Abstract
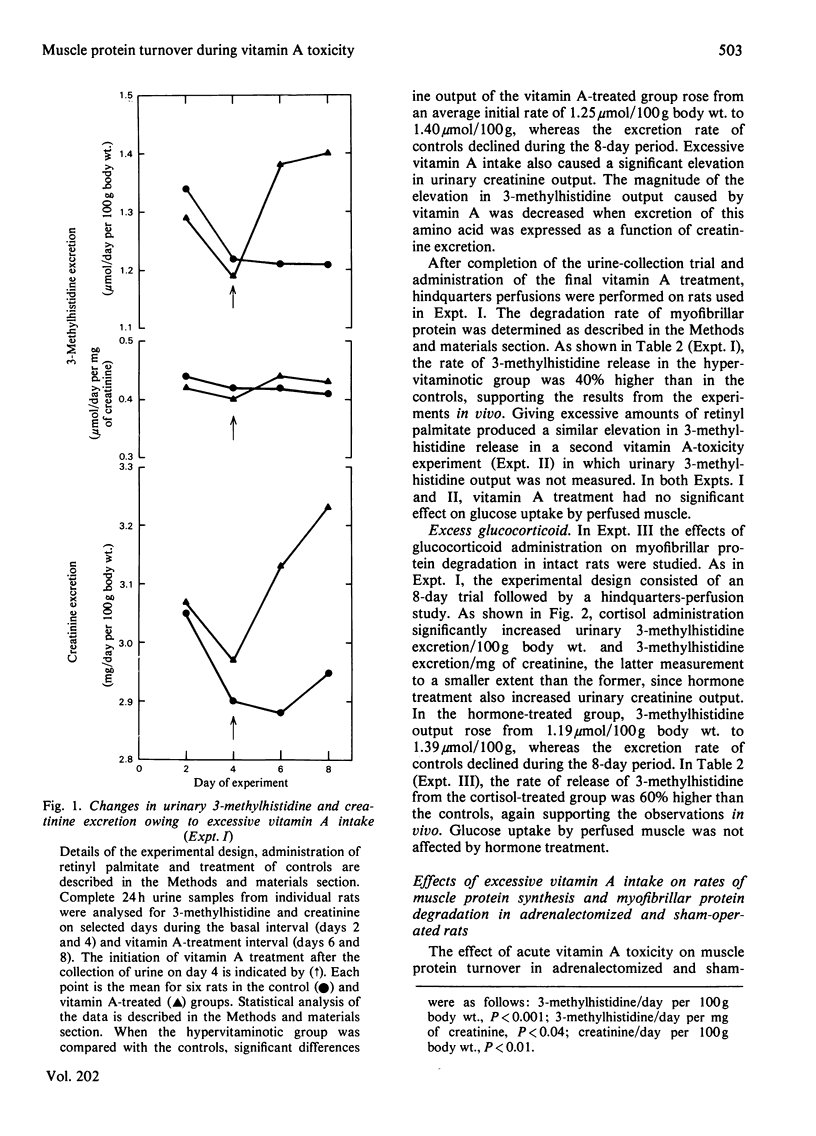
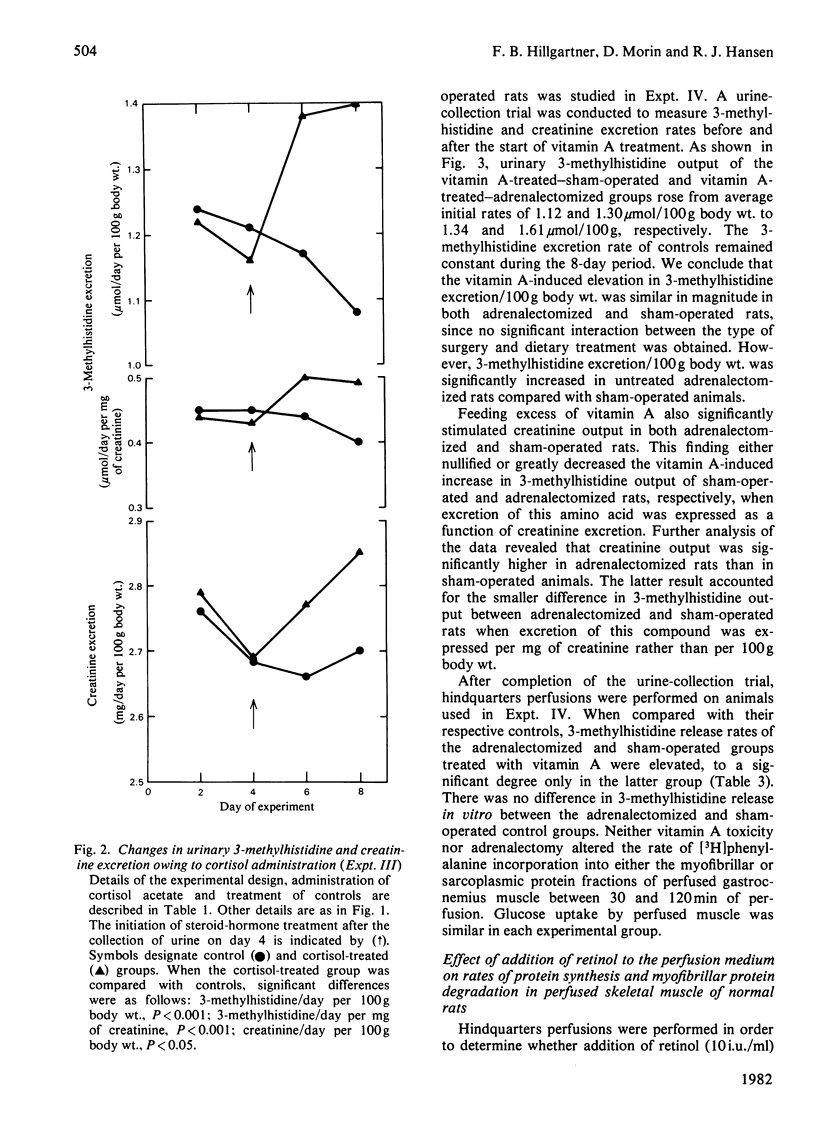
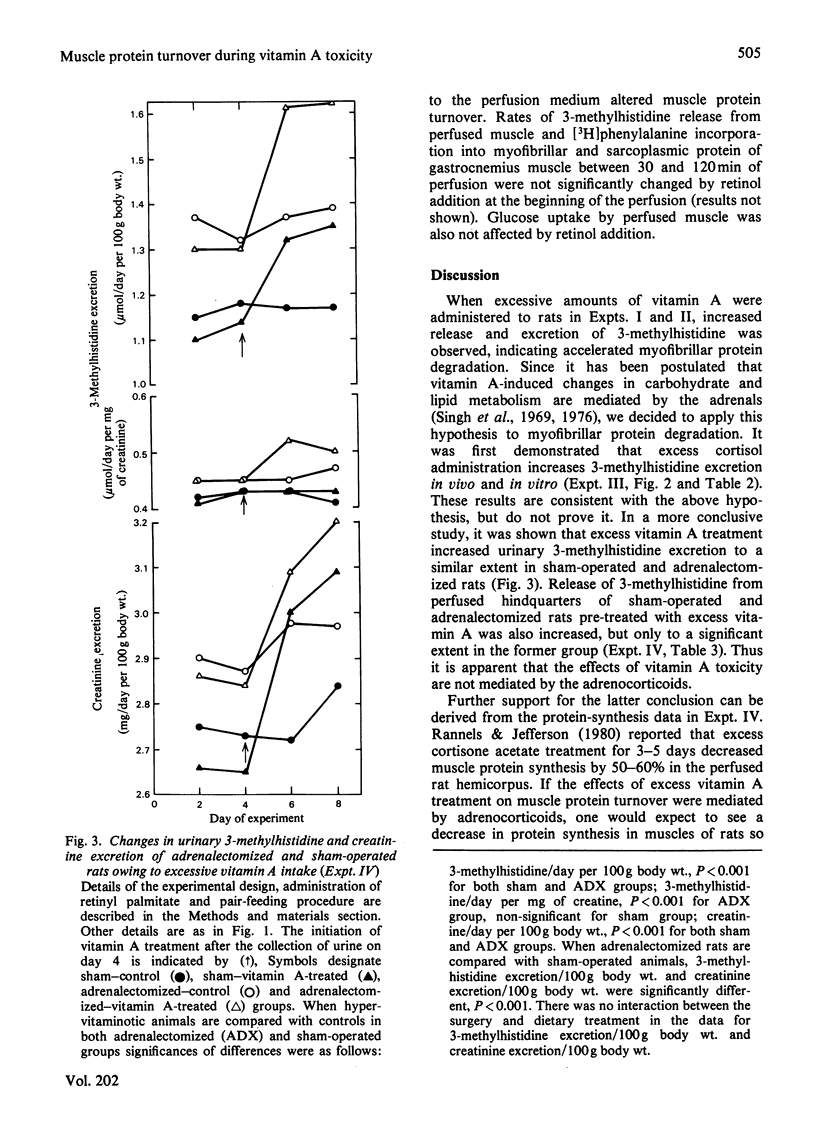
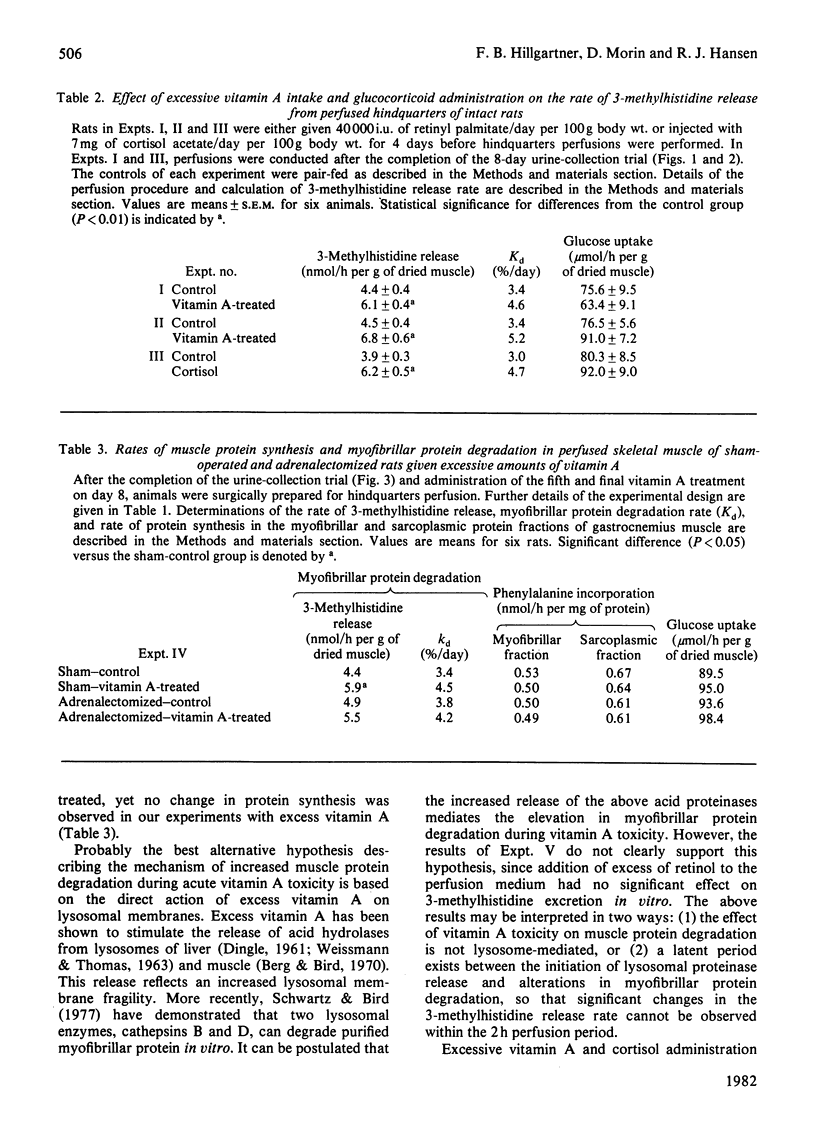
3-Methylhistidine excretion in vivo and in vitro was monitored in hypervitaminotic and pair-fed control rats. Feeding with excess of retinyl palmitate (40 000 i.u./day per 100 g body wt.) significantly increased urinary 3-methylhistidine and creatinine output during a 4-day treatment interval. 3-Methylhistidine release from perfused rat hindquarters was also elevated after 5 days of vitamin treatment. To determine whether the adrenals were involved in mediating the above response, a study was conducted on adrenalectomized and sham-operated rats. Excessive vitamin A intake stimulated 3-methylhistidine excretion in vivo and in vitro in both adrenalectomized and sham-operated animals, thus suggesting that the vitamin A-induced acceleration in myofibrillar protein breakdown was not mediated by the adrenals. In both groups of rats, vitamin A treatment had no effect on the rate of protein synthesis, on the basis of incorporation in vitro of [3H]phenylalanine into muscle protein. Additional studies revealed that the addition of excess retinol to the perfusion medium (10 i.u./ml) had no significant effect on the rates of 3-methylhistidine release or [3H]phenylalanine incorporation in vitro. Finally, high doses of cortisol (7 mg/day per 100g body wt.) administered to intact rats for 5 days significantly increased rates of 3-methylhistidine excretion, both in vivo and in vitro.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews T. M., Goldthorp R., Watts R. W. Fluorimetric measurement of the phenylalanine content of human granulocytes. Clin Chim Acta. 1973 Feb 12;43(3):379–387. doi: 10.1016/0009-8981(73)90477-4. [DOI] [PubMed] [Google Scholar]
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- Berg T., Bird J. W. Properties of muscle and liver lysosomes in adrenalectomized rats. Acta Physiol Scand. 1970 Jul;79(3):335–350. doi: 10.1111/j.1748-1716.1970.tb04733.x. [DOI] [PubMed] [Google Scholar]
- COWGILL R. W., FREEBURG B. The metabolism of methylhistidine compounds in animals. Arch Biochem Biophys. 1957 Oct;71(2):466–472. doi: 10.1016/0003-9861(57)90059-0. [DOI] [PubMed] [Google Scholar]
- DINGLE J. T. Studies on the mode of action of excess of vitamin A. 3. Release of a bound protease by the action of vitamin A. Biochem J. 1961 Jun;79:509–512. doi: 10.1042/bj0790509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan K. N., Singh V. N., Ramachandran C. K. Early effects of hypervitaminosis A on gluconeogenic activity and amino acid metabolizing enzymes of rat liver. J Nutr. 1977 Oct;107(10):1809–1815. doi: 10.1093/jn/107.10.1809. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Flaim K. E., Li J. B., Jefferson L. S. Protein turnover in rat skeletal muscle: effects of hypophysectomy and growth hormone. Am J Physiol. 1978 Jan;234(1):E38–E43. doi: 10.1152/ajpendo.1978.234.1.E38. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Ruderman N. B., Aoki T. T. Glucose and amino acid metabolism in perfused skeletal muscle. Effect of dichloroacetate. Diabetes. 1978 Nov;27(11):1065–1074. doi: 10.2337/diab.27.11.1065. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Munro H. N., Young V. R. Isolation and quantitation of Ntau-methylhistidine in actin and myosin of rat skeletal muscle: use of pyridine elution of protein hydrolysates on ion-exchange resins. Biochim Biophys Acta. 1974 Nov 5;371(1):226–237. doi: 10.1016/0005-2795(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NERURKAR M. K., SAHASRABUDHE M. B. Metabolism of calcium, phosphorus and nitrogen in hypervitaminosis A in young rats. Biochem J. 1956 Jun;63(2):344–349. doi: 10.1042/bj0630344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels S. R., Jefferson L. S. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol. 1980 Jun;238(6):E564–E572. doi: 10.1152/ajpendo.1980.238.6.E564. [DOI] [PubMed] [Google Scholar]
- Rao P. R., Joseph P. K., Rao A. B., Ramakrishnan S. Effect of excess vitamin A intake on glucose metabolism in liver & kidney cortex of rat. Indian J Biochem Biophys. 1977 Jun;14(2):167–171. [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W., Bird J. W. Degradation of myofibrillar proteins by cathepsins B and D. Biochem J. 1977 Dec 1;167(3):811–820. doi: 10.1042/bj1670811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Pennington R. J. The effect of cortisone on protein breakdown and synthesis in rat skeletal muscle. Mol Cell Endocrinol. 1977 Jan;6(3):159–169. doi: 10.1016/0303-7207(77)90082-x. [DOI] [PubMed] [Google Scholar]
- Singh M., Singh V. N., Venkitasubramanian T. A. Early effects of excessive retinol intake on gluconeogenesis. Involvement of adrenals in the increased activities on Gluconeogenic Enzymes of rat. Arch Biochem Biophys. 1976 Mar;173(1):82–92. doi: 10.1016/0003-9861(76)90237-x. [DOI] [PubMed] [Google Scholar]
- Singh N., Singh V. N., Venkitasubramanian T. A. Effect of hypervitaminosis A on adrenal corticosteroids. Indian J Biochem Biophys. 1972 Dec;9(4):313–314. [PubMed] [Google Scholar]
- Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17(4):381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- Strebel R. F., Girerd R. J., Wagner B. M. Cardiovascular calcification in rats with hypervitaminosis A. Arch Pathol. 1969 Mar;87(3):290–297. [PubMed] [Google Scholar]
- Thompson E. B., Lippman M. E. Mechanism of action of glucocorticoids. Metabolism. 1974 Feb;23(2):159–202. doi: 10.1016/0026-0495(74)90113-9. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. II. The effect of cortisone on the release of acid hydrolases from a large granule fraction of rabbit liver induced by an excess of vitamin A. J Clin Invest. 1963 May;42:661–669. doi: 10.1172/JCI104757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Alexis S. D., Baliga B. S., Munro H. N., Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3-methylhistidine and its N-acetyl derivative. J Biol Chem. 1972 Jun 10;247(11):3592–3600. [PubMed] [Google Scholar]



