Abstract
Background
All individuals with Down Syndrome (DS) will develop full-blown Alzheimeŕs disease (AD) pathology by age 40, decades before the occurrence of sporadic late-onset AD. Understanding this strong biological relation between age and AD pathology risk in DS is important to accelerate diagnostics, disease monitoring, and treatment. Several genes encoded in chromosome 21 including dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) have been proven to contribute to the pathology. A recently validated plasma immunoassay to measure tau phosphorylation at threonine-212 (p-tau212) has very high diagnostic accuracy in detecting AD. P-tau212 is also very sensitive to DYRK1A phosphorylation and is increased in DSAD brain lysates. Here, we assessed the potential of this biomarker in DSAD and sporadic AD.
Methods
Using Simoa technology, we tested p-tau212 and p-tau181 (n=245 for plasma, n=114 matching cerebrospinal fluid (CSF) samples). We used AUC-ROC to examine diagnostic performance and the DeLong test to compare the AUC-ROC differences between methods. Spearman correlation is used to examine correlations. Fold changes relative to median levels were calculated for their respective asymptomatic groups. ANCOVA followed by Tukey posthoc test was used to calculate differences across groups. LOESS was used to determine the temporality of plasma biomarker changes.
Results
We have confirmed that p-tau212 has extremely high accuracy in detecting AD-related changes in euploid controls. For the DS population, we observed a strong correlation between plasma and CSF p-tau212 (r=0.867; p<0.001). In prodromal DS (pDS) and dementia DS (dDS), we observed significantly elevated levels of p-tau212 in reference to asymptomatic DS (aDS). The diagnostic accuracy to differentiate between aDS and dDS was AUC=0.91 and AUC = 0.86 in discriminating between DS amyloid positive and amyloid negative participants. Plasma p-tau212 started increasing approximately when people became amyloid PET-positive.
Conclusions
We have confirmed that the levels of plasma p-tau212 are increased in the DS population and sporadic AD cases including prodromal and MCI states. Plasma p-tau212 might have utility for theragnostic, monitoring therapy efficacy, and as a target engagement biomarker in clinical trials both in sporadic and DSAD.
Keywords: Alzheimeŕs disease, Down Syndrome, plasma biomarkers, p-tau212, DYRK1A, DABNI, SPIN, Simoa, CSF biomarkers
Introduction
A triplication of chromosome 21 causes Down syndrome (DS)(1). All individuals with DS develop full-blown Alzheimer’s Disease (AD) pathology by age 40(2) and the lifetime risk of developing AD exceeds 90% in the seventh decade(3). The estimated age of onset of AD dementia in this population is 53.8 years, decades before the occurrence of late-onset AD in the general population(4). Understanding this strong DSAD relationship is important to accelerate diagnostics and treatment. This ultra-high risk is mainly due to the triplication of the amyloid precursor protein (APP), which leads to the overproduction of amyloid-β (Aβ) peptides, and increased Aβ plaques formation(5). Although other genes encoded in chromosome 21 such as dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) and RCAN1 contribute to the pathology(6–9). DYRK1A is a dose-sensitive gene that overexpression contributes to DS cognitive dysfunction(10). This protein phosphorylates different targets involved in AD development and progression for instance Glycogen synthase kinase-3β (GSK-3β)(11), Presenilin1(12), APP(6), and tau(13).
Diagnosing DSAD is challenging due to the association of DS with cognitive dysfunction(14,15). Recent advances allow us to recognize ante-mortem AD pathology using blood-based biomarkers(16–19). These reflect the disease almost as accurately as more expensive and less available CSF and imaging biomarkers(20). Several plasma p-tau biomarkers have been found to reflect both Aβ and tau pathology(21). Recently validated plasma p-tau212 has shown very high performance for detecting those pathologies and AD diagnosis(22). That phosphorylation site might also have the strongest biological correlation with AD pathology in DS since threonine-212 is a primary target for DYRK1A in tau protein(23). Intensified phosphorylation at thr-212 induces tau aggregation, reduces tau binding to microtubules, and increases cell toxicity in in-vitro studies(24). Levels of p-tau212 are highly elevated in reference to AD and control participants in human AD-DS brains(25). Additionally, a major tau phosphatase – Protein phosphatase 1A (PP1A) is not dephosphorylating p-tau212 derived from AD brains(26), suggesting that this epitope might be vulnerable to very subtle changes related to AD pathology. Knowing that direct biological association, we hypothesized that p-tau212 is an accurate tau species to reflect AD pathology in the DS population. To test this hypothesis, we used Single molecule array (Simoa) immunoassays to measure plasma and cerebrospinal fluid (CSF) p-tau212 concentrations in asymptomatic DS (aDS), prodromal DS (pDS), and dementia DS (dDS) individuals, as well as in sporadic AD patients both in Mild Cognitive impairment (MCI) and dementia states and we compare the results with a validated biomarker.
Methods
Study design and participants
We performed a cross-sectional cohort study of adults with DS, and euploid individuals along the Alzheimer’s disease continuum in the Hospital of Sant Pau, Barcelona (Spain), Adults with DS in Barcelona were recruited from a population-based health plan designed to screen for AD dementia, which includes yearly neurological and neuropsychological assessments. Those subjects interested in research studies are included in the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort(14,15). We also recruited euploid controls and sporadic Alzheimer’s disease patients from the Sant Pau Initiative on Neurodegeneration (SPIN cohort)(27).
Biomarker measurements
Amyloid-β peptides (Aβ40, Aβ42) analyses were performed on the LUMIPULSE G600II, and the cut-off for positivity was defined as in the previous publication(28). All other plasma and CSF biomarker assays were performed on the Simoa HD-X platform at the University of Gothenburg. P-tau212 and p-tau181 concentrations were measured using published and validated in-house assays(16,22). For p-tau181, the AT270 antibody (Invitrogen) was used as a capture antibody and was paired with n-terminal antibody for detection (Tau12; BioLegend). For p-tau212, a sheep monoclonal antibody was used for capture paired with Tau12 as the detector. Coefficients of variation for 3 different internal quality controls for plasma were 5.3–12% for within-plate variation and 6.4%−12% for between-plate variation. For CSF, these values were 10.8%−13.3% and 12.9%−15.5%, respectively.
Statistical Analysis:
We used AUC-ROC to examine diagnostic performance and the DeLong test to compare the AUC-ROC differences between methods. Spearman correlation is used to examine correlations. Fold changes relative to median levels are calculated for their respective asymptomatic groups. Age-adjusted analysis of covariance (ANCOVA) followed by Tukey post-hoc test is used to calculate differences across groups. A First-degree locally estimated scatterplot smoothing curve (LOESS) is independently used in controls and adults with Down Syndrome to determine the temporality of plasma biomarker changes. For Down participants we set the mean age of symptoms onset at 53.8 years according to a previous study from our group(29). All significance tests were two-sided, and significance was set at p<0.05.
Results
Cohort characteristics
We tested n=245 plasma samples for p-tau212 and p-tau181. A subset of participants had amyloid PET data. Table 1. shows the demographics, cognitive, and plasma biomarkers across groups of all participants included in the analyses. N=114 (47%) participants had CSF biomarker measurements. Demographics for the CSF subset are shown in Supplementary Table 1.
Table 1.
Study Participants
| aDS | pDS | dDS | CN | MCI-AD | AD | |
|---|---|---|---|---|---|---|
| n= Female/Male | 36/56 (39.1/60.9%) | 5/3 (62.5/37.5%) | 8/9 (47.1/52.9%) | 31/15 (67.4/32.6%) | 35/27 (56.5/43.5%) | 12/8 (60/40%) |
| AGE (years)-range | 18–62 | 41–60 | 46–59 | 18–75 | 56–83 | 53–83 |
| AGE (years)-mean(sd) | 42.2 (9.78) | 51.5 (7.41) | 51.8 (3.75) | 50 (14.8) | 72.3 (6.03) | 70.5 (8.94) |
| AGE (years)-median[IQR] | 44 [35.8–49] | 52 [46.2–57] | 52 [49–55] | 46.5 [39–63.8] | 72 [68–76.8] | 71 [64.8–77.5] |
| APOE4-/APOE4+ | 68/23 (74.7/25.3%) | 7/1 (87.5/12.5%) | 13/4 (76.5/23.5%) | 39/7 (84.8/15.2%) | 24/37 (39.3/60.7%) | 10/10 (50/50%) |
| Plasma p-tau212 n | 92 | 8 | 17 | 46 | 62 | 20 |
| Plasma p-tau212 range | 0.0435–1.69 | 0.666–2 | 0.404–2 | 0.0163–0.444 | 0.079–1.65 | 0.174–1.23 |
| Plasma p-tau212 mean (SD) | 0.404 (0.377) | 1.11 (0.445) | 0.929 (0.46) | 0.166 (0.0856) | 0.498 (0.28) | 0.629 (0.29) |
| Plasma p-tau212 median[IQR] | 0.245 [0.156–0.529] | 0.926 [0.8311.24] | 0.764 [0.627–1.23] | 0.151 [0.113–0.235] | 0.427 [0.33–0.632] | 0.65 [0.372–0.738] |
| Plasma p-tau181 range | 3.34–47.8 | 17–50.1 | 13.5–42.9 | 3.95–41.6 | 4.97–53.7 | 11.4–37.8 |
| Plasma p-tau181 mean (SD) | 15.9 (10.9) | 29 (11.8) | 23.5 (8.18) | 11.1 (7.03) | 22.3 (11.5) | 22.6 (7.01) |
| Plasma p-tau181 median[IQR] | 11.7 [7.54–20.9] | 24.7 [20.4–35.3] | 21.6 [18.9–27.7] | 9.02 [7.07–12.7] | 19.3 [14.6–27.9] | 22.5 [18.2–27.4] |
| Total CAMCOG-DS score-n | 73 | 3 | 12 | 0 | 0 | 0 |
| Total CAMCOG-DS score-range | 25–102 | 6–56 | 32–77 | - | - | - |
| Total CAMCOG-DS score-mean(sd) | 70.7 (20) | 37.7 (27.5) | 49.8 (13) | - | - | - |
| Total CAMCOG-DS score-median[IQR] | 74 [57–87] | 51 [28.5–53.5] | 52 [38.5–57.2] | - | - |
aDS – asymptomatic Down syndrome; pDS – prodromal Down syndrome; dDS – dementia Down syndrome; CN – cognitively normal; MCI-AD – mild cognitive impairment due to Alzheimer’s Disease; AD – Alzheimer’s disease dementia; IQR – interquartile range; SD – standard deviation. CAMCOG-DS The Cambridge Cognitive Examination adapted for individuals with Down Syndrome
Correlations with biomarkers and cognition
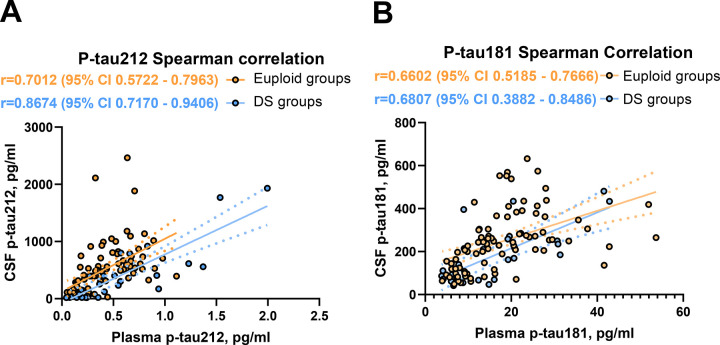
Plasma and CSF p-tau212 were highly correlated with each other within the cohort. We observed moderate correlation across sample types in all participants (r=0.712 p<0.0001) (Fig. 1A). However, the strongest correlation of plasma p-tau212 with CSF p-tau212 was observed in the DS-only subgroup (r=0.867, p<0.0001) (Fig 1A). This improved CSF-plasma correlation in DS groups was not seen for p-tau181, which showed similar correlations in the whole cohort and subgroups (r=0.652–0.681, p<0.0001) (Fig 1B). Those results indicate that plasma p-tau212 measurements accurately reflect AD-related p-tau level changes in CSF in sporadic and DS groups.
Figure 1. Spearman correlations for plasma and CSF p-tau212 and p-tau181.
The Figure shows correlations between plasma and CSF for A) p-tau212 and B) p-tau181 for euploid groups (n=88; p<0.001; r= 0.701 and r=0.660 respectively) and DS groups (n=26, p<0.001; r=0.867, r=0,681 respectively). The correlation between plasma and CSF measurements for all measurements (n=114, p<0.001) is 0.712 for p-tau212 and 0.652 for p-tau181. The fitted simple linear regression line is presented as a mean and error.
Both biomarkers were correlated with decrease in The Cambridge Cognitive Examination adapted for individuals with Down Syndrome (CAMCOG-DS) in plasma (r=−0.338; p=0.001 for p-tau212 and r=−0.328 for p-tau181; p=0.002;) and in CSF (r=−0.686; p<0.001 for p-tau212; and r=−0.511; p<0.001 for p-tau181).
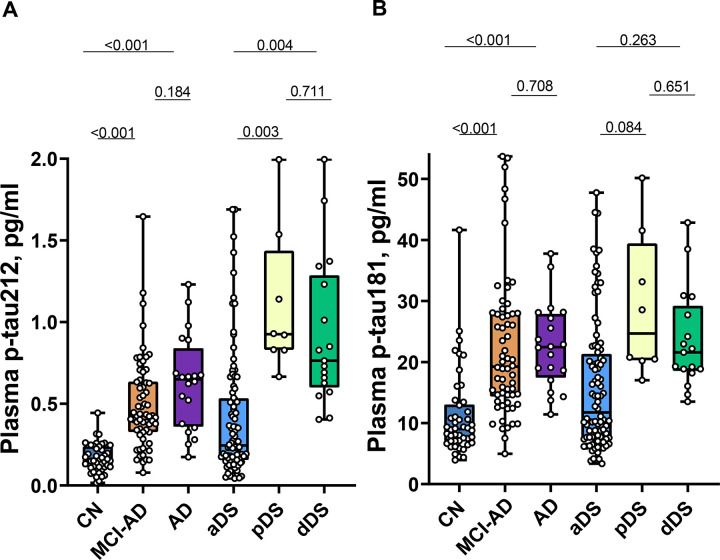
Plasma p-tau212 levels are increased in asymptomatic Down syndrome
Plasma p-tau212 concentration was 2.4x times higher in the aDS group compared with cognitively normal (CN) euploid people (p<0.001) whereas plasma p-tau181 was not significantly changed (p=0.052).
Plasma and CSF p-tau212 increase along the AD continuum in DSAD and sporadic AD
Both in individuals with DS and euploid participants, p-tau212 levels were increased in symptomatic patients in comparison with asymptomatic individuals. (Fig. 2A). For pDS we observed a 3.4x (p=0.003) mean fold increase in plasma and a 5.6x mean fold-fold increase in CSF in reference to aDS. For dDS we observed a 2.9x (p=0.004) mean fold increase in plasma and a 7.1x mean fold increase in CSF. For MCI-AD, compared with cognitively normal euploid people, we observed a 3.0x (p<0.001) mean fold increase in plasma and a 7.8x mean fold increase in CSF. AD dementia patients had a 3.8x mean fold increase in plasma (p<0.001) and a 9.1x mean fold increase in CSF. P-tau181 concentrations were also increased, but with a lower magnitude than p-tau212, and comparison between DS groups showed no significance (Fig. 2B). Both biomarkers kept the pattern of greater increases in CSF than in plasma (Supplementary Fig. 1A–B).
Figure 2. Plasma p-tau212 and p-tau181 levels in euploid and Down Syndrome (DS) groups.
Box plots represent median and IQR, and boundaries of the whiskers are minimum to maximum values for A) plasma p-tau212 and B) plasma p-tau181. Differences for euploid participants are calculated for Mild Cognitively impaired Alzheimer’s Disease (MCI-AD; n=62) and Alzheimer’s Disease Dementia (AD; n=20) in reference to Cognitively Normal (CN; n=46) participants. Differences for prodromal Alzheimer’s Disease in DS (pDS; n=8) and Alzheimer’s Disease dementia in DS (dDS; n=17) are calculated in reference to asymptomatic (aDS; n=92). Age-adjusted analysis of covariance (ANCOVA) followed by Tukey post-hoc test is used to calculate differences across groups.
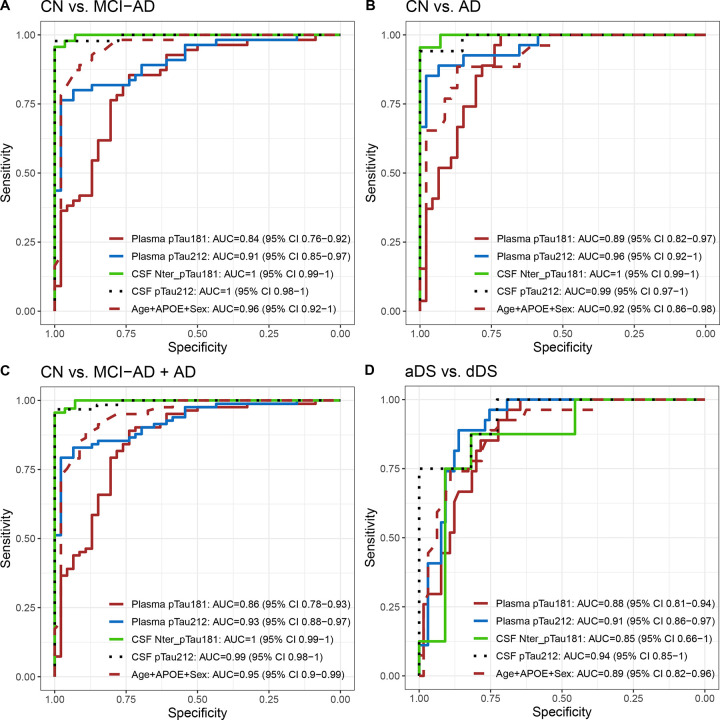
Plasma p-tau212 has greater diagnostic accuracy than p-tau181
ROC analysis was used to evaluate the diagnostic performance of plasma and CSF p-tau212 and p-tau181. AUCs were usually higher for biomarkers in CSF, and for p-tau212 than for p-tau181. In our comparisons, we included Age+ Apolipoprotein E4 (APOE4)+Sex since they have been shown to influence diagnostic accuracy(30). AUCs of Age+APOE+Sex were not significantly different from p-tau212 but better than p-tau181. Both biomarkers had high accuracy to differentiate between CN and AD in plasma AUC = 0.96 (95% CI 0.92–1) for p-tau212 and AUC = 0.89 (95% CI 0.84–0.95) for p-tau181 (Fig. 3B). For differentiating between CN and MCI-AD (Fig. 3A) or MCI-AD+AD (Fig. 3C), p-tau212 had accuracy of 0.91 (95% CI 0.85–0.97) and AUC=0.93 [95% CI 0.88–0.97] respectively. That accuracy was significantly higher than p-tau181 - (p=0.026 for both comparisons). Plasma p-tau212 reached AUC=0.91 (95% CI 0.86–0.97) to differentiate between aDS and dDS diagnosis (Fig. 3D).
Figure 3. Diagnostic accuracy of plasma and CSF biomarkers to discriminate between sporadic and DSAD groups.
P-tau212 and p-tau181 receiver operating characteristic curves (ROC) to discriminate between sporadic and DSAD groups. Plasma p-tau181, plasma p-tau212, CSF p-tau181, CSF p-tau212, and Age+APOE+Sex are on each graph. In A) ROC curves for differentiating Cognitively normal (CN) and Mild Cognitive Impairment - Alzheimer’s Dementia (MCI-AD). B) ROC curves to discriminate between CN and Alzheimer’s Disease (AD). C) ROC curves to differentiate between CN and MCI-AD combined with AD group. D) ROC curves to discriminate between asymptomatic Down syndrome (aDS) and prodromal Down Syndrome (pDS) + dementia Down syndrome (dDS).
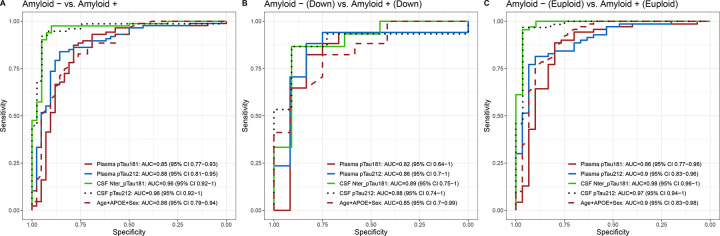
Plasma p-tau212 increases approximately parallel to amyloid PET positivity and has great accuracy in discriminating between Aβ+ and Aβ− participants
Both biomarkers had significant accuracy in discriminating between Aβ+ and Aβ− participants (Fig. 4). Plasma p-tau212 had numerically higher accuracy than p-tau181 both for DS (AUC = 0.86 (95%CI = 0.7–1) (Fig. 4B) and euploid participants AUC = 0.9 (0.83 – 0.96) (Fig. 4C).
Figure 4. Diagnostic accuracy of plasma and CSF biomarkers to discriminate between Aβ+ and Aβ− participants.
P-tau212 and p-tau181 receiver operating characteristic curves (ROC) to discriminate between amyloid positive (Aβ+) and amyloid negative (Aβ−) participants in sporadic and DSAD groups. Plasma p-tau181, plasma p-tau212, CSF p-tau181, CSF p-tau212, and Age+APOE+Sex are on each graph. In A) ROC curves for differentiating Aβ+ from Aβ− in whole cohort. B) ROC curves to discriminate Aβ+ and Aβ− in DS groups. C) ROC curves to differentiate between Aβ+ and Aβ− in euploid groups.
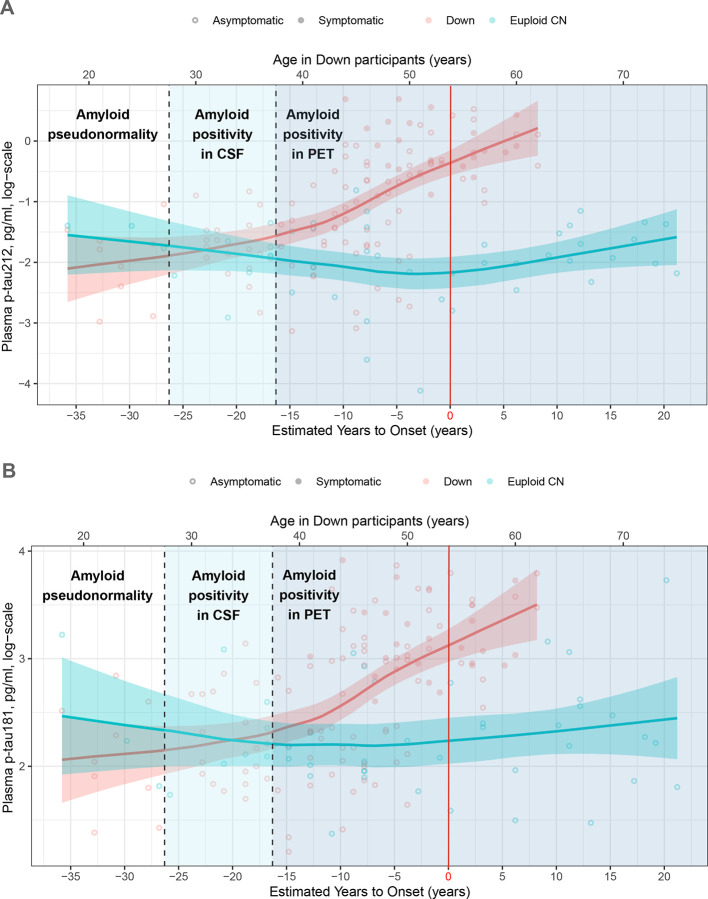
An early increase in plasma levels was observed many years before the onset of clinical AD symptoms in DS (Fig 5). For p-tau212 the increase started approximately when people became amyloid PET-positive, i.e., in their late 30s, and approximately 15 years before the disease onset (Fig. 5A). P-tau181 started increasing approximately 10 years before the estimated disease onset (Fig. 5B).
Figure 5. Age-related plasma p-tau212 and p-tau181 changes in Down syndrome and euploid controls.
Open Circles represent asymptomatic and filled circles represent symptomatic participants. Down syndrome population is represented in red and cognitively normal euploid people are represented in blue. Horizontal lines depict each group’s fitted loess model, and fainted bands display confidence intervals. The vertical red line represents the estimated years to symptom onset which is 53.8 for Down syndrome participants and was used as reference in euploid controls for comparison purposes.
Discussion
In this cross-sectional study, we show that p-tau212 serves as a biomarker to track AD-related changes in sporadic cases and DSAD. P-tau212 has a very high correlation between plasma and CSF. The strongest correlation was observed for the DS population, where p-tau212 reached a greater correlation than for euploid participants. For p-tau181 correlation in euploid and DS groups was similar, suggesting greater translation from CSF to plasma for p-tau212 in DS populations.
We observed elevated p-tau212 concentration in plasma and CSF in aDS compared with euploid CN. That elevation is greater than the p-tau181 elevation, which did not reach statistical significance. Additionally, other reported biomarkers, such as p-tau217 Glial fibrillary acidic protein (GFAP)(30,31), had lower increases. This suggests the effects of DYRK1A gene dose interacting with AD pathology on p-tau212 levels in DS and a better fit for the use of this biomarker in the DS population.
Levels of plasma p-tau212 were significantly higher across disease groups. Plasma p-tau212 reached significance levels to differentiate between aDS vs pDS and aDS vs dDS groups while p-tau181 failed to do it. However, we think this might be a limitation of the small sample size used in this cohort since p-tau181 levels were previously shown to be significantly increased in pDS and aDS(30,32). In MCI-AD and AD dementia groups, we observed significant increases for both biomarkers, concomitantly having fold changes higher for p-tau212 which confirms our previous findings(22).
The excellent performance of the assay was confirmed in discriminating patients according to the diagnosis in both DS and euploid groups. P-tau212 additionally has greater accuracy than p-tau181 in discriminating between MCI-AD and control groups, simultaneously reaching 0.96 AUC to differentiate CN from AD. Additionally, plasma p-tau212 acquired very high AUC-ROC in discriminating between Aβ+ and Aβ− participants in both euploid and DS groups. This accuracy was not different from CSF accuracy, supporting the high between-matrix translation of p-tau212 and providing additional reasoning to use plasma p-tau212 to recruit participants for clinical trials.
Plasma p-tau212 starts increasing in the 30s, approximately when people start being positive in amyloid PET scans, and 5 years before we observe an increase in p-tau181. Additionally, the biomarker increased further as AD progressed towards symptomatic stages. Therefore p-tau212 could be useful to monitor the progression of asymptomatic DS people to prodromal AD. Moreover, the onset of the increase comes along with the appearance of neurofibrillary tangles (NFTs) and Aβ plaques in brain(4,33).
This is (to our best knowledge) the first study to measure p-tau212 in CSF and plasma as a biomarker for DSAD however, the potential involvement of p-tau212 in this population and its association with DYRK1A was published more than 20 years ago(13). The kinase is perceived as a target in DS and neurodegenerative diseases(8,34). Its under- or overexpression leads to different clinical phenotypes including cognitive impairment(34). Since DYRK1A is a dose-sensitive protein in which down-regulation or up-regulation has a critical role, DYRK1A inhibitors have already been widely explored in clinical trials and have been proven to improve cognitive function in DS people(10,35,36). Importantly, p-tau212 has already been successfully used to test the efficacy of DYRK1A inhibitor in cell models(37). The use of the chosen inhibitor was further shown to reverse the upregulation of p-tau212 in hippocampal tissue and temporal cortex in mouse models(37). Our novel plasma p-tau212 immunoassay provides a simple-to-implement and cost-effective opportunity to monitor the efficacy of DYRK1A inhibitors, or in the future – enhancers, not allowing the activity of this kinase to be reduced or increased to levels that could cause more harm than good. This utility will be explored in our future research.
The major strength of this study is the confirmation that p-tau212 is increased in the DS population, and levels of this biomarker increase with progression to AD dementia. P-tau212 reaches very high accuracy to differentiate between control and disease groups and Aβ+ and Aβ− participants. The high correlation between plasma and CSF p-tau212 also supports a very high translation of the results from CSF to plasma. DeLong tests between DS groups did not show any significantly better performance of CSF p-tau212 compared with plasma p-tau212 providing further evidence that plasma measurements can be used for clinical evaluation of AD pathophysiological processes occurring in patients with suspected disease. Advantages would also be reflected in the economy, availability, and perception of the test, since lumbar punctures or PET scans are costly, require resources, and might be perceived as frightening(20). Next, plasma p-tau212 increased approximately when people are starting to be amyloid PET positive and 5 years before p-tau181, indicating the benefits in disease monitoring.
This study has a few limitations. First is a slightly low representation of prodromal-DS participants, which prohibits us from making better AUC-ROC analysis in that group. Ideally, longitudinal measurements of p-tau212 in the DS population would tell us more about the trajectories of this biomarker. The second limitation is that p-tau217 measurements are unavailable at the moment, however direct comparison between biomarkers was not a purpose of experiments presented in this article. Still, p-tau181 is the most commercialized and fully automated immunoassay, with great utility in AD.
Conclusions
In conclusion, we have confirmed that levels of plasma p-tau212 are increased in the DS population and sporadic AD cases including prodromal and MCI states. High accuracy in discriminating amyloid positive from amyloid negative people and increase in parallel to amyloid-PET positivity give the promise to evaluate ongoing pathophysiological AD processes many years before the disease onset in individuals with DS. This will also facilitate participants recrutation for clinical trials. This is a cost-effective application that provides higher chance to receive appropriate therapy. Plasma p-tau212 will also find high utility for theragnostic, to monitor therapy efficacy, and as a target engagement biomarker in clinical trials both in sporadic and DSAD.
Supplementary Material
Acknowledgements
We thank all the participants with Down syndrome, their families, and their carers for their support of, and dedication to this research. We also acknowledge Fundació Catalana Síndrome de Down for global support and the members of the Alzheimer Down Unit and the Memory Unit from Hospital de la Santa Creu i Sant Pau for their daily work and dedication.
Funding
PRK was funded by Demensförbundet and Anna Lisa and Brother Björnsson’s Foundation. LMG is supported by the Brightfocus Foundation (A2022015F), the Swedish Dementia Foundation, Gun and Bertil Stohnes Foundation, Åhlén-stifelsen, Alzheimerfonden (AF-968621) and Gamla Tjänarinnor Foundation. FG-O was funded by the Anna Lisa and Brother Björnsson’s Foundation. HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C), the Bluefield Project, Cure Alzheimer’s Fund, the Olav Thon Foundation, the Erling-Persson Family Foundation, Familjen Rönströms Stiftelse, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme - Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). KB is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, #AF-968270, and #AF-994551), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC), La Fondation Recherche Alzheimer (FRA), Paris, France, the Kirsten and Freddy Johansen Foundation, Copenhagen, Denmark, and Familjen Rönströms Stiftelse, Stockholm, Sweden. TKK was supported by the NIH (R01 AG083874, RF1 AG052525-01, P30 AG066468-04, R01 AG053952, R01 MH121619, R37 AG023651, RF1 AG025516-12A1, R01 AG073267, R01 MH108509, R01 AG075336, R01 AG072641, P01 AG025204), the Swedish Research Council (Vetenskåpradet; #2021-03244), the Alzheimer’s Association (#AARF-21-850325), the Swedish Alzheimer Foundation (Alzheimerfonden), the Aina (Ann) Wallströms and Mary-Ann Sjöbloms stiftelsen, and the Emil och Wera Cornells stiftelsen. J.F was supported by the Fondo de Investigaciones Sanitario, Carlos III Health Institute (INT21/00073, PI20/01473 and PI23/01786) and the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas Program 1, partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una Manera de Hacer Europa. This work was also supported by the National Institutes of Health grants (R01 AG056850; R21 AG056974, R01 AG061566, R01 AG081394 and R61AG066543), the Department de Salut de la Generalitat de Catalunya, Pla Estratègic de Recerca i Innovació en Salut (SLT006/17/00119). It was also supported by Fundación Tatiana Pérez de Guzmán el Bueno (IIBSP-DOW-2020-151) and Horizon 2020-Research and Innovation Framework Programme from the European Union (H2020-SC1-BHC-2018-2020).
Competing interests
MT and PH are employees of Bioventix Plc. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant or at advisory boards for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers. HZ and KB are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. D.A. participated in advisory boards from Fujirebio-Europe, Roche Diagnostics, Grifols S.A. and Lilly, and received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A. D.A. and JF declare a filed patent application (licensed to Adx, EPI8382175 J.F. reported receiving personal fees for service on the advisory boards, adjudication committees or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Eisai, Esteve, Eisai, Fujirebio, Ionis, Laboratorios Carnot, Lilly, Life Molecular Imaging, Lundbeck, Perha, Roche, and, outside the submitted work.The other authors declare no competing interest.
Abbreviations
- AD
Alzheimeŕs disease
- DS
Down Syndrome
- DYRK1A
dual-specificity tyrosine phosphorylation-regulated kinase 1A
- p-tauX
tau phosphorylated at amino acid X
- DSAD
Down Syndrome Alzheimeŕs disease
- CSF
Cerebrospinal Fluid
- AUC-ROC
Area Under the Curve and Receiver Operating Curves
- ANCOVA
Age-adjusted analysis of covariance
- LOESS
locally estimated scatterplot smoothing
- pDS
prodromal Down syndrome
- aDS
asymptomatic Down syndrome
- dDS
dementia Down syndrome
- PET
Positron Emission Tomography
- MCI
mild cognitive impairment
- Aβ
amyloid beta
- APP
amyloid precursor protein
- GSK-3β
Glycogen synthase kinase-3β
- PP1A
Protein phosphatase 1A
- DABNI
Down Alzheimer Barcelona Neuroimaging Initiative
- SPIN
Sant Pau Initiative on Neurodegeneration
- CN
cognitively normal
- IQR
interquartile range
- SD
standard deviation
- CAMCOG-DS
The Cambridge Cognitive Examination adapted for individuals with Down Syndrome
- APOE4
Apolipoprotein E4
- GFAP
Glial fibrillary acidic protein
- NFTs
Neurofibrillary tangles
Footnotes
Ethics approval and consent to participate
Study procedures were approved by the Sant Pau Ethics Committee (IIBSP-NGF-2018–36 and IIBSP-DOW-2014–30), following the standards for medical research in humans, as recommended in the Declaration of Helsinki. All participants or their legally authorized representative gave written informed consent before enrolment.
Availability of data and materials
Blinded Anonymized data is available on reasonable request from the corresponding author. Request will be reviewed by the investigators and respective institutions to verify if data transfer is in the agreement with EU legislation on the general data protection or is subject to any intellectual property or confidentiality obligations.
References
- 1.Lejeune J, Gautier M, Turpin R. [Study of somatic chromosomes from 9 mongoloid children]. Comptes Rendus Hebd Seances Acad Sci. 1959. Mar 16;248(11):1721–2. [PubMed] [Google Scholar]
- 2.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17(3):278–82. [DOI] [PubMed] [Google Scholar]
- 3.McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61(9):843–52. [DOI] [PubMed] [Google Scholar]
- 4.Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona-Iragui M. Down syndrome-associated Alzheimer’s disease: a genetic form of dementia. Lancet Neurol. 2021. Nov;20(11):930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down’s syndrome. Lancet Neurol. 2016. May 1;15(6):622–36. [DOI] [PubMed] [Google Scholar]
- 6.Kimura R, Kamino K, Yamamoto M, Nuripa A, Kida T, Kazui H, et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet. 2007. Jan 1;16(1):15–23. [DOI] [PubMed] [Google Scholar]
- 7.Branca C, Shaw DM, Belfiore R, Gokhale V, Shaw AY, Foley C, et al. Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell. 2017. Oct;16(5):1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegiel J, Kaczmarski W, Barua M, Kuchna I, Nowicki K, Wang KC, et al. Link between DYRK1A overexpression and several-fold enhancement of neurofibrillary degeneration with 3-repeat tau protein in Down syndrome. J Neuropathol Exp Neurol. 2011. Jan;70(1):36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ermak G, Harris CD, Battocchio D, Davies KJA. RCAN1 (DSCR1 or Adapt78) stimulates expression of GSK-3beta. FEBS J. 2006. May;273(10):2100–9. [DOI] [PubMed] [Google Scholar]
- 10.Duchon A, Herault Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front Behav Neurosci [Internet]. 2016. [cited 2021 Jul 14];10. Available from: https://www.frontiersin.org/articles/10.3389/fnbeh.2016.00104/full#B228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skurat AV, Dietrich AD. Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem. 2004. Jan 23;279(4):2490–8. [DOI] [PubMed] [Google Scholar]
- 12.Ryu YS, Park SY, Jung MS, Yoon SH, Kwen MY, Lee SY, et al. Dyrk1A-mediated phosphorylation of Presenilin 1: a functional link between Down syndrome and Alzheimer’s disease. J Neurochem. 2010;115(3):574–84. [DOI] [PubMed] [Google Scholar]
- 13.Woods YL, Cohen P, Becker W, Jakes R, Goedert M, Wang X, et al. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J. 2001. May 1;355(Pt 3):609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortea J, Carmona-Iragui M, Benejam B, Fernández S, Videla L, Barroeta I, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018. Oct 1;17(10):860–9. [DOI] [PubMed] [Google Scholar]
- 15.Fortea J, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. The Lancet. 2020. Jun 27;395(10242):1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020. May;19(5):422–33. [DOI] [PubMed] [Google Scholar]
- 17.Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020. Aug 25;324(8):772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol (Berl). 2021. May 1;141(5):709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kac PR, Gonzalez-Ortiz F, Simrén J, Dewit N, Vanmechelen E, Zetterberg H, et al. Diagnostic value of serum versus plasma phospho-tau for Alzheimer’s disease. Alzheimers Res Ther. 2022. May 11;14(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Ortiz F, Kac PR, Brum WS, Zetterberg H, Blennow K, Karikari TK. Plasma phospho-tau in Alzheimer’s disease: towards diagnostic and therapeutic trial applications. Mol Neurodegener. 2023. Mar 16;18(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson-Carlgren N, Janelidze S, Bateman RJ, Smith R, Stomrud E, Serrano GE, et al. Soluble P-tau217 reflects amyloid and tau pathology and mediates the association of amyloid with tau. EMBO Mol Med. 2021. Jun 8;13(6):e14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kac PR, González-Ortiz F, Emeršič A, Dulewicz M, Koutarapu S, Turton M, et al. Plasma p-tau212 antemortem diagnostic performance and prediction of autopsy verification of Alzheimer’s disease neuropathology. Nat Commun. 2024. Mar 23;15(1):2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, et al. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008. Sep;22(9):3224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso AD, Di Clerico J, Li B, Corbo CP, Alaniz ME, Grundke-Iqbal I, et al. Phosphorylation of Tau at Thr212, Thr231, and Ser262 Combined Causes Neurodegeneration. J Biol Chem. 2010. Oct 1;285(40):30851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawa M, Overk C, Becker A, Derse D, Albay R, Weldy K, et al. Impact of increased APP gene dose in Down syndrome and the Dp16 mouse model. Alzheimers Dement. 2022;18(6):1203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman A, Grundke-Iqbal I, Iqbal K. Phosphothreonine-212 of Alzheimer abnormally hyperphosphorylated tau is a preferred substrate of protein phosphatase-1. Neurochem Res. 2005. Feb;30(2):277–87. [DOI] [PubMed] [Google Scholar]
- 27.Alcolea D, Clarimón J, Carmona-Iragui M, Illán-Gala I, Morenas-Rodríguez E, Barroeta I, et al. The Sant Pau Initiative on Neurodegeneration (SPIN) cohort: A data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement N Y N. 2019;5:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcolea D, Pegueroles J, Muñoz L, Camacho V, López-Mora D, Fernández-León A, et al. Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on Lumipulse. Ann Clin Transl Neurol. 2019. Sep;6(9):1815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iulita MF, Garzón Chavez D, Klitgaard Christensen M, Valle Tamayo N, Plana-Ripoll O, Rasmussen SA, et al. Association of Alzheimer Disease With Life Expectancy in People With Down Syndrome. JAMA Netw Open. 2022. May 23;5(5):e2212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montoliu-Gaya L, Alcolea D, Ashton NJ, Pegueroles J, Levin J, Bosch B, et al. Plasma and cerebrospinal fluid glial fibrillary acidic protein levels in adults with Down syndrome: a longitudinal cohort study. eBioMedicine. 2023. Mar 30;90:104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janelidze S, Christian BT, Price J, Laymon C, Schupf N, Klunk WE, et al. Detection of Brain Tau Pathology in Down Syndrome Using Plasma Biomarkers. JAMA Neurol. 2022. Aug 1;79(8):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lleó A, Zetterberg H, Pegueroles J, Karikari TK, Carmona-Iragui M, Ashton NJ, et al. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer’s disease in adults with Down syndrome. Nat Commun. 2021. Jul 14;12(1):4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lott IT, Head E. Dementia in Down syndrome: unique insights for Alzheimer disease research. Nat Rev Neurol. 2019. Mar;15(3):135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deboever E, Fistrovich A, Hulme C, Dunckley T. The Omnipresence of DYRK1A in Human Diseases. Int J Mol Sci. 2022. Aug 19;23(16):9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AJ, Wilton SD, Aung-Htut MT, McIntosh CS. Down syndrome and DYRK1A overexpression: relationships and future therapeutic directions. Front Mol Neurosci. 2024. Jul 24;17:1391564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De la Torre R, De Sola S, Pons M, Duchon A, de Lagran MM, Farré M, et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014. Feb;58(2):278–88. [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Gao X, Li X, Yu C, Liu W, Qiu J, et al. Discovery of ZJCK-6–46: A Potent, Selective, and Orally Available Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A Inhibitor for the Treatment of Alzheimer’s Disease. J Med Chem. 2024. Aug 8;67(15):12571–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Blinded Anonymized data is available on reasonable request from the corresponding author. Request will be reviewed by the investigators and respective institutions to verify if data transfer is in the agreement with EU legislation on the general data protection or is subject to any intellectual property or confidentiality obligations.