Abstract
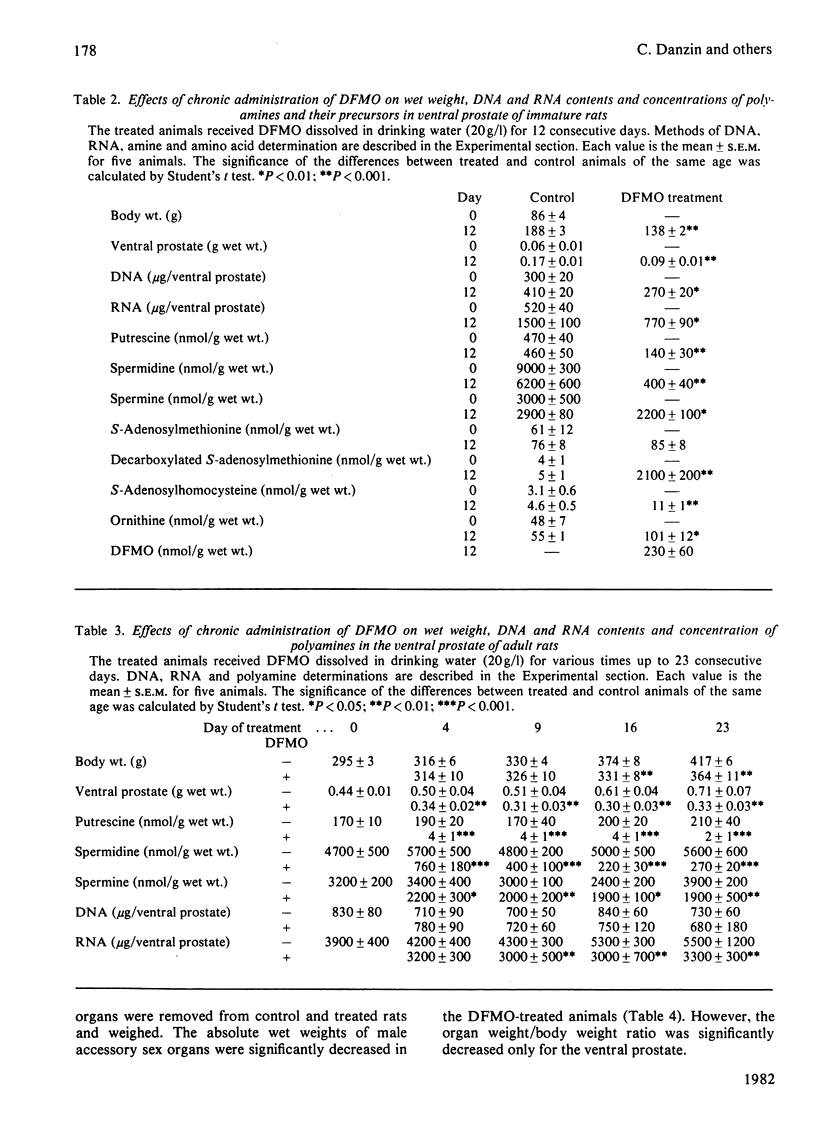
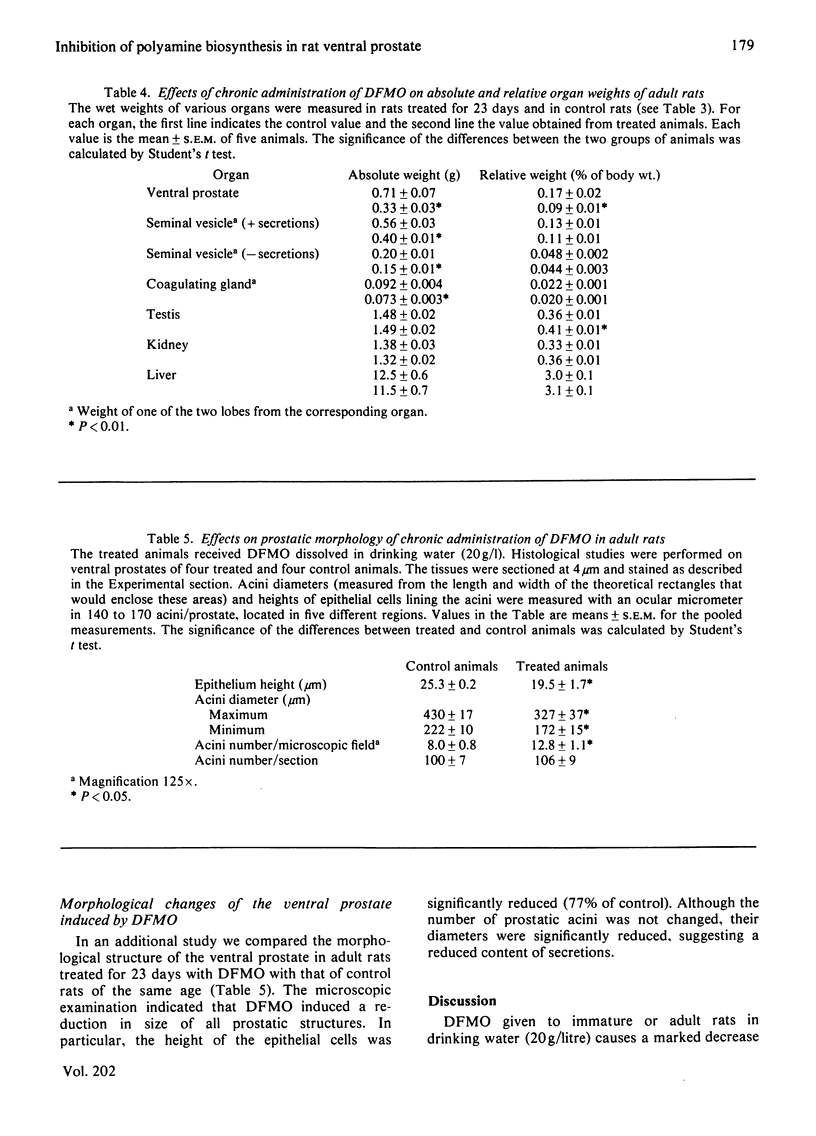
2-Difluoromethylornithine (DFMO), an enzyme-activated irreversible inhibitor of ornithine decarboxylase, causes marked changes in the polyamine metabolism of ventral prostate when given to adult rats in drinking water (20 g/l) for 3 consecutive days. A 90% inhibition of ornithine decarboxylase activity is accompanied by approx. 80% decreases of the concentrations of putrescine and spermidine and by a 36% decrease in spermine. Concomitantly, S-adenosylmethionine decarboxylase activity increases 7-fold and the concentration of decarboxylated S-adenosylmethionine 450-fold. When DFMO is given to immature rats for 12 consecutive days the above described changes are accompanied by a marked reduction in the age-dependent increases of the wet weight and RNA and DNA contents of the ventral prostate. In adult rats DFMO decreases the weight and RNA content of the ventral prostate within 4 days by 32% and 24% respectively and maintains them constant for the next 19 days. After 23 days of treatment, the prostatic weight is 46% of that of control animals of the same age, whereas the weights of other organs are only slightly decreased. Cytological studies carried out at this time show that DFMO reduces the size of both prostatic acini and the epithelial cells lining the acini.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Topper Y. J. Relationships between spermidine, glucocorticoid and milk proteins in different mammalian species. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1131–1135. doi: 10.1016/0006-291x(79)91153-7. [DOI] [PubMed] [Google Scholar]
- Danzin C., Jung M. J., Claverie N., Grove J., Sjoerdsma A., Koch-Weser J. Effects of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor or ornithine decarboxylase, on testosterone-induced regeneration of prostate and seminal vesicle in castrated rats. Biochem J. 1979 Jun 15;180(3):507–513. doi: 10.1042/bj1800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D. J., Donaldson L. J., Thomas G. H. Ornithine decarboxylase activity and: [125I]iododeoxyuridine incorporation in rat prostate. Biochem J. 1975 Sep;150(3):557–559. doi: 10.1042/bj1500557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns W., Van Damme B., De Moor P. Secretion of prostatic binding protein by rat ventral prostate: influence of age and androgen. Endocrinology. 1978 Oct;103(4):1090–1095. doi: 10.1210/endo-103-4-1090. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Hoffman J. L., Pegg A. E. Decarboxylated S-adenosylmethionine in mammalian cells. J Biol Chem. 1980 Jul 25;255(14):6675–6678. [PubMed] [Google Scholar]
- Houdebine L. M., Devinoy E., Delouis C. Role of spermidine in casein gene expression in the rabbit. Biochimie. 1978;60(8):735–741. doi: 10.1016/s0300-9084(78)80018-2. [DOI] [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Lee P. L. Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med. 1974 Jun;10(2):107–121. doi: 10.1016/0006-2944(74)90013-1. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan F. R., Pegg A. E., Mainwaring I. P. A reappraisal of the effects of adenosine 3':5'-cyclic monophosphate on the function and morphology of the rat prostate gland. Biochem J. 1973 May;134(1):129–142. doi: 10.1042/bj1340129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton L. J., Heby O., Wilson C. B., Lee P. L. An automated micromethod for the quantitative analysis of di- and polyamines utilizing a sensitive high pressure liquid chromatographic procedure. FEBS Lett. 1974 Apr 15;41(1):99–103. doi: 10.1016/0014-5793(74)80963-4. [DOI] [PubMed] [Google Scholar]
- Ono M., Inoue H., Suzuki F., Takeda Y. Studies on ornithine decarboxylase from the liver of thioacetamide-treated rats. Purification and some properties. Biochim Biophys Acta. 1972 Sep 19;284(1):285–297. doi: 10.1016/0005-2744(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J., Wilson J., Williams-Ashman H. G. Androgenic regulation of 5'-deoxy-5'-methylthioadenosine concentrations and methylthioadenosine phosphorylase activity in relation to polyamine metabolism of rat prostate. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1861–1868. doi: 10.1016/s0006-291x(80)80116-1. [DOI] [PubMed] [Google Scholar]
- Shull K. H., McConomy J., Vogt M., Castillo A., Farber E. On the mechanism of induction of hepatic adenosine triphosphate deficiency by ethionine. J Biol Chem. 1966 Nov 10;241(21):5060–5070. [PubMed] [Google Scholar]
- Takyi E. E., Fuller D. J., Donaldson L. J., Thomas G. H. Deoxyribonucleic acid and polyamine synthesis in rat ventral prostrate. Effects of age of the intact rat and androgen stimulation of the castrated rat with testosterone, 5 alpha-dihydrotestosterone and 5 alpha-androstane-3 beta, 17 beta-diol. Biochem J. 1977 Jan 15;162(1):87–97. doi: 10.1042/bj1620087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyi E. E., Thomas G. H. Ornithine decarboxylase activity during neonatal development of the rat ventral prostate. Biochim Biophys Acta. 1977 May 26;497(3):652–656. doi: 10.1016/0304-4165(77)90285-9. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Tadolini B., Wilson J., Corti A. Polynucleotide polymerizations and prostate proliferation. Vitam Horm. 1975;33:39–60. doi: 10.1016/s0083-6729(08)60950-4. [DOI] [PubMed] [Google Scholar]


