Abstract
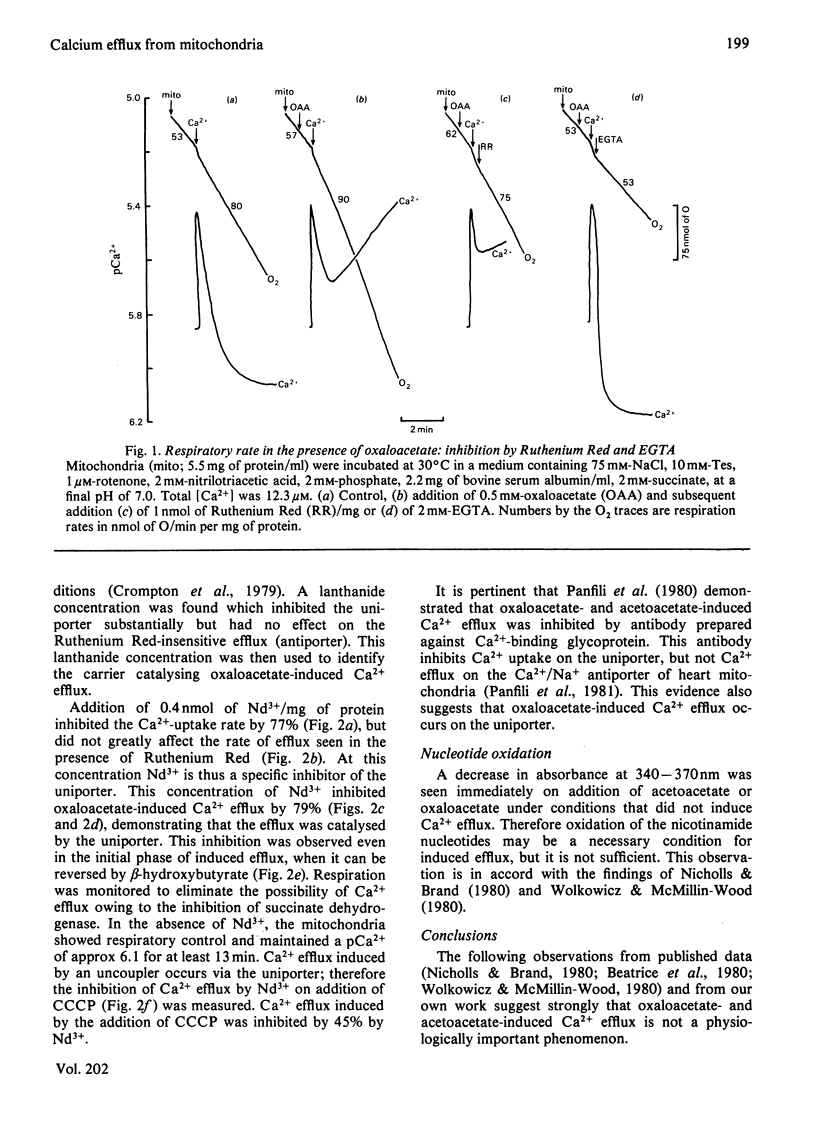
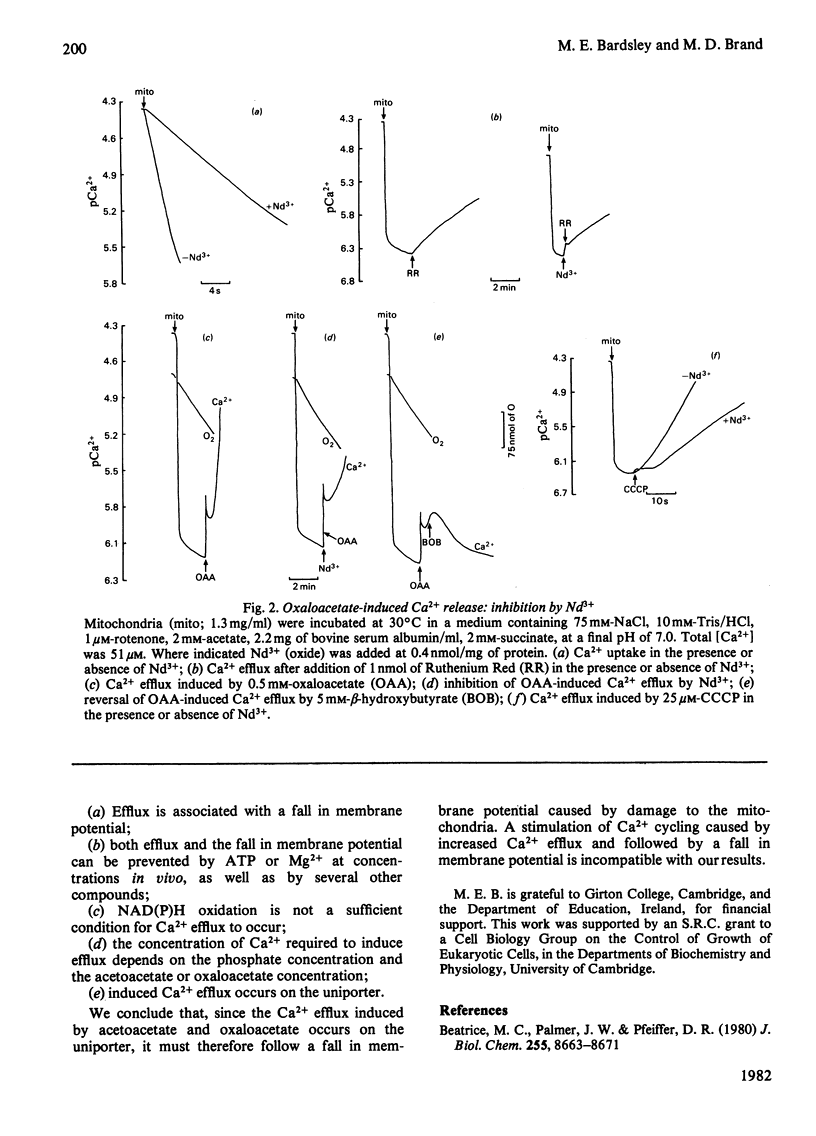
1. Addition of oxaloacetate or acetoacetate to isolated rat liver mitochondria results in an efflux of Ca2+. Concomitant with this efflux is an immediate oxidation of endogenous nicotinamide nucleotides, a fall in the mitochondrial membrane potential and an increase in the rate of respiration. The primary effect in this sequence may be either (a) physiologically important stimulation of a Ca2+-efflux carrier, followed by Ca2+ re-uptake, a fall in membrane potential and increased respiration, or (b) physiologically unimportant damage to mitochondrial integrity, followed by a fall in membrane potential, increased respiration and Ca2+ efflux. 2. Ruthenium Red and EGTA will restore the increased respiratory rate to one approximating to the control rate of respiration. However, addition of lanthanide, at a concentration which inhibits the uptake but not the normal efflux of Ca2+, inhibits the rate of Ca2+ efflux induced by oxaloacetate or acetoacetate. Therefore the observed efflux is occurring by a reversal of the uptake pathway (uniporter) and thus follows the fall in membrane potential. 3. From these results we conclude that the decrease in membrane potential and increase in the rate of respiration seen during oxaloacetate- or acetoacetate-induced Ca2+ efflux cannot be accounted for by rapid Ca2+ cycling, but are due to damage to mitochondrial integrity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatrice M. C., Palmer J. W., Pfeiffer D. R. The relationship between mitochondrial membrane permeability, membrane potential, and the retention of Ca2+ by mitochondria. J Biol Chem. 1980 Sep 25;255(18):8663–8671. [PubMed] [Google Scholar]
- Crompton M., Heid I., Baschera C., Carafoli E. The resolution of calcium fluxes in heart and liver mitochondria using the lanthanide series. FEBS Lett. 1979 Aug 15;104(2):352–354. doi: 10.1016/0014-5793(79)80850-9. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans. 1980 Jun;8(3):266–268. doi: 10.1042/bst0080266. [DOI] [PubMed] [Google Scholar]
- Fiskum G., Lehninger A. L. Regulated release of Ca2+ from respiring mitochondria by Ca2+/2H+ antiport. J Biol Chem. 1979 Jul 25;254(14):6236–6239. [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- Nicholls D. G., Brand M. D. The nature of the calcium ion efflux induced in rat liver mitochondria by the oxidation of endogenous nicotinamide nucleotides. Biochem J. 1980 Apr 15;188(1):113–118. doi: 10.1042/bj1880113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980 Mar 10;111(2):261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfili E., Crompton M., Sottocasa G. L. Immunochemical evidence of the independence of the Ca2+/Na2+ antiporter and electrophoretic Ca2+ uniporter in heart mitochondria. FEBS Lett. 1981 Jan 12;123(1):30–32. doi: 10.1016/0014-5793(81)80012-9. [DOI] [PubMed] [Google Scholar]
- Panfili E., Sottocasa G. L., Sandri G., Liut G. The Ca2+-binding glycoprotein as the site of metabolic regulation of mitochondrial Ca2+ movements. Eur J Biochem. 1980 Mar;105(1):205–210. doi: 10.1111/j.1432-1033.1980.tb04490.x. [DOI] [PubMed] [Google Scholar]
- Prpić V., Bygrave F. L. On the inter-relationship between glucagon action, the oxidation-reduction state of pyridine nucleotides, and calcium retention by rat liver mitochondria. J Biol Chem. 1980 Jul 10;255(13):6193–6199. [PubMed] [Google Scholar]
- Wolkowicz P. E., McMillin-Wood J. Dissociation between mitochondria calcium ion release and pyridine nucleotide oxidation. J Biol Chem. 1980 Nov 10;255(21):10348–10353. [PubMed] [Google Scholar]



