Abstract
1. The breakdown of the adenine nucleotide pool provoked by the replacement of the O2/CO2 gas phase by N2/CO2 was studied in isolated rat hepatocytes with the purpose of defining the pathway of the catabolism of AMP in anoxic conditions. 2. Approx. 40% of the adenine nucleotide pool was lost after 40–60 min of anoxia. In hepatocytes from fed rats there was a slow disappearance of ATP. This is explained by the presence of glycogen stores, allowing the generation of ATP by anaerobic glycolysis. In hepatocytes from 24h-starved rats, ATP almost completely disappeared within 5 min, and was partly replaced by an accumulation of AMP. This indicates that another mechanism protects the adenine nucleotide pool in the starved state. In both conditions, the loss of adenine nucleotides was mainly accounted for by an accumulation of uric acid, owing to the oxygen-dependence of urate oxidase. 3. Incubation of the hepatocytes before the suppression of O2 with coformycin at concentrations known to inhibit selectively adenosine deaminase did not result in an accumulation of adenosine and did not influence the formation of uric acid. This indicates that the degradation of AMP does not proceed by way of 5′-nucleotidase under these conditions. In the presence of coformycin at concentrations which are inhibitory to AMP deaminase, however, the formation of uric acid was nearly suppressed, demonstrating that the initial degradation of AMP was catalysed by the latter enzyme. 4. The accumulation of AMP in the starved state can be explained by the pronounced decrease in ATP, the major stimulator of AMP deaminase, and the enhanced increase in Pi, one of its physiological inhibitors. The modifications of these effectors can also explain the increased inhibition of the cytoplasmic 5′-nucleotidase, shown by the accumulation of IMP in the absence of coformycin, in hepatocytes from starved rats. 5. Reoxygenation of the hepatocytes after 20 min of anoxia induced a prompt regeneration of ATP, which reached concentrations equal to the pre-existing concentration of AMP. 6. No explanation was found for the accumulation of IMP observed after preincubation of the hepatocytes with 0.1μm-coformycin, since the activities of the IMP-metabolizing enzymes were not influenced by this inosine analogue.
Full text
PDF

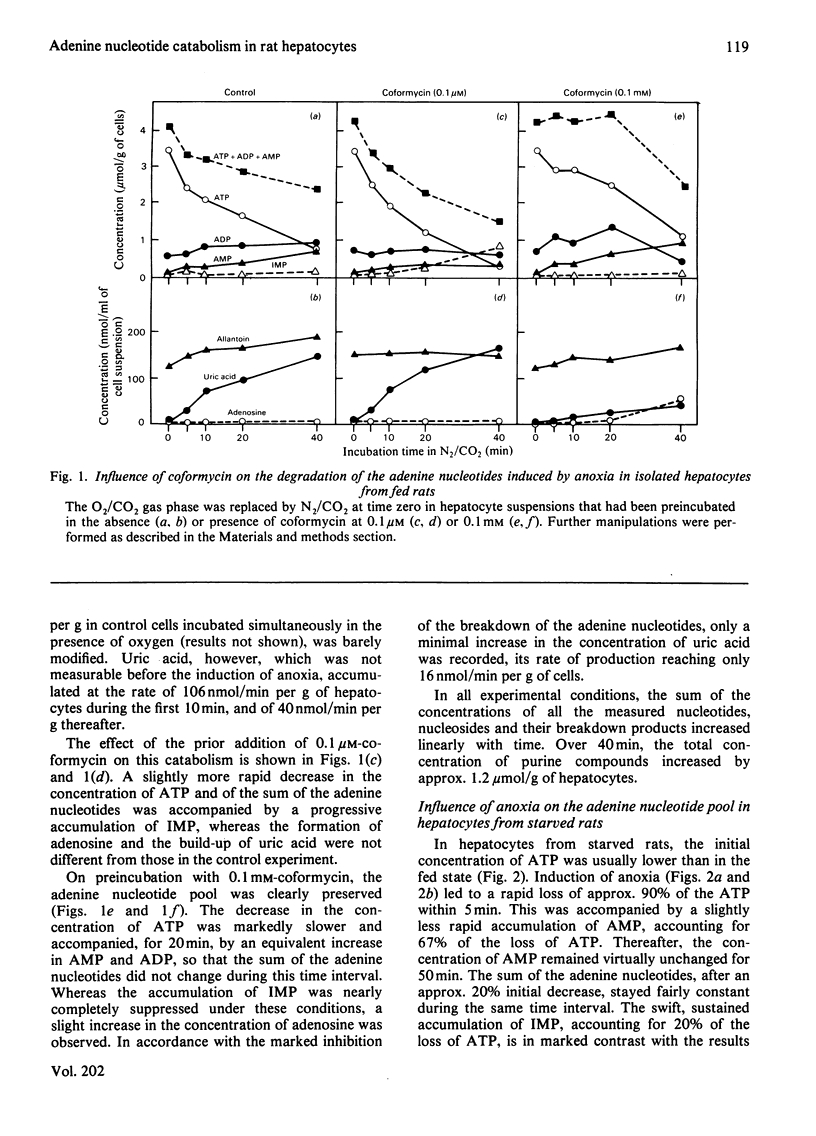
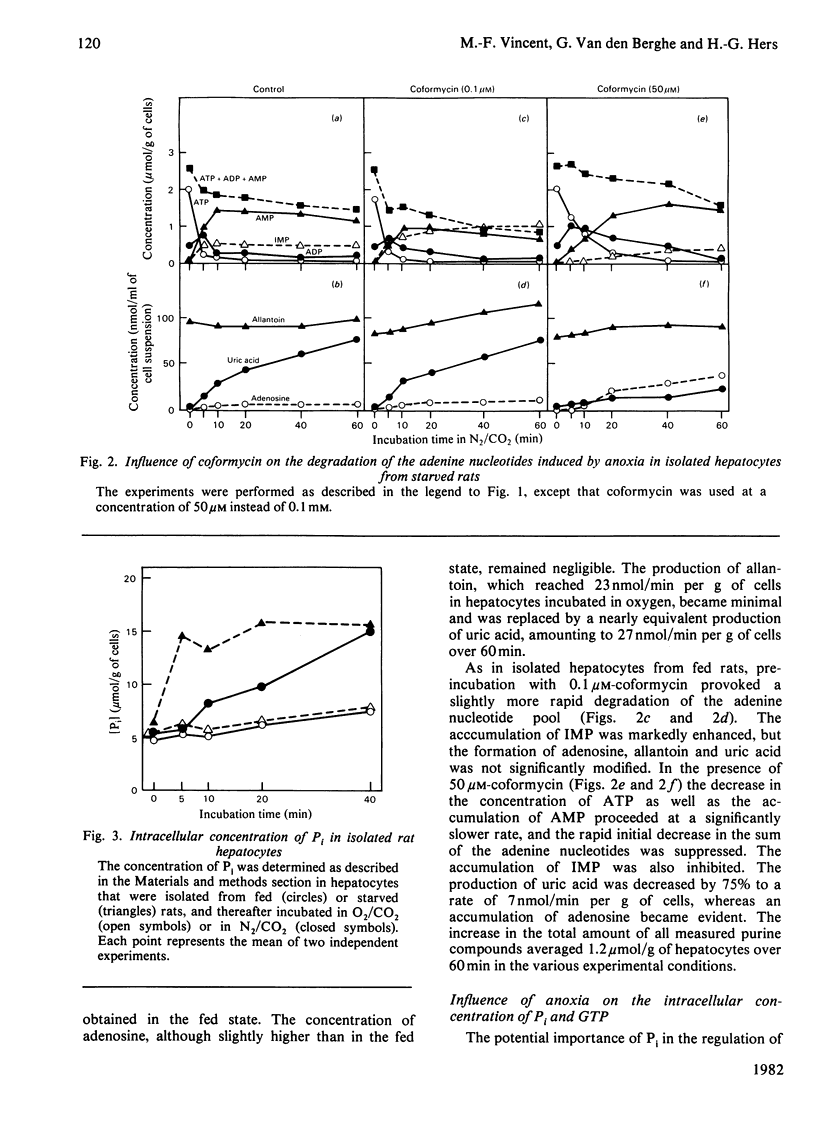
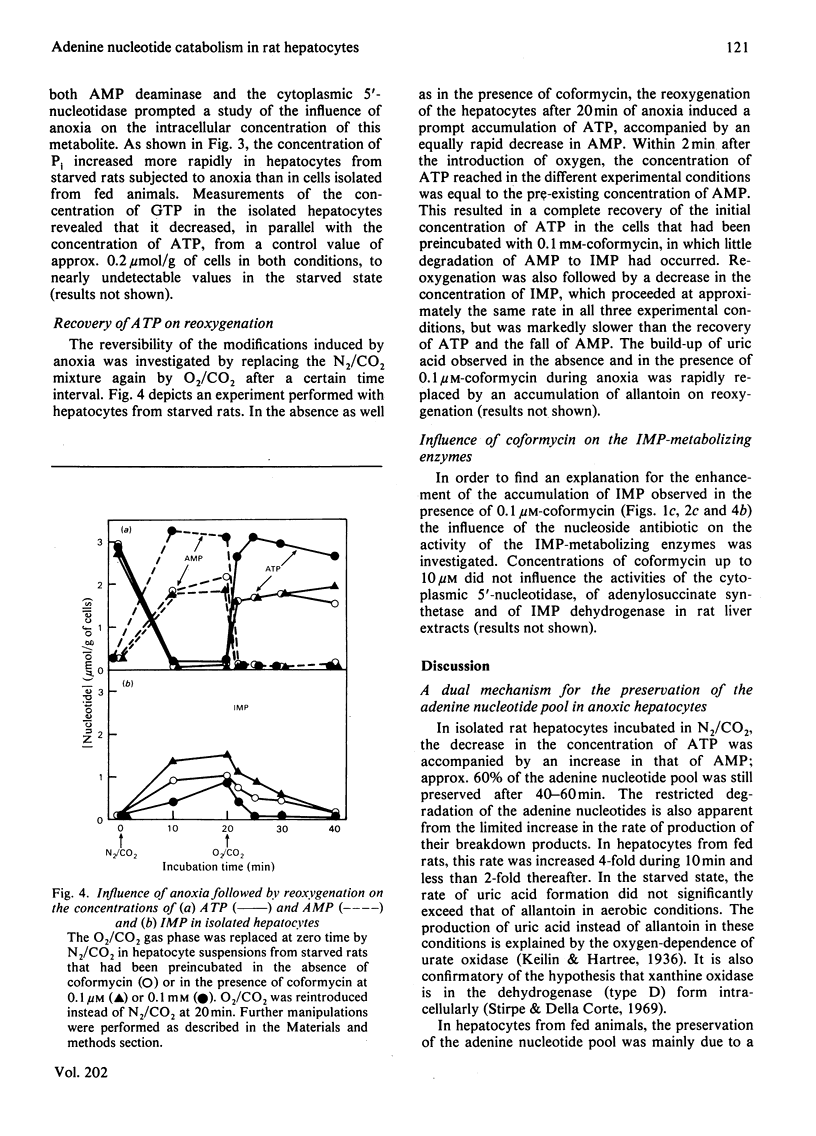


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E. Potent inhibition of muscle 5'-AMP deaminase by the nucleoside antibiotics coformycin and deoxycoformycin. Biochem Pharmacol. 1977 Apr 1;26(7):663–666. doi: 10.1016/0006-2952(77)90046-6. [DOI] [PubMed] [Google Scholar]
- Agarwal R. P., Sagar S. M., Parks R. E., Jr Adenosine deaminase from human erythrocytes: purification and effects of adenosine analogs. Biochem Pharmacol. 1975 Mar 15;24(6):693–701. doi: 10.1016/0006-2952(75)90245-2. [DOI] [PubMed] [Google Scholar]
- Bogusky R. T., Lowenstein L. M., Lowenstein J. M. The purine nucleotide cycle. A pathway for ammonia production in the rat kidney. J Clin Invest. 1976 Aug;58(2):326–335. doi: 10.1172/JCI108476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch E. W., von Borcke I. M., Martinez B. Abbauwege und Abbaumuster der Purinnucleotide in Herz-, Leber-, und Nierengewebe von Kaninchen nach Kreislaufstillstand. Biochim Biophys Acta. 1968 Sep 24;166(2):547–556. [PubMed] [Google Scholar]
- Deuticke B., Gerlach E., Dierkesmann R. Abbau freier Nucleotide in Herz, Skeletmuskel, Gehirn und Leber der Ratte bei Sauerstoffmangel. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(3):239–254. [PubMed] [Google Scholar]
- Frieden C., Gilbert H. R., Miller W. H., Miller R. L. Adenylate deaminase: potent inhibition by 2'-deoxycoformycin 5'-phosphate. Biochem Biophys Res Commun. 1979 Nov 14;91(1):278–283. doi: 10.1016/0006-291x(79)90614-4. [DOI] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970 Nov;120(1):105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R., Usami C., Nishino T., Tsushima K. Kinetic properties of cytosol 5'-nucleotidase from chicken liver. Biochim Biophys Acta. 1978 Sep 11;526(1):154–162. doi: 10.1016/0005-2744(78)90300-5. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Boritzki T. J., Morris H. P., Weber G. Purine and pyrimidine ribonucleotide contents of rat liver and hepatoma 3924A and the effect of ischemia. Life Sci. 1976 Nov 15;19(10):1531–1536. doi: 10.1016/0024-3205(76)90098-9. [DOI] [PubMed] [Google Scholar]
- Reibel D. K., Rovetto M. J. Separation of myocardial purine nucleosides and nucleotides by one-dimensional thin-layer chromatography. J Chromatogr. 1978 Nov 21;161:406–409. doi: 10.1016/s0021-9673(01)85263-8. [DOI] [PubMed] [Google Scholar]
- Sawa T., Fukagawa Y., Homma I., Takeuchi T., Umezawa H. Mode of inhibition of coformycin on adenosine deaminase. J Antibiot (Tokyo) 1967 Jul;20(4):227–231. [PubMed] [Google Scholar]
- Sharma R. J., Rodrigues L. M., Whitton P. D., Hems D. A. Control mechanisms in the acceleration of hepatic glycogen degradation during hypoxia. Biochim Biophys Acta. 1980 Jul 3;630(3):414–424. doi: 10.1016/0304-4165(80)90290-1. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Henderson J. F. Alternative pathways of deoxyadenosine and adenosine metabolism. J Biol Chem. 1973 Aug 25;248(16):5899–5904. [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. W., Scrimgeour K. G. Properties of inosinic acid dehydrogenase from Bacillus subtilis. I. Purification and physical properties. Can J Biochem. 1973 Oct;51(10):1380–1390. doi: 10.1139/o73-181. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]



