Abstract
Background
The attention towards the relationship between chronic obstructive pulmonary disease (COPD) and dietary intake is escalating. However, the effects of dietary niacin on COPD in middle and older individuals remains unclear. This study aimed to illuminate the connection between dietary niacin intake and COPD.
Methods
This cross-sectional study analyzed 7,170 participants from the National Health and Nutrition Examination Survey (NHANES) spanning the years 2013 to 2018. Participants were categorized into four groups based on quartiles of dietary niacin intake. To examine the association between covariates, dietary niacin intake, and COPD, we employed univariate analysis and multivariate logistic regression equations. Additionally, restricted cubic splines were utilized to assess linearity. Furthermore, we conducted stratified and interaction analyses to evaluate the stability of the relationship in diverse subgroups.
Results
Among the 7,170 participants, 11.6% (834/7170) were diagnosed with COPD. The multivariable adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for COPD were 0.96 (95% CI: 0.77–1.19, p = 0.706), 0.78 (95% CI: 0.62–0.99, p = 0.038), and 0.76 (95% CI: 0.57–1.00, p = 0.047), respectively, when comparing the second, third, and fourth quartiles of niacin intake levels to the lowest quartile (p for trend = 0.017). An inverse association was observed between the occurrence of COPD and dietary niacin intake (nonlinear: p = 0.347). Stratified analyses revealed no significant differences or interactions.
Conclusion
Our findings suggest a potential link between increased dietary niacin intake and a decreased prevalence of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) encompasses a group of lung disorders marked by enduring respiratory symptoms and airflow limitation [1]. In the United States (US), COPD stands as the leading cause of disability and the third leading cause of death, imposing a substantial burden on clinical and healthcare resources [2, 3]. The anticipated increase in the burden of COPD in the coming decades, attributed to chronic exposure to risk factors and demographic shifts [4, 5]. As such, exploring avenues for the prevention and management of COPD is paramount. Beyond the established preventive measures like cessation of smoking and minimizing exposure to smoke, dietary factors are increasingly acknowledged for their potential influence in the onset and progression of chronic ailments such as COPD [6]. A diet high in vegetables, fruit, and fish is associated with a lower prevalence of COPD, while coffee, and vitamin D intake deficiency is associated with a higher prevalence of COPD [7–9]. As observed in various studies, vitamin supplementation could play a significant role in the management of COPD [10–13].
Niacin, or vitamin B3, is a precursor to nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), which are integral to cellular redox reactions and energy metabolism [14, 15]. Niacin supplementation has demonstrated benefits in experimental models related to cancer, cardiovascular disease, mental health, and oxidant lung injury [14] which are relevant to the systemic manifestations of COPD. Niacin’s role in inflammation and oxidative stress pathways is well-documented, and these pathways are known to be dysregulated in COPD [16]. A recent observational study investigated the relationship between dietary niacin intake and the COPD in the adult population of the United States [17]. Nonetheless, the specific link between niacin intake and COPD prevalence in people older than 40 years has not been extensively explored.
Therefore, the primary aim of this cross-sectional study was to investigate the association between dietary niacin intake and the prevalence of COPD among American middle-aged and older individuals. This study may provide insights for future COPD prevention and treatment strategies.
Materials and methods
Data sources
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning the years 2013 to 2018. NHANES is a de-identified and publicly available dataset, participants are selected through a stratified, multistage, probability sampling design. The survey involves home visits, screenings, and laboratory testing conducted by a mobile examination center (MEC). Detailed data can publicly accessible at https://www.cdc.gov/nchs/nhanes/index.htm.
Approval for all procedures in the survey was granted by the National Center for Health Statistics (NCHS) Research Ethics Review Board (Continuation of Protocol #201117), and written informed consent was obtained from all participants. Throughout data collection, we had no access to information that could identify individual participants. Additional Institutional Review Board approval was not required for the secondary analysis. Thus, The Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine agreed to strike the ethics of this study (No:2024–0006).
Study participants
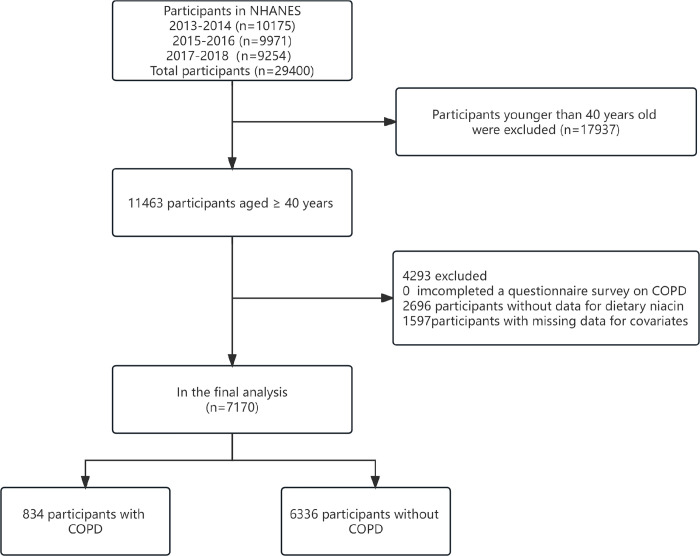
In this study, we used publicly available NHANES data generated from surveys conducted in 2013–2014, 2015–2016, and 2017–2018, in which a total of 29,400 people provided demographic data. We excluded individuals age < 40 years (n = 17937), missing data on COPD (n = 0), missing data on dietary niacin intake (n = 2696), we further excluded individuals missing data on covariates(n = 1597), which including family income (n = 831), body mass index (n = 87), education level (n = 4), marital status (n = 1), serum cotinine (n = 289), results of the questionnaire in hypertension (n = 8), high cholesterol (n = 63), diabetes (n = 280), coronary heart disease (n = 25), stroke (n = 6), cancer (n = 3). Ultimately, 7170 participants were included in the further analyses. The detailed inclusion and exclusion process is shown in Fig 1.
Fig 1. Flow diagram of study participants.
Abbreviations: NHANES, National Health and Nutrition Examination Survey; COPD, chronic obstructive pulmonary disease.
Measurement of dietary niacin intake
The dietary interview component, known as What We Eat in America (WWEIA), is a collaborative effort between the US Department of Agriculture (USDA) and the US Department of Health and Human Services (DHHS). It involves administering two 24-hour dietary recall interviews. The first dietary recall is conducted face-to-face at the Mobile Examination Center (MEC), while the second recall is carried out via a telephone interview approximately 3 to 10 days after the initial recall.
To calculate the nutrients and food components in all reported food items, the USDA’s Food and Nutrient Database for Dietary Studies (FNDDS) was employed. The dataset on total nutrient intakes provided a summary record of nutrient intake for each individual. Nutrients obtained from dietary supplements or medications were excluded from the nutrient estimates. This approach ensured a comprehensive and accurate assessment of dietary niacin intake from natural food sources without the confounding influence of supplemental sources. In this study, the daily niacin intake of participants was determined by calculating the average of their two dietary recalls.
COPD outcomes
The COPD outcome in this study was defined as a composite of three self-reported COPD conditions: emphysema, chronic bronchitis, and COPD itself [18–20]. A participant was categorized as having COPD if they responded "yes" to any of the three questions during the standardized medical condition questionnaire administered in the personal interview. The questions included, "Have you ever been told that you have emphysema/chronic bronchitis/chronic obstructive pulmonary disease?"
Assessment of covariates
Various potential covariates were included based on the published literatures [21–23]. We included demographic data, lifestyle, body measurements, chronic diseases, and laboratory examinations. The present study included the following demographic covariates: age, sex (female, male), race (Mexican American, non-Hispanic white, non-Hispanic black, others), education level (<high school, high school, >high school) [24], marital status (married, living with a partner, living alone), and family income. The poverty income ratio (PIR) categorized family income into three groups: low (PIR ≤ 1.3), medium (1.3 < PIR ≤ 3.5), and high (PIR > 3.5) [25]. In lifestyle, smoking status were categorized as never smoked (smoked less than 100 cigarettes in their lives), former smoker (quit smoking after smoking more than 100 cigarettes), or current smoker [26]. Recreational physical activities were categorized as sedentary, moderate, vigorous (vigorous recreational activities were defined as yes to the question: do you do any vigorous-intensity sports, fitness, or recreational activities that cause a small increase in breathing or heart rate) [22]. Body measurements included body mass index (BMI), which was calculated by dividing weight (kg) by height in square meters, and it was categorized as less than 25, 25 to 30, and more than 30kg/m2 [27]. Chronic diseases include hypertension, high cholesterol diabetes, stroke, coronary heart disease, and cancer, defined as yes or no. Information regarding total energy intake was acquired through the 24-hour dietary recall. laboratory examinations included serum cotinine(ng/mL).
Statistical analysis
Categorical variables were presented as absolute values (n) or percentages (%), while continuous variables were described using the mean ± standard deviation (mean ± SD) or median (interquartile range, IQR). Group differences were assessed through one-way analyses of variance for normal distribution, Kruskal–Wallis tests for skewed distribution, and chi-square tests for categorical variables.
Univariate logistic regression analyses were employed to explore risk factors for COPD prevalence. Multivariate logistic regression models were utilized to determine odds ratios (OR) and 95 percent confidence intervals (95% CIs) regarding the relationship between dietary niacin intake and COPD. The crude model was unadjusted. Model 1 incorporated adjustments for age, sex, and race/ethnicity. Model 2 further adjusted for family income, physical activity, smoking status, education level, marital status, body mass index, serum cotinine, and total energy. Model 3 represented a fully adjusted model, including sex, age, race/ethnicity, family income, physical activity, smoking status, education level, marital status, body mass index, serum cotinine, total energy, hypertension, high cholesterol, diabetes, coronary heart disease, stroke, and cancer.
Restricted cubic spline analysis examined nonlinear associations between dietary niacin intake and COPD, adjusting for all potential confounding variables in model 3, with four knots positioned at the 5th, 35th, 65th, and 95th percentiles.
Interaction and subgroup analyses were conducted to explore potential confounding factors influencing the association between niacin intake and COPD. Subgroup analyses were stratified by sex (female or male), age (<65 or ≥65 years), smoking status (never, former, current), hypertension (yes or no), and diabetes (yes or no).
To assess the robustness of the results, we performed several sensitivity analyses. To assess the impact of the outlier on our findings, we excluded participants with extreme energy intake (<500 or >5000 kcal per day) to mitigate the influence of potential outliers(25, 27). We also excluded individuals whose niacin intake exceeded the sample population mean ± 3 standard deviations (SD).
Furthermore, we employed multiple imputation (MI) methods by chained equations method to address the missing data, followed by a multifactor regression analysis of the imputed dataset. Finally, given that the NHANES database utilizes a complex multi-stage sampling methodology, we performed a weighted regression analysis to test the robustness of our main findings. The sampling weights for 2013–2018 were calculated as follows: 1/3 × WTDR2D.
All statistical analyses were performed using R software (version 4.2.2) and Free Statistics software version 1.9 [28]. Two-sided p < 0.05 was considered for statistical significance.
Results
Baseline characteristics of participants
The baseline characteristics of 7,170 subjects according to their niacin intake quartiles were shown in Table 1. The COPD adults accounted for 11.6% (834/7170). The mean age of the population in this study was 59.5 ± 11.5 years, males constituted 47.5% of the study population, and 43.3% were White. Higher niacin intake is likely to be younger age, male gender, marital status of being married, never smokers, have higher education, have higher body mass index. Additionally, these individuals were less likely to report histories of coronary heart disease, stroke, hypertension, diabetes, or cancer. The baseline characteristics of participants were also summarized in S1 Table according to whether they have COPD. Members in COPD group were more likely older (62.9 ± 11.5 years vs 59.1 ± 11.9 years, p< 0.001), and had a lower average daily niacin intake (22.2 ± 11.2mg/d vs 24.0 ± 11.7 mg/d, p< 0.001).
Table 1. Population characteristics by quartiles of dietary niacin intake.
| Characteristic | Total (n = 7170) | Niacin Intake, mg/d | p | |||
|---|---|---|---|---|---|---|
| Q1(≤16.08) (n = 1793) |
Q2(16.09–21.84) (n = 1792) |
Q3(21.85–28.97) (n = 1792) |
Q4 (≥28.98) (n = 1793) |
|||
| Sex, n (%) | < 0.001 | |||||
| Male | 3407 (47.5) | 487 (27.2) | 687 (38.3) | 917 (51.2) | 1316 (73.4) | |
| Female | 3763 (52.5) | 1306 (72.8) | 1105 (61.7) | 875 (48.8) | 477 (26.6) | |
| Age, years, Mean ± SD | 59.5 ± 11.9 | 61.4 ± 11.8 | 60.5 ± 12.1 | 59.5 ± 12.0 | 56.6 ± 11.2 | < 0.001 |
| Physical activity, n (%) | < 0.001 | |||||
| Sedentary | 3900 (54.4) | 1067 (59.5) | 978 (54.6) | 936 (52.2) | 919 (51.3) | |
| Moderate | 2048 (28.6) | 516 (28.8) | 522 (29.1) | 549 (30.6) | 461 (25.7) | |
| Vigorous | 1222 (17.0) | 210 (11.7) | 292 (16.3) | 307 (17.1) | 413 (23.0) | |
| Smoking status, n (%) | < 0.001 | |||||
| Never | 3817 (53.2) | 992 (55.3) | 1012 (56.5) | 953 (53.2) | 860 (48.0) | |
| Former | 2145 (29.9) | 492 (27.4) | 515 (28.7) | 539 (30.1) | 599 (33.4) | |
| Current | 1208 (16.8) | 309 (17.2) | 265 (14.8) | 300 (16.7) | 334 (18.6) | |
| Race/ethnicity, n (%) | < 0.001 | |||||
| Mexican American | 926 (12.9) | 231 (12.9) | 234 (13.1) | 222 (12.4) | 239 (13.3) | |
| Non-Hispanic white | 3106 (43.3) | 700 (39.0) | 787 (43.9) | 807 (45.0) | 812 (45.3) | |
| Non-Hispanic black | 1501 (20.9) | 444 (24.8) | 380 (21.2) | 349 (19.5) | 328 (18.3) | |
| Others | 1637 (22.8) | 418 (23.3) | 391 (21.8) | 414 (23.1) | 414 (23.1) | |
| Education level, n (%) | < 0.001 | |||||
| Less than high school | 1402 (19.6) | 443 (24.7) | 353 (19.7) | 314 (17.5) | 292 (16.3) | |
| High school | 1670 (23.3) | 435 (24.3) | 413 (23.0) | 430 (24.0) | 392 (21.9) | |
| More than high school | 4098 (57.2) | 915 (51) | 1026 (57.3) | 1048 (58.5) | 1109 (61.9) | |
| Marital status, n (%) | < 0.001 | |||||
| Married | 4211 (58.7) | 949 (52.9) | 1036 (57.8) | 1110 (61.9) | 1116 (62.2) | |
| living with a partner | 331 (4.6) | 68 (3.8) | 82 (4.6) | 76 (4.2) | 105 (5.9) | |
| Living alone | 2628 (36.7) | 776 (43.3) | 674 (37.6) | 606 (33.8) | 572 (31.9) | |
| Family income, n (%) | < 0.001 | |||||
| Low (PIR<1.3) | 1970 (27.5) | 600 (33.5) | 464 (25.9) | 470 (26.2) | 436 (24.3) | |
| Medium (1.3≤PIR<3.5) | 2802 (39.1) | 714 (39.8) | 707 (39.5) | 696 (38.8) | 685 (38.2) | |
| High (PIR≥3.5) | 2398 (33.4) | 479 (26.7) | 621 (34.7) | 626 (34.9) | 672 (37.5) | |
| Body mass index, n (%) | 0.299 | |||||
| <25 kg/m2 | 1694 (23.6) | 406 (22.6) | 453 (25.3) | 408 (22.8) | 427 (23.8) | |
| 25 to <30 kg/m2 | 2432 (33.9) | 594 (33.1) | 593 (33.1) | 617 (34.4) | 628 (35) | |
| ≥30 kg/m2 | 3044 (42.5) | 793 (44.2) | 746 (41.6) | 767 (42.8) | 738 (41.2) | |
| Total energy, Kcal, Mean ± SD | 2028.3 ± 921.1 | 1352.2 ± 549.2 | 1813.0 ± 627.3 | 2142.0 ± 671.5 | 2806.0 ± 1061.6 | < 0.001 |
| Serum cotinine, ng/mL, Median (IQR) | 0.03 (0.01, 0.51) | 0.03(0.01,0.56) | 0.02 (0.01, 0.29) | 0.02 (0.01, 0.30) | 0.03 (0.01,1.97) | < 0.001 |
| Hypertension, n (%) | 3465 (48.3) | 926 (51.6) | 883 (49.3) | 876 (48.9) | 780 (43.5) | < 0.001 |
| High cholesterol, n (%) | 3391 (47.3) | 840 (46.8) | 849 (47.4) | 884 (49.3) | 818 (45.6) | 0.162 |
| Diabetes, n (%) | 1470 (20.5) | 420 (23.4) | 346 (19.3) | 365 (20.4) | 339 (18.9) | 0.003 |
| Coronary heart disease, n (%) | 466 (6.5) | 126 (7) | 122 (6.8) | 111 (6.2) | 107 (6) | 0.53 |
| Stroke, n (%) | 389 (5.4) | 134 (7.5) | 92 (5.1) | 87 (4.9) | 76 (4.2) | < 0.001 |
| Cancer, n (%) | 1013 (14.1) | 258 (14.4) | 291 (16.2) | 238 (13.3) | 226 (12.6) | 0.011 |
| Niacin Intake, mg/d, Mean ± SD | 23.8 ± 11.6 | 12.1 ± 2.9 | 19.0 ± 1.6 | 25.2 ± 2.0 | 38.9 ± 11.6 | < 0.001 |
| COPD, n (%) | 834 (11.6) | 254 (14.2) | 221 (12.3) | 189 (10.5) | 170 (9.5) | < 0.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; PIR, family poverty income ratio; Q1–Q4, quartiles based on dietary niacin intake.
Univariate analyses
Table 2 displays the results of the univariate analysis, indicating significant associations between COPD and various factors including age, gender, smoking habits, race/ethnicity, marital status, economic status, level of physical activity, and history of hypertension, diabetes, stroke, and cancer.
Table 2. Association of covariates and COPD risk.
| Variable | OR_95CI | p-Value |
|---|---|---|
| Sex, n (%) | ||
| Male | 1 (reference) | |
| Female | 1.23 (1.07~1.43) | 0.005 |
| Age, years | 1.03 (1.02~1.03) | <0.001 |
| Physical activity, n (%) | ||
| Sedentary | 1 (reference) | |
| Moderate | 0.6 (0.5~0.71) | <0.001 |
| Vigorous | 0.31 (0.23~0.4) | <0.001 |
| Smoking status, n (%) | ||
| Never | 1 (reference) | |
| Former | 2.81 (2.35~3.36) | <0.001 |
| Current | 4.71 (3.89~5.68) | <0.001 |
| Race/ethnicity, n (%) | ||
| Mexican American | 1 (reference) | |
| Non-Hispanic white | 3.35 (2.49~4.52) | <0.001 |
| Non-Hispanic black | 1.81 (1.3~2.51) | <0.001 |
| Others | 1.5 (1.08~2.1) | 0.016 |
| Education level, n (%) | ||
| Less than high school | 1 (reference) | |
| High school | 1.02 (0.83~1.25) | 0.837 |
| More than high school | 0.66 (0.55~0.79) | <0.001 |
| Marital status, n (%) | ||
| Married | 1 (reference) | |
| living with a partner | 1.32 (0.93~1.87) | 0.12 |
| Living alone | 1.82 (1.57~2.11) | <0.001 |
| Family income, n (%) | ||
| Low (PIR<1.3) | 1 (reference) | |
| Medium (1.3≤PIR<3.5) | 0.61 (0.52~0.72) | <0.001 |
| High (PIR≥3.5) | 0.31 (0.25~0.38) | <0.001 |
| Body mass index, n (%) | ||
| <25 kg/m2 | 1 (reference) | |
| 25 to <30 kg/m2 | 0.83 (0.68~1.02) | 0.083 |
| ≥30 kg/m2 | 1.4 (1.17~1.69) | <0.001 |
| Total energy, kcal, | 1 (1~1) | 0.041 |
| Serum cotinine | 1 (1~1) | <0.001 |
| Niacin Intake, mg/d | 0.99 (0.98~0.99) | <0.001 |
| Hypertension (Yes vs No) | 1.73 (1.49~2.01) | <0.001 |
| High cholesterol (Yes vs No) | 1.5 (1.3~1.74) | <0.001 |
| Diabetes (Yes vs No) | 1.72 (1.47~2.03) | <0.001 |
| Coronary heart disease (Yes vs No) | 3.94 (3.19~4.87) | <0.001 |
| Stroke (Yes vs No) | 2.29 (1.78~2.94) | <0.001 |
| Cancer (Yes vs No) | 1.83 (1.53~2.2) | <0.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; PIR, family poverty income ratio; Ref: reference.
Associations between dietary niacin intake and COPD
We conducted four logistical regression models (crude model, model 1–3) to explore the association between dietary niacin intake and COPD. As presented in Table 3. In comparison to individuals with lower dietary niacin intake in Q1 (≤16.08 mg/day), the adjusted odds ratios (OR) for COPD in Q2 (16.09–21.84 mg/day), Q3 (21.85–28.97 mg/day), and Q4 (≥28.98 mg/day) were 0.96 (95% CI: 0.77–1.19, p = 0.706), 0.78 (95% CI: 0.62–0.99, p = 0.038), and 0.76 (95% CI: 0.57–1.00, p = 0.047), respectively.
Table 3. Multivariate logistic regression analysis of dietary niacin for risk of COPD.
| Variable | n. event (%) | Crude model | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | ||
| Quartile | |||||||||
| Q1 (≤16.08) | 254 (14.2) | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) | ||||
| Q2(16.09–21.84) | 221 (12.3) | 0.85 (0.7~1.03) | 0.106 | 0.85 (0.7~1.03) | 0.102 | 0.97 (0.78~1.19) | 0.742 | 0.96 (0.77~1.19) | 0.706 |
| Q3(21.85–28.97) | 189 (10.5) | 0.71(0.58~0.8) | 0.001 | 0.73 (0.59~0.9) | 0.003 | 0.79 (0.63~0.99) | 0.041 | 0.78 (0.62~0.99) | 0.038 |
| Q4 (≥28.98) | 170 (9.5) | 0.63(0.52~0.7) | <0.001 | 0.72 (0.57~0.9) | 0.004 | 0.76 (0.58~1.00) | 0.05 | 0.76 (0.57~1.00) | 0.047 |
| P for trend | <0.001 | 0.001 | 0.018 | 0.017 | |||||
Abbreviations: COPD, chronic obstructive pulmonary disease; Q, quartiles; OR, odds ratio; CI, confidence interval; Ref: reference.
The crude model was not adjusted for covariates.
Model I was adjusted for sex, age, race/ethnicity.
Model2 was adjusted for sex, age, race/ethnicity, family income, physical activity, smoking status, education level, marital status, body mass index, serum cotinine, total energy.
Model 3 was adjusted for sex, age, race/ethnicity, family income, physical activity, smoking status, education level, marital status, body mass index, serum cotinine, total energy, hypertension, high cholesterol, diabetes, coronary heart disease, stroke, cancer.
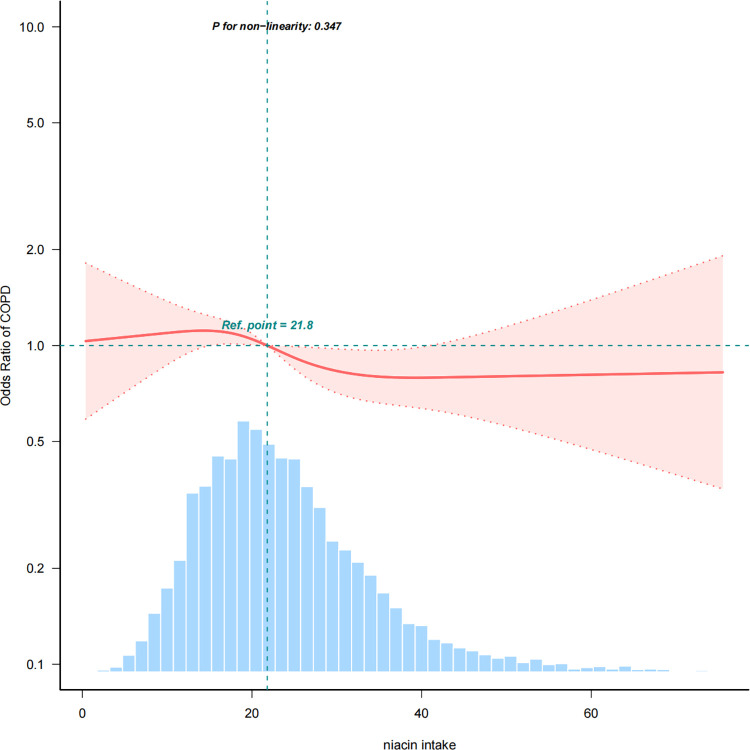
The restricted cubic spline analysis illustrated a negative association between dietary niacin intake and COPD incidence, considering all potential confounders (non-linearity: p = 0.347). Fig 2 provides a visual representation of the association between dietary niacin intake and COPD.
Fig 2. Association between dietary niacin intake and COPD odds ratio.
Solid and dashed lines represent the predicted value and 95% confidence intervals. They were adjusted for sex, age, race/ethnicity, family income, physical activity, smoking status, education level, marital status, body mass index, Serum cotinine, total energy, hypertension, high cholesterol, diabetes, coronary heart disease, stroke, cancer. only 99.5% of the data is shown.
Subgroup analysis
In this study, we performed analyses on subgroups and interactions to determine the consistency of the association between dietary niacin intake and the prevalence of COPD. As depicted in Fig 3, the stratification by age, sex, smoking status, hypertension, and diabetes did not reveal significant interactions, underscoring a stable inverse relationship between niacin intake and COPD risk across different subgroups (P for interaction > 0.05).
Fig 3. The relationship between dietary niacin intake and COPD stratified using different characteristics.
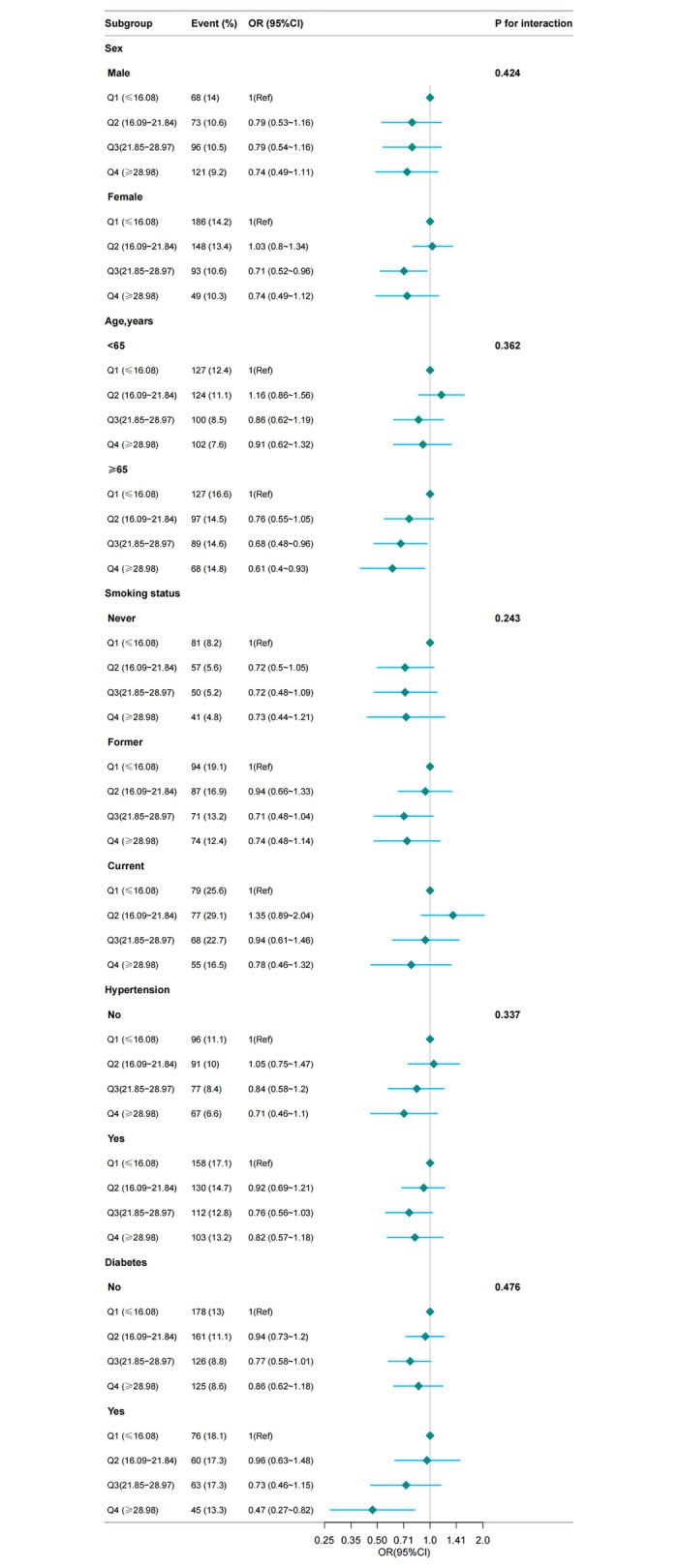
Except for the stratification component itself, each stratification factor was adjusted for all other variables (sex, age, race/ethnicity, family income, physical activity, smoking status, education level, marital status, body mass index, Serum cotinine, total energy, hypertension, high cholesterol, diabetes, coronary heart disease, stroke, cancer.).Abbreviations: COPD, chronic obstructive pulmonary disease; OR, odds ratio; 95% CI, 95% confidence interval.
Sensitivity analysis
Firstly, we excluded participants with extreme energy intake (<500 or >5000 kcal per day), the study cohort comprised 7,040 individuals, and the association between dietary niacin intake and COPD remained consistent. In comparison to individuals with lower niacin consumption in Q1 (≤16.14 mg/day), the adjusted odds ratios (OR) for COPD in Q3 (21.84–28.82 mg/day) and Q4 (≥28.83 mg/day) were 0.77 (95% CI: 0.61–0.98, p = 0.033) and 0.75 (95% CI: 0.57–0.99, p = 0.043) (S2 Table), respectively. We also excluded individuals whose niacin intake exceeded the sample population mean ± 3 standard deviations (SD) (S3 Table), the association between dietary niacin intake and COPD in 7057 individuals remained consistent.
Further validation through multiple imputation for missing covariates followed by multifactor regression analysis confirmed the robustness of these findings. This analysis, illustrated in S4 Table, maintained the inverse relationship between higher dietary niacin levels and decreased COPD prevalence.
As shown in S5 Table, the results of the weighted analyses were similar to the unweighted analyses, which confirmed the stability of the inverse association between dietary niacin intake and COPD, even after accounting for the complex sampling design.
Discussion
The present cross-sectional study, encompassing an analysis of 7,170 participants, has delineated an inverse association between dietary niacin intake and the prevalence of COPD, particularly among American middle-aged and older adults. The subgroup analyses and sensitivity analyses further underscored the robustness of this association. The insights from our study suggest that increasing dietary niacin may be a valuable strategy for families looking to manage COPD risk among their members.
Emerging evidence increasingly emphasizes the impact of diet on obstructive pulmonary disease [5, 29]. Adopting a diet abundant in vitamins, omega-3 fatty acids, fiber, fruits, and vegetables may contribute to a potential reduction in COPD prevalence [30–32]. Despite this, studies exploring the effects of dietary niacin intake on COPD remain scarce. Our study contributes to this evidence base by specifically highlighting the modulatory role of dietary niacin, a B vitamin with established functions in lipid regulation, anti-inflammatory, and antioxidative activities. The observed lower risk of COPD in individuals with higher niacin intake (OR: 0.76; 95% CI: 0.57~1) after adjustment for confounders provides preliminary support for the potential of dietary niacin in COPD prevention and management.
Our study had similar results to a recently published observational study [17], the difference is that previous study population was US adults and we were middle-aged and older adults over the age of 40 years; in addition, previous studies of niacin intake used only one 24-hour recall data, which may have had some effect on the stability of the results, whereas we averaged over the collection of two 24-hour recalls to minimize memory bias. In addition, we adjusted for more confounding and performed some sensitivity analyses to verify the stability of the results. Future prospective studies are warranted to delve deeper into the impact of dietary niacin intake on COPD.
Niacin, a member of the B vitamins group and a precursor of nicotinamide adenine dinucleotide (NAD+), has gained recognition for its use as a lipid-regulating medication to mitigate atherosclerosis [33]. Furthermore, multiple studies have delved into its anti-inflammatory and antioxidative properties [34, 35]. Niacin protects lung function impairment through the activation of SIRT1 and reduced pulmonary oxidative stress and lung inflammation [36]. A study indicated that niacin, vitamins A and D, and dietary fiber associated with on average better FEV1 in chronic smokers [37]. A high dose of niacin attenuated lung inflammation, suppressed proinflammatory cytokine release, reduced histologic lung damage, and improved survival [38]. While the exact molecular mechanism of the association between dietary niacin intake and the risk of COPD requires further investigation.
This study possesses several strengths. Firstly, it was a large-scale investigation examining the association between dietary niacin intake and the prevalence of COPD in the US middle-aged and older individuals. Secondly, stringent screening of measurement methods and protocols was conducted within the NHANES database. Thirdly, adjustments for potential confounders were made, ensuring robust and stable conclusions across different subgroups. Additionally, to assess the robustness of the results, we performed several sensitivity analyses.
Nevertheless, there are certain limitations to our research. Firstly, the NHANES population consists of Americans and may not be representative of other populations. Secondly, due to the cross-sectional study design of NHANES, establishing a causal relationship between dietary niacin intake and COPD is not feasible. Thirdly, the assessment of 24-hour dietary intake may not precisely reflect an individual participant’s lifetime diet. However, 24-hour dietary recall is widely acknowledged as the most suitable method for estimating the average intake of a population [39]. Additionally, we mitigated potential errors by averaging two 24-hour diet recall interviews. Fourthly, the diagnosis of COPD relies on self-reporting, introducing the possibility of recall bias.
Conclusion
This study established a reverse association between dietary niacin intake and COPD in the American population middle-aged and older individuals. Our results suggest that niacin may be a significant nutrient influencing COPD. Further prospective studies are necessary to validate and corroborate our findings.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We gratefully thank Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for his contribution to advise manuscript.
Data Availability
The data underlying the results presented in our study are from the National Health and Nutrition Examination Survey (NHANES), a public data repository. The website for accessing the data is: https://www.cdc.gov/nchs/nhanes/index.htm.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet (London, England). 2022;399(10342):2227–42. doi: 10.1016/S0140-6736(22)00470-6 [DOI] [PubMed] [Google Scholar]
- 2.Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. The Lancet Respiratory medicine. 2022;10(5):447–58. doi: 10.1016/S2213-2600(21)00511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahnert K, Jörres RA, Behr J, Welte T. The Diagnosis and Treatment of COPD and Its Comorbidities. Deutsches Arzteblatt international. 2023;120(25):434–44. doi: 10.3238/arztebl.m2023.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fazleen A, Wilkinson T. Early COPD: current evidence for diagnosis and management. Therapeutic advances in respiratory disease. 2020;14:1753466620942128. doi: 10.1177/1753466620942128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scoditti E, Massaro M, Garbarino S, Toraldo DM. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients. 2019;11(6). doi: 10.3390/nu11061357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ (Clinical research ed). 2018;361:k2396. doi: 10.1136/bmj.k2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaluza J, Harris H, Wallin A, Linden A, Wolk A. Dietary Fiber Intake and Risk of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study of Men. Epidemiology (Cambridge, Mass). 2018;29(2):254–60. doi: 10.1097/EDE.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 8.Hanson C, Rutten EP, Wouters EF, Rennard S. Diet and vitamin D as risk factors for lung impairment and COPD. Translational research: the journal of laboratory and clinical medicine. 2013;162(4):219–36. doi: 10.1016/j.trsl.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 9.Kaluza J, Larsson SC, Orsini N, Linden A, Wolk A. Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax. 2017;72(6):500–9. doi: 10.1136/thoraxjnl-2015-207851 [DOI] [PubMed] [Google Scholar]
- 10.Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019;74(4):337–45. doi: 10.1136/thoraxjnl-2018-212092 [DOI] [PubMed] [Google Scholar]
- 11.van Iersel LEJ, Beijers R, Gosker HR, Schols A. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: a systematic review. Nutrition reviews. 2022;80(6):1434–44. doi: 10.1093/nutrit/nuab077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piscaer I, Wouters EFM, Vermeer C, Janssens W, Franssen FME, Janssen R. Vitamin K deficiency: the linking pin between COPD and cardiovascular diseases? Respiratory research. 2017;18(1):189. doi: 10.1186/s12931-017-0673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim T, Oak CH, Jung MH, Jang TW, Kim J. High Serum Folate Concentration Is Associated with Better Lung Function in Male Chronic Obstructive Pulmonary Disease Patients Who Are Current Smokers: Analysis of Nationwide Population-Based Survey. Nutrients. 2020;12(8). doi: 10.3390/nu12082219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer-Ficca M, Kirkland JB. Niacin. Advances in nutrition (Bethesda, Md). 2016;7(3):556–8. doi: 10.3945/an.115.011239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revuelta JL, Buey RM, Ledesma-Amaro R, Vandamme EJ. Microbial biotechnology for the synthesis of (pro)vitamins, biopigments and antioxidants: challenges and opportunities. Microbial biotechnology. 2016;9(5):564–7. doi: 10.1111/1751-7915.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox biology. 2020;33:101544. doi: 10.1016/j.redox.2020.101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li WW, Ren KL, Yu J, Guo HS, Liu BH, Sun Y. Association of dietary niacin intake with the prevalence and incidence of chronic obstructive pulmonary disease. Scientific reports. 2024;14(1):2863. doi: 10.1038/s41598-024-53387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013;187(3):228–37. doi: 10.1164/rccm.201210-1843CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. International journal of chronic obstructive pulmonary disease. 2015;10:995–1013. doi: 10.2147/COPD.S82518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konietzke P, Wielpütz MO, Wagner WL, Wuennemann F, Kauczor HU, Heussel CP, et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. European radiology. 2020;30(5):2502–12. doi: 10.1007/s00330-019-06577-y [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Wang Q, Dong Z, Yu S. Dietary zinc intake and migraine in adults: a cross-sectional analysis of the National Health and Nutrition Examination Survey 1999–2004. Headache. 2023;63(1):127–35. doi: 10.1111/head.14431 [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Guo S, Yang J, Wang Q, Lu X. Association between niacin intake and depression: A nationwide cross-sectional study. Journal of affective disorders. 2023;340:347–54. doi: 10.1016/j.jad.2023.08.053 [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Li S, Wan J, Nan W, He B. Association between ethylene oxide exposure and prevalence of COPD: Evidence from NHANES 2013–2016. The Science of the total environment. 2023;885:163871. doi: 10.1016/j.scitotenv.2023.163871 [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Mao Y, Lu M, Liu X, Sun Y, Li Z, et al. Association between serum Klotho levels and the prevalence of diabetes among adults in the United States. Frontiers in endocrinology. 2022;13:1005553. doi: 10.3389/fendo.2022.1005553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Wang L, Chen C, Dong Z, Yu S. Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. Nutrients. 2022;14(15). doi: 10.3390/nu14153052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Ma X, Liang H, Li H, Chen J, Fang L, et al. Dietary Magnesium Intake Affects the Association Between Serum Vitamin D and Type 2 Diabetes: A Cross-Sectional Study. Frontiers in nutrition. 2021;8:763076. doi: 10.3389/fnut.2021.763076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian S, Wu L, Zheng H, Zhong X, Liu M, Yu X, et al. Dietary niacin intake in relation to depression among adults: a population-based study. BMC psychiatry. 2023;23(1):678. doi: 10.1186/s12888-023-05188-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, Zheng J, Chen W, Chen X, Wen D, Chen W, et al. Association Between Preadmission Metformin Use and Outcomes in Intensive Care Unit Patients With Sepsis and Type 2 Diabetes: A Cohort Study. Frontiers in medicine. 2021;8:640785. doi: 10.3389/fmed.2021.640785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthon BS, Wood LG. Nutrition and respiratory health—feature review. Nutrients. 2015;7(3):1618–43. doi: 10.3390/nu7031618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varraso R, Willett WC, Camargo CA Jr. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. American journal of epidemiology. 2010;171(7):776–84. doi: 10.1093/aje/kwp455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celik F, Topcu F. Nutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokers. Clinical nutrition (Edinburgh, Scotland). 2006;25(6):955–61. doi: 10.1016/j.clnu.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 32.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–91. doi: 10.1136/thx.2006.074534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyers CD, Kamanna VS, Kashyap ML. Niacin therapy in atherosclerosis. Current opinion in lipidology. 2004;15(6):659–65. doi: 10.1097/00041433-200412000-00006 [DOI] [PubMed] [Google Scholar]
- 34.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202(1):68–75. doi: 10.1016/j.atherosclerosis.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 35.Zhou E, Li Y, Yao M, Wei Z, Fu Y, Yang Z. Niacin attenuates the production of pro-inflammatory cytokines in LPS-induced mouse alveolar macrophages by HCA2 dependent mechanisms. International immunopharmacology. 2014;23(1):121–6. doi: 10.1016/j.intimp.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 36.Wu NC, Wang JJ. Niacin Pretreatment Attenuates Lung Ischemia and Reperfusion-Induced Pulmonary Barrier Function Impairment by Reducing Oxidative Stress and Activating SIRT1 in an Isolated-Perfused Rat Lung Model. Transplantation proceedings. 2018;50(9):2834–8. doi: 10.1016/j.transproceed.2018.04.047 [DOI] [PubMed] [Google Scholar]
- 37.Leng S, Picchi MA, Tesfaigzi Y, Wu G, Gauderman WJ, Xu F, et al. Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. International journal of chronic obstructive pulmonary disease. 2017;12:3171–81. doi: 10.2147/COPD.S142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong KY, Suh GJ, Kwon WY, Kim KS, Jung YS, Kye YC. The therapeutic effect and mechanism of niacin on acute lung injury in a rat model of hemorrhagic shock: Down-regulation of the reactive oxygen species-dependent nuclear factor κB pathway. The journal of trauma and acute care surgery. 2015;79(2):247–55. doi: 10.1097/ta.0000000000000761 [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Hur J, Nguyen LH, Liu J, Song M, Wu K, et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. Journal of the National Cancer Institute. 2021;113(5):543–52. doi: 10.1093/jnci/djaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The data underlying the results presented in our study are from the National Health and Nutrition Examination Survey (NHANES), a public data repository. The website for accessing the data is: https://www.cdc.gov/nchs/nhanes/index.htm.