Abstract
Purpose:
The purpose of this study was to examine the association between determinants of health, medication engagement, and A1C levels in adults with type 2 diabetes (T2DM) receiving Tribal health and pharmacy services.
Methods:
A retrospective analysis of 2020–2021 electronic health record (EHR) data was conducted and included adult patients with T2DM using Choctaw Nation Health Services Authority (CNHSA) prescribed ≥1 non-insulin glucose-lowering medication in 2020, had ≥1 A1C value in 2020 and 2021, and a valid zip code in 2021. Patients receiving both insulin and other non-insulin glucose-lowering medication were included. The proportion of days covered (PDC) was used to calculate medication engagement. Statistical analyses included bivariate analysis and linear regression.
Results:
There were 3787 patients included in the analyses; 62.5% were considered engaged (PDC≥0.8). The mean 2020 A1C level was 8.0 (64 mmol/mol) ±1.8; 33% had an A1C of <7%, 42% had an A1C 7–9%, and 25% had an A1C >9%. The mean A1C in 2021 was 7.9 (63 mmol/mol) ±1.7; 34% had an A1C of <7%, 44% had an A1C 7–9%, and 22% had an A1C >9%. Older age was weakly correlated with higher engagement; higher engagement was associated with lower A1C levels while adjusting for covariates.
Conclusions:
Medication engagement was associated with lower A1C levels, older age was weakly associated with higher engagement to non-insulin glucose-lowering medications, consistent with previous literature. No determinants of health were significantly associated with A1C levels while adjusting for covariates.
Taking medication is often a vital aspect of managing chronic diseases. Low engagement to diabetes medications has an estimated annual economic impact of over $5 billion in the United States (U.S.).1 Low medication engagement can be affected by social determinants of health (SDOH)2 and influence A1C levels3 and risk for diabetes-related complications such as cardiovascular disease and death.4 The determinants of medication engagement and the relationship between engagement and A1C levels are not well understood in American Indian adults with type 2 diabetes (T2DM), especially those who receive health care and pharmacy services within a rural Tribal health care system.
Medication engagement, a term in line with person-centered language in diabetes care5, will be used to describe what has been historically referred to as medication adherence or medication compliance in the scientific literature. According to a systematic review, engagement with oral glucose-lowering medication reportedly ranged from 36% to 93% in patients with diabetes6; however, these studies seldom included American Indian or other Indigenous peoples. Little research has examined medication engagement among patients served by Tribal health care systems or the Indian Health Service (IHS). Members of federally-recognized Tribes are entitled to federally funded health care under treaties negotiated between the U.S. government and their respective nations.7 Therefore, services and medications provided through IHS or Tribal health care systems do not require patient co-payments.
Our 2017–2018 preliminary study used pharmacy fill data in a Tribal health system, and found that medication engagement was associated with lower A1C levels.8 Also in this study, the majority of patients met the threshold for medication engagement (PDC ≥ 80%) for all included oral glucose lowering medication classes (62–83%).8 In a non-rural commercial health care system, engagement with oral glucose-lowering medication was lower and A1C levels were higher among American Indian adults compared to non-Hispanic white adults.9 Glucose-lowering medication engagement was reported as low to medium in several studies among American Indian adults who used the same self-report medication engagement measure.10–12
Previous studies have identified numerous factors that influence medication engagement. Although overall findings have been mixed,3,13 age and sex or gender may influence medication engagement. Among American Indian adults, not taking diabetes medication as prescribed has been associated with younger age and female sex or gender.11 Also, use of T2DM-related primary care and diabetes care and education specialist visits may present opportunities for patient education3 that can lead to higher medication engagement. Although the evidence is inconsistent, travel barriers may be associated with reduced or delayed medication use14 whereas higher socioeconomic status (SES) has been associated with improved medication engagement.13
The electronic health record (EHR)-derived determinants of health were examined which included age, sex, frequency of visits with a diabetes care and education specialist and T2DM-related primary care provider as well as SDOH which included travel distance to the nearest clinic and SES. The World Health Organization (WHO) defines SDOH as the non-medical factors that impact a person’s health outcomes.15 Guided by the WHO SDOH framework,15 we developed a conceptual framework for this study shown in Figure 1.
Figure 1. Conceptual Framework.
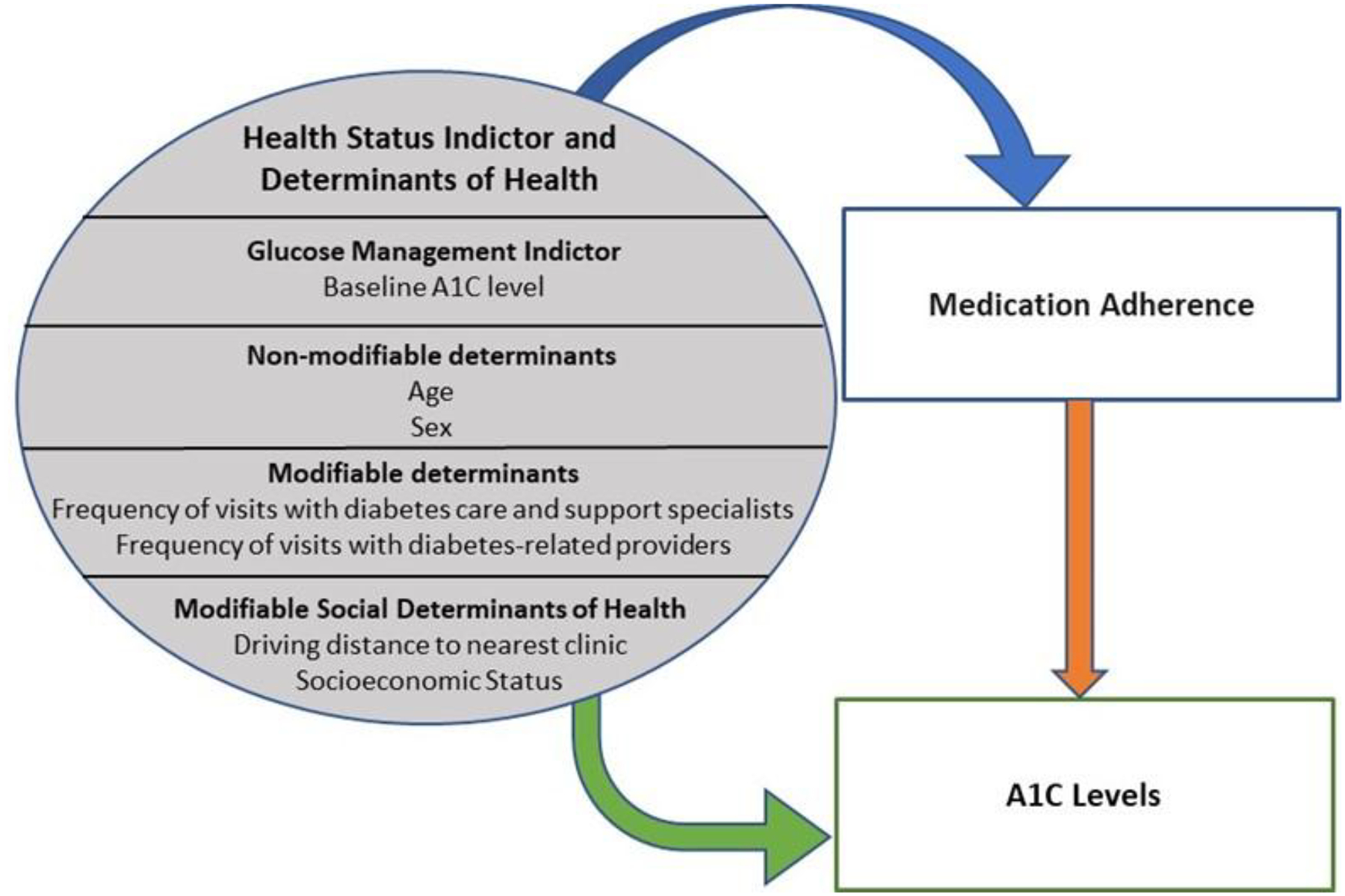
Copyrighted by Tarah Nelson
The purpose of this study was to examine the association among EHR-derived determinants of health, medication engagement, and A1C levels in American Indian adults with T2DM receiving Tribal health and pharmacy services. Our hypothesis was that higher non-insulin glucose-lowering medication engagement would be associated with male sex, older age, closer distance to the nearest clinic, more frequent primary care and diabetes care and education specialist visits, higher SES, and lower A1C levels.
Methods
Study Design
A retrospective, correlational study design was used to answer the research question, what is the relationship between medication engagement, A1C levels, and EHR-derived determinants of health., which was based on theory15,16 and literature-based evidence.3,11,13,14,17 We analyzed the 2020–2021 EHR data from an available 2017–2021 limited dataset provided by the Choctaw Nation Health Services Authority (CNHSA). Medication engagement and baseline A1C were assessed in 2020 while the remaining variables were assessed in 2021. The Choctaw Nation of Oklahoma (CNO) Institutional Review Board and University of Florida IRB approved the study. Due to Tribal data sovereignty, we do not have permission to share the dataset.
Sample
Adults ≥18 years of age on the first date of the overall 2017–2021 dataset, January 1, 2017, with a documented diagnosis of T2DM, at least one A1C level drawn in 2020 and in 2021 at CNHSA, and a valid zip code in 2021 were included in the study. Most patients who use CNHSA are members of CNO or another federally recognized tribe. A small proportion of CNHSA patients are family members of a Tribal member or other patients authorized to use CNHSA for limited services. Study inclusion and exclusion criteria are listed in Table 1. Patients diagnosed with end-stage renal disease (ESRD) were excluded due to the unreliability of A1C and numerous changes to medication that often take place in the setting of ESRD.18 Patients who were only dispensed insulin were excluded due to difficulty calculating an accurate measure of insulin engagement.19
Table 1.
Inclusion and Exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients 18 years or older | Patients diagnosed with end-stage renal disease (ESRD) |
|
Dispensed ≥1 non-insulin glucose-lowering medication in 2020 from CNHSA pharmacy Met the TEN-SPIDERS-derived criteria (Table 2)29 Patients dispensed insulin in addition to other non-insulin glucose-lowering medication were included but engagement to insulin was not calculated |
Patients with a diagnosis code of pure hyperglyceridemia, other specified diabetes mellitus, and diabetes mellitus due to underlying condition with diabetic chronic kidney disease Patients who were only dispensed insulin |
Setting
CNO consists of an 11,000 square mile area that includes 10.5 counties in Southeastern Oklahoma.20 There are over 225,000 Choctaw Nation enrolled tribal members.21 The CNHSA consists of a 44-bed hospital, 8 outlying clinics, and pharmacies with a service area spanning 10.5 counties.22,23
Data Extraction
CNHSA staff members extracted a dataset that included the International Classification of Diseases, Tenth Revision (ICD-10) codes,24 sociodemographic data, laboratory A1C values, pharmacy dispensing data, and T2DM-related health care visit information. Except for zip codes, the data were de-identified.
Measures
Medication engagement.
PDC was used to assess engagement to medications in 2020. PDC was analyzed as a continuous variable with a range of 0–1. The PDC threshold endorsed by the Pharmacy Quality Alliance was used: ≥80% engaged and <80% not engaged.25 PDC is one of the primary engagement measures for research and is considered a more conservative measure of medication engagement than another widely used measure called Medication Possession Ratio (MPR) since it does not include overlapping days of medication.26 Although an estimate of the proportion of days that patients have access to a medication over a specified period of time, variation in approaches to calculating PDC may yield different outcomes.27,28
Medication engagement was calculated for patients who were dispensed non-insulin glucose-lowering medication through CNHSA pharmacies. The TEN-SPIDERS tool was used to develop the PDC parameter definitions that were adapted for this study and outlined in Table 2.29
Table 2.
Approach used to calculate the medication engagement measure called the proportion of days covered (PDC) using the TEN-SPIDERS tool.29
| Parameter | Our Definition |
|---|---|
| Threshold |
|
| Eligibility criteria for inclusion in sample |
|
| Numerator and denominator |
|
| Survival |
|
| Pre-supply |
|
| In-hospital supply |
|
| Dosing information |
|
| Early refills |
|
| Switching |
|
Copyrighted by the T2DM and Cardiometabolic Control Research Team
A1C levels.
Glycemic status was assessed using reliable and validated EHR-derived laboratory values.30 The mean 2020 A1C value for each patient was used as the baseline measure of A1C (health status indicator) while the mean of the 2021 A1C value was used as the outcome variable.
Determinants of Health.
The dataset included the following modifiable and non-modifiable determinants of health including SDOH.
SES.
Medical Assistance (Medicaid status) as listed in the EHR for patients was used as a surrogate for SES. Medicaid status is a common proxy for SES in EHR-derived research. EHR data often offer limited self-reported SES measures.31 An individual or family qualifies for Medicaid if their income is at or below the 138% Federal Poverty Level (FPL), eligibility for which is determined by income and household size.32 SES was defined as low (active Medicaid) or not low (no active Medicaid). Medicaid expansion benefits began in Oklahoma on July 1, 2021 for those with income at or below 138% of the FPL.33 Therefore, each patient’s final Medicaid status listed in the EHR in 2021 was used for this study.
Driving distance to the nearest CNHSA primary care clinic.
The 2021 EHR-derived individual zip codes were used to generate the geographic centroid of each zip code at baseline.34 The geographic centroid of each zip code, in turn, was used to estimate the location of each patient’s primary residence and the exact addresses for each CNHSA clinic. One way driving distance in miles to the nearest CNHSA clinic was calculated using Google Maps™ on the basis of the geocoded latitude and longitudes of individual zip codes and clinic addresses.34
Frequency of visits with diabetes care and education specialists.
The 2021 visit information was used to determine the number of diabetes care and education specialist visits for all patients in 2021.
Frequency of T2DM-related primary care visits.
The 2021 EHR-derived ICD-10 codes and visit information were used to determine the number of T2DM-related visits with a T2DM-related primary care provider for all patients in 2021. In CNHSA, both primary care providers and endocrinologists provide T2DM-related primary care. Some providers also worked in the Emergency Department (ED) so ED visits may also have been included in this calculation. Of note, minimal CNHSA telehealth visits occurred during the COVID-19 pandemic.
Patient characteristics.
Age, sex, and race was assessed using the first value in the 2021 EHR dataset.
Statistical Analyses
Medication engagement and baseline A1C were assessed in 2020 while the remaining variables were assessed in 2021. Sex, age, and race were collected at baseline in 2021, mean A1C levels were collected in 2020 and 2021, and pharmacy dispensing records collected in 2020. The number of T2DM-related primary care and diabetes care and education specialist visits was collected in 2021, and the last recorded Medicaid status in 2021 was used to determine the SES variable.
All statistical analyses were performed using SAS software version 9.4. Descriptive statistics were used to describe the determinants of health and A1C levels. Chi-square tests of independence were used for categorical data. Continuous and ordinal data were examined using independent t tests, Pearson’s correlations, and logistic regression. The association between PDC and A1C was assessed using a linear regression model while controlling for covariates. Statistical significance was set at a two-sided alpha of 0.05.
Results
The study sample included 3,787 patients of 10,506 patients with T2DM in the 2017–2021 CNHSA EHR dataset. Of the 10,506 patients, those who met the following criteria were excluded: <18 years of age, a diagnosis of ESRD (N18.6), hyperglyceridemia (E78.1), a diagnosis of other specified diabetes mellitus (E13), a diagnosis of diabetes mellitus due to underlying condition with diabetic chronic kidney disease (E08.22). Medication engagement and baseline A1C were assessed in 2020 while the remaining variables were assessed in 2021. First, patients with data in 2021 were examined and after applying these exclusions, 8,244 patients remained with EHR data in 2021. An additional 217 patients who did not have valid zip code data in 2021 (2.6%), 2,721 patients who did not have a A1C level drawn in 2020 or 2021 (33%), and 1,519 patients who did not meet the criteria (Table 2) to have engagement measured for ≥1 non-insulin glucose-lowering medication in 2020 (18.4%) were excluded.
The sample was 53.2% female and 99.8% American Indian. Mean age was 59.0±12.5 years; 14.9% received Medicaid Assistance, indicating low SES. The average one-way commute to the nearest CNHSA primary care clinic was 31±77 miles with a median of 13.5 with an interquartile range of 3.9 to 28.9 miles. The mean number of primary care visits in 2021 was 3.0±1.9 and the mean number of diabetes care and education specialist visits in 2021 was 0.67±0.96. The mean PDC in 2020 was 0.8±0.2. More detailed descriptive statistics as well as descriptive statistics by PDC engagement levels (<0.8 versus ≥0.8) can be found in Table 3. The bivariate associations between medication engagement (PDC) and age, sex, SES, driving distance to the nearest CNHSA clinic, and number of primary care visits, were statistically significant (Table 3).
Table 3.
Sample characteristics by mean proportion of days covered (PDC) calculated for each patient in 2020 (N=3787).
| 2020 Mean PDC | ||||
|---|---|---|---|---|
| Characteristics n (%) | Total n (%) | <0.8 | ≥0.8 | P-value |
| Total patients | 3787 | 1422 (37.5) | 2365 (62.5) | |
| Age (y) mean (SD) | 59.0 (12.5) | 56.0 (12.9) | 60.9 (11.8) | <0.001a |
| Patient sex | <0.001b | |||
| Female | 2016 (53.2) | 811 (40.2) | 1205 (59.8) | |
| Male | 1771 (46.8) | 611 (34.5) | 1160 (65.5) | |
| Racec | ||||
| American Indian | 3778 (99.8) | 1419 (37.6) | 2359 (62.4) | |
| Otherd | 7 (0.2) | 2 (28.6) | 5 (71.4) | |
| SES | <0.001b | |||
| Low SES | 565 (14.9) | 275 (48.7) | 290 (51.3) | |
| Not low SES | 3222 (85.1) | 1147 (35.6) | 2075 (64.4) | |
| Driving distance to nearest clinic | <0.001e | |||
| <5 miles | 1536 (40.6) | 637 (41.5) | 899 (58.5) | |
| 5–<25 miles | 1173 (31.0) | 412 (35.1) | 761 (64.9) | |
| 25–<50 miles | 556 (14.7) | 201 (36.2) | 355 (63.8) | |
| ≥50 miles | 522 (13.8) | 172 (33.0) | 350 (67.0) | |
| PC visits per year | <0.001e | |||
| 0 | 160 (4.2) | 66 (41.2) | 94 (58.8) | |
| 1–2 | 1540 (40.7) | 642 (41.7) | 898 (58.3) | |
| 3–5 | 1696 (44.8) | 584 (34.4) | 1112 (65.6) | |
| >5 | 391 (10.3) | 130 (33.2) | 261 (66.8) | |
| DCES visits | 0.18b | |||
| Yes | 1800 (47.5) | 656 (36.4) | 1144 (63.6) | |
| No | 1987 (52.5) | 766 (38.6) | 1221 (61.4) | |
Abbreviations: DCES, diabetes care and education specialist; PC, primary care; SES, socioeconomic status.
Independent t-test.
Chi-Square test.
2 participants’ data were missing.
Limited services available to non-eligible patients.
Logistic Regression.
A1C Levels
The mean 2020 A1C level was 8.0 (64 mmol/mol) ±1.8 with a range of 4.9% (30 mmol/mol) to 17.2% (164 mmol/mol). In 2020, 33% of the participants had a A1C of <7%, 42% had a A1C 7–9%, and a total of 25% had a A1C >9%. The mean A1C in 2021 was 7.9 (63 mmol/mol) ±1.7 (range 4.6% (27 mmol/mol) to 16.2% (154 mmol/mol)). Thirty-four percent of patients had a A1C of <7%, 44% had a A1C 7–9%, and a total of 22% had a A1C >9%.
Engagement to Non-Insulin Glucose-Lowering Medications
The most frequently prescribed medication classes in 2020 were biguanides (81.1%), sulfonylureas (36.5%), and GLP-1 receptor agonists (17.0%) (Table 4). Patients were most likely to be engaged to DD4-inhibitors (72%) and meglitinides (71%) and least likely to be engaged to biguanides (62%) and alpha-glucosidase inhibitors (65%). The non-insulin glucose lowering medication classes with the highest mean PDC were DPP-4 inhibitors (0.84 ± 0.20) and TZDs (0.84 ± 0.19) and the medication with the lowest mean PDC were biguanides (0.80 ± 0.21).
Table 4.
Non-insulin glucose lowering medications dispensed and medication engagement in 2020.
| PDC in 2020 | |||
|---|---|---|---|
| Medication class | Medications dispensed, n (%) | % ≥0.80a | Mean ± SD |
| Alpha-Glucosidase Inhibitors | 40 (1.1) | 65% | 0.83±0.17 |
| Biguanides | 3073 (81.1) | 62% | 0.80±0.21 |
| DPP-4 Inhibitors | 629 (16.6) | 72% | 0.84±0.20 |
| GLP-1 Receptor Agonists | 645 (17.0) | 67% | 0.81±0.20 |
| Meglitinides | 17 (0.4) | 71% | 0.83±0.24 |
| SGLT-2 Inhibitors | 203 (5.4) | 70% | 0.83±0.20 |
| Sulfonylureas | 1381 (36.5) | 66% | 0.82±0.20 |
| TZDs | 481 (12.7) | 69% | 0.84±0.19 |
Abbreviations: PDC, proportion of days covered; TZDs, Thiazolidinediones.
PDC≥0.8=threshold for medication engagement.
Determinants of Health, Medication Engagement, and A1C Levels
There was a weak, positive correlation between age and PDC (r=.21 P<.001). Also, a weak, negative correlation between age and 2020 mean A1C (r=−.17, P<.001) as well as age and mean A1C in 2021 (r=−.15, P<.001), and a weak, positive correlation between age and number of primary care visits (r=.20, P<.001), were observed (Table 5). There was a weak, positive correlation between diabetes care and education specialist visits and primary care visits (r=.19, P<.001) and a weak, negative correlation with distance to the nearest clinic (r=−.12, P<.001). PDC was weakly, negatively correlated with both A1C in 2020 (r=−.23, P<.001) and with A1C in 2021 (r=−.24, P<.001). There was a strong, positive correlation between A1C in 2020 and in 2021 (r=.70, P<.001).
Table 5.
Correlations among study variables.
| Age | Distance to nearest clinica | PC visits | DCES visits | 2020 mean A1C | 2021 mean A1C | |
|---|---|---|---|---|---|---|
| Distance to nearest clinica | .02 | |||||
| PC visits | .20*** | −.07*** | ||||
| DCES visits | .02 | −.12*** | .19*** | |||
| 2020 mean A1C | −.17*** | −.03* | .10*** | .16*** | ||
| 2021 mean A1C | −.15*** | −.05** | .06*** | .14*** | .70*** | |
| 2020 PDC | .21*** | .06*** | .08*** | .009 | −.23*** | −.24*** |
Abbreviations: DCES, diabetes care and education specialist; PC, primary care; PDC, proportion of days covered.
log transformed.
P < 0.05;
P < 0.01;
P < 0.001.
Medication Engagement and A1C Levels
The association of medication engagement with A1C levels is described in Table 6. Nonengaged patients (PDC<0.8) had a higher mean A1C (8.42 (69 mmol/mol) ±1.98) compared to the mean A1C (7.65 (60 mmol/mol) ±1.46) for engaged patients (PDC≥0.8) (P<.001). Higher engagement was also associated with lower A1C levels (β=−.67, P<.001) in a linear regression model while adjusting for covariates (Table 7). Mean A1C in 2020, a proxy for prior T2DM severity, was positively associated with mean A1C levels in 2021 (β=.66, P<.001) while adjusting for other covariates. No determinants of health were significantly associated with mean A1C levels in 2021 adjusting for other covariates.
Table 6.
Association between medication engagement and A1C levels.
| PDCa | n (%) | 2021 A1C (Mean±SD) | 95% CI | P-value |
|---|---|---|---|---|
| <0.80 | 1422 (37.5) | 8.42 (69 mmol/mol) ±1.98 | (8.32,8.53) | <0.001b |
| ≥0.80 | 2365 (62.5) | 7.65 (60 mmol/mol) ±1.46 | (7.59,7.71) |
Abbreviations: PDC, proportion of days covered.
PDC≥0.8=threshold for medication engagement.
Independent t-test.
Table 7.
Association between a health status indicator (2020 mean A1C), medication engagement, determinants of health, and 2021 A1C levels.
| Health status indicator, medication engagement & determinants of Health | Estimate | SE | t | P-value |
|---|---|---|---|---|
| 2020 mean A1C | 0.659 | 0.012 | 55.4 | <0.001* |
| 2020 PDC | −0.667 | 0.102 | −6.54 | <0.001* |
| Age | −0.003 | 0.002 | −1.62 | 0.105 |
| Female sex | −0.036 | 0.040 | −0.91 | 0.363 |
| Male sexa | ||||
| Not low SES | −0.043 | 0.057 | −0.74 | 0.459 |
| Low SESa | ||||
| Nearest clinic <5 miles | 0.082 | 0.063 | 1.30 | 0.195 |
| Nearest clinic 5–<25 miles | 0.035 | 0.065 | 0.54 | 0.590 |
| Nearest clinic 25–<50 miles | −0.033 | 0.075 | −0.44 | 0.663 |
| Nearest clinic ≥50 milesa | ||||
| No PC visits | −0.110 | 0.116 | −0.95 | 0.343 |
| 1–2 PC visits | −0.013 | 0.071 | −0.19 | 0.852 |
| 3–5 PC visits | −0.018 | 0.069 | −0.26 | 0.796 |
| >5 PC visitsa | ||||
| No DCES visits | −0.072 | 0.041 | −1.77 | 0.773 |
| DCES visitsa |
Abbreviations: DCES, diabetes care and education specialist; PC, primary care; PDC, proportion of days covered; SES, socioeconomic status.
reference group.
P <0.05.
Discussion
Medication engagement was associated with lower A1C levels in American Indian adults with T2DM receiving non-insulin glucose-lowering medication without copayments. These findings are consistent with our hypothesis as well as other studies.35,36 The percentage of the sample that were not engaged (37.5%) was similar to the mean poor medication engagement rate (37.8%) in a metanalysis examining the association between engagement to glucose lowering medications and outcomes in patients with T2DM.37 In our bivariate analysis, older age was weakly associated with increased engagement to non-insulin glucose-lowering medications. None of the included EHR-derived determinants of health were significantly associated with A1C after controlling for medication engagement.
The established engagement threshold of PDC ≥ 80% was used to assess the characteristics of patients engaged and not engaged to non-insulin glucose-lowering medication. Evidence supports that patients who achieve or surpass this threshold have lower hospitalization rates as well as lower health care costs overall.38,39 Consistent with the hypothesis, patients who met or exceeded the threshold for engagement had an older mean age. Also consistent with the hypothesis, a lower proportion of women were engaged compared to men. This finding is consistent with some prior studies but overall the evidence is generally inconclusive.11,13,40 The proportion of patients that were engaged versus not engaged was similar for patients deemed low SES, whereas a much larger proportion of patients with higher SES were engaged versus not engaged. Higher income may be a facilitator of medication engagement.13,40
Although there was a weak correlation between distance and medication engagement, living close to a CNHSA clinic was weakly associated with lower engagement to non-insulin glucose-lowering medication. This finding suggests that a longer driving distance was not associated with lower medication engagement in the sample. This lack of association may be attributed to the availability of a mail-order pharmacy option at CNHSA, which has been associated with medication engagement in previous studies.40 Further analysis is needed to examine engagement for those who use mail-order pharmacy services compared to those who pick up medications. Patients with ≥3 primary care visits were more likely to be engaged than those with fewer (≤2) visits. A possible explanation may be that more frequent T2DM-related primary care visits provide opportunities to optimize treatment or provide education, which may facilitate engagement. While we expected patients with diabetes care and education specialist visits to have higher medication engagement, we found a similar proportion of those with at least one diabetes care and education specialist visit were engaged compared to those without a diabetes care and education specialist visit. This may be due to diabetes care and education specialist referrals are often for patients who have A1C levels above target or need T2DM-related education.41 The facilitators and barriers of medication engagement should be assessed from the patient perspective in future studies in the context of the local community.
The most commonly prescribed non-insulin glucose-lowering medication classes were biguanides, sulfonylureas, and GLP-1 receptor agonists. The finding that GLP-1 receptor agonists are a commonly dispensed medication is consistent with more recent medication guidelines for patients with T2DM.42 Patients were most engaged to DPP-4 inhibitors, which are generally well-tolerated medications.43 The medication class with the lowest percentage that met the engagement threshold was biguanides. A metanalysis noted similar findings and partly attributed low engagement to metformin to gastrointestinal side effects and high engagement to DPP-4 inhibitors to fewer side effects.44
Limitations
In addition to medication engagement, other factors that affect A1C including diet and exercise could not be assessed in a retrospective study using EHR data. The data spanned the height of the COVID-19 pandemic, which likely affected the typical number of patient encounters, medication engagement, laboratory draws (A1C levels) as well as primary care provider and diabetes care and education specialist visits. Minimal telehealth visits were conducted due to unreliable internet service. Many patients in the sample were excluded which may have reduced the representativeness of the sample. SES is a multidimensional construct and using Medicaid status may not have captured all patients who would otherwise be considered having low SES. This approach does not include patients who qualify for Medicaid but do not receive benefits, although it is not clear how prevalent this is in the CNHSA patient population. Due to the Medicaid expansion, many patients were newly eligible and may have not yet enrolled. Also, American Indian and Alaska Native people may be at increased risk for some barriers to Medicaid enrollment such as limited access to internet or as skepticism towards governmental programs.45 One limitation to engagement measures based on prescription refill data, like PDC, is that it cannot be confirmed from the EHR that the medication was consumed, only that the medication was picked up from the pharmacy.46 Necessary assumptions in the operationalization of PDC (Table 2) were made which may affect the accuracy of the PDC calculation. The exclusion of patients taking only insulin and not assessing engagement to insulin, limits the generalizability of the findings. We also did not have access to hospitalization data or survival data. It is possible that some gaps in engagement were due to hospitalizations.
There are many determinants of health not addressed due to their limited availability in EHR data. Specifically, housing and food insecurity and adverse SDOH collectively were associated with worse engagement to medication in a recent meta-analysis.2 There is evidence that incorporating individual-level SDOH into the EHR can aid in improving medication engagement.47 Future studies should focus on integrating SDOH into EHR systems in a culturally and linguistically manner appropriate to the local community.48
Conclusion
Engagement to non-insulin glucose-lowering medications was associated with lower A1C levels among patients receiving medication and health services without copayments from a Tribal health care system. Future studies should compare these findings to other years unimpacted by the COVID-19 pandemic. In the bivariate analysis, older age was weakly associated with engagement to non-insulin glucose-lowering medications, which is consistent with previous literature. No determinants of health were significantly associated with A1C levels. Future studies may need to go beyond the EHR to examine additional SDOH such as access to food, quality of care, transportation, and housing. This study helps to guide future research on determinants of health, medication engagement, and A1C levels in American Indian adults with T2DM using rural Tribal health care systems.
Acknowledgements
The authors would like to thank Choctaw Nation of Oklahoma for their support and contribution to this study. The authors would also like to acknowledge Dr. Michael Weaver at the University of Florida for his statistical support for this study.
Financial Support
This research was made possible by Grant Numbers 1R01NR020386-01 from the National Institutes of Health (NIH) and National Institute of Nursing Research (NINR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NINR. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy. The authors would also like to acknowledge the College of Nursing (CON) at the University of Florida (UF) for PhD scholarship support.
Footnotes
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
Contributor Information
Tarah Nelson, University of Florida, College of Nursing, Gainesville, FL.
Diana J. Wilkie, University of Florida, College of Nursing, Gainesville, FL.
Yingwei Yao, University of Florida, College of Nursing, Gainesville, FL.
Richard Segal, University of Florida, College of Pharmacy, Gainesville, FL.
Ashley DeVaughan-Circles, Choctaw Nation of Oklahoma, Choctaw Nation Health Services Authority, Talihina, OK.
William T. Donahoo, University of Florida, College of Medicine, Gainesville, FL.
R. Turner Goins, Western Carolina University, College of Health and Human Sciences, Cullowhee, NC.
Spero M. Manson, University of Colorado Anschutz Medical Campus, Centers for American Indian and Alaska Native Health, Aurora, CO.
Anatolia B. Legaspi, University of Florida, College of Pharmacy, Gainesville, FL
Lisa Scarton, University of Florida, College of Nursing, Gainesville, FL.
References
- 1.Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). Aug 2012;31(8):1836–46. doi: 10.1377/hlthaff.2011.1198 [DOI] [PubMed] [Google Scholar]
- 2.Wilder ME, Kulie P, Jensen C, et al. The Impact of Social Determinants of Health on Medication Adherence: a Systematic Review and Meta-analysis. J Gen Intern Med. May 2021;36(5):1359–1370. doi: 10.1007/s11606-020-06447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capoccia K, Odegard PS, Letassy N. Medication Adherence With Diabetes Medication: A Systematic Review of the Literature. Diabetes Educ. Feb 2016;42(1):34–71. doi: 10.1177/0145721715619038 [DOI] [PubMed] [Google Scholar]
- 4.Kim YY, Lee JS, Kang HJ, Park SM. Effect of medication adherence on long-term all-cause-mortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Sci Rep. Aug 15 2018;8(1):12190. doi: 10.1038/s41598-018-30740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson JK, Guzman SJ, Maryniuk MD, et al. The Use of Language in Diabetes Care and Education. Diabetes Care. Dec 2017;40(12):1790–1799. doi: 10.2337/dci17-0041 [DOI] [PubMed] [Google Scholar]
- 6.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. May 2004;27(5):1218–24. doi: 10.2337/diacare.27.5.1218 [DOI] [PubMed] [Google Scholar]
- 7.Skinner D The Politics of Native American Health Care and the Affordable Care Act. J Health Polit Policy Law. 2016;41(1):41–71. doi: 10.1215/03616878-3445601 [DOI] [PubMed] [Google Scholar]
- 8.Scarton L, Nelson T, Yao Y, et al. Association of Medication Adherence With HbA1c Control Among American Indian Adults With Type 2 Diabetes Using Tribal Health Services. Diabetes Care. Jun 1 2023;46(6):1245–1251. doi: 10.2337/dc22-1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmittdiel JA, Steiner JF, Adams AS, et al. Diabetes care and outcomes for American Indians and Alaska natives in commercial integrated delivery systems: a SUrveillance, PREvention, and ManagEment of Diabetes Mellitus (SUPREME-DM) Study. BMJ Open Diabetes Res Care. 2014;2(1):e000043. doi: 10.1136/bmjdrc-2014-000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs A, Kemppainen JK, Taylor JS, Hadsell C. Beliefs About Diabetes and Medication Adherence Among Lumbee Indians Living in Rural Southeastern North Carolina. J Transcult Nurs. 2014;25(2):167–175. doi: 10.1177/1043659613515718 [DOI] [PubMed] [Google Scholar]
- 11.Aronson BD, Sittner KJ, Walls ML. The Mediating Role of Diabetes Distress and Depressive Symptoms in Type 2 Diabetes Medication Adherence Gender Differences. Health Educ Behav. Jun 2020;47(3):474–482. doi: 10.1177/1090198119885416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiyanbola OO, Nelson J. Illness perceptions, beliefs in medicine and medication non-adherence among South Dakota minority women with diabetes: a pilot study. S D Med. Oct 2011;64(10):365–8. [PubMed] [Google Scholar]
- 13.Gast A, Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst Rev. May 10 2019;8(1):112. doi: 10.1186/s13643-019-1014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. Oct 2013;38(5):976–93. doi: 10.1007/s10900-013-9681-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Social Determinants of Health. Accessed September 23, 2022, https://www.who.int/health-topics/social-determinants-of-health
- 16.Peh KQE, Kwan YH, Goh H, et al. An Adaptable Framework for Factors Contributing to Medication Adherence: Results from a Systematic Review of 102 Conceptual Frameworks. J Gen Intern Med. Sep 2021;36(9):2784–2795. doi: 10.1007/s11606-021-06648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratner NL, Davis EB, Lhotka LL, Wille SM, Walls ML. Patient-Centered Care, Diabetes Empowerment, and Type 2 Diabetes Medication Adherence Among American Indian Patients. Clin Diabetes. Dec 2017;35(5):281–285. doi: 10.1155/2018/2742565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinaki Z, Kapnisi S, Theodorelou-Charitou SA, Nikas IP, Paschou SA. Type 2 diabetes mellitus management in patients with chronic kidney disease: an update. Hormones (Athens). Dec 2020;19(4):467–476. doi: 10.1007/s42000-020-00212-y [DOI] [PubMed] [Google Scholar]
- 19.Stolpe S, Kroes MA, Webb N, Wisniewski T. A Systematic Review of Insulin Adherence Measures in Patients with Diabetes. J Manag Care Spec Pharm. Nov 2016;22(11):1224–1246. doi: 10.18553/jmcp.2016.22.11.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choctaw Nation of Oklahoma. Choctaw Nation Reservation. Accessed October 5, 2023. https://www.choctawnation.com/about/reservation/
- 21.Choctaw Nation of Oklahoma. About the Choctaw Nation. Accessed March 29, 2024, https://www.choctawnation.com/about/
- 22.Choctaw Nation of Oklahoma. Family Medicine Residency Program: About Choctaw Nation Health Services Authority. Accessed March 29, 2024, https://www.choctawnation.com/services/residency/
- 23.Choctaw Nation of Oklahoma. About: Health. Accessed March 29, 2024, https://www.choctawnation.com/about/health/
- 24.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Accessed October 9, 2023. https://icd.who.int/browse10/2019/en#/
- 25.Pharmacy Quality Alliance. Proportion of Days Covered: Diabetes All Classes (PDC-DR). https://www.pqaalliance.org/measures-overview#pdc-dr
- 26.Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. Jan 2009;43(1):36–44. doi: 10.1345/aph.1K671 [DOI] [PubMed] [Google Scholar]
- 27.Prieto-Merino D, Mulick A, Armstrong C, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract. Dec 29 2021;14(1):113. doi: 10.1186/s40545-021-00385-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pednekar PP, Ágh T, Malmenäs M, et al. Methods for Measuring Multiple Medication Adherence: A Systematic Review-Report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health. 02 2019;22(2):139–156. doi: 10.1016/j.jval.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 29.Dalli LL, Kilkenny MF, Arnet I, et al. Towards better reporting of the proportion of days covered method in cardiovascular medication adherence: A scoping review and new tool TEN-SPIDERS. Br J Clin Pharmacol. Oct 2022;88(10):4427–4442. doi: 10.1111/bcp.15391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights. 2016;11:95–104. doi: 10.4137/BMI.S38440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casey JA, Pollak J, Glymour MM, Mayeda ER, Hirsch AG, Schwartz BS. Measures of SES for Electronic Health Record-based Research. Am J Prev Med. Mar 2018;54(3):430–439. doi: 10.1016/j.amepre.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medicaid. Medicaid Eligibility. Accessed November 8, 2022, https://www.medicaid.gov/medicaid/eligibility/index.html
- 33.Oklahoma Health Care Authority. Understanding the basics of soonercare adult expansion. https://oklahoma.gov/content/dam/ok/en/okhca/docs/providers/training/2021/Understanding%20the%20Basics%20of%20SoonerCare%20Expansion.pdf
- 34.Weiss AJ PG, Roemer M. Methods for Calculating Patient Travel Distance to Hospital in HCUP Data. Quality. USAfHRa; 2021. December 6, 2021. Accessed November 28, 2022. www.hcup-us.ahrq.gov/reports/methods/methods.jsp. [Google Scholar]
- 35.Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. May 2014;48(5):562–70. doi: 10.1177/1060028014526362 [DOI] [PubMed] [Google Scholar]
- 36.Patel S, Abreu M, Tumyan A, Adams-Huet B, Li X, Lingvay I. Effect of medication adherence on clinical outcomes in type 2 diabetes: analysis of the SIMPLE study. BMJ Open Diabetes Res Care. 2019;7(1):e000761. doi: 10.1136/bmjdrc-2019-000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khunti K, Seidu S, Kunutsor S, Davies M. Association Between Adherence to Pharmacotherapy and Outcomes in Type 2 Diabetes: A Meta-analysis. Diabetes Care. Nov 2017;40(11):1588–1596. doi: 10.2337/dc16-1925 [DOI] [PubMed] [Google Scholar]
- 38.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). Jan 2011;30(1):91–9. doi: 10.1377/hlthaff.2009.1087 [DOI] [PubMed] [Google Scholar]
- 39.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. Jun 2005;43(6):521–30. doi: 10.1097/01.mlr.0000163641.86870.af [DOI] [PubMed] [Google Scholar]
- 40.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. Apr 2015;38(4):604–9. doi: 10.2337/dc14-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown-Podgorski BL, Shi Y, Vest JR. Patient need and provider referrals to diabetes self-management education. Am J Manag Care. Jun 1 2021;27(6):e201–e207. doi: 10.37765/ajmc.2021.88669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers. Clin Diabetes. Jan 2020;38(1):10–38. doi: 10.2337/cd20-as01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makrilakis K The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int J Environ Res Public Health. Jul 30 2019;16(15)doi: 10.3390/ijerph16152720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. Apr 2018;20(4):1040–1043. doi: 10.1111/dom.13160 [DOI] [PubMed] [Google Scholar]
- 45.Medicaid and Chip Payment and Access Commission. Medicaid’s Role in Health Care for American Indians and Alaska Natives. Accessed August 25, 2023, https://www.macpac.gov/wp-content/uploads/2021/02/Medicaids-Role-in-Health-Care-for-American-Indians-and-Alaska-Natives.pdf
- 46.Campbell PJ. Adherence: An Important Metric to Assess Pharmacist Value in Quality Care. J Manag Care Spec Pharm. Oct 2019;25(10):1044–1045. doi: 10.18553/jmcp.2019.25.10.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M, Tan X, Padman R. Social determinants of health in electronic health records and their impact on analysis and risk prediction: A systematic review. J Am Med Inform Assoc. Nov 1 2020;27(11):1764–1773. doi: 10.1093/jamia/ocaa143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andermann A Taking action on the social determinants of health in clinical practice: a framework for health professionals. Cmaj. Dec 6 2016;188(17–18):E474–e483. doi: 10.1503/cmaj.160177 [DOI] [PMC free article] [PubMed] [Google Scholar]


