Abstract
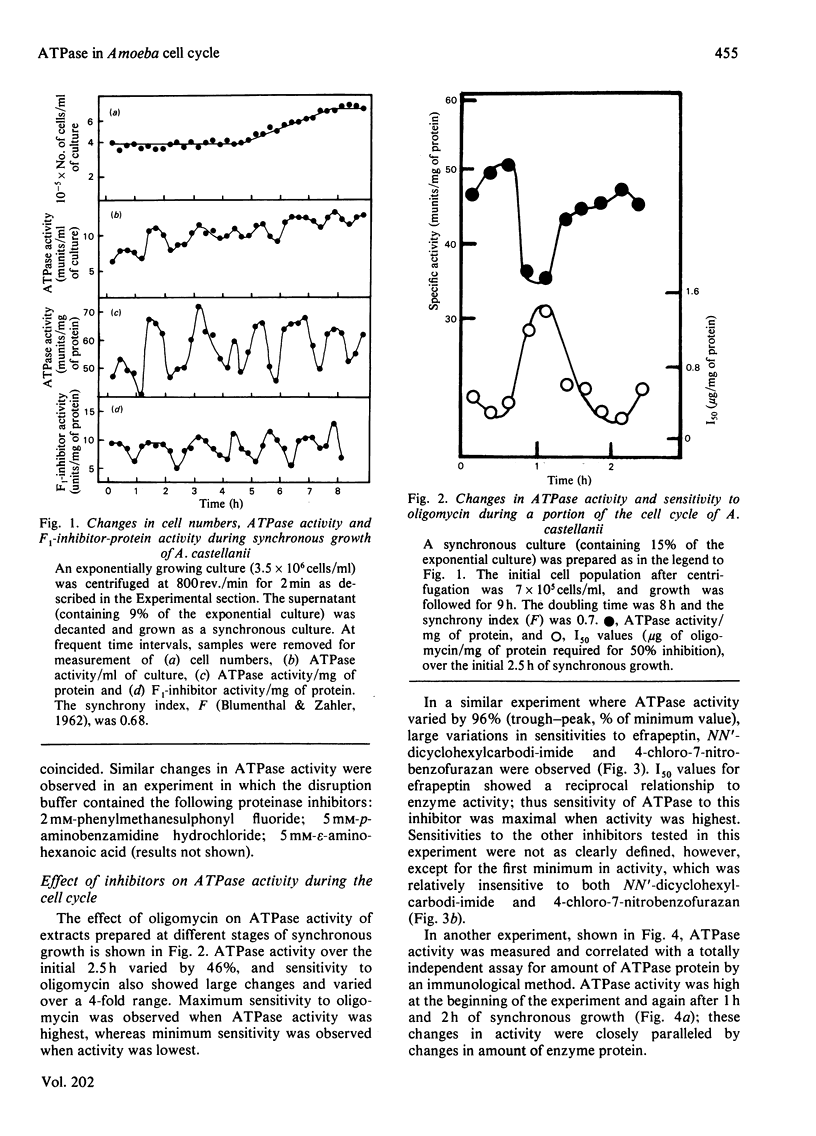
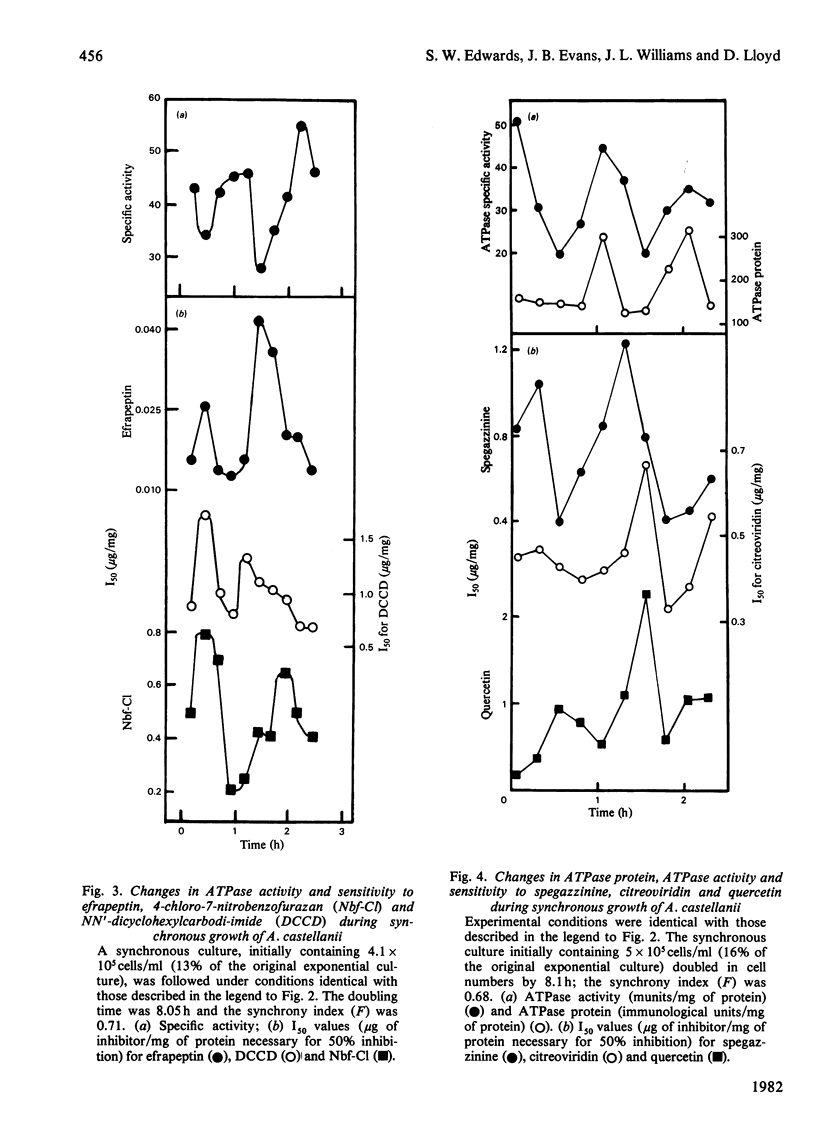
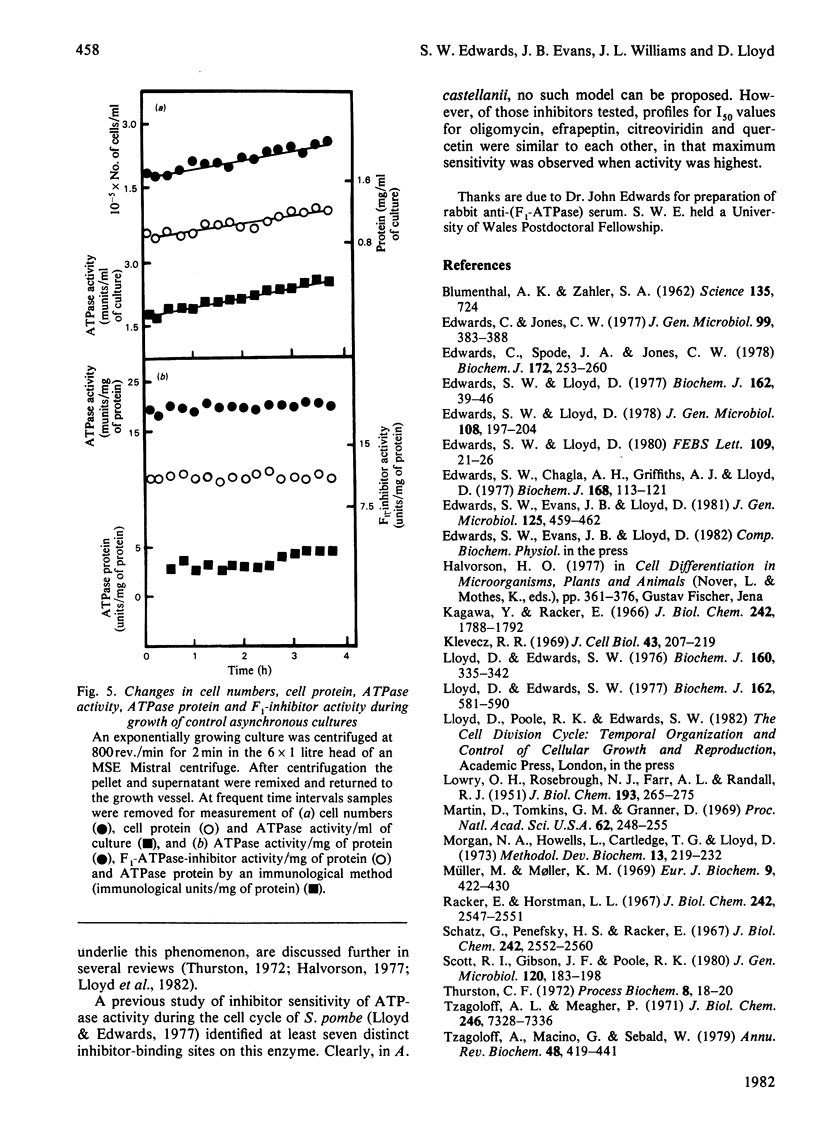
1. The mitochondrial ATPase of Acanthamoeba castellanii accumulated discontinuously in synchronous cultures prepared by a minimally perturbing size-selection technique. 2. Enzyme activity per ml of culture doubled overall during one cell cycle time of 8 h, but oscillated to give seven maxima during this period. Similar oscillations were observed in the specific activities of ATPase and of the naturally occurring inhibitor protein. 3. These variations in enzyme activity reflected changes in amount of enzyme protein as assayed by an immunological technique. 4. Large variations in I50 values (micrograms of inhibitor/mg of protein necessary for 50% inhibition of inhibitor-sensitive activity) for inhibition of ATPase activity by seven different inhibitors of energy conservation were observed. Activity was more sensitive to inhibition by oligomycin, efrapeptin, citreoviridin and quercetin when values were highest. 5. The results are discussed in relation to the phased organization of biosynthesis and degradation of cellular components known to occur during the cell cycle of this organization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL L. K., ZAHLER S. A. Index for measurement of synchronization of cell populations. Science. 1962 Mar 2;135(3505):724–724. doi: 10.1126/science.135.3505.724. [DOI] [PubMed] [Google Scholar]
- Edwards C., Spode J. A., Jones C. W. The properties of adenosine triphosphatase from exponential and synchronous cultures of Alcaligenes eutrophus H16. Biochem J. 1978 May 15;172(2):253–260. doi: 10.1042/bj1720253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Chagla A. H., Griffiths A. J., Lloyd D. The cytochromes of Acanthamoeba castellanii. Biochem J. 1977 Oct 15;168(1):113–121. doi: 10.1042/bj1680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Mitochondrial adenosine triphosphatase of the fission yeast, Schizosaccharomyces pombe 972h-. Changes in activity and oligomycin-sensitivity during the cell cycle of catabolite-repressed and -de-repressed cells. Biochem J. 1977 Jan 15;162(1):39–46. doi: 10.1042/bj1620039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Lloyd D. Oscillations in protein and RNA content during synchronous growth of Acanthamoeba castellanii. Evidence for periodic turnover of macromolecules during the cell cycle. FEBS Lett. 1980 Jan 1;109(1):21–26. doi: 10.1016/0014-5793(80)81302-0. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzine oxidative phosphorylation. XII. The H-2-18-O-inorganic phosphate and H-2-18-O-adenosine triphosphate exchange reactions in submitochondrial particles from beef heart. J Biol Chem. 1967 Apr 25;242(8):1788–1792. [PubMed] [Google Scholar]
- Klevecz R. R. Temporal order in mammalian cells. I. The periodic synthesis of lactate dehydrogenase in the cell cycle. J Cell Biol. 1969 Nov;43(2):207–219. doi: 10.1083/jcb.43.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd D., Edwards S. W. Mitochondrial adenosine triphosphatase of the fission yeast Schizosaccharomyces pombe 972h-. Changes in inhibitor sensitivities during the cell cycle indicate similarities and differences in binding sites. Biochem J. 1977 Mar 15;162(3):581–590. doi: 10.1042/bj1620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D., Edwards S. W. Mitochondrial adenosine triphosphatase of the fission yeast, Schizosaccharomyces pombe 972h-. Changes in activity and inhibitor-sensitivity in response to catabolite repression. Biochem J. 1976 Nov 15;160(2):335–342. doi: 10.1042/bj1600335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Jr, Tomkins G. M., Granner D. Synthesis and induction of tyrosine aminotransferase in synchronized hepatoma cells in culture. Proc Natl Acad Sci U S A. 1969 Jan;62(1):248–255. doi: 10.1073/pnas.62.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Horstman L. L. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem. 1967 May 25;242(10):2547–2551. [PubMed] [Google Scholar]
- Schatz G., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XIV. J Biol Chem. 1967 May 25;242(10):2552–2560. [PubMed] [Google Scholar]
- Scott R. I., Gibson J. F., Poole R. K. Adenosine triphosphatase activity and its sensitivity to ruthenium red oscillate during the cell cycle of Escherichia coli K12. J Gen Microbiol. 1980 Sep;120(1):183–198. doi: 10.1099/00221287-120-1-183. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Macino G., Sebald W. Mitochondrial genes and translation products. Annu Rev Biochem. 1979;48:419–441. doi: 10.1146/annurev.bi.48.070179.002223. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]


