Abstract
Non-typhoidal Salmonella serovars, such as Salmonella enterica serovar Typhimurium (STm), are a leading cause of inflammatory diarrhea in otherwise healthy individuals. Among children, the elderly, and immunocompromised individuals, STm can spread to systemic sites and cause potentially lethal bacteremia. Phagocytic cells and the immune complement system are pivotal to preventing the dissemination of STm. PgtE, an STm outer membrane protease, has been previously described to cleave over a dozen mammalian protein substrates in vitro, including complement protein C3. However, these activities have mostly been observed with mutant, avirulent strains with a truncated O-antigen that renders bacteria sensitive to complement killing. Here, we report that virulent STm utilizes PgtE to evade complement-mediated killing in vivo. The wild-type pathogen increases pgtE expression and PgtE proteolytic function within macrophages and in macrophage-like in vitro growth conditions, concomitant with physiologic O-antigen shortening in these environments. Furthermore, we found that wild-type STm’s resistance to complement-mediated serum and neutrophil killing is PgtE-dependent. We propose that PgtE promotes the systemic spread of STm by acting as a second line of defense against complement when STm escapes from a macrophage.
INTRODUCTION
Infections with non-typhoidal Salmonella (NTS) are among the leading causes of gastrointestinal disease worldwide (1). Clinically, NTS infection presents with inflammatory diarrhea (2), characterized by localized gastrointestinal inflammation and neutrophil influx in the intestinal mucosa (3). In healthy individuals, NTS infection remains localized to the gut (2). However, approximately 5% of patients infected with NTS develop bacteremia, a serious and potentially fatal complication (2). Children and the elderly are at risk for developing bacteremia (4), and additional risk factors include leukemia, chemotherapy, and HIV infection prior to the advent of antiretroviral therapy (5–8). In recent years, invasive non-typhoidal Salmonella (iNTS) strains have emerged as a prominent cause of bloodstream infection in sub-Saharan Africa (9), with serovars Typhimurium (STm) and Enteritidis implicated in 91% of iNTS cases (10). Important risk factors for iNTS disease in Africa are HIV infection, malaria, and malnutrition (9). Furthermore, complicated iNTS infections present a challenge for antibiotic treatment due to increased multidrug resistance (2, 11). It is thus imperative to elucidate mechanisms by which STm can evade host immune defenses to cause bacteremia.
Neutrophils are thought to play a crucial role in preventing NTS bacteremia through limiting dissemination of the pathogen from the mucosa to systemic sites. Neutropenia in patients with HIV (7) or cancer (6), as well as defective production of reactive oxygen species (ROS) in patients with chronic granulomatous disease (12), heightens the risk of NTS bacteremia. Experiments in mice, largely conducted with STm, corroborate these clinical observations, as neutrophil depletion leads to increased pathogen dissemination (13). Even with a fully functional immune system, macrophages are less effective at killing STm due to the pathogen’s numerous strategies for survival and replication within these cells. Within the macrophage phagosome, STm uses the two-component regulatory system PhoPQ to sense acidification, Mg2+-limiting conditions, and cationic antimicrobial peptides, which together induce the expression of Salmonella Pathogenicity Island 2 (SPI2) effector genes (14–18). The SPI2-encoded type-3 secretion system delivers a plethora of effector proteins that prevent the fusion of the phagosome with lysosomes, allowing STm to persist in Salmonella-containing vacuoles (SCVs) within macrophages (19–21).
Protected inside the macrophage compartment, STm can spread to the liver, spleen, and blood while evading extracellular host defenses (22–25). In the extracellular environment, Salmonella is more vulnerable to complement opsonization, which contributes to host protection during bacteremia (26, 27) by mechanisms that are not completely elucidated. Long O-antigen chains of lipopolysaccharide on Salmonella play a crucial role in steric inhibition of complement, reducing effective membrane attack complex (MAC) formation. Consequently, STm lacking O-antigen (rough mutants) are susceptible to serum complement killing (28) and are avirulent (29, 30). Resistance to complement is also mediated by the outer membrane proteins TraT and Rck (31–33). A third outer membrane protein, PgtE, is a promiscuous protease described to cleave a dozen different substrates in vitro (34–39), including complement-associated proteins. Increased expression of pgtE has also been proposed to promote survival and dissemination of iNTS (39). Nevertheless, it is unknown whether the cleavage of complement proteins promotes STm virulence in vivo.
All previous studies investigating PgtE function in vitro used rough mutants, because the long O-antigen in wild-type strains sterically inhibits PgtE function (35–37, 39, 40). We thus sought to unravel the in vivo role of PgtE in wild-type, virulent strains with an intact O-antigen (smooth strains). Here we show that an STm pgtE mutant is attenuated in wild-type mice, but is rescued in complement-deficient mice. Mechanistically, we found that wild-type STm cleaves complement C3 in a PgtE-dependent manner when inside macrophages or cultured in media mimicking the SCV, environments where STm expresses a shorter O-antigen. Unexpectedly, however, PgtE-mediated disruption of complement did not promote STm survival in macrophages, but rather enhanced serum resistance and evasion of neutrophil killing, thereby contributing to bacteremia.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Bacterial strains used in this study are listed in Supplementary Table 1. Plasmids used in this study are listed in Supplementary Table 2. Most of the in vitro and all of the in vivo work was performed with Salmonella enterica serovar Typhimurium (STm) strain IR715, a fully virulent, nalidixic acid-resistant derivative of strain ATCC 14028s, as well as an isogenic pgtE mutant of IR715. For some in vitro experiments, we employed the Salmonella enterica serovar Typhimurium sequence type ST313 strain D23580 and its isogenic pgtE mutant (39).
IR715 and D23580 strains were cultured on LB agar plates that were supplemented with 50 μg/ml nalidixic acid or 30 μg/ml chloramphenicol, respectively. IR715 and E. coli XL1-Blue strains transformed with a low-copy plasmid (pWSK29) encoding wild-type pgtE (pPgtE) or a pgtE inactive mutant (pPgtE-D206A) were grown on LB agar plates supplemented with 100 μg/ml carbenicillin. For each inoculum, three colonies were cultured overnight in 5ml of medium without antibiotic selection. All bacteria were cultured with shaking/rolling, unless otherwise stated. For animal infections, all strains were cultured in L broth (LB; 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl) aerobically at 37 °C, overnight. For in vitro experiments, strains were cultured in either LB or SPI2-inducing phosphate-carbon-nitrogen (PCN) liquid media supplemented with low magnesium (InSPI2 LowMg2+) (41), aerobically at 37 °C, overnight.
Generation of bacterial mutants
Primers used in this study are listed in Supplementary Table 3. The STm pgtE mutant was constructed by allelic exchange with the plasmid pGP704 containing a tetracycline resistance cassette flanked by 1 kb regions upstream and downstream of the pgtE gene. Primers were used to PCR amplify 1kb upstream (left border, LB) and downstream (right border, RB) of the pgtE gene. The resulting products were fused in a fusion PCR and cloned into vector pCR-Blunt II-TOPO (Invitrogen). The resulting plasmid, pCRII::pgtE-LBRB, was sequenced and subsequently cut with SalI and EcoRV. The pgtE-LBRB fragment was gel purified and cloned into the SalI and EcoRV digested vector pGP704 and transformed into E. coli CC118 λpir. The resulting plasmid, pGP704::pgtE-LBRB, was cut with XbaI, and an NheI-digested tetracycline resistance cassette (tetRA) from pSPN23 was ligated into the plasmid and again transformed into CC118 λpir. The resulting plasmid, pGP704::pgtE-LBRB::tetRA, was transformed into E. coli S17–1 λpir, then the strain was conjugated with STm IR715, generating strain IR715 ΔpgtE via after selecting and screening for double-crossover events from homologous recombination. The integration of the resistance cassette and the deletion of the pgtE gene were confirmed by Southern blot using a probe for the 1kb region upstream of pgtE, and the North2South Chemiluminescent Hybridization and Detection kit (Thermo Fisher). D23580 ΔpgtE was constructed by transducing the pgtE deletion from IR715 to D23580 with P22 HT105/1 int-201.
For constitutive expression of the mCherry fluorescent protein, STm strains were transduced with a P22 lysate derived from STm SL1344 glmS::Ptrc-mCherryST::Cm (42), followed by removal of the CmR cassette using pCP20 (43).
For clean insertion of the FLAG sequence at the C-terminus of the chromosomal pgtE gene, primers for Gibson assembly were designed with the NEBuilder Assembly Tool (https://nebuilder.neb.com/#!/). FLAG_Downstream_Fwd and FLAG_Upstream_Rev primers respectively carried the FLAG sequence extension (GAC TAC AAG GAC GAC GAT GAC AAG) and the reverse complement of the FLAG sequence. Chromosomal IR715 DNA was PCR-amplified with the primer pairs of FLAG_Upstream_Fwd and FLAG_Upstream_Rev, and FLAG_Downstream_Fwd and FLAG_Downstream_Rev by PCR with High-Fidelity PCR Master Mix with HF buffer (New England Biolabs #M0531S) per manufacturer’s instructions. The plasmid pRDH10 was digested with the restriction enzymes NruI (New England Biolabs #R3192S) and SphI-HF (New England Biolabs #R3182S) per manufacturer’s instructions. All three products were then run on a 1% agarose gel, purified with a Zymoclean Gel DNA recovery kit (Zymo Research #D4001), and assembled with NEBuilder Hifi DNA assembly master mix at a 2:1 molar ratio (New England Biolabs #E5520S) following manufacturer’s instructions.
An aliquot of 100 μL of chemically competent CC118 λpir was thawed on ice then incubated with 2 μL of Gibson assembly product on ice for 30 minutes. Cells were then incubated at 42 °C in a water bath for 45 seconds, incubated on ice for 5 minutes, diluted with 1 mL of LB, and cultured for 1 hour aerobically at 37 °C. Cells were then spread-plated on LB agar plates that were supplemented with 30 μg/ml chloramphenicol, incubated overnight at 37 °C, then screened for tetracycline resistance the following day. After confirming correct Gibson assembly via sequencing of the plasmid by Primordium Labs, chemically competent S17–1 λpir cells were transformed as above with pRDH10::pgtE-FLAG isolated via QIAprep Spin Miniprep kit (Qiagen #27106) from CC118 λpir pRDH10::pgtE-FLAG. The resulting strain was used to conjugate the plasmid to STm IR715. Following conjugation, cells were incubated on LB agar plates to screen for resistance to both nalidixic acid and chloramphenicol. Cells that had undergone plasmid integration into the chromosome (single crossover events) were then counter-selected using Nutrient Broth with 7% sucrose (sacB gene residing in pRDH10). Clean insertion of chromosomal pgtE-FLAG was confirmed by PCR with primer pair FLAG_Verification_Fwd and FLAG_Verification_Rev, followed by sequencing by Primordium.
Complementation and reporter plasmids
To construct the PgtE complementation plasmid, the pgtE region was PCR-amplified from STm genomic DNA. A 300 bp region upstream of the coding sequence was amplified to include relevant regulatory elements. The PCR product was cloned into plasmid pCR-Blunt II-TOPO using the Zero Blunt TOPO PCR Cloning Kit (Invitrogen) following the manufacturer’s protocol. The product was then subcloned into the multiple cloning site of low-copy plasmid pWSK29 using XhoI and EcoRV to generate plasmid pWSK29::pgtE (pPgtE). A missense point mutation was introduced into pWSK29::pgtE using the QuikChange Site-Directed Mutagenesis Kit (Agilent) to create pWSK29::pgtE-D206A. Sequences were confirmed by Sanger sequencing (Eton Bioscience) or Oxford Nanopore Technology (Primordium Labs).
To construct the pgtE reporter plasmid, the pgtE promoter was amplified from STm SL1344 genomic DNA with the oligos PpgtE-XbaI-F (engineered restriction sites are underlined) and PpgtE-SmaI-R. The amplicon was digested with XbaI/SmaI and ligated into XbaI/SmaI-digested pGFPmut3.1, then the pgtE-gfpmut3.1 cassette was excised by XbaI/ApaI digestion, and ligated into the corresponding sites of pMPM-A3ΔPlac.
Serum and serum treatments
Normal human serum (NHS; #NHS), C3-depleted human serum (#A314), and cobra venom factor (CVF; #A150) were procured from Complement Technology. For mouse serum, blood was collected from uninfected C3+/+ and C3−/− mice through cardiac puncture with a 25-gauge needle. Mouse serum was subsequently recovered by centrifugation of blood for 5 minutes at 10,000 × g using Serum Gel Polypropylene Microtubes (Sarstedt, #41.1378.005). The serum was then pooled from several mice, aliquoted, and stored at −80 °C. Both human and mouse sera were used after thawing a maximum of one time.
Mice
The Institutional Animal Care and Use Committee (IACUC) at UC San Diego approved all mouse experiments perfomed at the institution (protocol #S17107). The IACUC at Washington State University approved mouse bone marrow collection for the generation of bone marrow-derived macrophages (protocol #6785). Mice were housed under specific pathogen-free conditions and were provided with an irradiated 2020X Teklad diet (Envigo). Furthermore, mice were randomly grouped in cages, with a maximum of five animals per cage.
The study utilized C57BL/6 wild-type mice, C3−/− mice (44), and Cybb-deficient mice (The Jackson Laboratory #002365) (45). For in vivo experiments depleting complement with CVF, six-to-eight-week-old female C57BL/6J mice (The Jackson Laboratory) were intraperitoneally injected with 0.1ml of phosphate-buffered saline (PBS) or 12.5 (one experiment) or 25 (two experiments) μg/ml CVF one day before bacterial infection (46). For all other experiments, six-to-ten-week-old female and male mice, bred and housed at UC San Diego, were used in the experiments, with similar numbers of female and male mice in each experimental group. For experiments with C3−/− mice, we used wild-type littermate control mice from the same colony (C57BL/6 background). Cybb-deficient mice were bred homozygous (CybbX−/X− females) or hemizygous (CybbX−/Y males).
For all in vivo experiments, STm strains were cultured aerobically in LB at 37 °C overnight. Mice were intraperitoneally infected with Ǘ104 colony-forming units (CFUs) of STm. Blood was collected via cardiac puncture with a 25-gauge needle and syringe pre-coated with 0.5M EDTA to prevent coagulation. Liver and spleen tissues were homogenized in PBS, and samples were plated on LB agar supplemented with 50 μg/ml nalidixic acid.
Cell culture reagents
For cell culture media, we primarily used RPMI 1640 medium with L-glutamine and Phenol Red (Gibco #11875093). In luminol assays, we employed RPMI 1640 medium with no glutamine and no phenol red (Gibco #32404014). As indicated in the respective sections, RPMI was supplemented with the following components, depending on the experiment: heat-inactivated Fetal Bovine Serum (HI-FBS) (Gibco #A3840001), Antibiotic-Antimycotic solution (Gibco #15240062), Gentamicin (Gibco #15710064), HEPES (Gibco #15630080), EDTA (Fisher Scientific #S311–500). Dulbecco’s PBS (DPBS; Gibco #14190) was used for dislodging bone marrow-derived macrophages and for the neutrophil Enrichment Kit isolation medium.
Bone marrow isolation and bone marrow-derived macrophage culture conditions
Murine bone marrow-derived macrophages (BMDMs) were prepared by maturing freshly isolated bone marrow cells from femurs and tibias. Bone marrow cells were isolated with a 21-gauge needle, filtered through a 70 μm filter, then subjected to Ammonium-Chloride-Potassium (ACK) lysis (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) buffer to remove excess red blood cells. For BMDMs used in fluorescent microscopy, cells were cultured for 5 days in RPMI 1640 medium with L-glutamine supplemented with 20% supernatant from L929 cells, and 10% HI-FBS. BMDMs were then re-seeded two days prior to infection. For BMDMs used to assess Salmonella burden and PgtE function, cells were then cultured for 7 days in RPMI 1640 medium with L-glutamine supplemented with 30% supernatant from L929 cells, 10% HI-FBS, and 1x Antibiotic-Antimycotic in Sigma culture dishes (Z358762). 18 hours prior to infection, cold DPBS was used to dislodge the cells, and BMDMs were seeded in RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS in 24-well plates (Corning #3524) at a density of 5×105 cells/well or 6-well plates at a density of 2×106 cells/well (Corning #3516).
Murine macrophage infection for bacterial enumeration
For macrophage infection experiments, STm strains were grown statically in LB media in an aerobic environment at 37 °C overnight. A concentration of 1.67×107 CFU/ml of STm was incubated in 20% mouse serum (opsonized) or PBS (non-opsonized) for 30 minutes at room temperature. Subsequently, STm was diluted 1:10 in RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS for an inoculum of 2% mouse serum with 1.67×106 CFU/ml STm. An aliquot of 300uL of this inoculum was added to BMDMs in a 24-well plate to reach an MOI of 1. The plate was centrifuged at 360 × g for 5 minutes at room temperature then transferred to a 37 °C tissue culture incubator. After 30 minutes of infection, BMDMs were washed with PBS then treated with RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 100 μg/ml gentamicin for 30 min before replacement with RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 20 μg/ml gentamicin for the remainder of the assay. BMDMs were washed with PBS then lysed with 1% Triton X-100 surfactant (EMD Millipore #EM-9400) in PBS at 30 minutes, 8 hours, and 24 hours post-infection. CFUs were enumerated by plating aliquots of serially diluted lysates onto LB agar supplemented with 50 μg/ml nalidixic acid.
Western blot detection of PgtE-FLAG and PgtE-dependent C3 cleavage
To assess PgtE-dependent cleavage of C3 in vitro, strains of STm and E. coli XL1-Blue were cultured overnight in LB or in InSPI2 LowMg2+ media in an aerobic environment at 37 °C. Bacteria were then incubated with 20% normal human serum (NHS) in PBS at 1.67×109 CFU/ml for 8 hours. Samples were subsequently centrifuged at 10,000 × g for 5 minutes, and supernatants were collected for Western blotting.
To assess PgtE-dependent cleavage of C3 by intracellular STm isolated from BMDMs, STm strains were cultured by rotating in LB media in an aerobic environment at 37 °C overnight. STm was incubated in 20% mouse serum in PBS for 30 minutes at 37 °C at a concentration of 2×107 CFU/ml. STm was then diluted 1:40 in RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS, then added to BMDMs in a 6-well plate at an MOI of 10. Plates were centrifuged at 360 × g for 5 minutes at room temperature and then transferred to a 37 °C tissue culture incubator. After 30 minutes of infection, BMDMs were washed with PBS then treated with RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 100 μg/ml gentamicin for 30 min before replacement with RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 20 μg/ml gentamicin for 7.5 hours. Infected BMDMs were then washed with PBS and lysed with water for 10 minutes at 37 °C. Six infected wells were pooled together for each group, washed, resuspended in 100 μl of 20% NHS in PBS, then shaken at 300 rpm at 37 °C for 13 hours. Samples were then centrifuged at 10,000 × g for 5 minutes, and supernatants were collected for western blotting.
To assess PgtE protein production by in vitro cultures, STm WT and STm pgtE-FLAG (strain ML27) were cultured overnight in LB or in InSPI2 LowMg2+ media in an aerobic environment at 37 °C. 5×108 CFUs were washed twice in PBS; pellets were frozen at −80 °C for 30 minutes, then resuspended in 50 μl of lysis buffer (2% 2-Mercaptoethanol, 2% SDS, 10% glycerol, and 0.1M TrizmaHCl in water adjusted to pH 6.8). Samples were incubated at 95 °C for 20 minutes then spun down for 10 minutes at 10,000 × g.
For electrophoresis, samples were prepared with RunBlue LDS Sample Buffer (Expedeon #NXB31010) and 5mM dithiothreitol (Thermo Scientific #R0861). Electrophoresis was conducted using a Mini Gel Tank (Invitrogen #A25977), Novex Tris-Glycine Mini Protein Gel 4–12% (Invitrogen #XP04125BOX), WesternSure Pre-stained Chemiluminescent Protein ladder (Li-Cor #926–98000) and MES SDS Running Buffer (Invitrogen #B0002) at 90 volts for 80 minutes. Semi-dry transfer was performed with a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad), Immun-Blot PVDF membrane (Bio-Rad #1620177), and Whatman GB003 gel blotting papers (Whatman #10427806) at 20 volts for 1 hour.
Membranes were blocked with 5% (w/v) Nonfat dry milk (LabScientific #M0841) in Tris-buffered saline with 0.1% (w/v) Tween 20 (TBST) rocking for 2 hours at room temperature. For PgtE-dependent complement cleavage, membranes were then incubated with purified anti-complement C3/C3b/iC3b/C3d antibody (BioLegend #846302 clone 1H8/C3b) diluted to 1:5,000 in 5% milk in TBST rocking overnight at 4 °C. After 5 washes with TBST, membranes were then incubated with HRP goat anti-mouse IgG (BioLegend #405306) diluted to 1:20,000 in 5% milk in TBST rocking overnight at 4 °C. For detection, membranes were washed 5 times with TBST, incubated for 10 minutes in the dark with ECL Prime Western Blotting Detection Reagents (Amersham #RPN2232), and then imaged with an Azure 300 Chemiluminescent Western Blot Imager (Azure Biosystems #AZ1300–01).
For PgtE-FLAG tag analysis, after semi-dry transfer, PVDF membranes were cut in half at the 50 kDa protein ladder mark. The bottom half of the membrane was then incubated with purified rat anti-DYKDDDDK Tag antibody (anti-FLAG tag; BioLegend #637319 clone L5) diluted to 1:5,000 in 5% milk in TBST rocking overnight at 4 °C. After 5 washes with TBST, membranes were then incubated with HRP goat anti-rat IgG (BioLegend #405405) diluted to 1:5,000 in 5% milk in TBST rocking overnight at 4 °C. The top half of the membrane was incubated with mouse anti-DnaK (E. coli) antibody (Enzo #ADI-SPA-880-D clone 8E2/2) diluted to 1:10,000 in 5% milk in TBST, rocking overnight at 4 °C. After 5 washes with TBST, membranes were then incubated with HRP goat anti-mouse IgG antibody (BioLegend #405306) diluted to 1:10,000 in 5% milk in TBST rocking overnight at 4 °C. For detection, membranes were washed 5 times with TBST, incubated for 10 minutes in the dark with ECL Prime Western Blotting Detection Reagents (Amersham #RPN2232), and then imaged with a GeneGnome (Synoptics).
O-Antigen Staining
STm and E. coli XL1-Blue strains were cultured overnight in LB or in InSPI2 LowMg2+ media in an aerobic environment at 37 °C. 5×108 CFU was washed twice in PBS and then resuspended in 100 μl of lysis buffer (2% 2-Mercaptoethanol, 2% SDS, 10% glycerol, and 0.1M TrizmaHCl in water adjusted to pH 6.8). Samples were incubated at 95 °C for 10 minutes and then incubated with 1.25 μl of Proteinase K (20mg/ml; Viagen #501-PK) overnight at 55 °C. Lysates were prepared for electrophoresis with Laemmli Sample Buffer (Bio-Rad #1610747) and 7.5% 2-Mercaptoethanol. Electrophoresis was conducted using a Mini Gel Tank (Invitrogen #A25977), Novex Tris-Glycine Mini Protein Gel 4–12% (Invitrogen #XP04125BOX), and MES SDS Running Buffer (Invitrogen #B0002) at 25 mA for 2 hours. O-antigen staining was then performed with Pro-Q Emerald 300 Lipopolysaccharide Gel Stain Kit (Invitrogen #P20495) following the manufacturer’s instructions. Gels were imaged with the 302 nm UV transilluminator of an Azure 200 (Azure Biosystems #AZ1200–01).
Mouse neutrophil isolation
Fresh femur- and tibia-isolated bone marrow cells were isolated with a 21-gauge needle and filtered through a 70 μm filter. Neutrophils were isolated with the EasySep Mouse Neutrophil Enrichment Kit (Stemcell Technologies #19762) following the manufacturer’s instructions for the EasySep Magnet (Stemcell Technologies #18000). The isolation medium consisted of DPBS supplemented with 2% HI-FBS and 1 mM EDTA.
Neutrophil killing assay
Murine bone marrow neutrophils were resuspended in RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 1mM HEPES, then plated at 5×105 cells/well in a 96-well round bottom cell culture plate (Costar #3799). Neutrophils were incubated in a 37 °C tissue culture incubator for 30 minutes prior to infection.
STm strains were cultured overnight in LB or in InSPI2 LowMg2+ media in an aerobic environment at 37 °C. A concentration of 5×108 CFU/ml of STm was incubated in 20% mouse serum from C3+/+ and C3−/− mice (opsonized) or PBS (non-opsonized) for 30 minutes at room temperature. STm was then diluted 1:10 in RPMI 1640 medium with L-glutamine supplemented with 10% HI-FBS and 1mM HEPES, resulting in an inoculum of 5×107 CFU/ml STm with 2% mouse serum. Subsequently, 100 μl of inoculum was added to wells with 100 μl of medium or 100 μl of 5×105 neutrophils for an MOI of 10. After 2.5 hours in a 37 °C tissue culture incubator, 100 μl of 2% Triton X-100 surfactant in PBS was added to 100 μl of culture. CFUs were enumerated by plating aliquots of serially diluted lysates onto LB agar supplemented with 50 μg/ml nalidixic acid.
Luminol Assay
STm strains were grown aerobically overnight at 37 °C, then sub-cultured in LB (1:100 dilution) or in InSPI2 LowMg2+ media (1:10 dilution) and grown aerobically at 37 °C for 3 hours. A concentration of 1×108 CFU/ml of STm was then incubated in 20% mouse serum from C3+/+ and C3−/− mice for 30 minutes at room temperature. Murine bone marrow neutrophils were resuspended in RPMI 1640 medium with no glutamine and no phenol red supplemented with 2% HI-FBS and 1mM Luminol (Millipore Sigma #123072–2.5g) at 1.11×106 neutrophils/ml. 90 μl of 1.11×106 neutrophils/ml were added to a white opaque 96-well microplate (OptiPlate-96; Revvity #6005290). The plate was sealed with a Breathe-Easy sealing membrane (Diversified Biotek #BEM-1), and baseline luminescence was measured with a Synergy HTX Multi-Mode Microplate Reader (Agilent, formerly BioTek) at 37 °C. An aliquot of 10 μl of opsonized STm was then quickly added to each well for a final concentration of 106 neutrophils/ml, an MOI of 10, and a final concentration of 2% mouse serum, then resealed with Breathe-Easy sealing membrane. Luminescence was recorded every 2 minutes for 120 minutes.
Fluorescence Microscopy
Infected macrophages were fixed in 2.5% (w/v) paraformaldehyde at 37 °C for 10 min then washed three times in PBS. Monolayers were permeabilized in 10% (v/v) normal goat serum (Life Technologies), 0.2% (w/v) saponin in PBS for 20 min at room temperature, incubated with primary antibodies for 45 min at room temperature, washed three times with 0.2% (w/v) saponin in PBS, then incubated with secondary antibodies for 45 min at room temperature. Coverslips were washed in PBS, incubated with Hoechst 33342 (ThermoFisher Scientific) for 1 min to stain DNA, and then mounted onto glass slides in Mowiol (Calbiochem). Samples were viewed with a Leica DM4000 epifluorescence upright microscope for quantitative analysis or a Leica SP8 confocal laser-scanning microscope for image acquisition. Samples were blinded during the experiment. Representative confocal micrographs of 1024×1024 pixels were acquired and assembled using Adobe Photoshop CS6.
Statistical analysis of data
The experiments were not randomized. No statistical methods were used to predetermine the sample size. Prism 10 software (GraphPad) was used for statistical analysis. For in vivo experiments, outliers found by ROUT outlier analysis Q= 1% are removed. Data were analyzed by Kruskal-Wallis test (non-parametric, no pairing) followed by Dunn’s multiple comparison test. Serum killing assays were analyzed with a Two-way ANOVA followed by Sidak multiple comparison test. Neutrophil killing assays were analyzed with a One-way ANOVA Kruskal-Wallis test followed by Dunn’s comparison test. For luminol assays, Two-way ANOVA analysis was performed; the source of variation for significance is the Time × Column Factor.
RESULTS
PgtE promotes immune complement resistance in vivo.
Prior studies identified a potential role for PgtE in promoting STm colonization in mice and chickens (37, 39, 47) and described several potential proteolytic targets in vitro, including complement factor B, complement factor H, C3, C3b, C4b, and C5 (36, 38, 39).
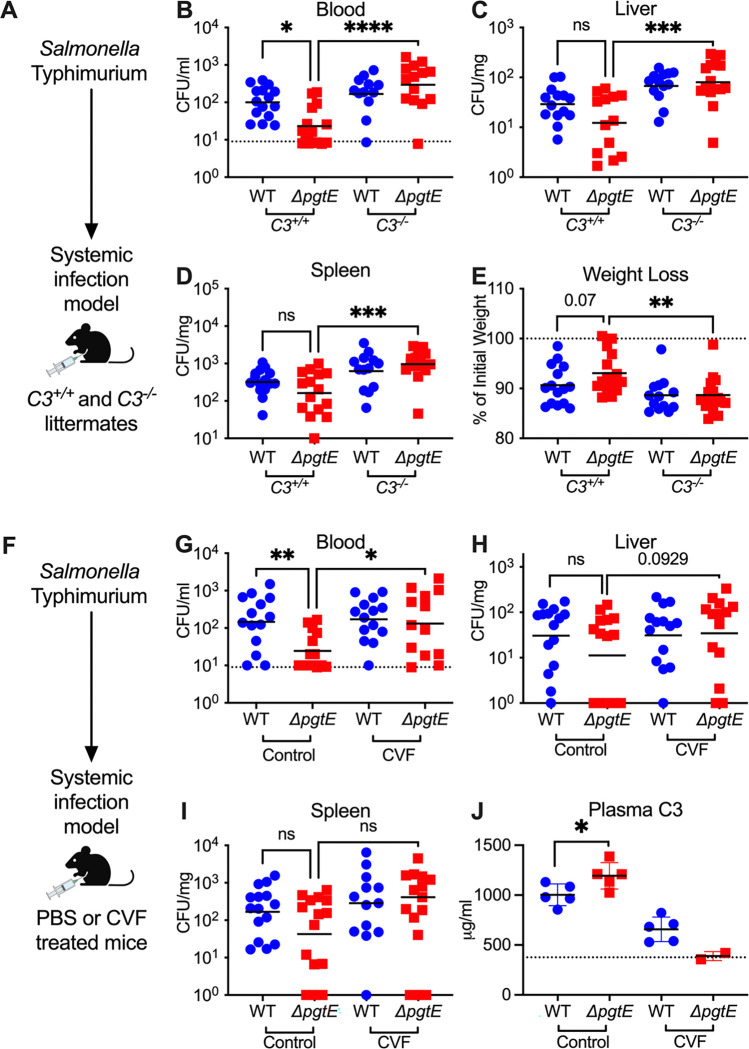
All three immune complement pathways converge at C3 (48). To elucidate whether PgtE enables STm to evade immune complement in vivo, we infected C3−/− mice and their C3+/+ littermates intraperitoneally with STm WT (strain IR715, a fully virulent NalR derivative of ATCC 14028s) or an isogenic ΔpgtE mutant (Fig. 1A–E). After 24 hours, we assessed bacterial burden in the blood (Fig. 1B), liver (Fig. 1C), and spleen (Fig. 1D). The ΔpgtE mutant was recovered at significantly lower levels than STm WT in the blood, but was fully rescued in C3−/− mice (Fig. 1B). Similar differences between STm WT and the ΔpgtE mutant were observed in the liver and spleen of C3+/+ mice, although they did not reach statistical significance. In all cases, while STm WT equally infected C3+/+ and C3−/− mice, the ΔpgtE mutant was recovered at much higher levels in the spleen and liver of C3−/− mice when compared to C3+/+ littermates (Fig. 1C, D). Furthermore, C3−/− mice infected with the ΔpgtE mutant exhibited significantly higher weight loss than the infected C3+/+ mice (Fig. 1E). Thus, PgtE enables STm to evade immune complement defense in vivo, particularly in the blood.
Figure 1. PgtE promotes smooth STm survival in vivo by evading complement C3.
(A-E) 6–10-week-old C3+/+ and C3−/− littermates were infected intraperitoneally (IP) with 104 CFU wild-type (WT) or isogenic PgtE-deficient (ΔpgtE) Salmonella strain IR715. Mice were euthanized 24 hours after infection and bacterial burden in the (B) blood, (C) liver, and (D) spleen were quantified. (E) Weight loss = (weight at 24 hours / weight at time of infection)*100%. (F-J) 6–8-week-old C57B6/J mice were IP-injected with PBS (Control) or Cobra Venom Factor (CVF). 24 hours after treatment, mice were infected IP with 104 CFU of either IR715 WT or IR715 ΔpgtE. Mice were euthanized 24 hours after infection and bacterial burden was assessed in the (G) blood, (H) liver, and (I) spleen. (J) Concentration of complement C3 in plasma measured by ELISA: dotted line represents average from 3 uninfected control mice. (B, G) Dotted line represents the limit of detection of STm CFU in blood. (B-E) N = 16–17 per group pooled from 6 independent experiments. (G-I) N = 15 per group pooled from 3 independent experiments. (J) ELISA from 1 representative experiment. (B-E, G-I) Outliers found by ROUT outlier analysis Q= 1% are removed. Data were analyzed by Kruskal-Wallis test (non-parametric, non-paired) followed by Dunn’s multiple comparison test. Adjusted p values from Dunn’s multiple comparison test: * p < 0.05. ** p < 0.01. *** p < 0.001. ns = not significant. Symbols represent data from individual mice. Bars represent the (B-D, G-I) geometric means or (E, J) mean.
We further investigated PgtE-dependent evasion of complement by infecting mice treated with cobra venom factor (CVF), a C3 convertase homolog which depletes complement (46) (Fig. 1F–J). Mice treated with PBS (control) or CVF for 24 hours were infected intraperitoneally with STm WT or the ΔpgtE mutant (Fig. 1F), and bacterial burden was assessed in the blood (Fig. 1G), liver (Fig. 1H), and spleen (Fig. 1I) at 24 hours. Similar to C3+/+ mice, the ΔpgtE mutant was recovered at significantly lower levels than STm WT in the blood of control-treated mice but was rescued in CVF-treated mice (Fig. 1G). No significant differences were observed in the liver (Fig. 1H) and spleen (Fig. 1I). To confirm that CVF treatment effectively depleted complement C3, we determined serum C3 concentration by ELISA. As expected, mice treated with CVF had reduced serum C3 compared to control-treated mice (Fig. 1J). Within the control-treated group, mice infected with STm WT had significantly less serum C3 compared to mice infected with the ΔpgtE mutant (Fig. 1J), suggesting that PgtE reduced serum C3 concentrations. Thus, PgtE enables STm to defend against immune complement in vivo.
Wild-type STm cleaves complement C3 in a PgtE-dependent manner when grown in conditions that mimic the phagosome or grown in macrophages
Previous in vitro studies used strains with a defective O-antigen, and thus were avirulent, to show PgtE-dependent cleavage of immune complement (36, 38, 39). As we identified a potential role for PgtE in cleaving C3 in vivo, we hypothesized that PgtE acts by a different mechanism in fully virulent STm.
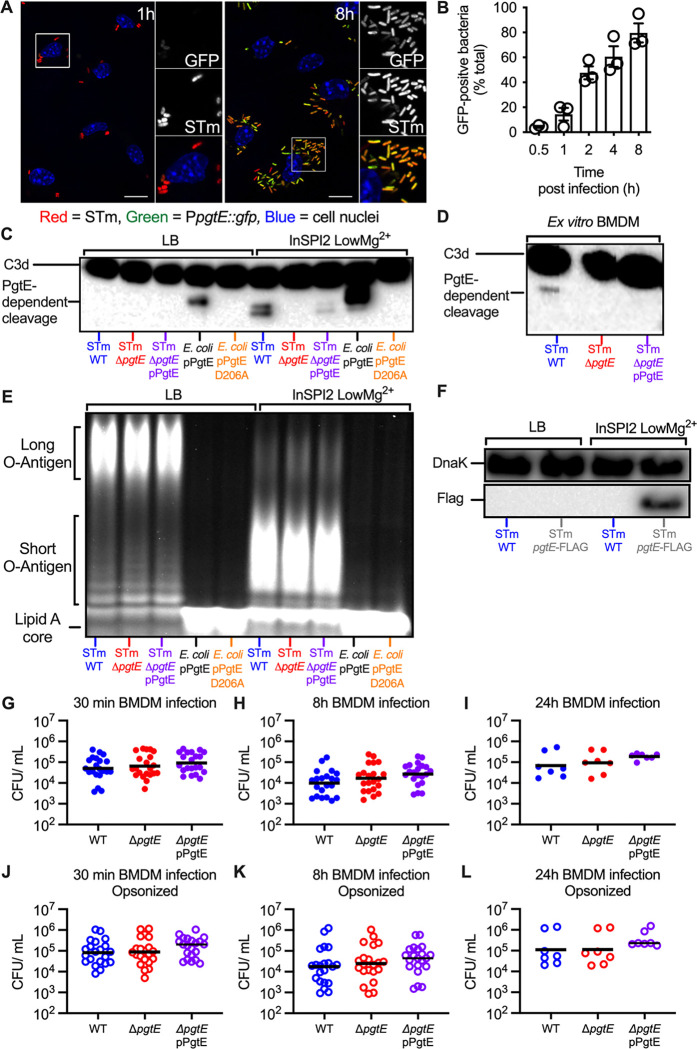
Transcriptome analysis has revealed that STm increases pgtE expression in infected murine macrophages (49), indicating that PgtE may function in these cells. To elucidate the time course of pgtE expression, we infected bone marrow-derived macrophages (BMDMs) with an STm strain carrying a chromosomally encoded Ptrc::mCherry, for constitutive expression of mCherry fluorescent protein and a plasmid encoding a PpgtE::gfp transcriptional reporter fusion (Fig. 2A). Monitoring GFP fluorescence over time by fluorescence microscopy revealed that 4.3% and 80% of bacteria were GFP-positive at 30 minutes and 8 hours post-infection, respectively (Fig. 2B). These results indicated a temporal induction of pgtE expression following STm infection of BMDMs.
Figure 2. PgtE expression and function are increased in macrophages but do not increase smooth STm survival in macrophages.
(A, B) Temporal and spatial distribution of PgtE-positive STm inside BMDMs. (A) BMDMs were infected with mCherry-STm carrying a plasmid encoding for a PpgtE::gfp transcriptional reporter fusion. Representative confocal microscopy images from 1 h and 8 h post-infection are displayed. GFP-positive bacteria (green), Salmonella (red), and the cell nuclei (DAPI; blue) are shown. Inset panels show 2x enlarged regions; scale bars are 10 μm. (B) Kinetics of intracellular pgtE expression in BMDMs. The number of GFP-positive bacteria at each timepoint was scored by fluorescence microscopy and reported as a percentage of total (red) bacteria (n = 3 experiments). (C, E) Smooth STm IR715 wild-type (WT), isogenic PgtE-deficient (ΔpgtE), and ΔpgtE complemented in trans (ΔpgtE pPgtE) or rough E. coli with a pWSK29 plasmid containing a functional pgtE gene (pPgtE) or a pgtE gene with a single point mutation PgtE (pPgtE D206A) were cultured overnight in (Left) LB or (Right) InSPI2 LowMg2+ minimal media. (D) Alternatively, STm was isolated from BMDMs 8 hours after infection. STm and E. coli were then incubated with normal human serum for (C) 8 hours or (D) 13 hours. PgtE-dependent complement cleavage in supernatants was assessed by western blot analysis with anti-complement C3/C3b/iC3b/C3d antibody. (E) Alternatively, after overnight culture, STm and E. coli were lysed, run on a 4–12% Tris-Glycine gel, and stained with Pro-Q Emerald 300 Lipopolysaccharide Gel Stain Kit to assess O-antigen chain length. (F) Western blot analysis of STm WT or STm pgtE-FLAG cultured overnight in LB or InSPI2 LowMg2+ minimal media. The bottom half of the membrane was stained with anti-FLAG tag antibody. The top half of the membrane was stained with anti-DnaK as a loading control. (G-L) BMDMs were infected at an MOI = 1 with IR715 WT, ΔpgtE, and ΔpgtE pPgtE that were either (G-I) not opsonized or (J-L) opsonized with normal mouse serum. (G, J) 30 minutes after infection, BMDM were lysed with 1% Triton-X 100 and STm CFUs were enumerated. Alternatively, BMDM were incubated with 100 μg/mL gentamicin for 30 minutes, followed by (H, K) 7 hours or (I, L) 23 hours with 20 μg/mL gentamicin then lysed with 1% Triton-X 100. (G-L) N = 21 or 7 from 10 or 3 independent experiments. Symbols represent data from BMDMs from individual mice, bars represent the geometric means.
The phagosome’s environment can be modeled in vitro using minimal phosphate-carbon-nitrogen (PCN) media supplemented with low magnesium. This medium induces SPI2 expression and is thus referred to as “InSPI2 LowMg2+”. In alignment with the macrophage results, pgtE expression is also increased in this medium (41). We thus investigated whether PgtE activity in vitro was dependent on culture conditions. We grew the following strains in standard LB or in InSPI2 LowMg2+ media: STm WT, the ΔpgtE mutant, and the ΔpgtE mutant complemented with a plasmid encoding pgtE (STm ΔpgtE pPgtE). As controls, we used an O-antigen-deficient E. coli strain expressing either functional pgtE (E. coli pPgtE) or nonfunctional pgtE with a missense point mutation (E. coli pPgtE D206A). Each culture was then incubated with normal human serum (NHS), which contains complement, to investigate C3 cleavage by Western blot.
In line with previous studies (35, 36, 40), STm WT grown in LB was unable to cleave C3 in a PgtE-dependent manner (Fig. 2C, Left). The O-antigen-deficient E. coli cleaved C3 when expressing functional PgtE, consistent with the hypothesis that long O-antigen sterically inhibits PgtE function (Fig. 2C, Left). Strikingly, however, STm WT cultured in InSPI2 LowMg2+ media cleaved C3 in a PgtE-dependent manner, as shown by two C3 cleavage products that were absent from sera incubated with STm ΔpgtE (Fig. 2C, Right). Genetic complementation in trans recovered PgtE-dependent C3 cleavage, albeit to a lesser extent than STm WT.
As InSPI2 LowMg2+ media models the intraphagosomal environment, we next investigated whether STm WT could cleave C3 when grown inside macrophages. We infected BMDMs with STm strains (WT, the ΔpgtE mutant, and the complemented strain) for 8 hours, then lysed the infected cells to retrieve STm. Bacteria isolated from macrophages were then incubated with NHS to detect their ability to cleave C3. We detected a C3 fragment in serum incubated with STm WT isolated from macrophages, but not in serum incubated with the ΔpgtE mutant (Fig. 2D). In this experimental setting, genetic complementation did not restore detectable PgtE-dependent C3 cleavage. Comparing these results with those generated with STm cultured in InSPI2 LowMg2+ media (Fig. 2C, Right), where also one additional fragment was detected, we speculate that this discrepancy is attributable to the technical limitation of isolating substantially fewer STm from infected BMDMs than from overnight cultures. Nevertheless, our results demonstrate that PgtE is functional in STm with an intact O-antigen depending on the growth conditions, enabling the pathogen to cleave C3 when cultured in InSPI2 LowMg2+ media or when isolated from macrophages.
Growth conditions that model the phagosome’s environment increase PgtE expression and decrease O-antigen length
PgtE activity can be observed in vitro among strains with an intact O-antigen as long as they are cultured in media that mimics the intraphagosomal environment. As avirulent mutants lacking an O-antigen have previously been shown to exhibit PgtE function, and as in vitro culture conditions and growth in macrophages can alter O-antigen length in wild-type strains (50, 51), we sought to determine whether the O-antigen length of our virulent, smooth strains was being altered by these growth conditions. To this end, we extracted and stained the O-antigen from STm strains cultured in LB or in InSPI2 LowMg2+ media. All STm strains cultured in InSPI2 LowMg2+ media had shorter O-antigen compared to STm cultured in LB (Fig. 2E). As expected, the rough E. coli strain that we used to express PgtE lacked O-antigen polysaccharides. Consistent with the observation that steric hindrance conferred by the presence of an O-antigen impacts PgtE function, PgtE activity was greatest when the protease was expressed by the rough E. coli strain (Fig. 2C). By contrast, although the shorter O-antigen detected in smooth STm strains cultured in InSPI2 LowMg2+ media likely enabled PgtE’s ability to function at all, the intermediary PgtE activity observed is likely the consequence of lingering steric hindrance conferred by the still present, albeit shorter, O-antigen. Nevertheless, these results are consistent with the idea that the shorter O-antigen induced by growth in InSPI2 LowMg2+ media enables complement C3 cleavage by PgtE (Fig. 2C, E).
The absence of PgtE activity when wild-type STm is cultured in LB could be due to a lack of PgtE expression or it could be solely explained by the steric hindrance caused by the long O-antigen. To assess whether PgtE is expressed in LB, we constructed an STm strain with a chromosomal pgtE allele harboring a FLAG tag at the C-terminus (STm pgtE-FLAG). We found that the FLAG tag was detectable when STm pgtE-FLAG was cultured in InSPI2 LowMg2+ medium, but not in LB (Fig. 2F). As expected, no FLAG tag was detected in STm WT in either condition. Thus, growth in InSPI2 LowMg2+ media has a two-pronged effect: 1) increasing PgtE expression; 2) shortening O-antigen length, which enables PgtE function and cleavage of complement C3.
PgtE appears dispensable for STm survival in primary macrophages under tested conditions
Our findings suggest a role for PgtE to enable Salmonella survival inside of macrophages. Even though complement is generally known to opsonize and lyse pathogens in extracellular spaces, recent studies have identified a role for complement in intracellular compartments (52–54). We thus tested whether PgtE disrupts intracellular C3 signaling and promotes STm survival within macrophages by infecting BMDMs with STm WT, the ΔpgtE mutant, or the complemented ΔpgtE mutant. The strains were either nonopsonized (Fig. 2G–I) or opsonized with normal mouse serum (Fig. 2J–L). We recovered a similar number of each STm strain at each of the time points analyzed, from 30 minutes post-infection (when pgtE is not highly expressed; Fig. 2A, B) to 8 hours (high pgtE induction) and even 24 hours post-infection, in both the non-opsonized and the opsonized groups (Fig. 2G–I and Fig. 2 J–L). As such, PgtE did not enhance STm survival in BMDMs in these conditions, even though it is highly produced and cleaves C3 in these cells.
PgtE increases STm serum resistance
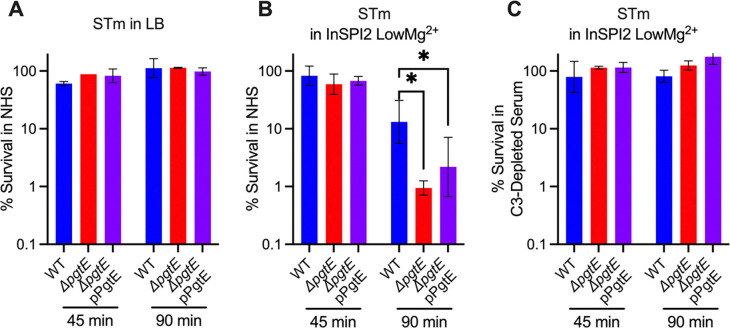
To determine whether PgtE promotes STm resistance to serum killing, we cultured STm WT, the ΔpgtE mutant, and the complemented ΔpgtE mutant in either LB or InSPI2 LowMg2+ media and exposed them to 20% normal human serum (NHS). When STm was cultured overnight in LB (Fig. 3A), all strains showed similar survival. However, when STm was cultured overnight in InSPI2 LowMg2+ media, STm WT survived significantly more than the ΔpgtE mutant, with the complemented strain showing an intermediate phenotype (Fig. 3B). To test whether the differences in serum resistance were dependent on PgtE-mediated C3 cleavage, the strains were incubated with C3-depleted human serum after overnight culture in InSPI2 LowMg2+ media. In the absence of C3, serum survival of the PgtE mutant was fully restored, and no difference in survival was detected between the three strains (Fig. 3C). Thus, PgtE enhanced STm serum survival by inhibiting the function of complement.
Figure 3. PgtE promotes survival of smooth, virulent STm in serum.
(A-C) Serum killing assays were performed with smooth STm IR715 wild-type (WT), isogenic PgtE-deficient (ΔpgtE), and ΔpgtE complemented in trans (ΔpgtE pPgtE). Strains were cultured overnight (A) in LB or (B, C) in InSPI2 LowMg2+ minimal media. STm at 106 CFU/mL was then incubated with (A, B) 20% normal human serum (NHS) or (C) 20% C3-depleted human serum at 37 °C shaking at 300 rpm. CFU were enumerated at 0 minutes, 45 minutes, and 90 minutes. % survival = (CFU at 45 minutes or 90 minutes / CFU at 0 minutes)*100%. (A, C) n = 2, (B) n = 6 from 2–3 independent experiments. Bar and error represent geometric mean and standard deviation. Data were analyzed by 2-way ANOVA followed by Sidak multiple comparison test. Adjusted p values from Sidak multiple comparison test: * p < 0.05.
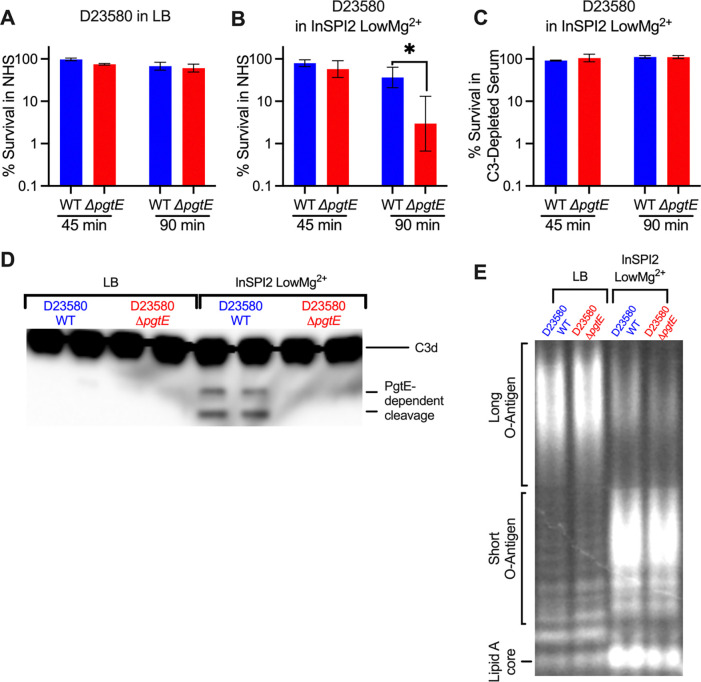
Many iNTS isolates display increased expression of pgtE (39). We next tested if PgtE played a similar role in increasing serum survival of iNTS sequence type ST313, a predominant etiologic agent of iNTS disease (55). Similar to what we observed with the ATCC 14028s strain IR715 (sequence type ST19), no significant difference in serum survival was seen between the ST313 strain D23580 wild-type and an isogenic ΔpgtE mutant when the strains were cultured overnight in LB (Fig. 4A). However, when cultured overnight in InSPI2 LowMg2+ media, D23580 WT survived significantly better than the isogenic ΔpgtE mutant in normal human serum (Fig. 4B) but not in C3-depleted human serum (Fig. 4C). Both D23580 WT and ΔpgtE strains exhibited shortened O-antigen chains when cultured overnight in InSPI2 LowMg2+ media compared to growth in LB (Fig. 4D), whereas only WT was able to cleave C3 (Fig. 4E). Thus, akin to the results with ST19 strains (Fig. 2C, 3), when an ST313 strain is cultured in media mimicking the SCV, PgtE-dependent inhibition of complement results in elevated serum survival (Fig. 4).
Figure 4. PgtE promotes survival of iNTS strain D23580 in serum when cultured in media mimicking the SCV luminal environment.
(A-C) Serum killing assays were performed with smooth STm D23580 wild-type (WT) and an isogenic PgtE-deficient mutant (ΔpgtE). Strains were cultured overnight (A) in LB or (B, C) in InSPI2 LowMg2+ minimal media. STm at 106 CFU/mL was then incubated with (A, B) 20% normal human serum (NHS) or (C) 20% C3-depleted human serum at 37 °C shaking at 300 rpm. CFUs were enumerated at 0 minutes, 45 minutes, and 90 minutes. % survival = (CFU at 45 minutes or 90 minutes / CFU at 0 minutes)*100%. (A, C) n = 2–3, (B) n = 6. Bar and error represent geometric mean and standard deviation. Data were analyzed by 2-way ANOVA followed by Sidak multiple comparison test. Adjusted p values from Sidak multiple comparison test: * p < 0.05. (D, E) D23580 WT and ΔpgtE were cultured overnight in (Left) LB or (Right) InSPI2 LowMg2+ minimal media. (D) After overnight culture, STm was lysed, supernatants were run on a 4–12% Tris-Glycine gel, and the gel was stained with Pro-Q Emerald 300 Lipopolysaccharide Gel Stain Kit to assess O-antigen chain length. (E) Alternatively, STm was then incubated with NHS for 8 hours. PgtE-dependent complement cleavage in supernatants was assessed by western blot analysis with anti-complement C3/C3b/iC3b/C3d antibody.
PgtE expression enables STm to evade complement-mediated neutrophil killing
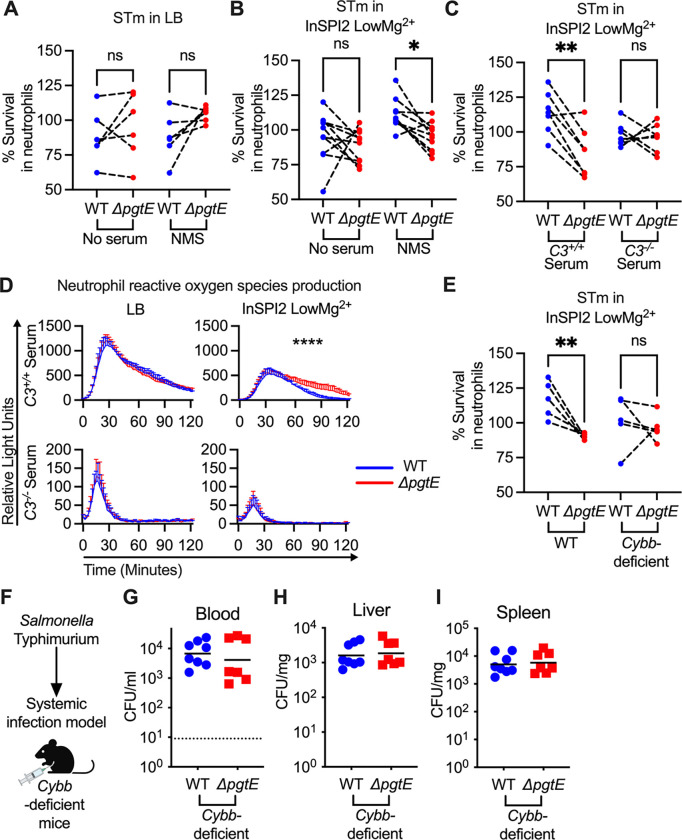
An important function of complement is to enhance neutrophil killing (48). To test whether PgtE-mediated complement cleavage enhances STm resistance to neutrophils, we cultured STm WT or the ΔpgtE mutant overnight in either LB (Fig. 5A) or InSPI2 LowMg2+ media (Fig. 5B–C) and infected neutrophils isolated from murine bone marrow. There was no difference in survival when the strains were grown in LB and either non-opsonized or opsonized with normal mouse serum (NMS) prior to the neutrophil infection (Fig. 5A). In contrast, when the strains were grown in InSPI2 LowMg2+ media and opsonized in NMS, STm WT survived significantly better than the ΔpgtE mutant in neutrophil killing assays (Fig. 5B). To assess if complement was the determinant factor in NMS for the difference in survival between STm WT and the ΔpgtE mutant, we opsonized the strains (cultured in InSPI2 LowMg2+ media) with serum from C3+/+ or C3−/− littermate mice. Here, the survival defect of the ΔpgtE mutant in neutrophils was rescued to STm WT levels when the strains were opsonized in serum from C3−/− mice (Fig. 5C), indicating that PgtE enables STm to evade complement-mediated neutrophil killing.
Figure 5. PgtE enhances STm survival in neutrophil killing assays and reduces complement-mediated neutrophil ROS response.
Neutrophils were isolated (Stem Cell EasySep kit) from bone marrow of (A-E) C57BL/6 mice and (E) Cybb-deficient mice. For neutrophil killing assays, smooth STm IR715 wild-type (WT) and an isogenic PgtE-deficient (ΔpgtE) strain were cultured overnight in (A) LB or (B-C, E) InSPI2 LowMg2+ minimal media. STm was then (A-B: Left) not opsonized or (A-B: Right, E) opsonized with normal mouse serum (NMS). (C) Alternatively, STm was opsonized with serum from C3+/+ and C3−/− littermates. (A-C, E) Neutrophils were then infected at an MOI = 10. STm CFU was enumerated 2.5 hours post-infection. % Survival in neutrophils = (CFU in wells with neutrophils at 2.5 hours/ CFU in control wells at 2.5 hours)*100%. (D) To determine neutrophil reactive oxygen species production, luminol assays were performed with STm cultured overnight in (Left) LB or (Right) InSPI2 LowMg2+ minimal media then opsonized with serum from (Top) C3+/+ and (Bottom) C3−/− littermates. Neutrophils were infected at an MOI = 10. Relative Light Unit reads were performed every 2 minutes with a BioTek Synergy HTX. Error bars represent mean + SD from 3 biological replicates from 1 of 3 representative experiments. (F-I) 8-week-old CybbX−/X− females or CybbX−/Y hemizygous males were infected IP with 104 CFU WT and ΔpgtE STm. Mice were euthanized 24 hours after infection and bacterial burden in the (G) blood, (H) liver, and (I) spleen was assessed. (A-C, E) N = 5–10 from 3–4 independent experiments. Symbols represent data with neutrophils from individual mice, bars represent the means. (A-C, E) Data were analyzed by One-way ANOVA Kruskal-Wallis test followed by Dunn’s comparison test. Adjusted p values from Dunn’s multiple comparison test: * p < 0.05, ** p < 0.01. (D) Data was analyzed by 2-way ANOVA. Time × Column Factor: **** p < 0.0001. (D) bar and error represent mean + SD. (G-I) Symbols represent data from individual mice, bars represent the geometric means. (G) Dotted line represents the limit of detection. (G-I) N = 7–8 from 2 independent experiments.
PgtE disrupts C3-induced neutrophil ROS production, helping STm to evade ROS-dependent neutrophil killing
Complement enhances the neutrophil respiratory burst in response to STm (56, 57). To determine if PgtE disrupts C3-mediated reactive oxygen species (ROS) production by neutrophils, we performed a luminol assay with STm WT and the ΔpgtE mutant opsonized with serum from C3+/+ or C3−/− mice (Fig. 5D). No differences were seen in neutrophil ROS production when the strains were grown in LB prior to opsonization with serum from C3+/+ mice (Fig. 5D). In contrast, neutrophils infected with the ΔpgtE mutant exhibited prolonged ROS production compared to neutrophils infected with STm WT when the strains were cultured in InSPI2 LowMg2+ media and were opsonized with serum from C3+/+ mice (Fig. 5D). Strains opsonized with complement-deficient serum induced lower levels of neutrophil ROS production, independent of PgtE expression (Fig. 5D). Thus, PgtE enables STm to evade the heightened ROS production that is triggered by C3 opsonization.
Next, we infected neutrophils isolated from wild-type or Cybb-deficient mice (Fig. 5E), which have defective ROS production (45). The ΔpgtE mutant exhibited comparable survival as STm WT in neutrophils from Cybb-deficient mice, indicating that PgtE promotes STm resistance to ROS-dependent neutrophil killing (Fig. 5E). When we infected Cybb-deficient mice intraperitoneally with STm WT or the ΔpgtE mutant (Fig. 5F), we recovered approximately 1–2 log more bacteria in comparison to WT mice in the blood, liver, and spleen (Fig 5; compare to Fig. 1). However, in Cybb-deficient mice, the ΔpgtE mutant was recovered to a similar level as STm WT in the blood (Fig. 5G), liver (Fig. 5H), and spleen (Fig. 5I). Thus, by disrupting C3-induced neutrophil ROS production, PgtE helps STm to evade ROS-dependent killing by neutrophils.
DISCUSSION
Bacteremia is a major complication of NTS infection, and the mechanisms by which the pathogen evades host immune defenses are not fully understood. Here, we show that PgtE is a virulence factor that helps STm to overcome complement-mediated host defenses, survive in serum, and evade ROS-dependent neutrophil killing.
PgtE is an outer membrane protease that has been hypothesized to promote STm virulence through multiple mechanisms. For instance, PgtE expressed in rough strains of bacteria has previously been shown to promote adhesion to matrigel (35), suggesting a role for PgtE in enhancing invasion. PgtE also inactivates α2-antiplasmin while activating plasmin (40) and mammalian matrix metalloproteinase-9 (MMP-9) (37). Macrophages use plasmin and MMP-9 to migrate through tissues, and therefore PgtE was hypothesized to promote the dissemination of STm within infected macrophages (37, 40). Furthermore, STm can cleave cationic antimicrobial peptides (34), and multiple components of immune complement in a PgtE-dependent manner (36, 38, 39). Using immortalized human macrophage-like cells, a recent study showed increased localization of human bactericidal/permeability-increasing protein to SCVs containing PgtE-deficient STm, suggesting that PgtE promotes STm persistence in SCVs (47).
Collectively, studies with data generated mostly in vitro have proposed that PgtE enables STm to evade antimicrobial peptides and immune complement while promoting an intracellular lifestyle within macrophages. However, to our knowledge, no prior studies have linked these observations to in vivo phenotypes and specific components of host immunity, which requires the use of knock-out mice. Our results show that a STm ΔpgtE mutant is attenuated in the blood of wild-type mice, but fully rescued in C3−/− mice (Fig. 1), in mice treated with CVF (Fig. 1), and in Cybb-deficient mice (Fig. 5), thus demonstrating that PgtE promotes STm evasion of complement component C3 and ROS in vivo.
Identifying where and how PgtE plays a role in vivo was not trivial, as virulent STm has multiple virulence factors that modulate resistance to immune complement. For instance, long O-antigen chains confer serum resistance, but also sterically inhibit PgtE function (40, 58). Therefore, prior studies used rough STm and rough E. coli mutants when studying PgtE in vitro (36, 38, 39). Additional mechanisms of STm serum resistance include Rck and TraT, outer membrane proteins that confer serum resistance in vitro to either smooth or rough E. coli and Salmonella (31, 32, 59) by disrupting the complement membrane attack complex (MAC) (60). The many proposed functions of PgtE, by contrast, were observed in rough, avirulent strains.
Our study indicates that PgtE in fact does function in vitro and in vivo with fully virulent, smooth strains, albeit only after the physiologic O-antigen shortening that follows growth inside the SCV (40, 50, 51) (Figs. 2, 4). A long O-antigen is a primary defense against an array of environmental insults, including immune complement activity. In environments where STm has a shortened O-antigen, such as in the SCV or having recently exited a phagocytic cell, PgtE likely represents a secondary line of defense to assist in protecting the more susceptible outer membrane.
Expression of pgtE and PgtE’s proteolytic function are enhanced in macrophages as well as in media that mimic the SCV lumen (40, 41, 49) (Figs. 2, 4). However, PgtE did not enhance STm survival in primary murine macrophages (Fig. 2), but did protect STm from C3-dependent serum killing (Figs. 3, 4). We obtained comparable results with the iNTS strain D23580 (clade ST313). When cultured in InSPI2 LowMg2+ media (mimicking the SCV lumen), strain D23580 exhibited reduced O-antigen length, cleaved C3 in a PgtE-dependent manner, and survived better in human serum (Fig. 4). These results are in agreement with a prior study that hypothesized that the increased expression of pgtE, due to a SNP in its promoter region, could enhance iNTS survival and dissemination (39).
A different study showed that, in response to serum exposure, multiple ST313 strains (including D23580), when cultured in LB, increased the expression of long O-antigen regulators but not of pgtE, rck, and traT (61). This suggests that when long O-antigen is present, STm continues to rely on the long O-antigen to resist complement killing. However, when the O-antigen is shortened (Fig. 2, 4), we demonstrate that PgtE defends against complement killing (Fig. 3, 4) and reduces neutrophil ROS production and killing (Fig. 5), thereby promoting bacteremia. Future studies will reveal whether PgtE also has other functions in vivo, and whether cleavage of other substrates contributes to STm pathogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the NIH grant AI145325. Additional support was provided by AMED grant JP233fa627003, by the Chiba University-University of California-San Diego (UCSD) Center for Mucosal Immunology, Allergy, and Vaccines, and by the UCSD Department of Pediatrics. M.H.L. was supported by T32 DK007202 and F32 AI169989. JC was supported by NIH grant AI129992. LAK was supported, in part, by a Burroughs Wellcome PATH award. APL was supported by the NIAID Mucosal Immunology Studies Team (MIST). GTW was supported by NIH training grant T32AI007036. We would like to thank Ferric Fang for sending us the D23580 wild-type strain.
Abbreviations etc.
- BMDMs
bone marrow-derived macrophages
- iNTS
invasive non-typhoidal Salmonella
- NTS
Non-typhoidal Salmonella
- STm
Salmonella enterica serovar Typhimurium
- SCVs
Salmonella-containing vacuoles
- SPI2
Salmonella Pathogenicity Island 2
- InSPI2 LowMg2+
SPI2-inducing PCN media supplemented with low magnesium
REFERENCES
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O’Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease “Burden of Illness” Studies. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. [DOI] [PubMed] [Google Scholar]
- 2.Hohmann EL. 2001. Nontyphoidal salmonellosis. Clin Infect Dis 32:263–269. [DOI] [PubMed] [Google Scholar]
- 3.Santos RL, Raffatellu M, Bevins CL, Adams LG, Tükel C, Tsolis RM, Bäumler AJ. 2009. Life in the inflamed intestine, Salmonella style. Trends Microbiol 17:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turgeon P, Ng V, Murray R, Nesbitt A. 2018. Forecasting the incidence of salmonellosis in seniors in Canada: A trend analysis and the potential impact of the demographic shift. PLoS One 13:e0208124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lester A, Eriksen NH, Nielsen H, Nielsen PB, Friis-Møller A, Bruun B, Scheibel J, Gaarslev K, Kolmos HJ. 1991. Non-typhoid Salmonella bacteraemia in Greater Copenhagen 1984 to 1988. Eur J Clin Microbiol Infect Dis 10:486–490. [DOI] [PubMed] [Google Scholar]
- 6.Noriega LM, Van der Auwera P, Daneau D, Meunier F, Aoun M. 1994. Salmonella infections in a cancer center. Support Care Cancer 2:116–122. [DOI] [PubMed] [Google Scholar]
- 7.Tumbarello M, Tacconelli E, Caponera S, Cauda R, Ortona L. 1995. The impact of bacteraemia on HIV infection. Nine years experience in a large Italian university hospital. J Infect 31:123–131. [DOI] [PubMed] [Google Scholar]
- 8.Hung C-C, Hung M-N, Hsueh P-R, Chang S-Y, Chen M-Y, Hsieh S-M, Sheng W-H, Sun H-Y, Huang Y-T, Lo Y-C, Hsiao C-F, Chang S-C. 2007. Risk of recurrent nontyphoid Salmonella bacteremia in HIV-infected patients in the era of highly active antiretroviral therapy and an increasing trend of fluoroquinolone resistance. Clin Infect Dis 45:e60–7. [DOI] [PubMed] [Google Scholar]
- 9.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uche IV, MacLennan CA, Saul A. 2017. A Systematic Review of the Incidence, Risk Factors and Case Fatality Rates of Invasive Nontyphoidal Salmonella (iNTS) Disease in Africa (1966 to 2014). PLoS Negl Trop Dis 11:e0005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Puyvelde S, Pickard D, Vandelannoote K, Heinz E, Barbé B, de Block T, Clare S, Coomber EL, Harcourt K, Sridhar S, Lees EA, Wheeler NE, Klemm EJ, Kuijpers L, Mbuyi Kalonji L, Phoba M-F, Falay D, Ngbonda D, Lunguya O, Jacobs J, Dougan G, Deborggraeve S. 2019. An African Salmonella Typhimurium ST313 sublineage with extensive drug-resistance and signatures of host adaptation. Nat Commun 10:4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Español T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia M-J, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. 2009. Chronic granulomatous disease: the European experience. PLoS One 4:e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conlan JW. 1996. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun 64:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpuche Aranda CM, Swanson JA, Loomis WP, Miller SI. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci U S A 89:10079–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalebroux ZD, Miller SI. 2014. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr Opin Microbiol 17:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensel M. 2000. Salmonella pathogenicity island 2. Mol Microbiol 36:1015–1023. [DOI] [PubMed] [Google Scholar]
- 17.Gal-Mor O, Elhadad D, Deng W, Rahav G, Finlay BB. 2011. The Salmonella enterica PhoP directly activates the horizontally acquired SPI-2 gene sseL and is functionally different from a S. bongori ortholog. PLoS One 6:e20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groisman EA, Duprey A, Choi J. 2021. How the PhoP/PhoQ System Controls Virulence and Mg2+ Homeostasis: Lessons in Signal Transduction, Pathogenesis, Physiology, and Evolution. Microbiol Mol Biol Rev 85:e0017620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele-Mortimer O. 2008. The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol 11:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchmeier NA, Heffron F. 1991. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun 59:2232–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach RG, Hensel M. 2007. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica. Berl Munch Tierarztl Wochenschr 120:317–327. [PubMed] [Google Scholar]
- 22.Vazquez-Torres A, Jones-Carson J, Bäumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804–808. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman D, Tevet Y, Trzebanski S, Rosenberg G, Vainman L, Solomon A, Hen-Avivi S, Ben-Moshe NB, Avraham R. 2021. A non-classical monocyte-derived macrophage subset provides a splenic replication niche for intracellular Salmonella. Immunity 54:2712–2723.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant AJ, Restif O, McKinley TJ, Sheppard M, Maskell DJ, Mastroeni P. 2008. Modelling within-host spatiotemporal dynamics of invasive bacterial disease. PLoS Biol 6:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gogoi M, Shreenivas MM, Chakravortty D. 2019. Hoodwinking the Big-Eater to Prosper: The Salmonella-Macrophage Paradigm. J Innate Immun 11:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest 118:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. 2010. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 107:3070–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman N, Schmetz MA, Foulds J, Klima EN, Jimenez-Lucho VE, Leive LL, Joiner KA. 1987. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol 169:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalsiamthara J, Kim JH, Lee JH. 2018. Engineering of a rough auxotrophic mutant Salmonella Typhimurium for effective delivery. Oncotarget 9:25441–25457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagy G, Dobrindt U, Hacker J, Emödy L. 2004. Oral immunization with an rfaH mutant elicits protection against salmonellosis in mice. Infect Immun 72:4297–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhen M, Sukupolvi S. 1988. The role of the traT gene of the Salmonella typhimurium virulence plasmid for serum resistance and growth within liver macrophages. Microb Pathog 5:275–285. [DOI] [PubMed] [Google Scholar]
- 32.Heffernan EJ, Reed S, Hackett J, Fierer J, Roudier C, Guiney D. 1992. Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella typhimurium virulence plasmid gene rck. J Clin Invest 90:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pramoonjago P, Kaneko M, Kinoshita T, Ohtsubo E, Takeda J, Hong KS, Inagi R, Inoue K. 1992. Role of TraT protein, an anticomplementary protein produced in Escherichia coli by R100 factor, in serum resistance. J Immunol 148:827–836. [PubMed] [Google Scholar]
- 34.Guina T, Yi EC, Wang H, Hackett M, Miller SI. 2000. A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol 182:4077–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukkonen M, Suomalainen M, Kyllönen P, Lähteenmäki K, Lång H, Virkola R, Helander IM, Holst O, Korhonen TK. 2004. Lack of O-antigen is essential for plasminogen activation by Yersinia pestis and Salmonella enterica. Mol Microbiol 51:215–225. [DOI] [PubMed] [Google Scholar]
- 36.Ramu P, Tanskanen R, Holmberg M, Lähteenmäki K, Korhonen TK, Meri S. 2007. The surface protease PgtE of Salmonella enterica affects complement activity by proteolytically cleaving C3b, C4b and C5. FEBS Lett 581:1716–1720. [DOI] [PubMed] [Google Scholar]
- 37.Ramu P, Lobo LA, Kukkonen M, Bjur E, Suomalainen M, Raukola H, Miettinen M, Julkunen I, Holst O, Rhen M, Korhonen TK, Lähteenmäki K. 2008. Activation of pro-matrix metalloproteinase-9 and degradation of gelatin by the surface protease PgtE of Salmonella enterica serovar Typhimurium. Int J Med Microbiol 298:263–278. [DOI] [PubMed] [Google Scholar]
- 38.Riva R, Korhonen TK, Meri S. 2015. The outer membrane protease PgtE of Salmonella enterica interferes with the alternative complement pathway by cleaving factors B and H. Front Microbiol 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammarlöf DL, Kröger C, Owen SV, Canals R, Lacharme-Lora L, Wenner N, Schager AE, Wells TJ, Henderson IR, Wigley P, Others. 2018. Role of a single noncoding nucleotide in the evolution of an epidemic African clade of Salmonella. Proceedings of the National Academy of Sciences 115:E2614–E2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lähteenmäki K, Kyllönen P, Partanen L, Korhonen TK. 2005. Antiprotease inactivation by Salmonella enterica released from infected macrophages. Cell Microbiol 7:529–538. [DOI] [PubMed] [Google Scholar]
- 41.Kröger C, Colgan A, Srikumar S, Händler K, Sivasankaran SK, Hammarlöf DL, Canals R, Grissom JE, Conway T, Hokamp K, Hinton JCD. 2013. An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe 14:683–695. [DOI] [PubMed] [Google Scholar]
- 42.Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C, Ernst RK, Steele-Mortimer O, Celli J, Vallance BA. 2014. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A 92:11490–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9:202–209. [DOI] [PubMed] [Google Scholar]
- 46.Belzer C, Liu Q, Carroll MC, Bry L. 2011. THE ROLE OF SPECIFIC IgG AND COMPLEMENT IN COMBATING A PRIMARY MUCOSAL INFECTION OF THE GUT EPITHELIUM. Eur J Microbiol Immunol 1:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatterjee R, Chowdhury AR, Nair AV, Hajra D, Kar A, Datey A, Shankar S, Mishra RK, Chandra N, Chakravortty D. 2023. Salmonella Typhimurium PgtE is an essential arsenal to defend against the host resident antimicrobial peptides. Microbiol Res 271:127351. [DOI] [PubMed] [Google Scholar]
- 48.Bjanes E, Nizet V. 2021. More than a Pore: Nonlytic Antimicrobial Functions of Complement and Bacterial Strategies for Evasion. Microbiol Mol Biol Rev 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canals R, Hammarlöf DL, Kröger C, Owen SV, Fong WY, Lacharme-Lora L, Zhu X, Wenner N, Carden SE, Honeycutt J, Monack DM, Kingsley RA, Brownridge P, Chaudhuri RR, Rowe WPM, Predeus AV, Hokamp K, Gordon MA, Hinton JCD. 2019. Adding function to the genome of African Salmonella Typhimurium ST313 strain D23580. PLoS Biol 17:e3000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis MJ, Tsai CN, Johnson JW, French S, Elhenawy W, Porwollik S, Andrews-Polymenis H, McClelland M, Magolan J, Coombes BK, Brown ED. 2019. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat Commun 10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Silva P, Manieri FZ, Herrera CM, Trent MS, Moreira CG. 2018. Novel Role of VisP and the Wzz System during O-Antigen Assembly in Salmonella enterica Serovar Typhimurium Pathogenesis. Infect Immun 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. 2008. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tam JCH, Bidgood SR, McEwan WA, James LC. 2014. Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345:1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, Arstila TP, Pekkarinen PT, Ma M, Cope A, Reinheckel T, Rodriguez de Cordoba S, Afzali B, Atkinson JP, Kemper C. 2013. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okoro CK, Barquist L, Connor TR, Harris SR, Clare S, Stevens MP, Arends MJ, Hale C, Kane L, Pickard DJ, Hill J, Harcourt K, Parkhill J, Dougan G, Kingsley RA. 2015. Signatures of adaptation in human invasive Salmonella Typhimurium ST313 populations from sub-Saharan Africa. PLoS Negl Trop Dis 9:e0003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westerman TL, Bogomolnaya L, Andrews-Polymenis HL, Sheats MK, Elfenbein JR. 2018. The Salmonella type-3 secretion system-1 and flagellar motility influence the neutrophil respiratory burst. PLoS One 13:e0203698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Bruggen R, Zweers D, van Diepen A, van Dissel JT, Roos D, Verhoeven AJ, Kuijpers TW. 2007. Complement receptor 3 and Toll-like receptor 4 act sequentially in uptake and intracellular killing of unopsonized Salmonella enterica serovar Typhimurium by human neutrophils. Infect Immun 75:2655–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hölzer SU, Schlumberger MC, Jäckel D, Hensel M. 2009. Effect of the O-antigen length of lipopolysaccharide on the functions of Type III secretion systems in Salmonella enterica.Infect Immun 77:5458–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackett J, Wyk P, Reeves P, Mathan V. 1987. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis 155:540–549. [DOI] [PubMed] [Google Scholar]
- 60.Krukonis ES, Thomson JJ. 2020. Complement evasion mechanisms of the systemic pathogens Yersiniae and Salmonellae. FEBS Lett 594:2598–2620. [DOI] [PubMed] [Google Scholar]
- 61.Ondari EM, Klemm EJ, Msefula CL, El Ghany MA, Heath JN, Pickard DJ, Barquist L, Dougan G, Kingsley RA, MacLennan CA. 2019. Rapid transcriptional responses to serum exposure are associated with sensitivity and resistance to antibody-mediated complement killing in invasive Salmonella Typhimurium ST313. Wellcome Open Res 4:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.