have shown that the pharmacological block of ASIC channels and genetic deletion of ASIC3 channels largely abolish mechanically evoked SA1 impulses on Aβ-afferent fibers. These results, for the first time, indicate that ASIC channels are essential in generating SA1 impulses at the MNCs in the glabrous skin, which strongly suggests an important role of ASIC channels in sensing touch by the glabrous skin in mammals.
The present study has focused on Aβ-afferent fibers that are LTMRs displaying SA1 impulses following tactile stimulation to the glabrous skin of rodent hindpaws. Previous studies have indicated that SA1 impulses originate from MNCs in the skin throughout the body and whisker hair follicles (Iggo and Muir, 1969; Halata et al., 2003; Handler and Ginty, 2021). Our recent study shows that ASIC channels are present at the postsynaptic sites of MNCs to mediate fast excitatory synaptic transmission, leading to the generation of SA1 impulses, and ASIC blockers block these channels to abolish SA1 impulses (Yamada et al., 2024). Therefore, in the present study, the inhibitory effects of ASIC channel blockers on SA1 impulses are most likely through their actions on ASIC channels at postsynaptic sites of MNCs in the glabrous skin, similar to our recent study on MNCs in rodent whisker hair follicles (Yamada et al., 2024). In addition to mechanical stimulation, we show that caffeine increases impulses in MNCs in the glabrous skin, and these impulses are also largely inhibited by the ASIC blocker amiloride. The same result was observed with caffeine and ASIC blockers in our previous study using whisker hair follicles (Yamada et al., 2024). In the present study with L4/L5 DRGs from mice, we have shown that ASIC3 channels are expressed in the DRG neurons with soma sizes ranging from small to large diameters and that ASIC3-ir is present in NF200-positive afferent neurons, suggesting that ASIC3 channels are expressed in different functional subtypes of DRG neurons very likely including Aβ-afferent LTMRs. Consistently, we have shown that Nav1.8-ChR2-EYFP–negative Aβ-afferent neurons in L4 DRGs are mostly sensitive to protons, and the application of acidified Krebs’ solution evokes inward currents mediated by ASIC channels. Nav1.8-ChR2-EYFP–negative Aβ-afferents innervating the glabrous skin of mice have been shown previously to be mainly SA1 LTMRs (Yamada et al., 2023). We have demonstrated with retrograde labeling that the Nav1.8-ChR2-EYFP–negative Aβ-afferents innervating the glabrous skin of mice were proton sensitive, and acidified Krebs’ solution could evoke inward currents. These results suggest that SA1 LTMRs may express ASIC channels and are sensitive to protons. Consistently, in the hindpaw glabrous skin of mice, ASIC3-ir was observed on the structures that were associated with Merkel cells in the present study and also in a previous study (Price et al., 2001), suggesting that ASIC channels are present at the postsynaptic sites of Aβ-afferent endings in the MNCs of the hindpaw glabrous skin of mice (Price et al., 2001). ASIC3 channels may also be present at the postsynaptic sites of Aβ-afferent endings in the MNCs of mouse hairy skin based on our immunohistochemical staining performed on the mouse back hairy skin. Interestingly, in both glabrous and hairy skin, ASIC3-ir was not found to be colocalized with NF200-ir in most parts of afferent nerves. This may suggest that ASIC3 expression is restricted to the postsynaptic endings in the MNCs, and its expression is very low or undetectable on other parts of the afferent fibers.
In the present study with ASIC3−/− mice, we show that SA1 impulses in the glabrous skin are significantly less in ASIC3−/− mice than in ASIC3+/+mice. The same results were observed in our previous study with whisker hair follicles of ASIC3−/− mice (Yamada et al., 2024). Collectively, there is a high similarity between our present study with glabrous skin and our recent study with whisker hair follicles (Yamada et al., 2024). This raises the possibility that, in the glabrous skin, ASIC channels mediate fast excitatory synaptic transmission with protons as transmitters to generate SA1 impulses at MNCs, similar to that in the whisker hair follicles (Yamada et al., 2024). However, by recording SA1 impulses in the skin–nerve preparations, a previous study suggested that norepinephrine, acting at β2-adrenergic receptors, mediated the tactile transmission at MNCs in the skin touch domes. The reason for the discrepancy between our studies and the previous study was unclear. To address this discrepancy in the future, patch-clamp recordings at the MNCs of the skin touch domes will be needed to determine whether ASIC channels, β2-adrenergic receptors, or other receptors mediate the excitatory synaptic transmission. It should be noted that fast excitatory synaptic transmission at MNCs may be modulated by chemical messengers. For example, serotonin, which was thought to be a synaptic transmitter, has recently been recognized as a synaptic modulator at the MNCs. Therefore, it is important to differentiate between synaptic transmission and synaptic modulation of the role of a chemical messenger in generating SA1 impulses at the MNCs of the skin.
The present study shows that the percentage of Aβ-afferent fibers displaying SA1 responses becomes lower, and RA becomes higher, in ASIC3−/− mice than in ASIC3+/+ mice. Furthermore, RA impulse frequency was higher in ASIC3−/− mice than in ASIC3+/+. These changes could be due to the conversion of SA1 impulses into RA-like impulses following the abolishment of SA1 impulses in their static phase in ASIC3−/− mice; a result was also observed in our recent study in whisker hair follicles (Yamada et al., 2024). RA impulses are generated in the Aβ-afferent endings in Meissner corpuscles in the glabrous skin and lanceolate endings in whisker hair follicles (Price et al., 2000; Handler and Ginty, 2021). Making patch-clamp recordings at Aβ-afferent endings in Meissner corpuscles and lanceolate endings will be helpful for better understanding the changes in RA impulses in ASIC3−/− mice.
Our finding that ASIC channels are essential for generating SA1 impulses in the MNCs has led ASICs to reemerge as a critical molecule for the sense of touch in mammals, which addresses the long-standing questions about the tactile functions of ASICs (Price et al., 2000; Page et al., 2004; Roza et al., 2004; Kang et al., 2012). Previous studies with ASIC KO mice, including ASIC3−/− mice, have produced controversial results, including no significant effects, decreases, or increases in tactile responses (Price et al., 2000; Page et al., 2004; Roza et al., 2004; Kang et al., 2012). The inconsistent tactile responses observed in previous studies with ASIC KO mice were thought to be at least partially due to functional compensation (Kang et al., 2012). Additionally, tactile tests used in the previous studies may be inappropriate in identifying the tactile functions of ASIC channels. We showed that ASIC3 contributes to the extent of firing, i.e., static phase of AS1 impulses, in response to the suprathreshold mechanical stimuli without affecting mechanical threshold. This may be also one of the reasons why previous behavioral studies with the von Frey test did not observe an increase in the mechanical threshold in ASIC3 KO mice (Price et al., 2001). Our recent study using the whisker tactile discrimination test has shown that ASIC channels are essential for tactile discrimination by whiskers in rodents (Yamada et al., 2024). In future studies with appropriate tactile behavioral tests, it will be interesting to know whether ASIC channels are also involved in tactile discrimination in the glabrous skin.
Pathological conditions such as diabetes and chemotherapy-induced peripheral neuropathy often result in the impairment of tactile functions or numbness in the fingertips and toes (Chang and Gu, 2019; Garcia-Mesa et al., 2021). This sensory dysfunction is a significant clinical problem that can severely lower the patient's quality of life. The essential role of ASIC channels in generating SA1 tactile impulses in Aβ-afferent fibers in the skin for tactile encoding and the sense of touch may provide new insights into the mechanisms underlying tactile dysfunctions in these pathological conditions.
References
- Bensmaia SJ, Craig JC, Yoshioka T, Johnson KO (2006) SA1 and RA afferent responses to static and vibrating gratings. J Neurophysiol 95:1771–1782. 10.1152/jn.00877.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM (2002) Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A 99:2338–2343. 10.1073/pnas.032678399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Johnson KO, Hsiao SS (1997) Monkey cutaneous SAI and RA responses to raised and depressed scanned patterns: effects of width, height, orientation, and a raised surround. J Neurophysiol 78:2503–2517. 10.1152/jn.1997.78.5.2503 [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ (1990) Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10:2638–2648. 10.1523/JNEUROSCI.10-08-02638.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Gu JG (2019) Impairment of tactile responses and Piezo2 channel mechanotransduction in mice following chronic vincristine treatment. Neurosci Lett 705:14–19. 10.1016/j.neulet.2019.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Kanda H, Ikeda R, Ling J, Gu JG (2017) Serotonergic transmission at Merkel discs: modulation by exogenously applied chemical messengers and involvement of Ih currents. J Neurochem 141:565–576. 10.1111/jnc.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YR, Jiang BY, Chen CC (2018) Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing. J Biomed Sci 25:46. 10.1186/s12929-018-0448-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60. 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Lingueglia E (2015) Acid-sensing ion channels and nociception in the peripheral and central nervous systems. Neuropharmacology 94:49–57. 10.1016/j.neuropharm.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556:691–710. 10.1113/jphysiol.2003.058693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE, Rice FL (2002) Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J Comp Neurol 449:103–119. 10.1002/cne.10277 [DOI] [PubMed] [Google Scholar]
- Garcia-Mesa Y, Feito J, Gonzalez-Gay M, Martinez I, Garcia-Piqueras J, Martin-Cruces J, Vina E, Cobo T, Garcia-Suarez O (2021) Involvement of cutaneous sensory corpuscles in non-painful and painful diabetic neuropathy. J Clin Med 10:4609–4614. 10.3390/jcm10194609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, et al. (2024) Distinct local and global functions of mouse Aβ low-threshold mechanoreceptors in mechanical nociception. Nat Commun 15:2911. 10.1038/s41467-024-47245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney SL, Goodman MB (2012) How we feel: ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 74:609–619. 10.1016/j.neuron.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z, Grim M, Bauman KI (2003) Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271:225–239. 10.1002/ar.a.10029 [DOI] [PubMed] [Google Scholar]
- Handler A, Ginty DD (2021) The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci 22:521–537. 10.1038/s41583-021-00489-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA (2018) Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100:1401–1413 e1406. 10.1016/j.neuron.2018.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR (1969) The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol 200:763–796. 10.1113/jphysiol.1969.sp008721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG (2014) Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell 157:664–675. 10.1016/j.cell.2014.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM, Caterina MJ (2024) Pheeling the pHorce. Neuron 112:1200–1202. 10.1016/j.neuron.2024.03.023 [DOI] [PubMed] [Google Scholar]
- Johnson KO (2001) The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11:455–461. 10.1016/S0959-4388(00)00234-8 [DOI] [PubMed] [Google Scholar]
- Kang S, Jang JH, Price MP, Gautam M, Benson CJ, Gong H, Welsh MJ, Brennan TJ (2012) Simultaneous disruption of mouse ASIC1a, ASIC2 and ASIC3 genes enhances cutaneous mechanosensitivity. PLoS One 7:e35225. 10.1371/journal.pone.0035225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L (2015) International union of basic and clinical pharmacology. XCI. Structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol Rev 67:1–35. 10.1124/pr.114.009225 [DOI] [PubMed] [Google Scholar]
- Krishtal O (2015) Receptor for protons: first observations on acid sensing ion channels. Neuropharmacology 94:4–8. 10.1016/j.neuropharm.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Lazdunski M (2013) Pharmacology of ASIC channels. WIREs Membr Transp Signal 2:155–171. 10.1002/wmts.88 [DOI] [Google Scholar]
- Maksimovic S, et al. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509:617–621. 10.1038/nature13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel F (1875) Tastzellen and Tastkoerperchen bei den Hausthieren und beim Menschen. Arch Mikrosc Anat 11:636–652. 10.1007/BF02933819 [DOI] [Google Scholar]
- Omerbasic D, Schuhmacher LN, Bernal Sierra YA, Smith ES, Lewin GR (2015) ASICs and mammalian mechanoreceptor function. Neuropharmacology 94:80–86. 10.1016/j.neuropharm.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Page AJ, et al. (2004) The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology 127:1739–1747. 10.1053/j.gastro.2004.08.061 [DOI] [PubMed] [Google Scholar]
- Price MP, et al. (2000) The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407:1007–1011. 10.1038/35039512 [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32:1071–1083. 10.1016/S0896-6273(01)00547-5 [DOI] [PubMed] [Google Scholar]
- Prigg T, Goldreich D, Carvell GE, Simons DJ (2002) Texture discrimination and unit recordings in the rat whisker/barrel system. Physiol Behav 77:671–675. 10.1016/S0031-9384(02)00917-4 [DOI] [PubMed] [Google Scholar]
- Roza C, Puel JL, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R (2004) Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol 558:659–669. 10.1113/jphysiol.2004.066001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin M, Ho CY, Abraira VE, Cassidy C, Bai L, Woodbury CJ, Ginty DD (2015) The cellular and molecular basis of direction selectivity of Aδ-LTMRs. Cell 160:1027. 10.1016/j.cell.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Sonekatsu M, Kanno S, Yamada H, Gu JG (2020) Selective impairment of slowly adapting type 1 mechanoreceptors in mice following vincristine treatment. Neurosci Lett 738:135355. 10.1016/j.neulet.2020.135355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386:173–177. 10.1038/386173a0 [DOI] [PubMed] [Google Scholar]
- Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA (2010) The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol 103:3378–3388. 10.1152/jn.00810.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, et al. (2014) Piezo2 is required for Merkel-cell mechanotransduction. Nature 509:622–626. 10.1038/nature13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Ling J, Yamada AI, Furue H, Gu JG (2024) ASICs mediate fast excitatory synaptic transmission for tactile discrimination. Neuron 112:1286–1301 e1288. 10.1016/j.neuron.2024.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Yamada AI, Ling J, Furue H, Luo W, Gu JG (2023) Properties of Nav1.8(ChR2)-positive and Nav1.8(ChR2)-negative afferent mechanoreceptors in the hindpaw glabrous skin of mice. Mol Brain 16:27. 10.1186/s13041-023-01015-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD (2014) The gentle touch receptors of mammalian skin. Science 346:950–954. 10.1126/science.1254229 [DOI] [PMC free article] [PubMed] [Google Scholar]