Abstract
Background:
Legacy brominated flame retardants have been recognized as risky factors leading to declined sperm quality. The widespread utilization of decabromodiphenyl ethane (DBDPE) as a replacement for decabromodiphenyl ether has given rise to considerable concern over its potential risks to reproductive health.
Objectives:
The objectives were to quickly determine whether DBDPE affects sperm quality upon ex vivo exposure, to reveal the reproductive outcomes and underlying molecular mechanisms using an in vivo zebrafish model exposed to DBDPE, and to validate the potential impact on DNA damage and energy metabolism balance in vitro.
Methods:
Zebrafish spermatozoa were treated with DBDPE (0.01, 0.1, 1, ) for 3 h, and the spermatozoa motility and fertilization ability with normal eggs were evaluated. Then adult male zebrafish were treated with DBDPE (0.1, 1, 10, and ) for 2 months, and their reproductive performance was examined. Four-dimensional label-free proteome and phosphoproteome were performed in zebrafish testes, and the findings were validated by multiple indicators. Finally, mouse spermatogonial GC-1 cells were treated with DBDPE (0.1, ) for 72 h, and DNA damage was examined, as well as the energy production of glycolysis and oxidative phosphorylation.
Results:
Ex vivo exposure to DBDPE caused lower motility and fertilization rates of zebrafish spermatozoa. In vivo exposure to DBDPE caused lower sperm motility and abnormal spermatogenesis in male zebrafish testes. Integrated whole-proteome and phosphoproteome analysis revealed DNA damage responses and energy metabolic disorders in zebrafish testes. A dosage window characterized by higher mitochondrial membrane potential (MMP) in combination with unchanged reactive oxygen species and apoptosis rates was observed in both zebrafish testes and GC-1 cells.
Discussion:
This study suggests that in zebrafish, DBDPE exposure could impair sperm quality and spermatogenesis, and the underlying mechanism could be related to DNA damage and energy metabolic reprogramming in testicular germ cells. https://doi.org/10.1289/EHP14426
Introduction
In recent decades, declining male reproductive capacity has become a global concern, with decreasing semen quality and quantity evident.1,2 Extensive evidence implicates environmental pollution rather than genetic factors as the leading risk factor for male reproductive health of various organisms.3,4 Among the growing list of pollutants, some brominated flame retardants (BFRs) are both persistent organic pollutants and endocrine-disrupting chemicals (EDCs) and have been repeatedly associated with declining sperm quality in both epidemiological and laboratory investigations.5,6 Governments have restricted the application of some BFRs in various plastics, as well as in electronic and textile products, and replacement chemicals are needed to ensure that products meet safety standards.7,8
Typically, decabromodiphenyl ethane (DBDPE) has been employed as an alternative to decabromodiphenyl ether (BDE209) since the 1990s. In a recent study in China, the major producer and exporter of BFRs,9,10 BDE209 and DBDPE were identified as the most predominant BFRs in the agricultural soils from different regions, and the DBDPE concentration increased rapidly between 2011 and 2021, with a doubling time of 6.84 y.11 In surface waters, the detected concentrations of DBDPE typically hover in the picograms-per-liter range, sometimes reaching the range of several to several tens of nanograms per liter,12–14 and occasionally reaching .15 Concentrations of DBDPE were detected at a concentration of up to in seepage water from a metal recycling site in Norway16 and up to in sewage outlets along the Dongjinang River in China.17 Furthermore, DBDPE has been widely detected in aquatic organisms worldwide,18,19 and the measured mean concentrations of DBDPE were lipid weight (lw) and lw in crucian carp (Carassius auratus) and tilapia (Oreochromis spp.) at an e-waste recycling site in South China.20 Notably, DBDPE tends to bioaccumulate at higher levels in humans living in more severely contaminated areas. It has been detected in serum and milk samples from nonoccupational populations living in BFR-producing areas21,22 and general city populations from no-novel-brominated-flame-retardant (NBFR)-producing areas.23–25 Serum levels of DBDPE in nonoccupational populations (detection frequency 98%) from a BFR-producing area were up to ( lw), with a median concentration of (lw).22 Therefore, DBDPE is considered a major BFR pollutant, and it may pose an increasing exposure risk to humans, as well as to domestic and wild animals, not only in China, but also across the globe.26–28
Paradoxically, to ensure good flame retardancy, alternatives usually share similar molecular structures with these legacy chemicals, thereby exhibiting similar chemical properties, environmental behavior, and even biological toxicity. This has resulted in widespread doubt about the safety of substitute BFRs.29 Typically, DBDPE persists in the environment, accumulates in organisms, and has been associated with thyroid disruption and neurotoxicity in vitro30 and in vivo (in nemotodes31 and zebrafish32), and these are typical features of BDE209.33,34 Researchers have reported fewer sperm, with diminished motility, in male rats following oral administration with DBDPE at 50 or , followed by DNA damage and apoptosis in testes.35–37 Our recent studies found that DBDPE exhibited typical characteristics of endocrine metabolism-disrupting chemicals (also called metabolic EDCS), which can demonstrate endocrine-disrupting effects and the capacity to affect cellular energy metabolism.37–39 Therefore, a more complicated underlying mechanism may contributed to DBDPE-induced toxic effects, given that these two processes are intricately interconnected.39,40 However, our current knowledge of the adverse impacts of DBDPE is far from adequate for properly assessing its potential risks to humans and animals. For one thing, the exposure doses in the abovementioned studies were much higher than the exposure levels in wild animals and humans. Furthermore, it is urgent to know whether DBDPE can have adverse effects on male reproduction at environmentally relevant concentrations. In addition, testes show some specificity because spermatogenic cells mainly use lactate as their energy substrate,41 a phenomenon that is similar to the Warburg effects reported in various cancer cells.42 Researchers have identified a variety of cancer/testis antigens that are specifically involved in the metabolic shift during spermatogenesis and tumorigenesis,43 which may pose more challenges in revealing the potential mechanism of male reproductive toxicity. Therefore, probing the responsive mechanisms in testes upon DBDPE exposure at environmentally relevant concentrations is a priority for properly assessing its environmental health risks.
Moreover, to keep pace with the rapidly increasing number of emerging pollutants, exploring new approach methodologies (NAMs) to expedite risk assessment has attracted global attention.39 High-throughput omics methods can provide comprehensive knowledge of the biological status at the molecular level in response to pollutants, and such approaches have been widely used to assess the toxic effects of new pollutants.44,45 The zebrafish is a valuable model for developing NAMs to investigate endocrine and metabolic disruption, allowing extrapolation to potential effects on wild fish and even human health.39 Researchers have also established spermatozoa exposure protocols that take advantage of external fertilization in fish.46,47 In this regard, DBDPE could be an appropriate example for exploring NAMs comprising in vitro, ex vivo, and non-rodent in vivo tests for future pollutant risk assessment. Thus, this study aimed to a) rapidly screen the potential effects of DBDPE on sperm using ex vivo exposure of zebrafish spermatozoa followed by artificial fertilization, b) evaluate whether DBDPE could impair spermatozoa quality and spermatogenesis in zebrafish following in vivo exposure, c) reveal the molecular responsive profiles in zebrafish testes using an integrated proteome and phosphoproteome approach, and d) validate the potential impact on DNA damage and energy metabolism balance using in vitro cells. The results not only expand our understanding of the toxic mechanisms of DBDPE but could also facilitate the development of NAMs in future studies.
Materials and Methods
Chemicals and Reagents
DBDPE [Chemical Abstracts Service (CAS): 84852-53-9; purity ] for fish exposure was obtained from Tokyo Chemical Industry Co. Ltd. Standards for chemical analysis, including DBDPE () and isotope-labeled -BDE-209 (CAS: 562099-68-7; purity ), were supplied by AccuStandard Inc. Dimethyl sulfoxide (DMSO; CAS: 67-68-5; purity ), methanesulfonate (MS-222), and lactate dehydrogenase (LDH) inhibitor oxamic acid (oxamate) were obtained from Sigma-Aldrich. Dulbecco’s Modified Eagle medium (DMEM) and fetal bovine serum (FBS) for cell culture were supplied by Shanghai VivaCell Biosciences Ltd. Penicillin and streptomycin were provided by Beijing Solarbio Science & Technology Co. Ltd. All other reagents used in this study were of chromatography or analytical grade.
Zebrafish Maintenance and Exposure
Wild-type zebrafish (AB strain) used in the present study were obtained from the China Zebrafish Resource Center of the National Aquatic Biological Resource Center (CZRC-NABRC). Six-month-old healthy male zebrafish were randomly selected and placed into glass tanks (20 fish/tank) containing of charcoal-filtered tap water with pH 7.0–7.4 and maintained at under a 14:10 light/dark cycle. The fish were fed fresh fairy shrimp twice a day. The care and use of zebrafish was performed under the approval of the institutional animal care and use committee of the Institute of Aquatic Biology, Chinese Academy of Sciences.
Measurement of Spermatozoa Motility in Ex Vivo Test
Zebrafish spermatozoa have been used as a good toxicological test system because of their simplicity and availability, and their motility changes can be used to predict reproductive success as an ecologically relevant effect.48,49 In this study, ex vivo zebrafish spermatozoa exposure was first performed to rapidly screen the potential effects of DBDPE on sperm. Ten unexposed male zebrafish (AB strain; 8 months old) were anesthetized with of MS-22250 and dried with paper towels, and their abdomens were gently massaged with fingers to release semen from the zebrafish cloaca. The semen samples were collected and pooled into a centrifuge tube containing Hanks’ balanced salt solution (HBSS; ), a solution in which zebrafish spermatozoa can stay in static status. Then the fish were placed into water to recover within 3–5 min.50 A total of 70 males were used to collect fresh semen, and seven tubes of fresh semen samples were obtained. Then the seven tubes of semen samples were divided into 14 samples (). The spermatozoa concentrations were estimated using a cell counting chamber (Marienfeld Superior) and adjusted to ensure equal concentrations among all samples at . In general, zebrafish spermatozoa can be maintained in a healthy state in HBSS for 6 h when stored at . To determine exposure concentrations and durations in the formal experiment, we performed an incubated exposure experiment.48,49 In the incubated exposure experiment, spermatozoa suspension was first incubated in HBSS containing a series of DBDPE concentrations (0.01, 0.1, 1, ) for 3 or 6 h on ice, with subsequent analysis of sperm motility (detailed methods are described below).
Based on the results of the pre-experiment, we finally exposed zebrafish spermatozoa to a series of DBDPE concentrations (0.01, 0.1, 1, ) for 3 h on ice. The lowest concentration () of DBDPE was on the same order of magnitude as the highest concentration () detected in the serum samples from the nonoccupational population from a BFR-producing area.22 A blank control (HBSS) and a solvent control (DMSO) were also included. The solvent control and all DBDPE treatments received 0.05% DMSO. To minimize errors due to pre-exposure sperm itself (e.g., status of males), semen samples in different treatments were from the same batches of male zebrafish. The detailed protocol and procedures for preparing the exposure solution and the exposure system are as follows: DBDPE was first dissolved in DMSO to configure a DBDPE stock solution, which was then diluted in a gradient with DMSO to obtain a series of DBDPE working solutions with concentrations of 20,000, 2,000, 200, and . One microliter of DMSO or DBDPE working solution was added to of HBSS to configure a exposure solution, to be used within 1 d. Five microliters of each well-prepared semen sample in HBSS and of the exposure solution were mixed in a tube at 4°C to obtain the exposure system, and the final concentrations contained .
After 3 h of exposure, each spermatozoa suspension sample was used for sperm motility analysis. Briefly, of spermatozoa suspension was placed on a slide (Hamilton Thorne; #223120), which was placed on a CX41 microscope (Olympus), and of deionized water was added and mixed quickly to activate the spermatozoa (induction of spermatozoa movement due to osmoticity changes), followed by the addition of a coverslip. Then six sperm motility parameters (see Table S1 for definitions), including a) total motility (TM; in percentage), b) progressive motility (PM; in percentage), c) average path velocity (VAP; in micrometers per second), d) velocity of spermatozoa linear motion (VSL; in micrometers per second), e) velocity of sperm (VCL; in micrometers per second), and f) sperm linearity (LIN; in percentage) were immediately measured using an IVOS Sperm Analyzer (Hamilton Thorne) and HT CASA II Animal Motility Software (version 1.8; Hamilton Thorne).51 The computer-assisted sperm analysis (CASA) input parameters used in this study are shown in Table S2, with slight modifications based on previous studies.19,48 A minimum of 300 sperm cells were tracked per field, at least three fields captured for each sample, and each sample was measured at least twice.
Measurement of Offspring Development in Ex Vivo Test
Fresh semen samples (/group) exposed for 3 h in the same way and with the same doses as described above were used for artificial fertilization with eggs from unexposed female zebrafish (AB strain; 8-month-old), according to a previously described method.51 Briefly, on the day before egg collection, unexposed females were isolated from the males (AB strain; 8-month-old). Egg collection and artificial fertilization were performed at the end of sperm exposure in the morning. Before egg stripping, four females were fully anesthetized with MS-222 and gently dried with paper towels, and their abdomens were gently massaged with fingers to collect unfertilized eggs into a Petri dish. Pooled eggs from four fish were mixed gently, then evenly divided into six Petri dishes (corresponding to 6 exposed groups) as quickly as possible, and semen samples exposed to DBDPE for 3 h (another subset of exposed semen samples from the abovementioned ex vivo zebrafish spermatozoa exposure experiment) were added to the six Petri dishes containing the eggs. The ratio of eggs to spermatozoa was : . A volume of charcoal-filtered tap water (pH 7.0–7.4) was added to each Petri dish and blown evenly with a straw to activate the sperm, ensuring full contact with eggs. After incubating at room temperature for 5 min, the fertilized eggs in each Petri dish were washed three times with charcoal-filtered tap water and transferred to Petri dishes to remove possible residual DBDPE. The obtained embryos were then cultured at under a 14:10 light/dark cycle until 120 h post-hatching (hpf).
The embryos were observed under a SMZ745T dissecting microscope (Nikon). Unfertilized eggs were distinguished at about 3 h by phenotypes of whiteness, opacity, and failure of embryonic development,51,52 and the fertilization rate was determined. Four end points were recorded daily as indicators of lethality: a) coagulation of embryos, b) lack of somite formation, c) nondetachment of the tail, and d) lack of heartbeat.52 Malformation phenotypes were observed daily, including, for example, yolk sac edema, pericardial edema, uninflated swim bladder, and spinal scoliosis.53 The survival rate was determined at 24, 48, and 120 hpf, and the malformation rate was determined at 120 hpf. All these development indicators of zebrafish offspring were repeated three times.
In Vivo Exposure
After 2 wk of acclimatization, adult male fish (6-month-old) were exposed to a series of DBDPE concentrations (0, 0.1, 1, 10, ; corresponding to 0, 97.1, 971.2, 9,712.0, ) for 2 months. This exposure window was chosen taking into account the fact that male zebrafish exhibit their peak reproductive capacity during the age of 6 to 8 months. The selected exposure concentrations included an environmentally relevant concentration () in surface waters, where DBDPE was detected, with concentrations at several to several tens of nanograms per liter and as high as .12,14,15 There were three replicate tanks for each exposure group, and the exposure solution was 100% renewed daily. At the end of exposure, 21 fish from each group were randomly chosen for subsequent measurement of reproductive behavior. Then, all the fish were euthanized with of MS-222, body length and body weight were measured, and condition factors were calculated [wet body weight (in grams)/total body length ]. The testes were isolated, weighed, and then stored at for subsequent analysis.
Cell Culture and Treatment
The in vitro test was performed to verify the observations of DNA damage and energy reprogramming in zebrafish testes upon DBDPE exposure. Mouse spermatogonial GC-1 cells purchased from FuHeng Cell Center were cultured in DMEM with 10% FBS, 100 IU/mL of penicillin, and of streptomycin, and incubated at in a humidified 5% carbon dioxide ()/95% air incubator. Cells were seeded in 96-well (20,000 cells/well) or 6-well (400,000 cells/well) plates overnight to adhere, then treated with a series of concentrations of DBDPE (0.1 and ) and the LDH inhibitor oxamic acid (oxamate; )54 for 72 h. Before this formal experiment, we performed a pre-experiment by exposing GC-1 cells to a series of concentrations of DBDPE (0.01, 0.1, 1, ) for 48 h to investigate its effects on MMP and adenosine triphosphate (ATP) content. The exposure concentrations and duration of DBDPE used in the formal experiment were set based on our pre-experiment (results are shown in the “Results” section). A oxamate stock solution was prepared with water, stored at , to be used within 7 d. All the treatment groups and the control group received 0.1% DMSO.
Reproductive Behavior and Offspring Development in Zebrafish
At the end of the exposure, 21 exposed male zebrafish from each group were randomly selected and paired with unexposed females for reproductive behavior tests according to a previously described method.55 One exposed male and one unexposed female were placed into a tank (length: , width: , height: ) and separated by a divider overnight. The next morning between 08:00 and 09:00, the divider was removed when the lights were turned on, and reproductive behavior was monitored for 30 min using an FDR-AX60 video camera (Sony) mounted above the tank. The obtained videos were imported into EthoVision XT 14 video tracking software (Noldus Inc.) and mating-related parameters of zebrafish reproductive behavior, including proximity, proximity frequency, body contact, body contact frequency, and body contact cumulative duration, were analyzed. Mating behavior was defined as a distance of no more than between male and female fish and sustained movement for s. After behavior assessment, the resulting eggs were collected immediately in clean water, transferred into clean charcoal-filtered tap water, and cultured in an incubator at under a 14:10 light/dark cycle. The fertilization rate, hatching rate at 72 hpf, and both malformation rate and survival rate at 120 hpf were determined as described above.
Quantification of DBDPE Contents in Zebrafish Testes
Testes from three exposed zebrafish from the same replicate tank were pooled as one replicate sample ( replicates), and DBDPE contents were determined according to our previous method.32 After freeze-drying, each testes sample was spiked with -BDE209 as the internal standard and then homogenized with of -hexane. After ultrasound sonication for 60 min, all samples were centrifuged at () for 10 min, and the supernatants were subsequently collected. The samples were extracted once more with of -hexane, and the supernatants from two extractions were combined. The supernatant was blown dry under nitrogen gas, and the lipid content was determined by gravimetric analysis. The extracts were fully redissolved in of -hexane, and of concentrated sulfuric acid was added to remove the lipid. After being thoroughly vortexed and allowed to stand for 2 h, the supernatants were subsequently collected and extracted once more with of -hexane. The supernatants were combined and further purified by passing them through a multilayered silica column that was packed with neutral silica, acid silica, and anhydrous sodium sulfate, arranged from the bottom to the top. The purified samples were then eluted with -hexane. The effluent was collected, nitrogen dried, and reconstituted in of methylbenzene. Then the samples were analyzed and quantified with an Agilent 7890A-5975C gas chromatograph–mass spectrometer (GC–MS) using the electron-capture negative ionization mode in the selected ion monitoring mode, employing a DB-5HT capillary column ( length, diameter, thickness; J&W Scientific). The column flow rate was . A pulsed non-shunt injection was used with an injection volume of . The injection temperature, interface temperature, ion source, and quadrupole were set at , , , and , respectively. For DBDPE quantification, oven temperature was programmed to hold at for 1 min before ramping at to and holding for 3.7 min. Helium (99.999%) was used as the carrier gas, with a constant flow rate of , and methane (99.999%) was used as the reaction gas. Ion fragments m/z 79 and 81, m/z 494.7 and 496.7 were monitored for DBDPE and -BDE209, respectively. The limit of detection (LOD) and limit of quantitation of were 10 and , respectively. The mean recovery of surrogate -BDE209 in the samples was . The recovery of spiked DBDPE was between 82% and 118%.
Spermatozoa Motility and Ultrastructure in In Vivo Test
Ten semen samples (each collected from one individual fish; of semen per fish) in each group were collected and diluted with HBSS () for measurement of spermatozoa motility, as described above. The ultrastructure of spermatozoa was observed by scanning electron microscopy, as previously described.56 First, of fresh semen was collected from at least 10 fish in each group and fixed with of 2% glutaraldehyde overnight. After centrifugation (, 5 min), sperm cells were diluted to with phosphate-buffered saline (PBS) and stored at . Each spermatozoa sample () was gradually dehydrated with ethanol and dried with gas on a glass coverslip. The coverslips were glued and gold sputtered, then examined under an S3000 N scanning electron microscopy instrument (Hitachi Ltd.). Approximately 100 random zebrafish spermatozoa scanning electron microscopy micrographs were captured. Images were analyzed using Image-Pro Plus software (version 6.0; https://mediacy.com/image-pro/) to determine the head length, head width, and tail length of the zebrafish spermatozoa.
Gonadal Histological Analysis in Zebrafish
After being anesthetized with of MS-222 until gill movements had slowed,56 intact testicular tissues were isolated and weighed to calculate the gonado–somatic index []. Testes ( fish/group; 3 fish from each replicate tank) were fixed in 4% Paraformaldehyde Fix Solution (PFA) overnight at , then dehydrated, paraffin-embedded, and sectioned into sections. Three tissue sections per fish were collected. The tissue sections were large enough to allow for equal amounts of space between the leading edge of the tissue and the midline of the gonad. The sections were stained with hematoxylin and eosin (H&E) and photographed under a BX53 microscope (Olympus) at a magnification. A total of six random fields without overlap of each section were photographed and quantified. The relative percentages of testicular germ cells at different stages were quantitated by a blind and experienced pathologist by measuring their areas using Image-Pro Plus software (version 6.0; https://mediacy.com/image-pro/).
Four-Dimensional Label-Free Proteome and Phosphoproteome Analysis in Testes
The whole-proteome and phosphoproteome analyses were performed to find clues for possible mechanisms underlying DBDPE-induced reproductive toxicity. Except for the control, only the highest concentration group was selected, based on the result that the zebrafish from this group showed the most significant differences from control in gonad histopathology and that the findings would be further validated by determining multiple indicators at all the test concentrations. Specifically, total protein from zebrafish testicular tissues pooled from nine fish from the same tank (; 9 fish/replicate) was extracted with a lysis buffer containing 4% sodium dodecyl sulfate (SDS), dithiothreitol (DTT), and Tris-hydrogen chloride, pH 8.0 (i.e., STD lysis buffer), boiled for 3 min, ultrasonicated for 2 min, and centrifuged at for 20 min at . Protein concentrations in the supernatants were determined using the bicinchoninic acid (BCA) method (Beyotime; #P0009). Protein ( per sample) digestion was performed with a filter-aided sample preparation method, as previously described.57 First, the detergent (SDS), DTT, and indole-3-acetic acid (IAA) in UA buffer (8 M urea, 150 mM Tris HCL, pH 8.0) were added to block reduced cysteine. The protein suspension was digested with trypsin (Promega) at a ratio of 50:1 overnight at 37°C, followed by centrifugation ( for 15 min) and peptide collection. The peptides were desalted with C18 StageTip, and the concentrations of peptides were determined with OD280 by Nanodrop One spectrometer. Phosphopeptides were enriched from digested protein samples using a High-Select Titanium Dioxide Phosphopeptide Enrichment kit (Thermo Fisher Scientific). Subsequent proteomic and phosphoproteomic sequencing and analysis were performed by Shanghai Bioprofile Technology.
After protein extraction and digestion, the obtained peptides were analyzed by liquid chromatography–tandem MS (LC-MS/MS) in an Easy nLC 1200 instrument (Thermo Scientific) coupled online to a hybrid trapped ion mobility spectrometry (TIMS) quadrupole time-of-flight mass spectrometer (timsTOF Pro; Bruker Daltonics). The peptides were separated on a C18 reversed-phase column ( long, internal diameter, ; Dr. Maisch GmbH) at a flow rate of . The mobile phases A and B used in this assay were 0.1% formic acid and 0.1% formic acid/80% acetonitrile, respectively. The percentage of B rose linearly from 2% to 22% within 90 min, then increased to 35% within 10 min, and further increased to 80% within the next 8 min before the last 12 min 80% process. Then, the separated peptides were analyzed on a hybrid TIMS quadrupole timsTOF Pro (Bruker Daltonics). The MS was used in a data-dependent acquisition coupled with parallel accumulation serial fragmentation (DDA-PASEF) mode. The accumulation and ramp times were set to 2 and 100 ms, respectively, and mass spectra were collected in the m/z 100–1,700 range in positive electrospray mode. Intensity threshold at 5,000 and ion mobility range were scanned from 0.6 to . Dynamic exclusion time for precursor ions reaching a target value of 20,000 counts was set at 0.4 min. Quadrupole isolation width ranged from 2 m/z for m/z ratios to 3 m/z for m/z ratios . Each acquisition cycle of 1.1 s comprised 1 full MS scan and 10 PASEF MS/MS scans. The acquired raw data were searched with MSFragger (version 3.4; https://msfragger.nesvilab.org/). Maximum missed cleavages was set to 2 when searching the database. The mass tolerance of for precursor ions and for fragment ions were defined for database search. Carbamidomethylation of cysteines was defined as fixed modification, whereas acetylation of the protein N-terminal and oxidation of methionine were set as variable modifications for database searching. The database search results were filtered and exported with false discovery rate (FDR) both at peptide-spectrum-matched level and protein level. PeptideProphet and ProteinProphet in Philosopher (version 2.2.0; https://philosopher.nesvilab.org/) were used to filter all phosphosites, peptide-spectrum matches, peptides, and proteins. Label-free quantification was conducted with IonQuant (version 1.1.0; Nesvilab). Site quantitation analysis was performed only for phosphorylation sites confidently localized with a localization probability of . Protein annotation was performed against the zebrafish database in UniProt [https://www.uniprot.org/taxonomy/7955; release: uniprot-Danio rerio (Zebrafish) (Brachydanio rerio) [7955]-62026-20220504]. Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.genome.jp/kegg/; release 103.1, 1 September 2022) and Gene Ontology (GO; https://geneontology.org/; release 18:31, 2 July 2018) analyses were performed for interpretation of the sequences. Fisher’s exact test was applied to conduct KEGG and GO enrichment analyses, and FDR correction was also conducted for multiple testing. Identification of differentially expressed proteins (DEPs) with (fold change) was performed using R Studio software (https://posit.co/products/open-source/rstudio/). Proteins with and fold change ratios were considered DEPs. Differences in phosphosites between DBDPE treatment and control groups were considered significant at and fold change ratios . Protein–protein interaction (PPI) networks were constructed using the STRING database (version 12.0; https://cn.string-db.org/) together with Cytoscape software (version 3.9.1; https://cytoscape.org/).
Quantitative Real-Time Polymerase Chain Reaction Analysis
To see whether the biological pathways proposed by proteome and phosphoproteome in testes of fish exposed to DBDPE were also affected at lower concentrations, the transcriptional profiles of a list of candidate genes in those pathways were detected by quantitative real-time polymerase chain reaction (qRT-PCR), as previously described.38 Testes from three exposed zebrafish from the same replicate tank were pooled as one replicate sample ( replicates; the DBDPE-exposed group was excluded owing to insufficient samples), and total RNA was extracted with RNAiso Plus reagent (Takara Bio Inc.; #9109), followed by determination of their quality and concentrations on a Nanodrop 2000 platform (Thermo Fisher Scientific). One microgram of total RNA was used as the template for complementary DNA synthesis using a commercial reverse transcription kit (Yeasen Biotech Co., Ltd.; #11141ES60). qRT-PCR was carried out in a total volume of using SYBR Green Master Mix (Yeasen Biotechnology Co., Ltd.; #11201ES08) and analyzed on a CFX384 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). The programs used are provided in Excel Table S15. Primer sequences of genes are listed in Table S3. The relative transcriptional levels of target genes were normalized using the method with the protein-coding gene ribosomal protein L8 (rpl8) as the reference gene.
Seahorse Assay
The GC-1 cells were pretreated with DBDPE (0.1 and ; ), oxamate (LDH inhibitor, ; ),54 and DMSO control (0.1% DMSO; ) for 72 h and then seeded into poly l-lysine-coated XF24 cell culture microplates (Agilent; #100777-004) in Seahorse XP RPMI medium at a density of 35,000 cells/well. The culture medium contained glucose (Sigma-Aldrich; #G8270-100G), sodium pyruvate, and l-glutamine (Thermo Fisher Scientific; #25030081). Before detection, the probe plates were hydrated overnight using high-performance LC (HPLC)–grade water and then calibrated with XF Calibrant for at least 1 h at . The ATP production rates of glycolysis and mitochondrial oxidative phosphorylation were measured using an Agilent Seahorse XF Real-Time ATP Rate Assay Kit (Agilent; #103592–100) and an XFe24 Extracellular Flux Analyzer (Agilent), following the manufacturer’s instructions.
Biochemical Measurement
Zebrafish testicular tissues (3 testes from 3 fish pooled as 1 replicate sample) and GC-1 cells were collected, homogenized in PBS, centrifuged at for 20 min at , and supernatants were collected for subsequent biochemical assays. ATP content (#S0027), mitochondrial transmembrane potential (MMP; #C2006), and reactive oxygen species (ROS; #S0033S) levels were determined using commercial kits from Beyotime Biotechnology. LDH content was measured using commercial kits (#ml911206V) from Enzyme-Linked Biotechnology Co. Ltd. LDH activity (#A020-2) and pyruvate (#A154-1-1) and lactate (#A081-1-1) levels were measured using commercial kits from Nanjing Jiancheng Bioengineering Institute. The nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide reduced (/NADH) ratio was determined using an NAD/NADH-Glo Assay kit (Promega; #G9071). All the investigated indicators were measured according to the instructions of manufacturers and normalized against protein content if necessary. Protein concentrations were determined by the BCA method.
Terminal Deoxynucleotidyl Transferase Deoxynucleotide Triphosphate Nick-End Labeling Assay and Flow Cytometry
Freshly isolated zebrafish testes ( testes; 1 testis randomly isolated from each fish) were immediately fixed in 4% PFA overnight at . After washing three times in PBS for 10 min each time, testes were infiltrated in 30% sucrose at overnight and then embedded in tissue freezing medium [i.e., optimal cutting temperature compound (OCT)], followed by cryosectioning using a cryostat (Leica; #CM1860). Terminal deoxynucleotidyl transferase (TdT) deoxynucleotide triphosphate (dUTP) nick-end labeling (TUNEL) assays were conducted using an in situ cell death detection kit (Roche; #11684795910), following the manufacturer’s protocols. Images were obtained, and TUNEL-positive cell numbers were counted and normalized against to the total area of 4′,6-diamidino-2′-phenylindole (DAPI) stain in each testicular section.
Apoptosis assays of GC-1 cells ( wells each group) were performed using an Annexin V-FITC Apoptosis Detection Kit (Dojindo; #AD10), according to the manufacturer’s instructions. GC-1 cells in 6-well plates were trypsinized with ethylenediaminetetraacetic acid–free trypsin (Servicebio; #G4013) and collected into centrifuge tubes. The cells were washed three times with PBS and resuspended in of binding buffer. Subsequently, of Annexin V-FITC and of propidium iodide solution were added to each sample. After incubation in darkness for 15 min at room temperature, 20,000 cells of each sample were counted and analyzed using flow cytometry on a Cytoflex S instrument (Beckman Coulter). Data were analyzed by CytExpert software (version 2.4; Beckman).
Immunostaining
Frozen slices of zebrafish testes were obtained as above; then the frozen sections were baked in an oven at 37°C for 10 to 20 min, fixed in 4% PFA for 30 min, and washed with PBS (pH 7.4) three times. Next, the slides were treated for antigen retrieval using citric acid antigen repair solution (Beyotime; #G1202). After washing with PBS, the slides were blocked with 3% bovine serum albumin (BSA; Beyotime; #GC305010) for 30 min at room temperature. The primary antibody rabbit anti- H2AX (1:500; GeneTex; #GTX127342) was added to the testes and incubated overnight at , followed by washing with PBS (pH 7.4) before incubation with a secondary antibody [1:300; Cy3-conjugated goat antirabbit immunoglobulin (IgG); Servicebio; #GB21303] for 50 min in darkness at room temperature. To label the nuclei, the testicular sections were stained with of DAPI (Servicebio; #G1012) at for 10 min after rinsing with PBS. Immunostained testicular tissues were washed, dried, and mounted in 75% antifade mounting medium (, 75% glycerol, 2% -propyl gallate). The slices were viewed under a TCS SP8 time-lapse confocal microscope (Leica) and photographed using a Leica LAS X software imaging system (version 3.7.4; Leica). The positive signal was quantified as the ratio of the fluorescence intensity of target positive signals to the corresponding fluorescence intensity of DAPI in each field. The area was measured using ImageJ software.58 A series of testicular sections from six individual zebrafish () were examined for image analysis. In zebrafish, the -H2AX signal can be found in spermatocytes at both the leptotene and the zygotene stages owing to meiotic recombination, with the signals being stronger at the leptotene stage than at the zygotene stage.59 To avoid the interference of meiotic recombination, we distinguished two different stages of spermatocytes (SPC-I: zygotene spermatocytes, signals dispersed within the nucleus; SPC-II: leptotene spermatocytes, strong signals concentrated within in the nucleus) by their shape and density of immunostaining signals,59 then quantified and compared their expression of -H2AX, separately.
Western Blotting Analysis
Total protein lysates were isolated from pooled zebrafish testicular tissues from three fish of the same replicate (; testes of three fish from the same tank were pooled as one replicate sample) or cultured GC-1 cells (; cells each group) with ice-cold radioimmunoprecipitation assay buffer (Beyotime; #P0013B), followed by centrifugation at for 15 min at . Supernatants were collected, and protein concentrations were determined using the BCA method. The isolated proteins were separated by 10% SDS−polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto a polyvinylidene fluoride (PVDF) membrane (Immobilon-P), blocked in 5% nonfat milk on a shaker at room temperature for 2 h, and incubated at overnight with primary antibodies, including anti-phospho-c-jun N-terminal kinase (anti-p-JNK; 46/54 KDa; 1:1,000; Cell Signaling Technology; #4668T), anti-cleaved-poly(adenosine diphosphosphate [ADP]-ribose) polymerase (anti-cleaved-PARP; 89 KDa; 1:1,000; Cell Signaling Technology; #5625T), anti-cleaved caspase-3 (17 KDa; 1:1,000; Cell Signaling Technology; #9661T), anti-Caspase-3 (32/17 KDa; 1:1,000; Abcam; #Ab13585), anti-caspase-8 (53/40 KDa; 1:1,000; Proteintech; #13423-1-AP), anti-receptor-interacting serine-threonine kinase 3 (anti-RIPK3; 56 KDa; 1:1,000; Affinity; #DF7339), anti-RIPK1 (76 KDa; 1:1,000; Affinity; #AF7877), anti-mixed lineage kinase domain-like protein (anti-MLKL; 54 KDa; 1:1,000; Proteintech; #66675-1-Ig), anti-p-MLKL (54 KDa; 1:1,000; Affinity; #AF7420), and anti-P53 (53 KDa; 1:1,000; Cell Signaling Technology; #2524). After being washed five times with Tris buffered saline with Tween 20 (TBST) solution for 5 min each time, membranes were incubated with secondary antibodies (horseradish peroxidase–conjugated affinipure goat antimouse/antirabbit IgG; 1:10,000; Boster; #BA1051/BA1054) on a shaker at room temperature for 2 h. Enhanced chemiluminescence (ECL) reagent (Pierce) was applied to visualize protein expression, and ImageJ software58 was used to analyze the expression of target proteins.
Statistical Analysis
Statistical analysis was performed with SPSS software (version 22.0; IBM), and graphs were generated by GraphPad Prism (version 8.0; Graphpad). Data normality and homogeneity of variance were first evaluated by Shapiro−Wilk and Levene’s tests, respectively. Then the differences in mean values among control and treatment groups were analyzed by one-way analysis of variance (ANOVA), followed by the post hoc least significant difference (LSD) test. The standard for significance was set as . Data are presented as errors of the mean (SEMs). A Pearson correlation analysis was conducted between the DBDPE-exposure group and the oxamate-exposure group for the investigated parameters in GC-1 cells.
Results
Spermatozoa Motility and Offspring Development in Ex Vivo
First, we performed a pre-experiment to determine exposure concentrations and durations in the formal experiment. The results showed that the 3-h–exposed spermatozoa demonstrated significantly lower TM and PM at 0.01, 0.1, and (Figure S1). However, when exposure duration was prolonged to 6 h, significantly lower PM and LIN of zebrafish spermatozoa were observed only in the DBDPE-exposed zebrafish spermatozoa when compared with control spermatozoa (Figure S1). The pre-experiment results suggested that the spermatozoa seemed to be more sensitive to 3 h of exposure than to 6 h of exposure. Based on the pre-experiment results, ex vivo zebrafish spermatozoa exposure to DBDPE for 3 h, followed by artificial fertilization, were performed to evaluate the potential toxicity of DBDPE on spermatozoa and the development of offspring in the formal experiment (Figure 1A). After 3 h of exposure, TM (0.1 and ) and PM () of zebrafish spermatozoa were significantly lower than those of the control, but spermatozoa LIN was not significantly different (Figure 1B–D). No significant differences were observed in spermatozoa velocity parameters, including VAP, VCL, and VSL (Figure S2A−C). In addition, when 3-h–exposed zebrafish spermatozoa were artificially fertilized with unexposed zebrafish eggs, significantly lower fertilization rates were observed in the 0.1, 1, and DBDPE-exposure groups when compared with the control group (Figure 1E), and survival rates at most time points displayed a concentration-dependent trend toward lower survival, but this was not statistically significant (Figure 1F). Malformation rates (Figure 1G) and hatching rates (Figure S2D) were also not different from control.
Figure 1.
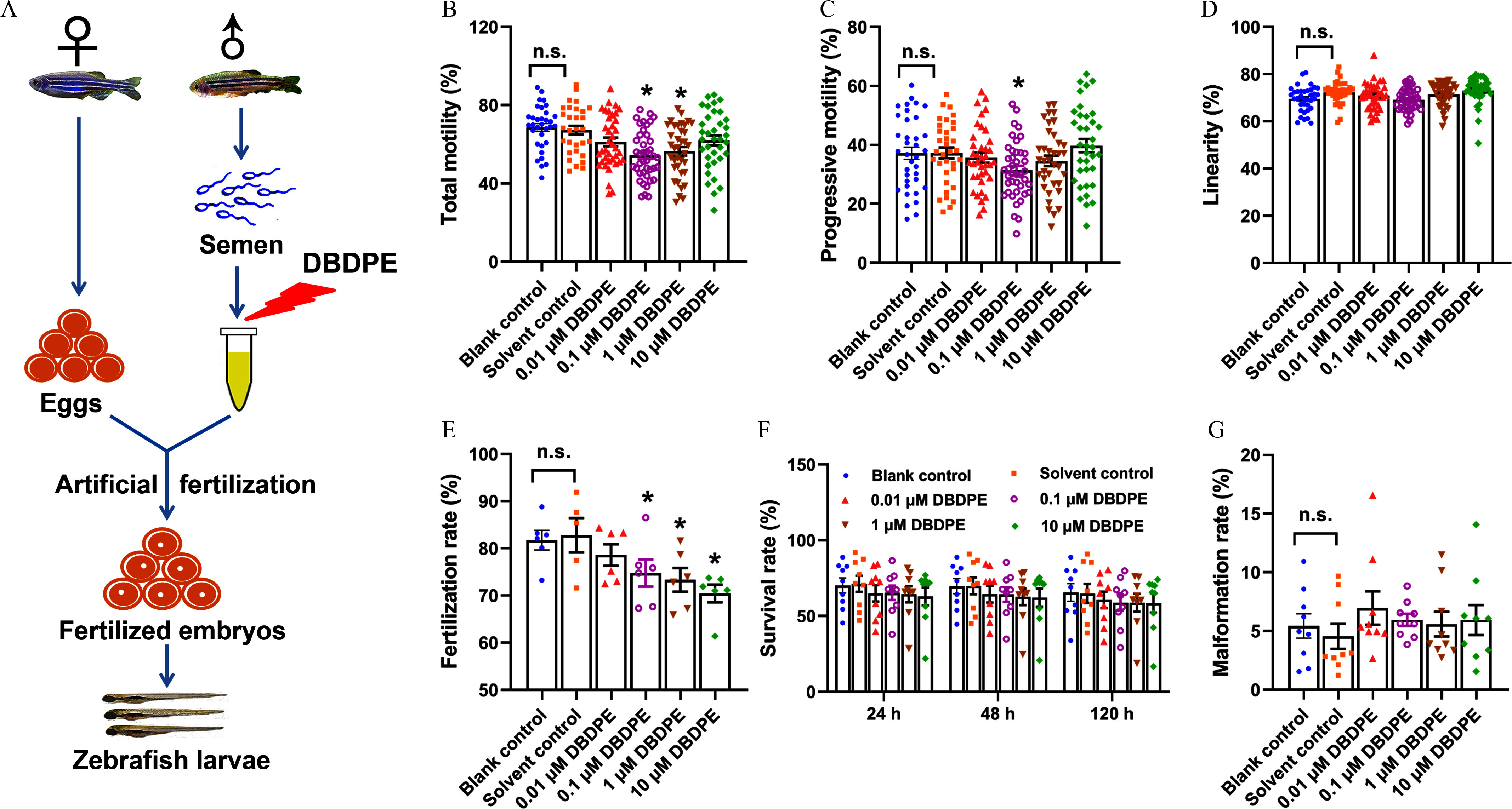
Effects of DBDPE ex vivo exposure on zebrafish spermatozoa motility and development of offspring obtained by artificial fertilization. (A) Schematic diagram for ex vivo exposure of zebrafish spermatozoa and artificial fertilization. (B) Total motility (TM) of zebrafish spermatozoa. (C) Progressive motility (PM) of zebrafish spermatozoa. (D) Linearity of zebrafish spermatozoa. Each dot in (A–D) represents one replicate data point (mean value of each test sample). The dot numbers represent the data size (/group) for statistical analysis. (E) Fertilization rate of zebrafish embryos derived by artificial fertilization using unexposed eggs and DBDPE-exposed sperm (/group). (F) Survival rate determined at 24 h, 48 h, and 120 h post-hatching (hpf) of zebrafish embryos obtained by artificial fertilization (/group). (G) Malformation rate determined at 120 h post-hatching of zebrafish embryos obtained by artificial fertilization (/group). Results are represented as errors of the mean (SEMs). See Table S1 for definitions of total and progressive motility and linearity of spermatozoa. Data are reported in Excel Table S1. Note: DBDPE, decabromodiphenyl ethane; DMSO, dimethyl sulfoxide. * indicates a significant difference between DBDPE exposure and solvent control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test; n.s. indicates no significant difference between blank control and solvent control (0.05% DMSO) groups.
DBDPE Contents in Zebrafish Testes
The contents of DBDPE in testes from 2-month–exposed zebrafish were , , , and lw in the 0.1, 1, 10 and DBDPE-exposed groups, respectively. The concentrations of DBDPE in the control testes were below the LOD (Table S4).
Reproductive Behavior and Offspring Development in Zebrafish
After DBDPE exposure, significantly greater body length and condition factor were observed in male zebrafish from the -exposure group, and significantly higher body weight was observed in those from the -exposure group, whereas no significant differences were observed in GSI in all DBDPE-exposure groups, when compared with the control group (Table S5). When DBDPE-exposed male zebrafish were paired with unexposed female zebrafish, all commonly used reproductive end points, including reproductive behavior (Figure S3) and egg production (Table S5), were unaffected. In F1 offspring larvae, a significantly lower hatching rate was observed only in the -exposure group compared with the control group, whereas other parameters, such as fertilization, malformation, and survival rates, were not different from control (Table S5).
Spermatozoa Motility, Ultrastructure, and Gonadal Histology in Zebrafish
After exposure to DBDPE for 2 months, significantly lower TM and PM of zebrafish spermatozoa were observed at all the tested concentrations when compared with the control (Figure 2A,B). Meanwhile, when compared with the control, significantly lower VAP and VCL of zebrafish spermatozoa were observed in fish exposed to DBDPE, significantly lower VSL was observed in fish exposed to both 0.1 and DBDPE, and the fish exposed to DBDPE demonstrated significantly lower spermatozoa LIN (Figure 2C–F). Furthermore, the scanning electron microscopy results showed no obvious effects on spermatozoa tail length following DBDPE exposure (Figure 2I), but the males exposed to 1 and DBDPE demonstrated significantly shorter spermatozoa head length than those in the control group (Figure 2J), and significantly greater spermatozoa head width was observed in fish exposed to DBDPE at all investigated concentrations (Figure 2K).
Figure 2.
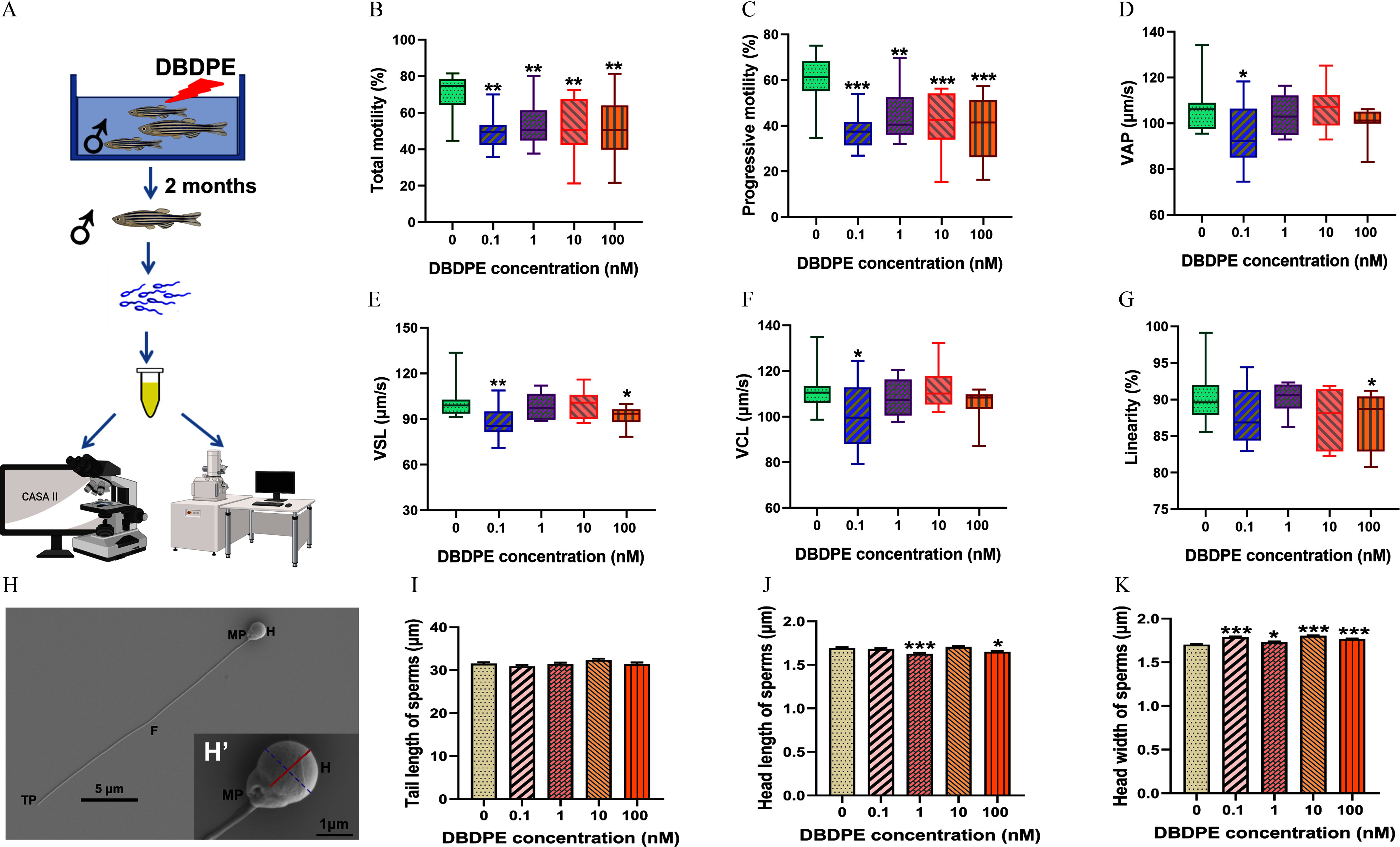
Effects of DBDPE in vivo exposure on motility parameters and ultrastructure morphology of zebrafish spermatozoa. (A) Schematic diagram of collecting sperm after in vivo exposure of zebrafish for spermatozoa motility and ultrastructural imaging. The illustration was in part created in BioRender (2024) https://BioRender.com/o11a377. (B) Total motility (TM; %) of spermatozoa (). (C) Progressive motility (PM; %) of spermatozoa (). (D) Average path velocity (VAP; ) of spermatozoa (). (E) Straight-line velocity (VSL; ) of spermatozoa (). (F) Curvilinear velocity (VCL; ) of spermatozoa (). (G) Linearity (LIN; %) of spermatozoa (). Box plots represent the median values with upper and lower quartiles; whiskers extend to the maximum to minimum. (H) Typical spermatozoa (normal spermatozoa from control group) morphology observed by scanning electron microscope. (H′) Magnification of a typical zebrafish spermatozoa head. The solid red line indicates head length, and the dotted blue line indicates head width measured in the present study. (I) Spermatozoa tail length measured in each group. (J) Spermatozoa head length measured in each group. (K) Spermatozoa head width measured in each group. Ten semen samples were assessed for different spermatozoa motility parameters in each group. Zebrafish spermatozoa ( spermatozoa/group) were randomly selected for measurement of tail length, head length, and head width in each group. Results are represented as errors of the mean (SEMs). See Table S1 for definitions of TM, PM, VAP, VSL, VCL, and LIN. Data are reported in Excel Table S2. Note: CASA, computer-assisted sperm analysis; DBDPE, decabromodiphenyl ethane; F, flagellum; H, head; MP, midpiece; TP, terminal piece. *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
H&E staining showed that germ cells at different developmental stages were present in the testes of each group, including spermatogonia (SG), spermatocytes (SPC), and spermatozoa (SPD; Figure 3A–E). A slight wider interstitial space was observed especially upon DBDPE exposure at higher concentrations (1, 10, and ), although no significant difference was observed compared with control (Figure 3F and Figure S4; , 0.056, and 0.116, respectively). Semiquantitative measurement showed that the DBDPE-exposure group demonstrated a significantly lower percentage of relative areas occupied by SPD compared with the control group, whereas both the 1 and DBDPE-exposure groups showed significantly higher percentages of relative areas occupied by spermatogonia (SPG) (Figure 3F).
Figure 3.
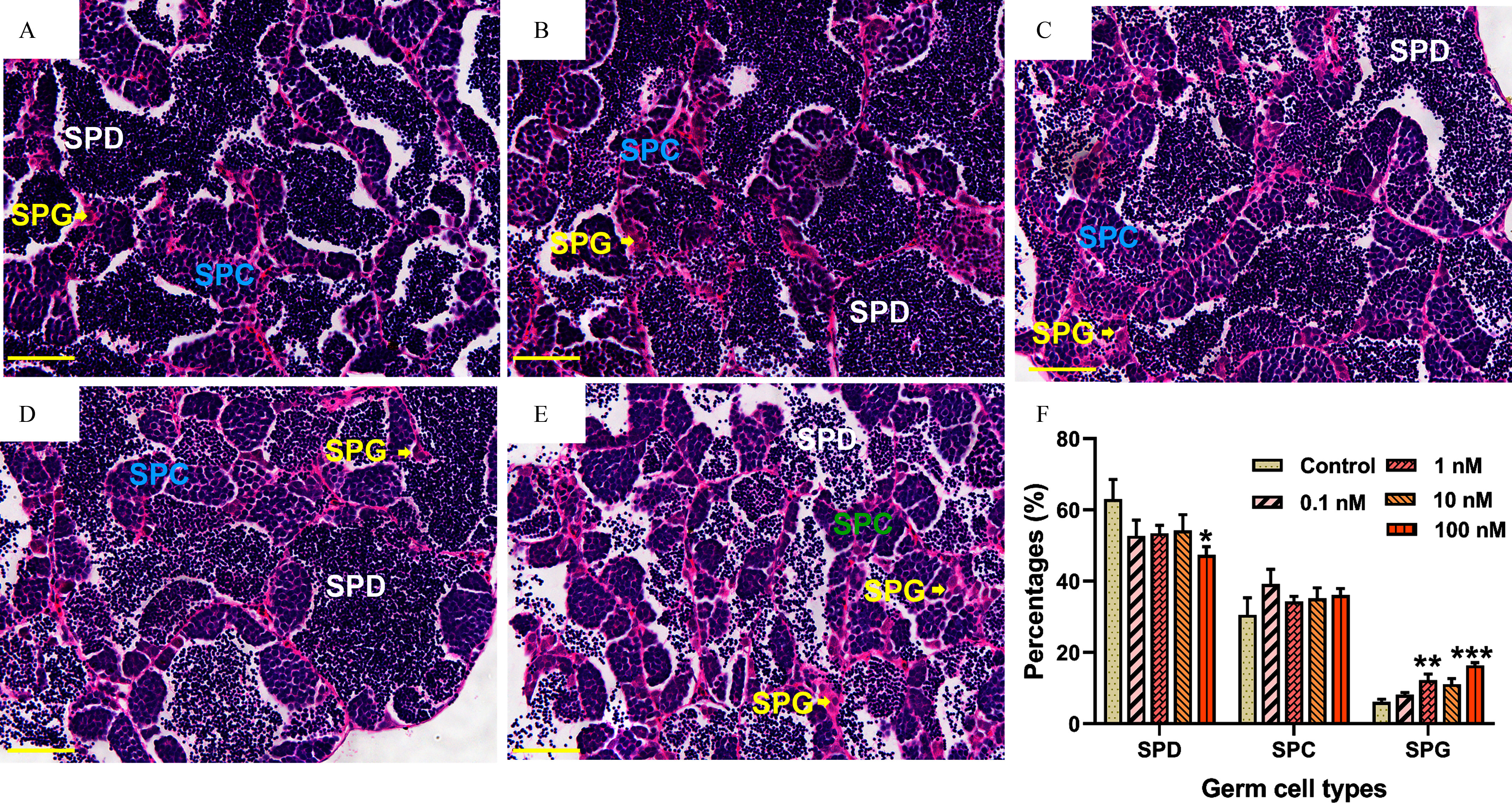
Histological observations of male zebrafish testes after 2-month in vivo exposure to DBDPE. (A−E) Typical hematoxylin/eosin–stained sections of testes in 0, 0.1, 1, 10, and -exposure groups, respectively. (F) Proportion quantification of germ cells at different developmental stages in testes ( fish/group). Results are represented as errors of the mean (SEMs). Scale bar: . Data are reported in Excel Table S3. Note: DBDPE, decabromodiphenyl ethane; SPC, spermatocytes; SPD, spermatozoa; SPG, spermatogonia. *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
Whole-Proteome and Phosphoproteome Analyses in Zebrafish Testes
Quantitative proteomics integrating whole-proteome and phosphoproteome approaches were applied to explore differences in expression and phosphorylation profiles of global proteins between control and DBDPE-exposed zebrafish testes (Figure 4A). In total, we obtained 10,274 proteins from whole-proteome analysis and 7,250 phosphorylation sites on 4,418 proteins from phosphoproteome ( FDR) analysis. Phosphorylation sites are shown in Figure S5A. Specifically, 457 DEPs (226 up-regulated and 231 down-regulated) and 2,110 phosphosites (1,527 on 487 proteins up-regulated and 583 on 239 proteins down-regulated) were identified in DBDPE-treated testes compared with controls (Figure 4B; Figure S5B,C).
Figure 4.
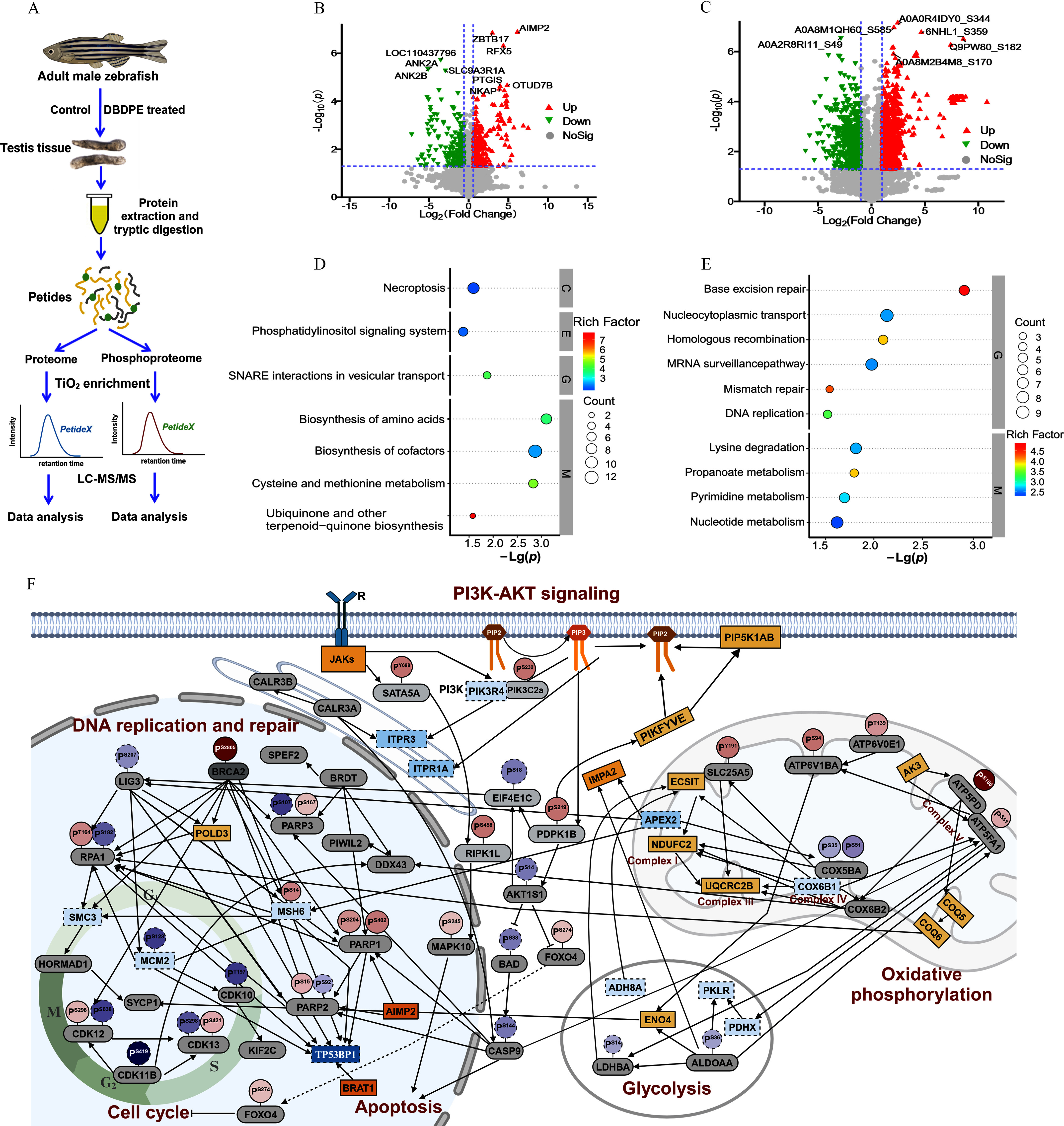
Whole-proteome and phosphoproteome analysis of male zebrafish testes after in vivo exposure to DBDPE. (A) Schematic diagram of proteome and phosphoproteome analysis. The illustration was in part created in BioRender (2024) https://BioRender.com/x32w463. (B) Volcano plot of quantified proteins in DBDPE-treated zebrafish testes. (C) Volcano plot of quantified phosphosites in DBDPE-treated zebrafish testes. Upward triangle (filled in red; on the upper right corner) and upside-down triangle (filled in green; on the upper left corner) in (B) and (C) indicate up-regulation and down-regulation, respectively; gray dots indicate no significant difference. (D) Significantly enriched KEGG pathways of differentially expressed proteins (DEPs) from whole-proteome data. (E) Significantly enriched KEGG pathways of proteins harboring differentially phosphorylated sites from phosphoproteome data. The size of the dots in (D) and (E) represents the number of DEPs in the pathway, and the color of the dots represents the enrichment factors of KEGG pathway. (F) Protein–protein interaction (PPI) network after exposure to DBDPE. The illustration was created in BioRender (2023) https://BioRender.com/o93k219. Each large oval represents protein expression level, and smaller ovals with a tail on top represent phosphorylation levels of specific phosphorylation sites of the protein. Solid line boxes and dotted line boxes respectively indicate up-regulation and down-regulation in the proteome; gray-filled ovals indicate proteins without significant differences. Solid line circles and dotted line circles respectively indicate up-regulation and down-regulation in the phosphoproteome. Color intensity is proportional to (fold change). Data are reported in Excel Table S4. Note: DBDPE, decabromodiphenyl ethane; KEGG, Kyoto Encyclopedia of Genes and Genomes; PI3K-AKT, phosphoinositide 3-kinase/protein kinase B; SNARE, soluble -ethylmaleimide-sensitive factor attachment protein receptor; , titanium dioxide.
KEGG analysis of DEPs revealed that DBDPE exposure was associated with proteins in the necroptosis, phosphatidylinositol signaling system, ubiquinone, and other terpenoid–quinone biosynthesis categories (Figure 4D). KEGG enrichment analysis of phosphoproteins harboring differentially phosphorylated sites revealed that pathways, including base excision repair, homologous recombination, mismatch repair, and DNA replication, were associated with DBDPE exposure (Figure 4E). GO analysis of both whole-proteome and phosphoproteome data revealed that organelle organization and cellular component organization were enriched (Figure 5E; Figure S5D). Other biological processes, such as histone deacetylation and protein deacetylation, were enriched in proteome data according to GO analysis (Figure S5D), and chromosome organization, chromatin organization, and DNA repair were also enriched in phosphoproteome data (Figure S5E).
Figure 5.
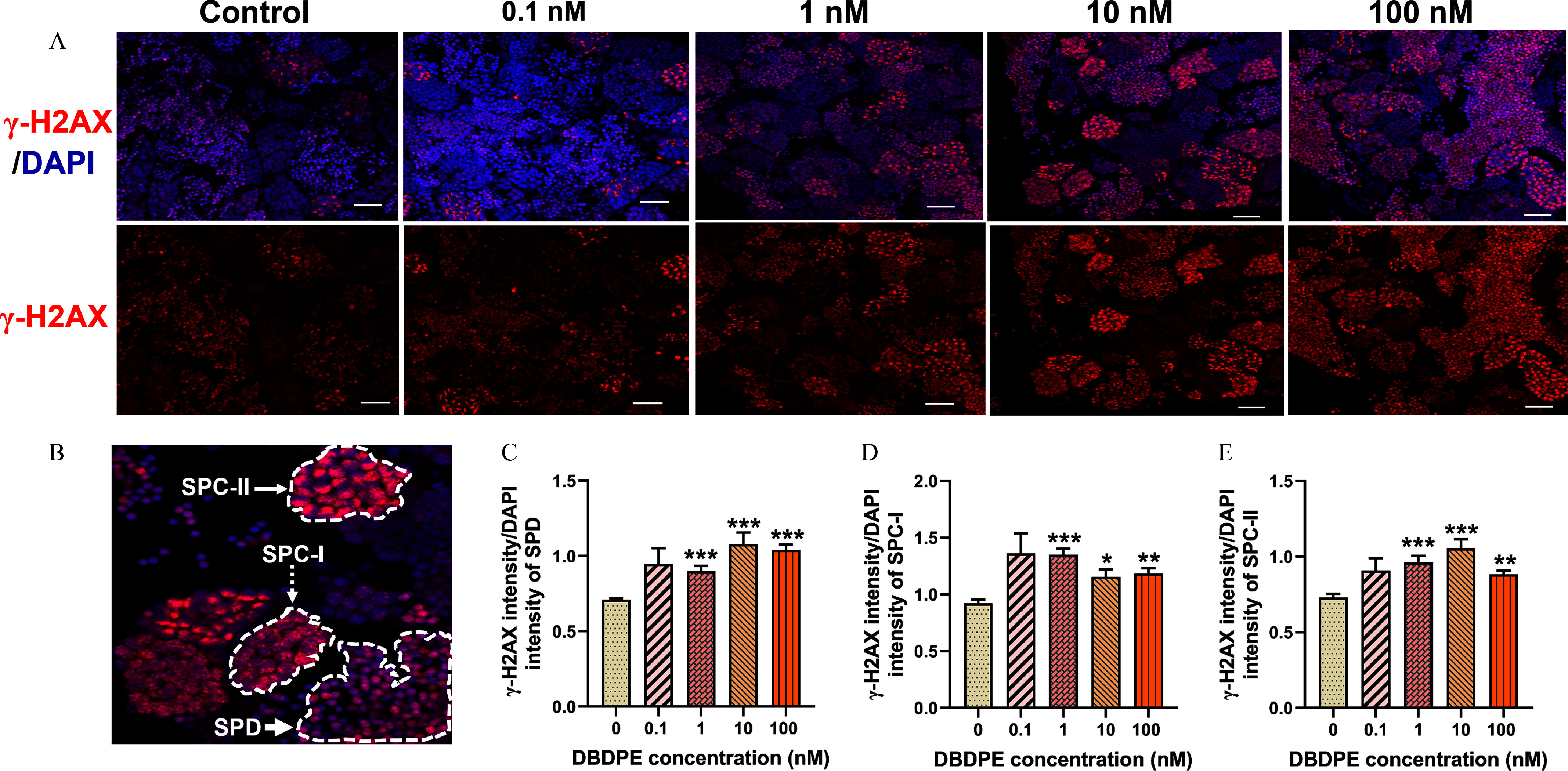
DNA damage in zebrafish testes exposed to DBDPE in vivo. (A) DNA damage in zebrafish testes detection by immunofluorescence staining against the histone protein -H2AX. The representative images show DAPI-stained (blue) nuclei with nuclear -H2AX foci in red. Scale bar: . (B) Representative positive signals detected in SPD (indicated by white bold arrow), SPC-I (indicated by white dashed arrow; zygotene spermatocytes, signals dispersed within the nucleus), and SPC-II (indicated by white thin arrow; leptotene spermatocytes, strong signals concentrated within in the nucleus). (C) Ratio of fluorescence intensity of -H2AX to the corresponding fluorescence intensity of DAPI in SPD. (D) Ratio of fluorescence intensity of -H2AX signals to the corresponding fluorescence intensity of DAPI in SPC-I. (E) Ratio of fluorescence intensity of -H2AX signals to the corresponding fluorescence intensity of DAPI in SPC-II. Results are represented as errors of the mean (SEMs), testes. Data are reported in Excel Table S5. Note: DAPI, 4′,6-diamidino-2′-phenylindole; DBDPE, decabromodiphenyl ethane; SPC, spermatocytes; SPD, spermatozoa. *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
As indicated in the PPI network (Figure 4F; Excel Table S4), expression levels of inositol 1,4,5-trisphosphate receptors ITPR1A [] and ITPR3 [] along the inositol triphosphate/calcium ion (IP3/) signaling pathway were significantly lower than in control, and proteins related to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) decomposition [inositol monophosphatase 2 (IMPA2); ] and PIP2 synthesis [phosphatidylinositol-4-phosphate 5-kinase, type I, alpha, b (PIP5K1AB); ; 1-phosphatidylinositol-3-phosphate 5-kinase (PIKFYVE); ] were significantly higher, but the phosphorylation level of AKT1 substate 1 [AKT1S1; ] was significantly lower. In addition, less glycolysis was observed in DBDPE-exposed fish testes, as indicated by lower expression levels of the dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex [PDHX; ] and pyruvate kinase L/R [PKLR; ] and lower phosphorylation levels of lactate dehydrogenase Ba [LDHBA; ] and aldolase a [ALDOAA; ]. Greater oxidative phosphorylation was observed in DBDPE-exposed fish testes, as shown by higher expression levels of proteins such as NADH dehydrogenase (ubiquinone) 1 subunit C2 [NDUFC2; ] and ubiquinol-cytochrome C reductase core protein 2b [UQCRC2B; ] and higher phosphorylation levels of proteins, such as ATP synthase peripheral stalk subunit d [ATP5PD; ] and solute carrier family 25 member 5 [SLC25A5; ].
Transcriptional Profiles in Zebrafish Testes
The transcriptional profiles of representative genes in the proposed pathways from the proteome and phosphoproteome analysis were determined in zebrafish testes by qRT-PCR to see whether those pathways were also affected at lower concentrations (0.1, 1, and ). As shown in Figure S6 and Excel Table S15, exposure to DBDPE at concentrations of 1 and resulted in significantly lower transcription levels of the cell death-associated gene caspase9, whereas the transcription of ripk3, another crucial gene in cell death pathways, demonstrated higher levels at 0.1 and . Among genes associated with the DNA damage response, the transcription of parp1, parp2, parp3, pold1, pold3, lig3, and tp53bp1 demonstrated an upward trend after exposure to lower concentrations of DBDPE; the transcription of brca2, brat1, and mcm2 demonstrated an upward trend at , but a downward trend at . The transcription levels of genes (cdk10, cdk11b, cdk12, cdk13) regulating the cell cycle and genes (jak3, akt1s1, pik3c2a, and pik3r1) related to the Janus kinase/phosphoinositide 3-kinase/protein kinase B (JAK/PI3K/AKT) pathways were higher after exposure to DBDPE. The transcription levels of genes related to oxidative phosphorylation (ndufc2, uqcrc2b, atp6voe1, atp5l, slc25a5, coq5, and coq6) were higher after exposure to DBDPE, whereas the transcription levels of the glycolysis-related genes pkma and adh8a demonstrated a downward trend in these exposure groups.
DNA Damage and Apoptosis in Zebrafish Testes
We used a typical marker (the histone protein -H2AX) for DNA double-strand breaks (DSBs) to test whether DBDPE exposure was associated with DNA damage in the testicular germ cells via immunofluorescence staining. Significantly higher relative florescence density of -H2AX was observed in SPD, SPC-I, and SPC-II in the testes of fish exposed to 1, 10, and DBDPE (Figure 5A–E). We subsequently found a significantly higher number of TUNEL-positive cells in the testes of zebrafish exposed to DBDPE compared with those in the control (Figure 6A,B). We also evaluated key proteins mediating the responses to DNA damage (cleaved PARP) and those contributing to cell apoptosis (cleaved caspase-3, P-JNK) by Western blotting (Figure 6C), and DBDPE-exposed zebrafish testes presented significantly higher expression levels of cleaved PARP, cleaved caspase-3, and both P-JNK-1 and P-JNK-2 compared with control fish (Figure 6D).
Figure 6.
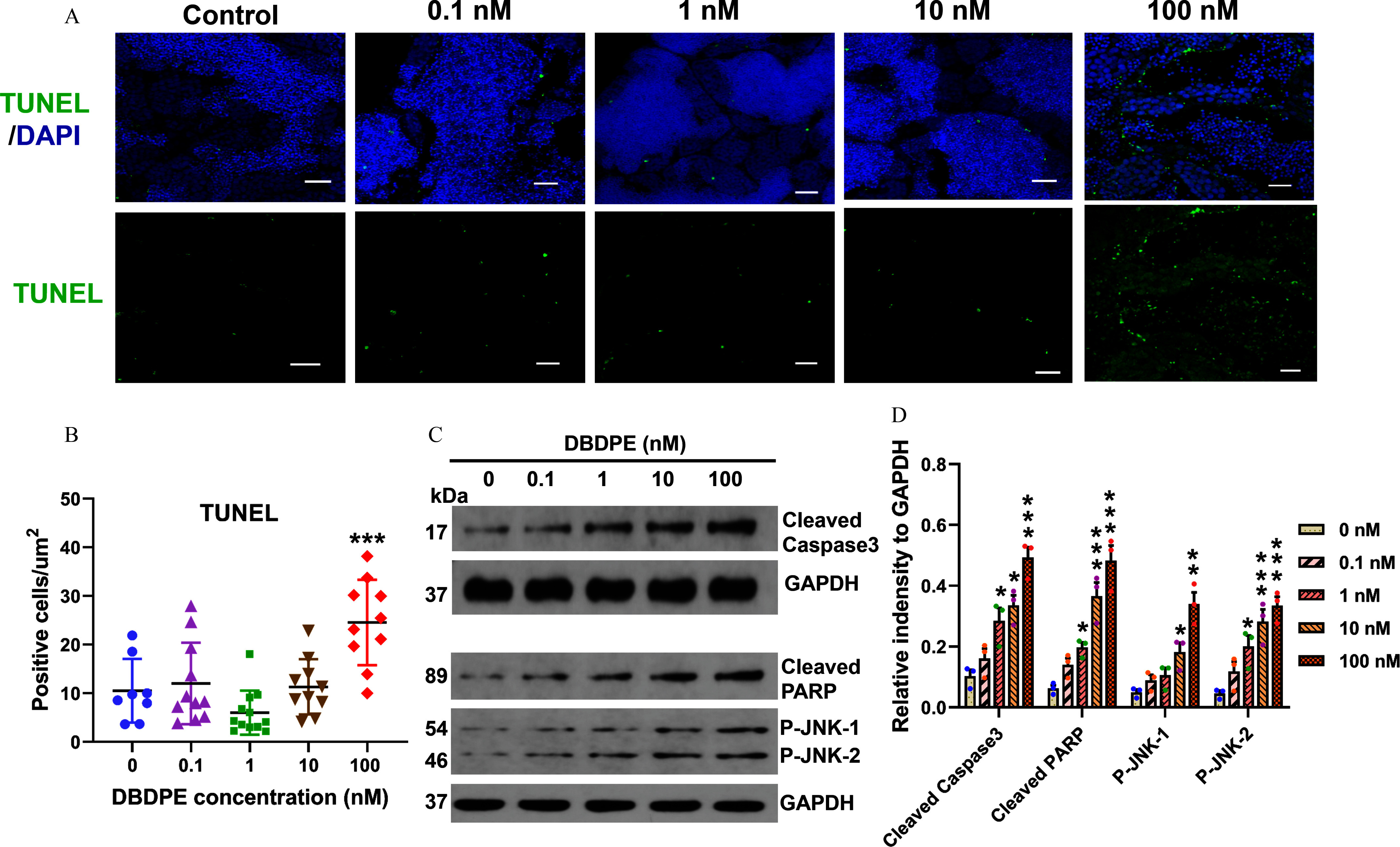
Germ cell apoptosis and expression of proteins related to cell apoptosis in zebrafish testes exposed to DBDPE in vivo. (A) Apoptosis detection in zebrafish testes by immunofluorescence staining using TUNEL assay. The representative images show DAPI-stained (blue) nuclei with TUNEL-positive signals in green. Scale bar: . (B) Statistical analysis of the total number of TUNEL-positive cells relative to the section areas. Each dot in (B) represents one replicate data point (mean value of each testis). The dot numbers represent the data size () for statistical analysis. (C) Western blotting analyses using antibodies against cleaved caspase-3, cleaved PARP, p-JNK, and GAPDH in testes tissues. The numbers on the left represent molecular weight. (D) Quantification of the abundances of proteins relative to GAPDH (). Results are represented as errors of the mean (SEMs). Data are reported in Excel Table S6. Note: DAPI, 4′,6-diamidino-2′-phenylindole; DBDPE, decabromodiphenyl ethane; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PARP, poly(adenosine diphosphosphate-ribose) polymerase; p-JNK, phospho-c-jun N-terminal kinase; TUNEL, terminal deoxynucleotidyl transferase deoxynucleotide triphosphate nick-end labeling (assay). *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
Energy Metabolic Level in Zebrafish Testes
Integrated whole-proteome and phosphoproteome analysis also indicated energy metabolism differences in zebrafish testes following DBDPE exposure. The fish exposed to DBDPE at all investigated concentrations demonstrated a significantly lower /NADH ratio in testicular tissues compared with that in the control (Figure 7A). Significantly higher MMP in testes was observed in fish following exposure to 0.1, 10, and DBDPE compared with the control (Figure 7B). Exposure to DBDPE at 1 and also resulted in a significantly higher ATP content in testes (Figure 7C). However, no obvious differences were observed in ROS levels in testes following DBDPE exposure (Figure 7D). The glucose content in testes exhibited a dose-dependent accumulation upon DBDPE exposure, with significantly higher content observed at the highest concentration compared with that in the control (Figure 7E). A significantly lower lactate/pyruvate ratio was observed in testes following DBDPE exposure at all concentrations (Figure 7F). Furthermore, when compared with the control, lower levels of LDH content and LDH activity in testicular tissues were found in DBDPE-exposed fish, with significances observed at 10 and for LDH content, as well as at 1 and for LDH activity (Figure 7G,H).
Figure 7.
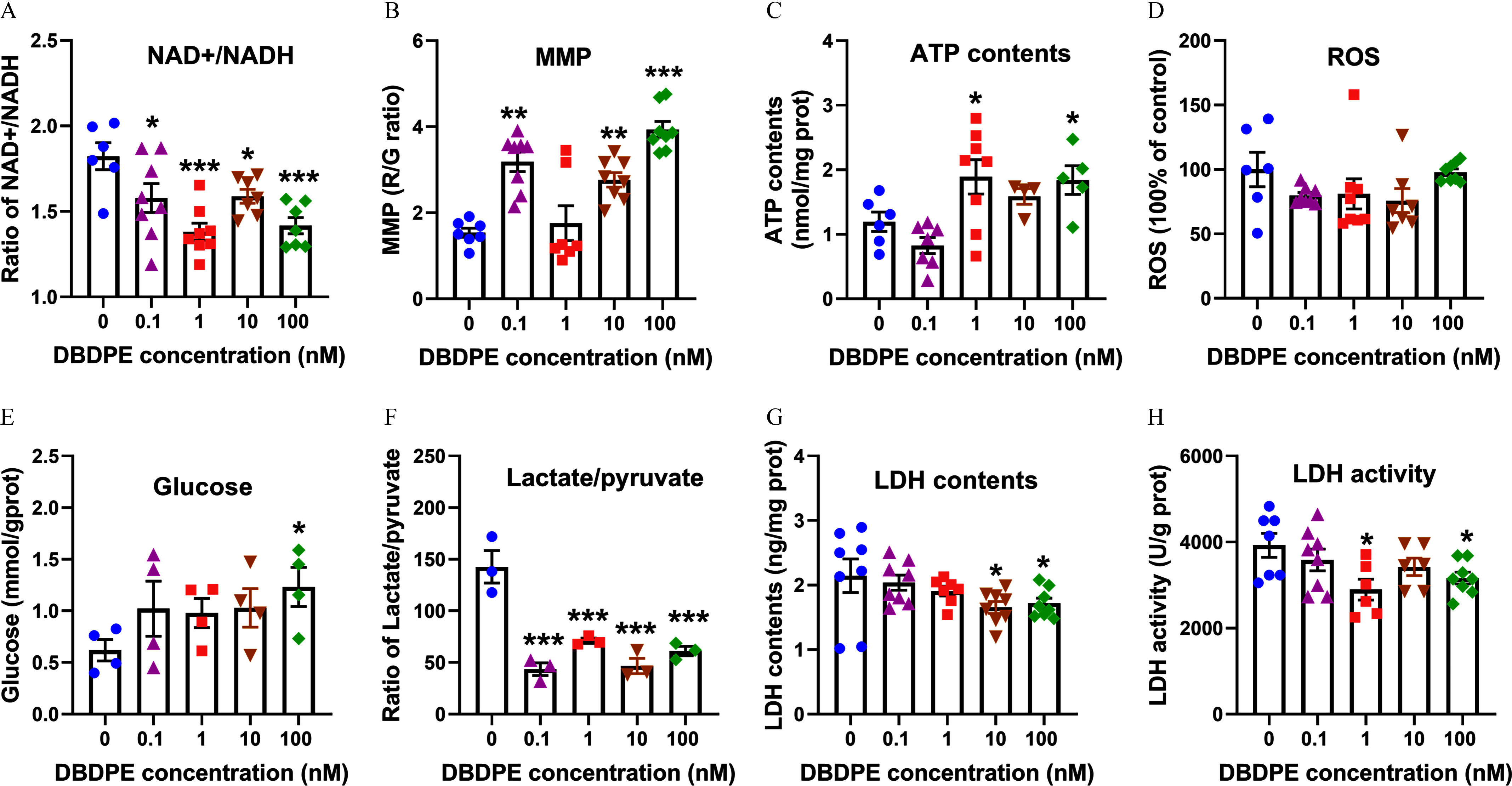
Energy metabolic level in zebrafish testes exposed to DBDPE in vivo. (A) /NADH ratio in testicular tissues (). (B) Mitochondrial membrane potential (MMP) of testicular tissues (). (C) ATP content in testicular tissues (). (D) ROS levels in testicular tissues (). (E) Glucose levels in testicular tissues (). (F) Intracellular lactate/pyruvate ratio in testicular tissues (). (G) LDH content in testicular tissues (). (H) LDH activity in testicular tissues (). Each dot in (A–H) represents one replicate data point. Results are represented as errors of the mean (SEMs). Data are reported in Excel Table S7. Note: ATP, adenosine triphosphate; DBDPE, decabromodiphenyl ethane; G, green; LDH, lactate dehydrogenase; , nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide reduced; R, red; ROS, reactive oxygen species. *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
DNA Damage and Energy Metabolism in GC-1 Cells
We further validated the DNA damage, apoptosis, and metabolic changes observed in zebrafish testicular tissues using mouse spermatogonial GC-1 cells. First, a pre-experiment was performed to determine exposure concentrations and duration by investigating effects of a series of concentrations of DBDPE () on MMP and ATP contents in GC-1 cells. After a 48-h exposure of DBDPE, MMP was significantly higher at 0.1 and , whereas ATP was significantly higher only at , with no significant differences observed at both 0.01 and (Figure S7). Therefore, two DBDPE concentrations (0.1 and ) were selected for further research, and the exposure duration was prolonged to 72 h. The results from the formal experiment showed that exposure to DBDPE and oxamate for 72 h resulted in significantly higher -H2AX foci formation compared with the control (Figure 8A,B). Consistent with these results, the expression levels of proteins in response to DNA damage, such as cleaved PARP and P53, were up-regulated by DBDPE and oxamate, and statistically significant differences were observed in GC-1 cells following exposure to both concentrations of DBDPE and oxamate for cleaved PARP (Figure 8D–G). Next, we evaluated cell apoptosis by flow cytometry (FCM), and no significant alterations were found in GC-1 cells following DBDPE or oxamate exposure (Figure 8C). However, Western blot analysis showed that expression levels of protein markers for cell apoptosis, such as cleaved caspase-8 and both P-JNK-1 and P-JNK-2, were significantly up-regulated in GC-1 cells upon exposure to DBDPE and oxamate, whereas expression levels of cleaved caspase-3 were also significantly up-regulated by oxamate exposure, and DBDPE up-regulated the expression levels of cleaved caspase-3, but not significantly (Figure 8D–G). Furthermore, several protein markers for necroptosis, including RIPK3, RIPK1, and p-MLKL, were also significantly up-regulated in GC-1 cells following exposure to DBDPE and oxamate, with the exception of RIPK1 at DBDPE (Figure 8H,I).
Figure 8.
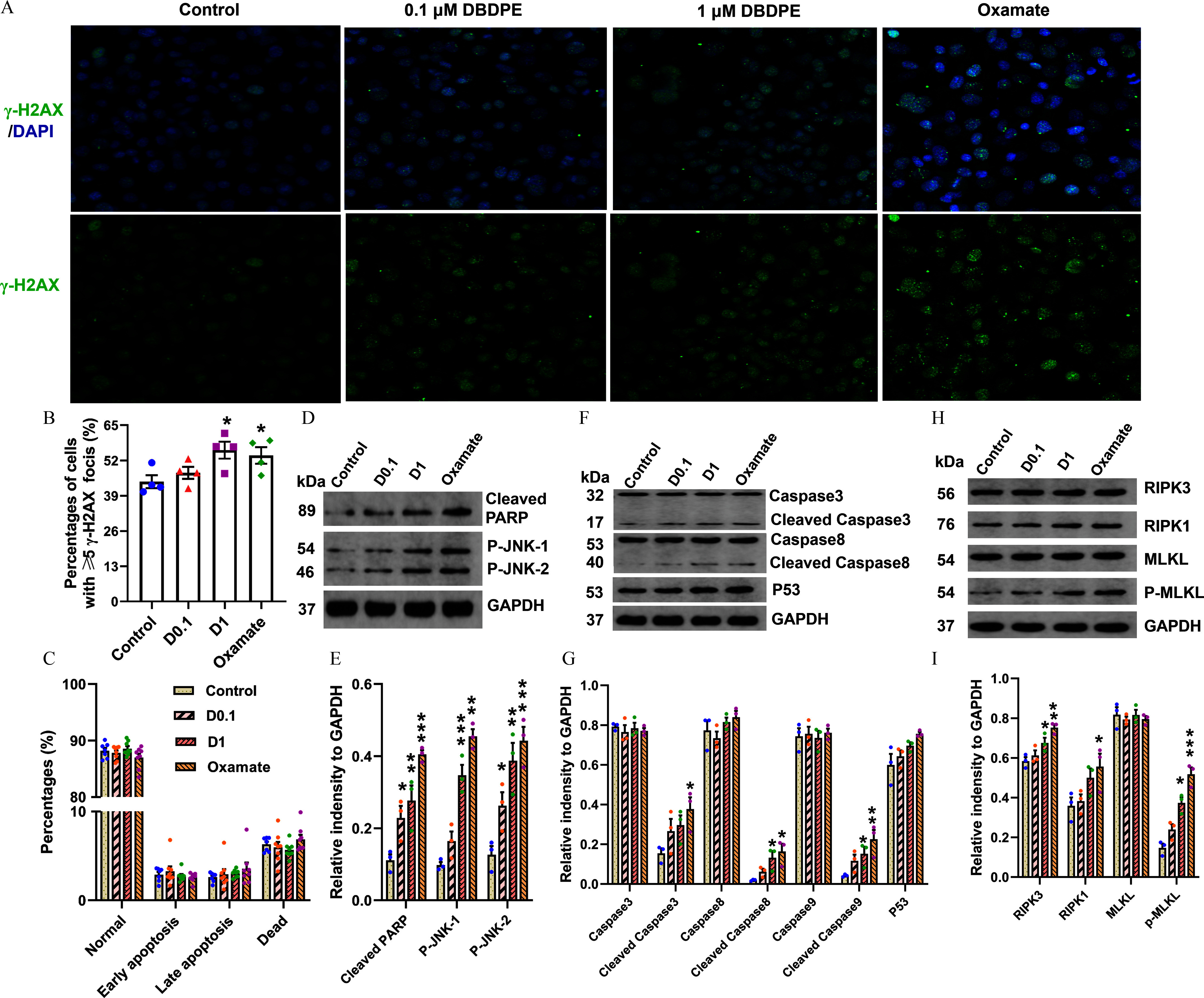
DNA damage and expression of proteins related to apoptosis or necroptosis in mouse spermatogonial GC-1 cells exposed to DBDPE and oxamate (LDH inhibitor) in vitro. (A) DNA damage in zebrafish testes detection by immunofluorescence staining against the histone protein -H2AX. The representative images show DAPI-stained (blue) nuclei with nuclear -H2AX foci in green. (B) Percentages of GC-1 cells with -H2AX foci (). (C) Cell apoptosis detection by flow cytometry (FCM) using an Annexin V-FITC Apoptosis Detection Kit (). (D) Western blotting analyses carried out with antibodies against caspase-3, cleaved caspase-3, caspase-8, cleaved caspase-8, P53, and GAPDH in GC-1 cells. (E) Western blotting analyses carried out with antibodies against cleaved PARP, p-JNK, and GAPDH in GC-1 cells. (F) Western blotting analyses carried out with antibodies against RIPK3, RIPK1, MLKL, p-MLKL, and GAPDH in GC-1 cells. (H–I) Quantification of the abundances of proteins relative to GAPDH (). Each dot in (E–I) represents one replicate data point (one well of cells/replicate). The dot numbers represent the data size for statistical analysis. Results are represented as errors of the mean (SEMs). Data are reported in Excel Table S8. Note: DBDPE, decabromodiphenyl ethane; FITC, fluorescein isothiocyanate; glyceraldehyde-3-phosphate dehydrogenase; LDH, lactate dehydrogenase; MLKL, mixed lineage kinase domain-like; PARP, poly(adenosine diphosphosphate-ribose) polymerase; p-JNK, phospho-c-jun N-terminal kinase; RIPK, receptor-interacting serine-threonine kinase 3. *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
We also investigated energy metabolism changes in GC-1 cells following DBDPE and oxamate exposure. Similar to the results observed in zebrafish testes, glucose content was higher in cells exposed to either DBDPE or oxamate, but this difference was not statistically significant (Figure 9A). In GC-1 cells exposed to DBDPE at the two investigated concentrations or oxamate, significantly lower LDH activity and higher MMP were observed compared with those in the control, but ROS levels were not different from control (Figure 9B–D), consistent with the results observed in zebrafish testes following DBDPE exposure. To measure the ATP content and further explore the effects of DBDPE and oxamate on glycolysis and oxidative phosphorylation, Seahorse Real-Time ATP Rate assays were performed (Figure 9E). The results suggested that oxamate exposure resulted in significantly higher basal respiration of GC-1 cells, but neither concentration of DBDPE was associated with differences in basal respiration (Figure 9F). However, consistent with the result of oxamate exposure, GC-1 cells exposed to both concentrations of DBDPE demonstrated a significantly higher mitoATP/glycoATP ratio (Figure 9G). The overall results indicate that the lower LDH activity might be driven by a metabolic shift from glycolysis to oxidative phosphorylation in GC-1 cells (Figure 9H). Furthermore, most of the investigated parameters in GC-1 cells were significantly different in the DBDPE- and oxamate-exposure groups, compared with controls, and the changing trends were similar. To investigate the relationship between these similarities, a Pearson correlation analysis was performed, and the correlation coefficients of most of the investigated parameters ranged from 0.804 to 0.999 (Figure S8), suggesting positive correlations for changes in the investigated parameters between DBDPE- and oxamate-exposure groups.
Figure 9.
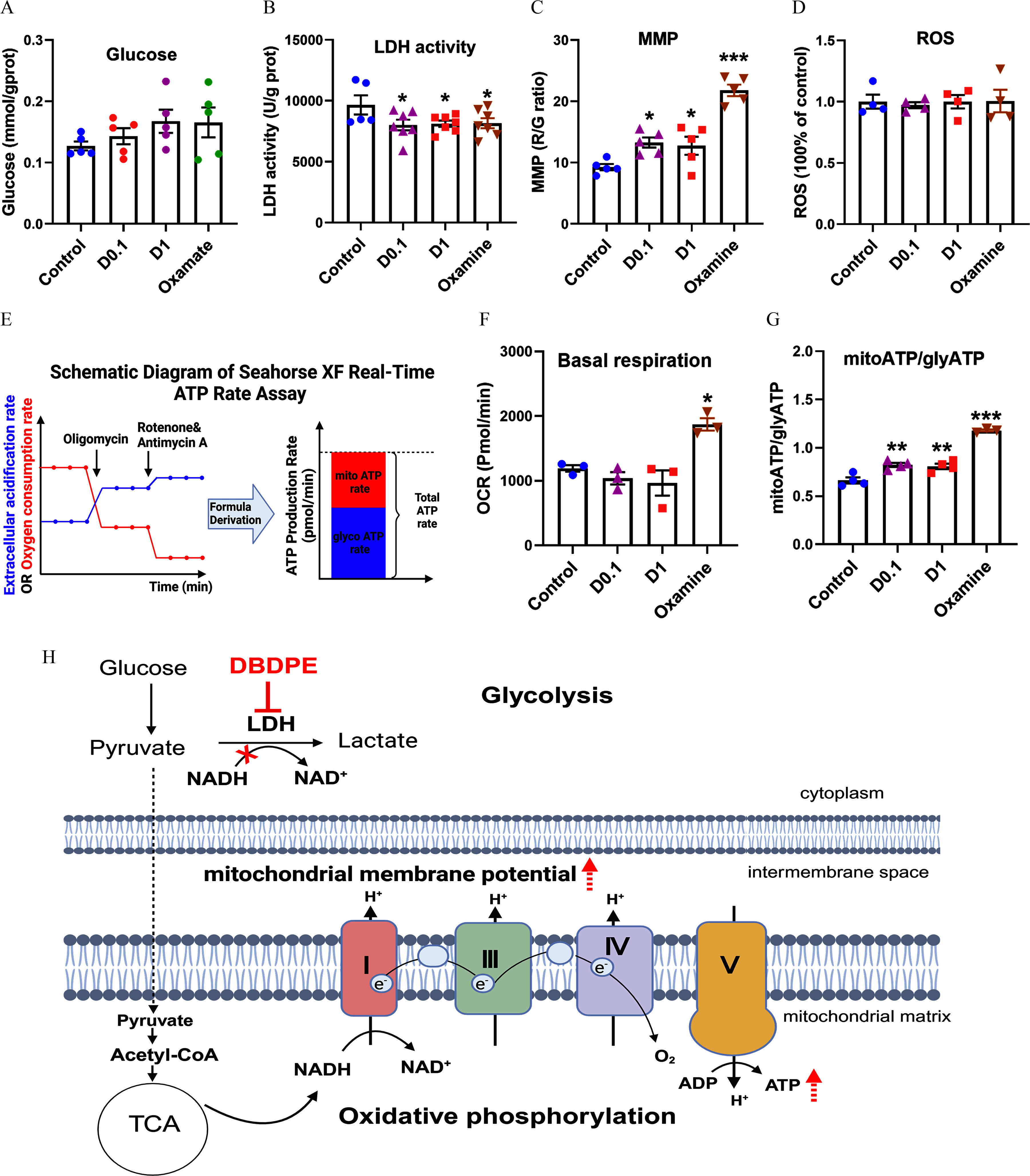
Energy metabolic levels in mouse spermatogonial GC-1 cells exposed to DBDPE and oxamate (LDH inhibitor) in vitro. (A) Glucose content in GC-1 cells treated for 72 h (). (B) LDH activity in GC-1 cells treated for 72 h (). (C) Mitochondrial membrane potential (MMP) of GC-1 cells treated for 72 h (). (D) ROS levels in GC-1 cells treated for 72 h (). (E) Schematic diagram of the Seahorse XF real-time ATP rate assay. The relative contributions of mitochondrial oxidative phosphorylation and glycolysis to ATP can be determined. The illustration was created in BioRender (2023) https://BioRender.com/p67n031. (F) Quantification of basal respiration in response to DBDPE or oxamate (LDH inhibitor) after 72 h of treatment (). (G) Glycolysis-derived ATP (glycoATP)/mitochondrial oxidative phosphorylation-derived ATP (mitoATP) ratio in GC-1 cells exposed to DBDPE ( and ) and oxamate (LDH inhibitor) for 72 h (). Each dot in (A–D,F,G) represents one replicate data point (one well of cells/replicate). (H) Proposed action of DBDPE on glycolysis and oxidative phosphorylation based on findings of the in vitro and in vivo studies. The illustration was created in BioRender (2023) https://BioRender.com/g29w956. Upward dashed arrows (in red) indicate elevation. The red cross indicates inhibition. Results are represented as errors of the mean (SEMs). Data are reported in Excel Table S9. Note: ATP, adenosine triphosphate; D, DBDPE, decabromodiphenyl ethane; G, green; LDH, lactate dehydrogenase; , nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide reduced; R, red; ROS, reactive oxygen species; TCA, tricarboxylic acid (cycle). *, **, and *** indicate significant differences between exposure and control groups, by one-way analysis of variance (ANOVA) followed by the post hoc least significant difference (LSD) test.
Discussion
DBDPE is among the most widely used and predominant NBFRs in various environments,9,60 and it poses significant exposure risks to humans,21,61 as well as to domestic62,63 and wild animals.64 In the present study, we found using an ex vivo zebrafish spermatozoa model that DBDPE-exposed spermatozoa demonstrated poorer quality, and this was confirmed in subsequent in vivo experiments. Analysis integrating whole-proteome and phosphoproteome approaches in zebrafish testes was employed to profile typical responses from DNA damage to cell death. Interestingly, we observed unexpected mitochondrial hyperpolarization (MHP; significantly higher MMP) and no differences in ROS levels in both zebrafish testicular tissues and GC-1 cells, suggesting the existence of a dosage window before DBDPE may lead to cell death. Moreover, energy reprogramming by shifting glycolysis to oxidative phosphorylation was demonstrated in GC-1 cells. The results suggest male reproductive toxicity of DBDPE and provide new insights into the underlying mechanisms.
Spermatozoa Motility and Offspring Development in Ex Vivo
Spermatozoa motility is an important indicator widely used to characterize male reproductive toxicity, and it is highly sensitive to xenobiotics exposure.48,65 Therefore, we first performed ex vivo zebrafish spermatozoa exposure followed by artificial fertilization to preliminarily evaluate the potential male reproductive toxicity of DBDPE. The TM and PM of zebrafish spermatozoa were significantly lower after 3 h of DBDPE exposure compared with those in control, suggesting impairment of sperm quality. However, the trajectory characteristics of zebrafish spermatozoa were not affected, possibly because they are less sensitive to chemicals owing to fish spermatozoa typically moving via a straight or slightly curved trajectory with a symmetrical flagellar wave.66 Fertilization success, which is closely related to sperm quality, has long been used as an important indicator of male reproductive toxicity.67 In the present study, when DBDPE-exposed zebrafish spermatozoa were used for artificial fertilization with healthy zebrafish eggs, fertilization rates were significantly lower, which can be explained by declining spermatozoa motility, as described above. Furthermore, the survival rates of offspring generated from DBDPE-exposed spermatozoa were lower, although this difference was not statistically significant. This may be related to the low concentrations and limited duration of DBDPE exposure, which are key elements determining the toxic effects.
Evaluation of Male Reproductive Toxicity in Vivo
Based on the ex vivo results, an in vivo DBDPE exposure experiment of male zebrafish was conducted to further investigate its male reproductive toxicity. After a 2-month exposure to DBDPE, we did not observe significant differences in several commonly used indicators, including mating behavior and reproductive success (egg production, fertilization rate, malformation rate, and survival rate of offspring larvae). These results suggest lower male reproductive toxicity of DBDPE compared with traditional BFRs.68–70 Regardless, the significantly lower hatching rate at 72 hpf indicated potential adverse effects on sperm quality given that hatching rate is the only characteristic inherited from exposed males. As expected, the TM and PM of zebrafish spermatozoa were also significantly lower, and the trajectory characteristics of spermatozoa, including VAP, VCL, and VSL, were also different than control. Furthermore, a smaller head length and larger head width of spermatozoa were observed following DBDPE exposure. These results suggested that DBDPE can impair spermatozoa quality in zebrafish. Similarly, DBDPE treatment (50 and ) significantly decreased the density and motility of sperm and increased the occurrence of abnormal sperm in male rats.36 Disrupted spermatogenesis is usually accompanied by a decline in semen quality following exposure to environmental pollutants.71,72 In this study, GSI and the interstitial space did not significantly differ between exposed and control fish. In contrast to our study, histopathological damage in testes in the form of seminiferous epithelium deletion was induced by exposure to DBDPE (50 and ) in the rat.36 In the present study, the proportion of SPG was significantly greater in the 1 and DBDPE-exposed fish, and that of SPD was significantly lower in the DBDPE-exposed fish, with a lower trend in the fish exposed to DBDPE, which might suggest inhibition of spermatogenesis following DBDPE exposure.
To comprehensively reveal the underlying mechanism of DBDPE-induced male reproductive toxicity, an integrated whole-proteome and phosphoproteome analysis was performed in zebrafish testes, comparing control and DEBDPE-exposed groups. KEGG analysis of DEPs revealed that the biological processes of necroptosis (, top 5; Excel Table S4) and the phosphatidylinositol signaling system (, top 7; Excel Table S4) were enriched. The PI3K/Akt signaling pathway is known to regulate cell cycle progression.73 Consistently, KEGG analysis of proteins harboring differentially phosphorylated sites revealed enrichment of several pathways, including base excision repair, homologous recombination, mismatch repair, and DNA replication, whereas GO analysis of the phosphoproteome highlighted chromosome organization, chromatin organization, and cytoskeleton organization in the DBDPE-exposed group. The qRT-PCR analysis in the zebrafish testes demonstrated that the proposed pathways (e.g., apoptosis and necroptosis, DNA damage and repair, cell cycle, PI3K-AKT pathways) from the proteome and phosphoproteome analysis at the highest concentration were also affected at lower concentrations (0.1, 1, ). All these enriched pathways are involved in DNA damage responses.74,75 In fact, DNA damage and apoptosis have been observed in testes of rats following oral administration of DBDPE at 50 or .35,36 Similarly, in the present study, DNA damage did occur in the SPD, SPG-I, and SPG-II as characterized by a higher expression of a biomarker (-H2AX) for DNA DSBs in testes of zebrafish following exposure to DBDPE at concentrations . Consistent with our results, oral administration of DBDPE at body weight per day to female mice for 30 d also resulted in significantly increased expression of -H2AX in both male and female pronuclei (PN) of zygotes obtained by mating the exposed females with sexually matured unexposed males.76 Together, these results suggest that DBDPE exposure can induce DNA damage. Interestingly, significantly more TUNEL-positive cells were only observed in the testes of zebrafish upon exposure to DBDPE. As the most serious form of DNA damage, DSBs have been well established as a common ultimate apoptosis-triggering lesion that arises from primary DNA lesions during DNA replication.77,78 Thus, we can infer that the testicular outcomes at DBDPE exposure may be driven by apoptosis due to DNA damage instead of the direct action of DBDPE. Furthermore, we also observed significantly higher expression levels of cleaved PARP, cleaved caspase-3, and P-JNK-1/2 via Western blotting, supporting apoptosis and DNA damage outcomes in DBDPE-exposed zebrafish testes. Although the apoptosis marker (TUNEL) was not observed to be higher in testes at lower concentrations, the transcription of genes associated with DNA damage responses (e.g., lig3, pold1, parp1, parp2, brca2, tp53bpa1, jak3, akt1s1, ripk3)75,79,80 and the expression of cleaved caspase-3 and P-JNK-1/280 demonstrated an upward trend or significantly higher levels, which suggested a status of DNA damage repair and possible triggering of pre-apoptosis. However, more studies are needed to investigate this hypothesis. Taken together, these results suggest that DNA integrity and stability in testicular germ cells are sensitive to DBDPE exposure, and DNA damage may trigger a series of cell cycle regulation processes associated with DNA damage repair.
Most previous studies have attributed pollutant-induced DNA damage to excessive ROS accumulation accompanied by mitochondrial impairment.81,82 In our previous study, exposure to DBDPE at (equal to ) was found to inhibit the activity of mitochondrial respiratory chain complexes, causing decreased MMP, reduced ATP synthesis, and increased ROS levels in zebrafish embryos/larvae.34 However, in the present study, we failed to observe significantly different ROS levels in zebrafish testes after exposure to DBDPE. This observation may indicate that the DNA damage (manifested by the increased expression of -H2AX) detected in our study was not related to ROS. Nuclear actin polymerization to form filamentous actin (F-actin) plays an important role in the responses to DNA damage and DNA replication stress to maintain genome stability.83 It has been reported that disruption of F-actin and the subsequent release of DNase I into the nucleus is a novel mechanism that cleaves DNA to generate -H2AX in response to chemical exposure.84 A recent study showed that DBDPE exposure of female mice to body weight per day resulted in DNA damage responses (increased expression of -H2AX) by affecting the assembly of the nuclear F-actin in the PN of zygotes.76 Our combined proteome and phosphoproteome analyses showed that key proteins involved in F-actin assembly were significantly less expressed in the DBDPE-exposed fish, which may contribute to the observed DNA damage. However, it is unclear whether this was a direct effect of the DBDPE or related to the cell death identified at this exposure. Although the /NADH ratio (an important indicator of the redox state) was lower, MMP was significantly higher, suggesting MHP in testicular cells. The occurrence of MHP is considered a prerequisite for apoptosis, but cells can remain viable under this condition.85 This may explain the lack of differences in ROS levels in the testes, possibly due to the exposure duration not being sufficient to induce more severe effects. Indeed, a recent study reported that MMP can serve as a sensitive indicator for screening mitochondrial toxicants, can be used for water quality monitoring, and can be used to evaluate a wide range of mitochondrial toxicants covering chemicals with diverse molecular initiating events (MIEs), given that multiple MIEs can be involved in MMP disruption.86
However, MMP is not the only determinant of ATP content, especially in spermatogenic cells using lactate as their energy substrate.41 Significantly lower LDH levels and less activity suggested an impaired capacity to convert pyruvate to lactate, supported by the observed lower lactate/pyruvate ratio and greater accumulation of glucose. Consistently, the PPI network analysis also suggested greater oxidative phosphorylation and less glycolysis. Hence, the greater ATP content could be explained by more efficient ATP production via oxidative phosphorylation. Taken together, the above results suggest possible energy reprogramming by shifting from glycolysis to oxidative phosphorylation in the testes of zebrafish following DBDPE exposure.
Validation of DNA Damage and Energy Metabolism in GC-1 Cells
To further confirm the existence of a dosage window and the occurrence of energy reprogramming upon DBDPE exposure, we conducted an in vitro study using the GC-1 spermatogonial cell line. For this purpose, pre–apoptosis-inducing doses (0.1 and ) were selected based on the altered MMP and ATP profiles. Furthermore, the primary role of LDH inhibition was also verified using oxamate as a positive control for glycolysis inhibition. As expected, cells exposed to oxamate or DBDPE demonstrated higher levels of -H2AX. In addition, the expression levels of proteins associated with DNA damage responses and cell death (PRAP, p-JNK1/2, RIPK3, p-MLKL, and cleaved caspase-3/8) were also significantly higher in both treatments. Except for higher MMP, we also observed significantly lower LDH activity, slightly higher glucose levels, and ROS levels that were not significantly different in GC-1 cells exposed to DBDPE or oxamate compared with control. However, the flow cytometry results showed that the early apoptosis, late apoptosis, and mortality rates of GC-1 cells did not differ significantly from control after exposure to any dose of DBDPE or oxamate. These results indicate that exposure to DBDPE at the selected concentrations was associated with DNA damage, mitochondrial hyperpolarization, and greater cell death signaling but not associated with a higher rate of apoptosis. These results are consistent with those obtained from in vivo experiments, thereby providing additional evidence for the existence of a dosage window for DBDPE before inducing germ cell apoptosis. The results may also suggest spermatogonia would be sensitive to direct exposure to environmental chemicals.
To further confirm the occurrence of energy programming, we determined the ATP content generated by the two pathways using Seahorse XF real-time ATP rate assays. A higher mitoATP/glyATP ratio suggested an energy metabolic shift from glycolysis to oxidative phosphorylation in GC-1 cells. We also noted that the changing profiles of most of the determined parameters showed positive correlations (correlation coefficients ranging from 0.804 to 0.999) between the DBDPE-treatment group and the oxamate-treatment group, demonstrating a similar mechanism of action for the two chemicals. Therefore, we speculate that DBDPE may suppress glycolysis by inhibiting LDH activity and enhance oxidative phosphorylation, thereby reprogramming the energy metabolic process in germ cells.
Environmental Implications
In the present study, the lowest concentration () of DBDPE for zebrafish exposure was comparable to the highest detected concentration () in surface water.15 This means that, at least in some cases, aquatic wild animals may face levels of external exposure risks similar to those of the test subjects in our in vivo experiment. Furthermore, the highest reported contents in wild crucian carp (Carassius auratus) ( lw)20 were even higher than those detected in testes from zebrafish after the 2-month DBDPE exposure (, , lw in the 0.1, 1, and DBDPE-exposure groups) in the present study. One possible explanation could be that wild fish spend their entire life time in water, so they have a longer time window to accumulate waterborne pollutants, leading to higher internal exposure risks. Specifically, exposure to DBDPE for 2 months decreased the TM and PM of zebrafish spermatozoa at all the tested concentrations and inhibited spermatogenesis at the highest concentrations, suggesting impaired functions in the male fish. These findings suggest potential risks for male reproductive toxicity in wild fish experiencing DBDPE exposure, and the multigenerational effects are of particular concern and need further research.
Further research revealed DNA damage both in zebrafish testes and mammalian cells (i.e., GC-1 cells) after exposure to DBDPE. Internal doses of up to lw have been reported in the serum of nonoccupational populations from a BFR-producing area.22 Despite the lack of exact dose data in testes from humans at present, it can be predicted that humans will be faced with long-term and persistent exposure to DBDPE due to the continued increase in the use and release of DBDPE into the environment. Given the evolutionary conservation of the mechanisms of DSBs (the observed DNA damage type in the present study) formation and repair in different species,87,88 our results may implicate potential inducing effects of DNA damage in humans and provide theoretical bases for speculating human health effects based on fish and cell experiment results. In addition, the present study also provides an example for establishing NAMs comprising in vitro, ex vivo, and non-rodent in vivo tests for pollutant risk assessment.
Research Limitation
However, certain limitations warrant comment. Notably, the proteomics/phospho-proteomics were limited to the highest concentration of DBDPE in zebrafish testes that exclusively demonstrated overt toxicity, including testicular cell apoptosis, which may not fully represent the effects of environmentally relevant exposure concentrations. Although the qRT-PCR analysis suggested that the proposed pathways at the highest concentration of DBDPE were also affected at lower concentrations, it may not suggest these responses would ultimately result in overt outcomes, such as cell death. Therefore, further research is needed to determine the specific impacts of these biological pathways and their correlation with testicular cell fate. Another limitation is about the difficulty of extrapolating the present results to human health. For one thing, there are many challenges when translating doses used in animal studies to humans, including the dearth of human exposure, toxicokinetic data, and differences in bioavailability for different exposure routes.89 These challenges underscore the importance of the collection of comprehensive data and information and call for further study. For another, mammalian spermatogenesis is more complex than that of zebrafish, possibly resulting in species-specific responses to DBDPE exposure. Thus, the extrapolation of the present findings to human health also warrants more investigation with optimal models (e.g., mice models).
Conclusions
In conclusion, our results suggest that DBDPE exposure could impair sperm quality and spermatogenesis, and the underlying mechanism could be attributed to DNA damage and energy metabolic reprogramming in testicular germ cells by shifting glycolysis to oxidative phosphorylation. Moreover, MMP could be used as a sensitive indicator of male reproductive impairment, both in vivo and in vitro. The dosage window characterized by greater MMP in combination with ROS and apoptosis that was not significantly different from controls may provide useful indications for early prediction and intervention of pollutant-induced cellular damage. The integrated in vitro, ex vivo, and in vivo analyses of male reproductive toxicity may provide new methodologies and expedite pollutant risk assessment in future studies.
Supplementary Material
Acknowledgments
L.Y. and Y.Z. contributed equally to this work. J. Hua, L.Y., and Y.Z. designed the study. J. Han, Y.Z., and G.S. performed ex vivo experiments. J. Han, Y.Z., L.Y., T.Z., X.R., and Z.X. performed animal experiments, Y.Z., J. Han, and N.Z. performed in vitro experiments. Y.Z., J. Han, and N.Z. performed bioinformatics analysis. Y.Z., L.Y., J. Han, T.Z., and F.L. performed data collection and analysis. J. Hua, L.Y., Y.Z., and B. Zhu provided experimental design guidance and interpretation of results. L.Y. and J.H. wrote the manuscript. L.Y., J. Hua, and B. Zhou edited the manuscript.
We thank Yuan Xiao, Yan Wang, Yuan Sun, Fang Zhou, Guangxin Wang, and Zhenfei Xing from the Analytical and Testing Center of Institute of Hydrobiology, Chinese Academy of Sciences, for their technical support in scanning and transmission electron microscopy (EM) analysis, flow cytometry, Seahorse assay, confocal microscope, and EM sample preparation.
This work was supported by the National Key Research and Development Program of China [grant 2022YFC3902102 (to L.Y.)] and the National Natural Science Foundation of China [grants 22006035 (to J. Hua.), 42377281 (to L.Y.), 42207332 (to B.Z.)].
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. 2010. World Health Organization reference values for human semen characteristics. Hum Reprod Update 16(3):231–245, PMID: 19934213, 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 2.Pizzol D, Foresta C, Garolla A, Demurtas J, Trott M, Bertoldo A, et al. 2021. Pollutants and sperm quality: a systematic review and meta-analysis. Environ Sci Pollut Res Int 28(4):4095–4103, PMID: 33196997, 10.1007/s11356-020-11589-z. [DOI] [PubMed] [Google Scholar]
- 3.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ 305(6854):609–613, PMID: 1393072, 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Sharma A, Thaker R. 2021. Air pollutants and impairments of male reproductive health—an overview. Rev Environ Health 36(4):565–575, PMID: 33544535, 10.1515/reveh-2020-0136. [DOI] [PubMed] [Google Scholar]
- 5.Albert O, Huang JY, Aleksa K, Hales BF, Goodyer CG, Robaire B, et al. 2018. Exposure to polybrominated diphenyl ethers and phthalates in healthy men living in the greater Montreal area: study of hormonal balance and semen quality. Environ Int 116: 165–175, PMID: 29684825, 10.1016/j.envint.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y-J, Lin B-G, Liang W-B, Li L-Z, Hong Y-D, Chen X-C, et al. 2018. Associations between PBDEs exposure from house dust and human semen quality at an e-waste areas in South China—a pilot study. Chemosphere 198:266–273, PMID: 29421738, 10.1016/j.chemosphere.2018.01.150. [DOI] [PubMed] [Google Scholar]
- 7.Stieger G, Scheringer M, Ng CA, Hungerbühler K. 2014. Assessing the persistence, bioaccumulation potential and toxicity of brominated flame retardants: data availability and quality for 36 alternative brominated flame retardants. Chemosphere 116:118–123, PMID: 24656972, 10.1016/j.chemosphere.2014.01.083. [DOI] [PubMed] [Google Scholar]
- 8.Zuiderveen EAR, Slootweg JC, de Boer J. 2020. Novel brominated flame retardants—a review of their occurrence in indoor air, dust, consumer goods and food. Chemosphere 255:126816, PMID: 32417508, 10.1016/j.chemosphere.2020.126816. [DOI] [PubMed] [Google Scholar]
- 9.Shen K, Li L, Liu J, Chen C, Liu J. 2019. Stocks, flows and emissions of DBDPE in China and its international distribution through products and waste. Environ Pollut 250:79–86, PMID: 30981938, 10.1016/j.envpol.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Ma T, Liu W, Yuan G, Pan X, Zhang M, et al. 2024. Brominated flame retardants (BFRs) in China over the past half-century: stocks, flows, fates, and ecological risks. Environ Sci Technol 58(31):13613–13623, PMID: 39051121, 10.1021/acs.est.4c00183. [DOI] [PubMed] [Google Scholar]
- 11.An Q, Yang L, Yang S, Wang Y, Shi L, Aamir M, et al. 2023. Legacy and novel brominated flame retardants in agricultural soils of eastern China (2011–2021): concentration level, temporal trend, and health risk assessment. J Hazard Mater 446:130631, PMID: 36586335, 10.1016/j.jhazmat.2022.130631. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Zhen X, Wang X, Zhang D, Sun L, Tang J. 2021. Spatio-temporal variations and input patterns on the legacy and novel brominated flame retardants (BFRs) in coastal rivers of North China. Environ Pollut 283:117093, PMID: 33857880, 10.1016/j.envpol.2021.117093. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Lai C, Xu F, Huang D, Zhang M, Zhou X, et al. 2023. A review of polybrominated diphenyl ethers and novel brominated flame retardants in Chinese aquatic environment: source, occurrence, distribution, and ecological risk assessment. Sci Total Environ 904:166180, PMID: 37562617, 10.1016/j.scitotenv.2023.166180. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Li Y, Zhang J, Niu D, Wang J, Tang J. 2023. Influence of hydrodynamic conditions on the fate of halogenated flame retardants along salinity gradients in a highly polluted micro-tidal estuary. Sci Total Environ 893:164716, PMID: 37301402, 10.1016/j.scitotenv.2023.164716. [DOI] [PubMed] [Google Scholar]
- 15.Zhen X, Tang J, Liu L, Wang X, Li Y, Xie Z. 2018. From headwaters to estuary: distribution and fate of halogenated flame retardants (HFRs) in a river basin near the largest HFR manufacturing base in China. Sci Total Environ 621:1370–1377, PMID: 29054623, 10.1016/j.scitotenv.2017.10.091. [DOI] [PubMed] [Google Scholar]
- 16.Nyholm JR, Grabic R, Arp HPH, Moskeland T, Andersson PL. 2013. Environmental occurrence of emerging and legacy brominated flame retardants near suspected sources in Norway. Sci Total Environ 443:307–314, PMID: 23201697, 10.1016/j.scitotenv.2012.10.081. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Y-H, Luo X-J, Sun Y-X, Yu L-H, Chen S-J, Mai B-X. 2011. [Concentration and emission fluxes of halogenated flame retardants in sewage from sewage outlet in Dongjiang River]. Huan Jing Ke Xue 32(10):2891–2895, PMID: 22279897. [PubMed] [Google Scholar]
- 18.Hou R, Lin L, Li H, Liu S, Xu X, Xu Y, et al. 2021. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments—a critical review. Water Res 198:117168, PMID: 33962238, 10.1016/j.watres.2021.117168. [DOI] [PubMed] [Google Scholar]
- 19.Hou R, Huang Q, Pan Y, Lin L, Liu S, Li H, et al. 2022. Novel brominated flame retardants (NBFRs) in a tropical marine food web from the South China Sea: the influence of hydrophobicity and biotransformation on structure-related trophodynamics. Environ Sci Technol 56(5):3147–3158, PMID: 35175039, 10.1021/acs.est.1c08104. [DOI] [PubMed] [Google Scholar]
- 20.Tao L, Zhang Y, Wu J-P, Wu S-K, Liu Y, Zeng Y-H, et al. 2019. Biomagnification of PBDEs and alternative brominated flame retardants in a predatory fish: using fatty acid signature as a primer. Environ Int 127:226–232, PMID: 30928846, 10.1016/j.envint.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Chen T, Yu D, Yang L, Sui S, Lv S, Bai Y, et al. 2019. Thyroid function and decabromodiphenyl ethane (DBDPE) exposure in Chinese adults from a DBDPE manufacturing area. Environ Int 133(pt A):105179, PMID: 31627134, 10.1016/j.envint.2019.105179. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Chen T, Yang B, Wang D, Sun W, Wang Y, et al. 2021. Serum levels of novel brominated flame retardants (NBFRs) in residents of a major BFR-producing region: occurrence, impact factors and the relationship to thyroid and liver function. Ecotoxicol Environ Saf 208:111467, PMID: 33080422, 10.1016/j.ecoenv.2020.111467. [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, Zhang L, Li J, Zhao Y, Sun Z, Zhou X, et al. 2016. Novel brominated flame retardants in food composites and human milk from the Chinese Total Diet Study in 2011: concentrations and a dietary exposure assessment. Environ Int 96:82–90, PMID: 27619751, 10.1016/j.envint.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Qiao L, Zheng X-B, Yan X, Wang M-H, Zheng J, Chen S-J, et al. 2018. Brominated flame retardant (BFRs) and dechlorane plus (DP) in paired human serum and segmented hair. Ecotoxicol Environ Saf 147:803–808, PMID: 28954370, 10.1016/j.ecoenv.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Huang M, Li J, Li J, Shi Z. 2019. Polybrominated diphenyl ethers and novel brominated flame retardants in human milk from the general population in Beijing, China: occurrence, temporal trends, nursing infants’ exposure and risk assessment. Sci Total Environ 689:278–286, PMID: 31276995, 10.1016/j.scitotenv.2019.06.442. [DOI] [PubMed] [Google Scholar]
- 26.Sala B, Garcia-Garin O, Borrell A, Aguilar A, Víkingsson GA, Eljarrat E. 2022. Transplacental transfer of plasticizers and flame retardants in fin whales (Balaenoptera physalus) from the North Atlantic Ocean. Environ Pollut 313:120168, PMID: 36115483, 10.1016/j.envpol.2022.120168. [DOI] [PubMed] [Google Scholar]
- 27.Barón E, Giménez J, Verborgh P, Gauffier P, De Stephanis R, Eljarrat E, et al. 2015. Bioaccumulation and biomagnification of classical flame retardants, related halogenated natural compounds and alternative flame retardants in three delphinids from Southern European waters. Environ Pollut 203:107–115, PMID: 25875161, 10.1016/j.envpol.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Aznar-Alemany Ò, Sala B, Jobst KJ, Reiner EJ, Borrell A, Aguilar À, et al. 2021. Temporal trends of halogenated and organophosphate contaminants in striped dolphins from the Mediterranean Sea. Sci Total Environ 753:142205, PMID: 33207472, 10.1016/j.scitotenv.2020.142205. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Yang Y, Dang C, Lu B, Luo Y, Fu J. 2023. Is it really safe to replace decabromodiphenyl ether (BDE209) with decabromodiphenyl ethane (DBDPE)? A perspective from hepatotoxicity. Environ Toxicol 38(4):844–856, PMID: 36660779, 10.1002/tox.23727. [DOI] [PubMed] [Google Scholar]
- 30.Dong L, Wang S, Zhang L, Liu D, You H. 2023. DBDPE and ZnO NPs synergistically induce neurotoxicity of SK-N-SH cells and activate mitochondrial apoptosis signaling pathway and Nrf2-mediated antioxidant pathway. J Hazard Mater 441:129872, PMID: 36084461, 10.1016/j.jhazmat.2022.129872. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Zeng L, Li Y, Shi C, Peng Y, Pan R, et al. 2022. Decabromodiphenyl ethane induces locomotion neurotoxicity and potential Alzheimer’s disease risks through intensifying amyloid-beta deposition by inhibiting transthyretin/transthyretin-like proteins. Environ Int 168:107482, PMID: 35998411, 10.1016/j.envint.2022.107482. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Ling S, Guan K, Luo X, Chen L, Han J, et al. 2019. Bioconcentration, biotransformation, and thyroid endocrine disruption of decabromodiphenyl ethane (DBDPE), a novel brominated flame retardant, in zebrafish larvae. Environ Sci Technol 53(14):8437–8446, PMID: 31188578, 10.1021/acs.est.9b02831. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Wang X, Sui S, Liu Z. 2023. Endocrine disrupting and carcinogenic effects of decabromodiphenyl ether. Front Endocrinol (Lausanne) 14:1183815, PMID: 37334308, 10.3389/fendo.2023.1183815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Xu Y, Wu H, Hou J. 2024. A critical review on BDE-209: source, distribution, influencing factors, toxicity, and degradation. Environ Int 183:108410, PMID: 38160509, 10.1016/j.envint.2023.108410. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Liu J, Zhou G, Sang Y, Zhang Y, Jing L, et al. 2021. BDE-209 and DBDPE induce male reproductive toxicity through telomere-related cell senescence and apoptosis in SD rat. Environ Int 146:106307, PMID: 33395949, 10.1016/j.envint.2020.106307. [DOI] [PubMed] [Google Scholar]
- 36.Xue J, Li X, Liu J, Zhang Y, Sang Y, Zhou G, et al. 2022. Decabromodiphenyl ethane induces male reproductive toxicity by glycolipid metabolism imbalance and meiotic failure. Ecotoxicol Environ Saf 246:114165, PMID: 36228355, 10.1016/j.ecoenv.2022.114165. [DOI] [PubMed] [Google Scholar]
- 37.Sun RB, Shang S, Zhang W, Lin BC, Wang Q, Shi Y, et al. 2018. Endocrine disruption activity of 30-day dietary exposure to decabromodiphenyl ethane in Balb/C mouse. Biomed Environ Sci 31(1):12–22, PMID: 29409581, 10.3967/bes2018.002. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Zhu B, Zhou S, Zhao M, Li R, Zhou Y, et al. 2023. Mitochondrial dysfunction was involved in decabromodiphenyl ethane-induced glucolipid metabolism disorders and neurotoxicity in zebrafish larvae. Environ Sci Technol 57(30):11043–11055, PMID: 37467077, 10.1021/acs.est.3c03552. [DOI] [PubMed] [Google Scholar]
- 39.Braeuning A, Balaguer P, Bourguet W, Carreras-Puigvert J, Feiertag K, Kamstra JH, et al. 2023. Development of new approach methods for the identification and characterization of endocrine metabolic disruptors—a PARC project. Front Toxicol 5:1212509, PMID: 37456981, 10.3389/ftox.2023.1212509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68:3–33, PMID: 27760374, 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boussouar F, Benahmed M. 2004. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab 15(7):345–350, PMID: 15350607, 10.1016/j.tem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Vaupel P, Schmidberger H, Mayer A. 2019. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol 95(7):912–919, PMID: 30822194, 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 43.Aurrière J, Goudenège D, Baris OR, Boguenet M, May-Panloup P, Lenaers G, et al. 2021. Cancer/testis antigens into mitochondria: a hub between spermatogenesis, tumorigenesis and mitochondrial physiology adaptation. Mitochondrion 56:73–81, PMID: 33220498, 10.1016/j.mito.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Jiang X, Shi P, Jiang L, Qiu J, Xu B, Pan Y, et al. 2022. In vivo toxicity evaluations of halophenolic disinfection byproducts in drinking water: a multi-omics analysis of toxic mechanisms. Water Res 218:118431, PMID: 35468502, 10.1016/j.watres.2022.118431. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Tang S, Peng H, Saunders DMV, Doering JA, Hecker M, et al. 2016. Combined transcriptomic and proteomic approach to identify toxicity pathways in early life stages of Japanese medaka (Oryzias latipes) exposed to 1,2,5,6-tetrabromocyclooctane (TBCO). Environ Sci Technol 50(14):7781–7790, PMID: 27322799, 10.1021/acs.est.6b01249. [DOI] [PubMed] [Google Scholar]
- 46.Żarski D, Horváth Á, Bernáth G, Palińska-Żarska K, Krejszeff S, Müller T, et al. 2014. Application of different activating solutions to in vitro fertilization of crucian carp, Carassius carassius (L.), eggs. Aquacult Int 22(1):173–184, 10.1007/s10499-013-9692-z. [DOI] [Google Scholar]
- 47.Shaliutina O, Materiienko A, Shaliutina-Kolešová A, Gazo I. 2021. Using fish spermatozoa in in vitro toxicity tests: a potential toxicology tool. Aquaculture 539:736647, 10.1016/j.aquaculture.2021.736647. [DOI] [Google Scholar]
- 48.Kollár T, Kása E, Ferincz Á, Urbányi B, Csenki-Bakos Z, Horváth Á. 2018. Development of an in vitro toxicological test system based on zebrafish (Danio rerio) sperm analysis. Environ Sci Pollut Res Int 25(15):14426–14436, PMID: 29525864, 10.1007/s11356-018-1613-2. [DOI] [PubMed] [Google Scholar]
- 49.Hatef A, Alavi SMH, Golshan M, Linhart O. 2013. Toxicity of environmental contaminants to fish spermatozoa function in vitro—a review. Aquat Toxicol 140–141:134–144, PMID: 23792626, 10.1016/j.aquatox.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Nordgreen J, Tahamtani FM, Janczak AM, Horsberg TE. 2014. Behavioural effects of the commonly used fish anaesthetic tricaine methanesulfonate (MS-222) on zebrafish (Danio rerio) and its relevance for the acetic acid pain test. PLoS One 9(3):e92116, PMID: 24658262, 10.1371/journal.pone.0092116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews JL, Murphy JM, Carmichael C, Yang H, Tiersch T, Westerfield M, et al. 2018. Changes to extender, cryoprotective medium, and in vitro fertilization improve zebrafish sperm cryopreservation. Zebrafish 15(3):279–290, PMID: 29369744, 10.1089/zeb.2017.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.OECD (Organisation for Economic Co-operation and Development). 2013. Test No. 236: Fish Embryo Acute Toxicity (FET) test, OECD Guidelines for the Testing of Chemicals, Section 2. Paris, France: OECD Publishing. 10.1787/9789264203709-en. [DOI] [Google Scholar]
- 53.Zhang S-Y, Gan X, Shen B, Jiang J, Shen H, Lei Y, et al. 2023. 6PPD and its metabolite 6PPDQ induce different developmental toxicities and phenotypes in embryonic zebrafish. J Hazard Mater 455:131601, PMID: 37182464, 10.1016/j.jhazmat.2023.131601. [DOI] [PubMed] [Google Scholar]
- 54.Notarangelo G, Spinelli JB, Perez EM, Baker GJ, Kurmi K, Elia I, et al. 2022. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377(6614):1519–1529, PMID: 36173860, 10.1126/science.abj5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong X, Chen R, Zhang L, Yan L, Li J, Zha J. 2022. Low doses and lifecycle exposure of waterborne antidepressants in zebrafish model: a survey on sperm traits, reproductive behaviours, and transcriptome responses. Sci Total Environ 832:155017, PMID: 35395305, 10.1016/j.scitotenv.2022.155017. [DOI] [PubMed] [Google Scholar]
- 56.Sáez-Espinosa P, Franco-Esclapez C, Robles-Gómez L, Silva WTAF, Romero A, Immler S, et al. 2022. Morphological and ultrastructural alterations of zebrafish (Danio rerio) spermatozoa after motility activation. Theriogenology 188:108–115, PMID: 35688040, 10.1016/j.theriogenology.2022.05.025. [DOI] [PubMed] [Google Scholar]
- 57.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. 2009. Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362, PMID: 19377485, 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 58.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675, PMID: 22930834, 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie H, Wang X, Jin M, Li L, Zhu J, Kang Y, et al. 2022. Cilia regulate meiotic recombination in zebrafish. J Mol Cell Biol 14(7):mjac049, PMID: 35981808, 10.1093/jmcb/mjac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong P, Yan X, Zhu Q, Qu G, Shi J, Liao C, et al. 2019. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ Sci Technol 53(23):13551–13569, PMID: 31682424, 10.1021/acs.est.9b03159. [DOI] [PubMed] [Google Scholar]
- 61.Liu M, Li A, Meng L, Zhang G, Guan X, Zhu J, et al. 2022. Exposure to novel brominated flame retardants and organophosphate esters and associations with thyroid cancer risk: a case–control study in eastern China. Environ Sci Technol 56(24):17825–17835, PMID: 36468700, 10.1021/acs.est.2c04759. [DOI] [PubMed] [Google Scholar]
- 62.Egloff C, Crump D, Chiu S, Manning G, McLaren KK, Cassone CG, et al. 2011. In vitro and in ovo effects of four brominated flame retardants on toxicity and hepatic mRNA expression in chicken embryos. Toxicol Lett 207(1):25–33, PMID: 21893176, 10.1016/j.toxlet.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 63.Zheng X-B, Luo X-J, Zeng Y-H, Wu J-P, Chen S-J, Mai B-X. 2014. Halogenated flame retardants during egg formation and chicken embryo development: maternal transfer, possible biotransformation, and tissue distribution. Environ Toxicol Chem 33(8):1712–1719, PMID: 24888473, 10.1002/etc.2588. [DOI] [PubMed] [Google Scholar]
- 64.Fernie KJ, Chabot D, Champoux L, Brimble S, Alaee M, Marteinson S, et al. 2017. Spatiotemporal patterns and relationships among the diet, biochemistry, and exposure to flame retardants in an apex avian predator, the peregrine falcon. Environ Res 158:43–53, PMID: 28599194, 10.1016/j.envres.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 65.Ozgur ME, Ulu A, Balcioglu S, Ozcan I, Okumus F, Koytepe S, et al. 2018. Investigation of toxicity properties of flower-like ZnO nanoparticles on Cyprinus carpio sperm cells using computer-assisted sperm analysis (CASA). Turk J Fish Aquat Sci 18:771–780, 10.4194/1303-2712-v18_6_03. [DOI] [Google Scholar]
- 66.Alavi SMH, Hatef A, Pšenička M, Kašpar V, Boryshpolets S, Dzyuba B, et al. 2012. Sperm biology and control of reproduction in sturgeon: (II) sperm morphology, acrosome reaction, motility and cryopreservation. Rev Fish Biol Fish 22(4):861–886, 10.1007/s11160-012-9270-x. [DOI] [Google Scholar]
- 67.Dietrich GJ, Dietrich M, Kowalski RK, Dobosz S, Karol H, Demianowicz W, et al. 2010. Exposure of rainbow trout milt to mercury and cadmium alters sperm motility parameters and reproductive success. Aquat Toxicol 97(4):277–284, PMID: 20044150, 10.1016/j.aquatox.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Han XB, Yuen KWY, Wu RSS. 2013. Polybrominated diphenyl ethers affect the reproduction and development, and alter the sex ratio of zebrafish (Danio rerio). Environ Pollut 182:120–126, PMID: 23906559, 10.1016/j.envpol.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 69.He J, Yang D, Wang C, Liu W, Liao J, Xu T, et al. 2011. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 20(8):1813–1822, PMID: 21695510, 10.1007/s10646-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y, Zhu G, Peng L, Ni W, Wang X, Zhang J, et al. 2015. Effect of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) on sexual behaviors and reproductive function in male zebrafish (Danio rerio). Ecotoxicol Environ Saf 111:102–108, PMID: 25450921, 10.1016/j.ecoenv.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 71.Vested A, Giwercman A, Bonde JP, Toft G. 2014. Persistent organic pollutants and male reproductive health. Asian J Androl 16(1):71–80, PMID: 24369135, 10.4103/1008-682X.122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brokken LJS, Lundberg PJ, Spanò M, Manicardi GC, Pedersen HS, Struciński P, et al. 2014. Interactions between polymorphisms in the aryl hydrocarbon receptor signalling pathway and exposure to persistent organochlorine pollutants affect human semen quality. Reprod Toxicol 49:65–73, PMID: 25084496, 10.1016/j.reprotox.2014.07.073. [DOI] [PubMed] [Google Scholar]
- 73.Liang J, Slingerland JM. 2003. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2(4):339–345, PMID: 12851486, 10.4161/cc.2.4.433. [DOI] [PubMed] [Google Scholar]
- 74.Stanic M, Mekhail K. 2022. Integration of DNA damage responses with dynamic spatial genome organization. Trends Genet 38(3):290–304, PMID: 34598804, 10.1016/j.tig.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 75.Chatterjee N, Walker GC. 2017. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58(5):235–263, PMID: 28485537, 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi F, Xu Y, Zhang S, Fu Z, Yu Q, Zhang S, et al. 2022. Decabromodiphenyl ethane affects embryonic development by interfering with nuclear F-actin in zygotes and leads to cognitive and social disorders in offspring mice. FASEB J 36(8):e22445, PMID: 35816173, 10.1096/fj.202200586R. [DOI] [PubMed] [Google Scholar]
- 77.Kaina B. 2003. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol 66(8):1547–1554, PMID: 14555233, 10.1016/s0006-2952(03)00510-0. [DOI] [PubMed] [Google Scholar]
- 78.Lorda-Diez CI, Solis-Mancilla ME, Sanchez-Fernandez C, Garcia-Porrero JA, Hurle JM, Montero JA. 2019. Cell senescence, apoptosis and DNA damage cooperate in the remodeling processes accounting for heart morphogenesis. J Anat 234(6):815–829, PMID: 30875434, 10.1111/joa.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida K, Miki Y. 2004. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 95(11):866–871, PMID: 15546503, 10.1111/j.1349-7006.2004.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roos WP, Kaina B. 2006. DNA damage-induced cell death by apoptosis. Trends Mol Med 12(9):440–450, PMID: 16899408, 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 81.Shaliutina O, Shaliutina-Kolešová A, Lebeda I, Rodina M, Gazo I. 2017. The in vitro effect of nonylphenol, propranolol, and diethylstilbestrol on quality parameters and oxidative stress in sterlet (Acipenser ruthenus) spermatozoa. Toxicol In Vitro 43:9–15, PMID: 28533019, 10.1016/j.tiv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, Liu JH, Ren LH, Wei JL, Duan JC, Zhang LF, et al. 2018. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Sci Total Environ 634:1435–1444, PMID: 29710643, 10.1016/j.scitotenv.2018.03.383. [DOI] [PubMed] [Google Scholar]
- 83.Wollscheid H-P, Ulrich HD. 2023. Chromatin meets the cytoskeleton: the importance of nuclear actin dynamics and associated motors for genome stability. DNA Repair (Amst) 131:103571, PMID: 37738698, 10.1016/j.dnarep.2023.103571. [DOI] [PubMed] [Google Scholar]
- 84.Zhao X, Yang G, Toyooka T, Ibuki Y. 2015. New mechanism of γ-H2AX generation: surfactant-induced actin disruption causes deoxyribonuclease I translocation to the nucleus and forms DNA double-strand breaks. Mutat Res Genet Toxicol Environ Mutagen 794:1–7, PMID: 26653977, 10.1016/j.mrgentox.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Iijima T. 2006. Mitochondrial membrane potential and ischemic neuronal death. Neurosci Res 55(3):234–243, PMID: 16716421, 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Lee J, König M, Braun G, Escher BI. 2024. Water quality monitoring with the multiplexed assay MitoOxTox for mitochondrial toxicity, oxidative stress response, and cytotoxicity in AREc32 cells. Environ Sci Technol 58(13):5716–5726, PMID: 38503264, 10.1021/acs.est.3c09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawale AS, Sung P. 2020. Mechanism and significance of chromosome damage repair by homologous recombination. Essays Biochem 64(5):779–790, PMID: 32756864, 10.1042/EBC20190093. [DOI] [PubMed] [Google Scholar]
- 88.Li Y, Wu Y-F, Jiang H-W, Khan R, Han Q-Q, Iqbal F, et al. 2021. The molecular control of meiotic double-strand break (DSB) formation and its significance in human infertility. Asian J Androl 23(6):555–561, PMID: 33586697, 10.4103/aja.aja_5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panlilio JM, Aluru N, Hahn ME. 2020. Developmental neurotoxicity of the harmful algal bloom toxin domoic acid: cellular and molecular mechanisms underlying altered behavior in the zebrafish model. Environ Health Perspect 128(11):117002, PMID: 33147070, 10.1289/EHP6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


