Abstract
Aims:
The study aimed to assess the antimicrobial effectiveness of green tea and neem extract compared to sodium hypochlorite (NaOCl) against various root canal microorganisms.
Materials and Methods:
Thirty patients with pulpal necrosis were selected, providing 60 samples before and after irrigation. Groups were assigned as follows: Group A: 3% NaOCl (control), Group B: green tea, and Group C: neem extract. Samples were collected before and after irrigation in two phases. Samples were collected maintaining a strict sterile condition and stored in buffer solution at −80°C for bacterial-load measurement through real-time Polymerase chain reaction (PCR). Statistical analysis included within-group comparisons using Wilcoxon’s test and the paired t-test and inter-group comparisons using the Kruskal–Wallis test with post hoc Dunn’s test and one-way analysis of variance with post hoc Tukey’s honestly significant difference test (P ≤ 0.05).
Results:
While no irrigant achieved complete bacterial eradication, all solutions exhibited significant antimicrobial activity postirrigation. NaOCl yielded the most effective results, with green tea nearly comparable, and neem extract demonstrating the lowest efficacy.
Conclusions:
Herbal irrigants, particularly green tea, can serve as effective alternatives to chemical solutions. However, neem extract proved less effective than both green tea and NaOCl, indicating its inferiority in root canal disinfection.
Keywords: Antimicrobial efficacy, green tea extract, herbal endodontic irrigants, neem extract, polymerase chain reaction, real-time polymerase chain reaction, root canal irrigants
INTRODUCTION
The root canal of a tooth presents a challenging environment due to its intricate anatomical features and colonization by microorganisms upon infection.[1] Mechanical instrumentation with rotary file systems, commonly used for initial cleaning, may not effectively debride the canal,[2] especially in cases of flat-oval or curved canals, leaving significant areas untouched.[3] Moreover, obligate anaerobic bacteria, predominant in endodontic infections,[4] pose a challenge for eradication. Enterococcus faecalis, a facultative bacterium, is frequently isolated from failed root canal treatments, underscoring the need for potent irrigation adjuncts.
Sodium hypochlorite (NaOCl), though widely used in concentration ranging from 0.5% to 5.25% for its antimicrobial properties, lacks systemic nontoxicity and may cause tissue irritation or complications if improperly handled with unappreciable taste, smell, and irritant to the human oral mucosa.[5] Herbal irrigants such as green tea and neem extract emerge as potential alternatives due to their antimicrobial and anti-inflammatory properties. Green tea (Camellia sinensis) contains polyphenols such as catechins, notably epigallocatechin gallate, which exhibit antibacterial activity against various pathogens.[6] Neem (Azadirachta indica) extract, rich in compounds such as nimbin, nimbidin, and nimbolide, offers bactericidal, fungicidal, and anti-inflammatory effects.[7] These compounds uncouple the mitochondrial oxidative phosphorylation by inhibiting the respiratory chain, leading to anti-adherence activity. This affects the microbial adhesion and colonization reducing the number of microorganisms in root canals.[8]
Conventional microbiological evaluation methods have limitations, prompting the adoption of molecular techniques such as polymerase chain reaction (PCR) for precise microbial identification.[9] Real-time PCR (qPCR) allows quantitative assessment of bacterial DNA, facilitating comprehensive analysis of root canal microorganisms.
This in vivo study aimed to compare green tea and neem extract with NaOCl through qPCR, assessing their antimicrobial efficacy against root canal microorganisms. The null hypothesis suggested no variation in antimicrobial efficacy among the herbal irrigants. By evaluating these herbal alternatives in clinical settings, this study contributes insights into safer, effective herbal alternatives for root canal treatment, potentially improving antimicrobial efficacy and reducing adverse effects linked to traditional chemical irrigants in clinical practice.
MATERIALS AND METHODS
Ethical clearance was obtained from the Institutional Ethics Committee (GNIDSR/IEC/21-24/05) and registered under the Clinical Trials Registry of India (CTRI/2023/09/057727).
The study comprised 60 samples across 6 groups, with 10 samples in each. Sample size determination utilized G*Power software (version 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany), ensuring 95% confidence level and 5% alpha error. Entire sample collection was conducted by a single operator, following taking patient consent, with no dropouts observed.
Inclusion criteria included patients aged 18–50 years with pulpal necrosis, with or without symptomatic or asymptomatic apical periodontitis including single or multirooted teeth (largest canal in multirooted). Exclusion criteria consisted of periodontal disease with pockets >4 mm, calcified canals, acute apical abscess, internal resorption, open apex, retreatment, recent antibiotic use within 3 months, pregnancy, and medical comorbidities.
Preparation of green tea
Green tea extract was prepared as per previous trials[10,11] using 3.5% powder extract from Tetrahedron Beverages Pvt. Ltd., Tamil Nadu, India. 3.5 g of extract was dissolved in 100 ml boiling distilled water, sterilized, and incubated at 37°C overnight.
Preparation of ethanolic neem extract
Ethanolic neem extract (ENE) was prepared,[12,13] and mature neem leaves were washed, dried, and powdered. The extract was made by macerating 15 g of powder with 30 ml 95% ethyl alcohol for a week, then filtered, and concentrated, resulting in a 50% solution in 99.99% dimethyl sulfoxide (DMSO).
Sample collection
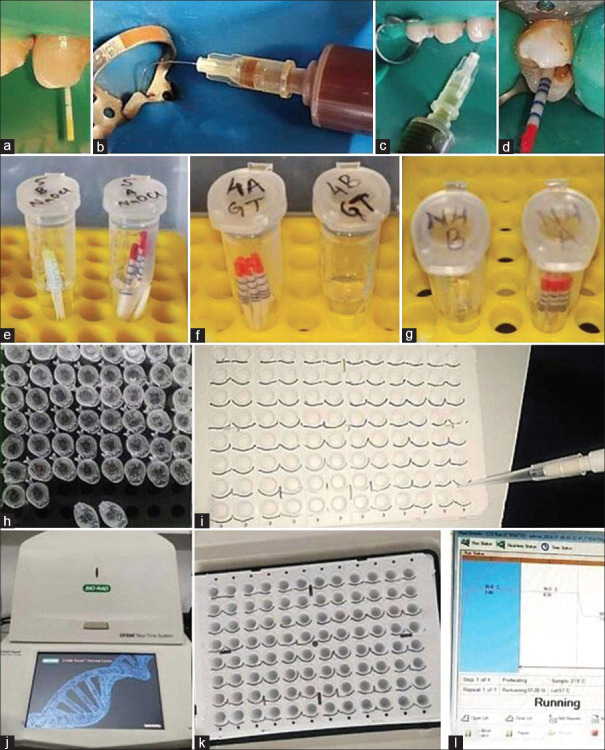
During single-visit root canal treatment, strict aseptic procedures were followed for sample collection. After oral prophylaxis and rubber dam isolation (COLTENE, India), the operative field was cleaned with 3% hydrogen peroxide and 3% NaOCl (Prime Dental Products Pvt. Ltd.) for 30 s.[14] This was followed by neutralization of the effect of NaOCl with 5% sodium thiosulfate. Access cavity preparation was conducted using sterile no. 2 and 4 round bur (Mani Inc., Japan) and Endo-Z bur (Dentsply Maillefer, Ballaigues, Switzerland) mounted to a high-speed handpiece (NSK Pana Air, Japan) under continuous saline irrigation. Canal patency was verified using a #10-15 K-file (Mani Inc., Japan), and working length was determined using an electronic apex locator (J Morita Corp., Kyoto, Japan) and confirmed using a digital radiograph. Samples were collected before and after irrigation in two stages, following a standardized procedure for each group. Before irrigation, three sterile paper points of ISO size 20 (Dentsply Maillefer, Ballaigues, Switzerland) were introduced consecutively into the canal [Figure 1a] soaked in normal saline (0.9% v/w, Lifusion, India) till working length keeping for 60 s and stored in 1.5 ml sterile DNase/RNase-free Eppendorf tube containing 10% sodium dodecyl sulfate and 10% Triton X100 as a buffer solution and initially stored at −20°C followed by final storage in −80°C.
Figure 1.
(a) Before-irrigation sample, (b) Irrigation with green tea, (c) Irrigation with neem, (d) After-irrigation sample, (e-g) Six groups stored in buffer solution, (h) Extracted bacterial 16-s-rDNA, (i) Each well containing final reaction mixture, (j) BIORAD thermocycler, (k) Sealed 96 well-plate inside thermocycler, (l) Real-time polymerase chain reaction operating by CFX-Maestro software
-
Group A: 3% NaOCl
A1 – Before irrigation sample (n = 10)
A2 – After irrigation sample (n = 10)
-
Group B: Green tea (Clonorchis sinensis)
B1 – Before irrigation sample (n = 10)
B2 – After irrigation sample (n = 10)
-
Group C: ENE (A. indica)
C1 – Before irrigation sample (n = 10)
C2 – After irrigation sample (n = 10).
To collect after-irrigation samples, canals were prepared using ProTaper Gold rotary system (Dentsply Maillefer, Ballaigues, Switzerland) to an apical size of #F3 (tip size 30 with a taper of 0.08 v) using a crown-down technique at 300 rpm and torque 2 N/cm and irrigated with the allocated irrigant. Each rotary file was discarded after preparation of five root canals. Between consecutive instrumentation, canals were irrigated with allocated solutions [Figure 1b and c] using a side-vented needle (Neoendo, Orikam, India) inserted 2 mm short of working length. Canals received a total of 15 mL of irrigant over 5 min. Manual dynamic irrigation with gutta-percha cone was performed before final flushing with 3 ml saline for 1 min. Sample collection and storage were done in a previous manner using three sterile F2 paper points (Dentsply Maillefer, Ballaigues, Switzerland) [Figure 1d] and later assessed using real-time PCR for microbial analysis. Each sample, both before and after irrigation with the three different irrigants, is illustrated in Figure 1e-g.
DNA extraction and real-time PCR
Bacterial genomic DNA was isolated [Figure 1h], purified, and standardized using the AllPrep DNA/RNA/miRNA Universal Kit (CAT#80224, QIAGEN, Venlo, The Netherlands), with final DNA concentration checked using NanoDrop Spectrophotometer (Thermo Fisher Scientific, USA), qPCR was conducted in 19 μL reaction mixtures [Figure 1i], comprising isolated DNA, sterile water, specific primer-probe sets, and universal PCR Master Mix (Cat# 1725124) (BIORAD Laboratories Inc., California, USA). PCR was carried out in 40 cycles using a 96-well plated system (CFX96 REAL-TIME SYSTEM, BIORAD) [Figure 1j and k], with conditions including initial denaturation at 95°C for 10 min, denaturation at 94°C for 25 s, annealing at 51°C for 25 s, and extension 72°C for 25 s operated by Bio-Rad CFX Maestro Software [Figure 1l]. Observations on cycle threshold (Ct) value and bacterial loading before and after irrigation with different solutions were tabulated in Excel for analysis.
Statistical analysis
The collected data underwent statistical analysis (SPSS, IBM, Chicago, IL, USA, software version 26.0). Shapiro–Wilk test and visual inspections assessed normality, revealing skewness in Ct variables and approximate normal distribution in bacterial count variables. Descriptive statistics presented mean and standard deviation for normally distributed data and median with interquartile range for skewed data. Inferential statistics employed parametric and nonparametric tests: Within-group comparisons utilized Wilcoxon’s signed rank test and paired samples t-test, while intergroup comparisons used Kruskal–Wallis test with post hoc Dunn’s test and one-way analysis of variance (ANOVA) with post hoc Tukey’s honestly significant difference test. Significance was set at P ≤ 0.05.
RESULTS
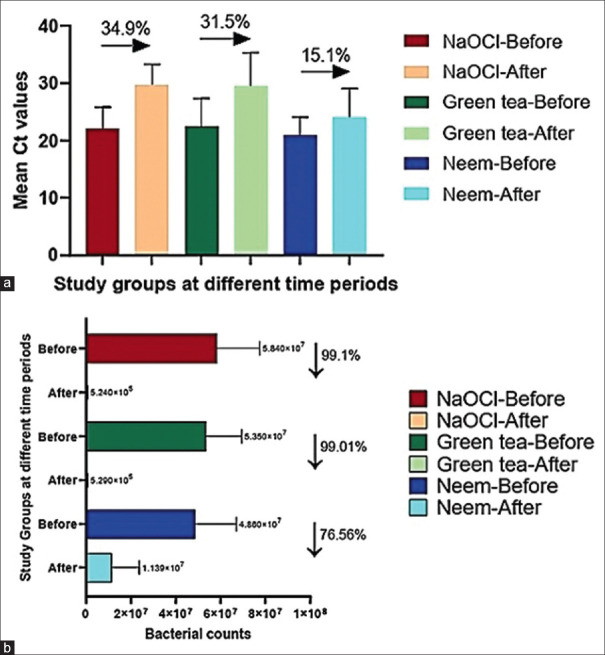
This study evaluated two parameters: Ct values, indicating amplification cycles needed for the fluorescent signal to cross the threshold in a positive sample, and bacterial counts. Wilcoxon’s signed rank test demonstrated significantly higher median Ct values [Table 1] postirrigation with NaOCl, green tea, and neem, indicating a decrease in bacterial load. Paired samples t-test revealed significantly lower mean bacterial counts postirrigation with all solutions [Table 2]. The Kruskal-Wallis test indicated that the increases in Ct values for green tea and NaOCl were comparable, with values of 31.5% and 34.9%, respectively. In contrast, neem exhibited a lower increase of 15% [Figure 2a]. Furthermore, one-way ANOVA demonstrated NaOCl and green tea’s effectiveness in bacterial-load reduction (99.1% and 99.01%, respectively) surpassing neem (76.5%) [Figure 2b]. This suggests NaOCl and green tea as more effective antibacterial agents than neem. In summary, the antibacterial effectiveness hierarchy based on median Ct values placed neem as the least effective, followed by green tea, with NaOCl showing the greatest impact. This order was consistent with the observed decrease in mean bacterial counts, where NaOCl again exhibited the strongest antibacterial action, green tea was moderately effective, and neem was the least effective.
Table 1.
Median cycle threshold values and interquartile range as a function of procedural stage and treatment groups
| Groups | Before irrigation | After irrigation | Difference | P § |
|---|---|---|---|---|
| NaOCl (n=10) | 21.88 (19.45–24.89) | 30.47 (28.44–31.81) | 7.79 (5.473–9.265)a | 0.0068* |
| Green tea (n=10) | 20.52 (19.1–24.21) | 29.51 (23.95–34.08) | 6.565 (4.978–10.47)a,b | |
| Neem (n=10) | 20.56 (18.73–21.97) | 21.49 (20.96–28.12) | 2.79 (0.4775–3.698)b |
*Statistically significant (P≤0.05), §Analyzed by Kruskal–Wallis test. Different lowercase letters imply significant differences. Values expressed as median (IQR), n: Sample size per group. IQR: Interquartile range
Table 2.
Mean bacterial count values and standard deviation as a function of procedural stage and treatment groups
| Groups | Before irrigation | After irrigation | Difference | P § |
|---|---|---|---|---|
| NaOCl (n=10) | 5.84×107±1.92×107 | 5.24×105±1.78×105 | 7.692±2.636a | 0.01* |
| Green tea (n=10) | 5.35×107±1.6×107 | 5.29×105±1.47×105 | 7.081±3.439a,b | |
| Neem (n=10) | 4.86×107±1.85×107 | 113.9×105±123.4×105 | 3.152±3.401b |
*Statistically significant (P≤0.05). §Analyzed by one-way ANOVA test. Different lowercase letters imply significant differences. Values expressed as mean ± standard deviation. n: Sample size per group. IQR: Interquartile range
Figure 2.
Bar graph: (a) Mean cycle threshold values and (b) bacterial counts before and after irrigation with total six study groups denoting the %-fold increase and decrease, respectively
DISCUSSION
The fundamental requirements of nonsurgical root canal treatment involve effective chemomechanical preparation and a three-dimensional fluid-tight seal of the root canal system.[15] NaOCl is considered the gold standard for irrigation due to its excellent antimicrobial and pulp-dissolving properties, despite concerns regarding adverse effects. Green tea extract, prepared at 3.5% concentration, demonstrated antimicrobial efficacy comparable to 2% chlorhexidine and low-concentration NaOCl, making it a potential alternative.[11] ENE dissolved in 30 ml of DMSO exhibited better antimicrobial activity compared to aqueous neem extract,[13] as neem contains phenol groups that dissolve in organic solvents better than water causing higher concentrations of bioactive compounds when compared with acetone, water, and methanol[16] with DMSO facilitating penetration without affecting active components of neem.[17] Aarti et al.[8] stated excellent antibacterial activity of ENE even in minimum inhibitory concentration of 1.88% compared to aqueous extract.
Rubber dam isolation was employed to prevent salivary contamination during treatment and sample collection.[18] The operative field was cleaned using 3% hydrogen peroxide and 3% NaOCl, ensuring disinfection.[12,14]
Irrigation with allocated irrigants followed established protocols in accordance with most of the previous clinical as well as in vitro studies,[10,11,12,13] with manual dynamic irrigation aiding irrigant flow. Saline flushing removed residual irrigants, providing a neutral medium for bacterial sampling. These standardized procedures ensure thorough root canal disinfection and microbial evaluation.
Quantitative analysis of antibacterial efficacy using qPCR reduces dependency on microbiology laboratories and offers faster, more precise results compared to culturing methods.[9] Both live and dead microorganisms can be detected, making qPCR highly sensitive.[19] Studies comparing qPCR to cultivation methods[20,21] have shown qPCR’s superiority in detecting and quantifying bacteria, particularly E. faecalis in endodontic infections.
In this study, none of the irrigants completely eliminated bacteria from the root canal, but all showed a statistically significant reduction in bacterial load before and after irrigation. The Ct values significantly increased after irrigation with NaOCl, green tea, and ENE. In addition, the mean bacterial counts significantly decreased after irrigation with each irrigant compared to before irrigation.
In intragroup comparisons, fold increases in Ct values and fold decreases in bacterial counts after irrigation were observed. Green tea and NaOCl groups showed comparable fold increases (31.5%- and 34.9%-fold increase for Ct value and 99.01%- and 99.1%-fold decrease for bacterial count), while ENE exhibited a lower fold increase in Ct value (15%) and fold decrease in bacterial count (76.5%), indicating its lesser efficacy in reducing bacterial count. These findings align with previous studies, which had demonstrated the antimicrobial efficacy of herbal irrigants compared to NaOCl. In a study done by Pujar et al.,[22] green tea polyphenols showed significant antibacterial activity against E. faecalis biofilms, while Persian green tea extract exhibited comparable efficacy to NaOCl against E. faecalis in another study by Fatemeh et al.[23] In addition, in a study by Dutta et al.,[24] ENE had shown promising antimicrobial properties in combination with NaOCl.
Intergroup comparisons revealed significant differences in Ct values and bacterial counts between NaOCl and neem extract groups, indicating varying antimicrobial efficacy. However, no significant difference was found between NaOCl and green tea groups, or between green tea and neem extract groups, consistent with previous findings.
Bhargava et al.[25] aimed to evaluate the antimicrobial efficacy of neem, green tea, Triphala, and 3% NaOCl against endodontic microflora. Contrary to some previous studies, no statistically significant difference was found between the antimicrobial activity of NaOCl and neem. However, the current study supports the rejection of the null hypothesis, indicating differences in antimicrobial efficacy among herbal irrigants used in root canal treatment. These findings suggest that herbal irrigants have the potential to serve as alternative antimicrobial agents in endodontic therapy. However, further clinical trials with larger samples and both quantitative as well as qualitative antimicrobial assessments are essential before endorsing herbal irrigants commercially for intracanal use.
CONCLUSIONS
Within the limitations of the study, both chemical (3% NaOCl) and herbal (green tea, neem) irrigants incompletely eradicate root canal micro-organisms. Green tea’s efficacy matches 3% NaOCl, proposing it as a viable alternative, whereas neem was less effective than green tea and significantly inferior to 3% NaOCl.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank the Department of Conservative Dentistry and Endodontics, Guru Nanak Institute of Dental Sciences and Research and West Bengal University of Health Sciences, for the opportunity, support, and academic environment to embark on the journey of scientific research.
REFERENCES
- 1.Kishen A, Peters OA, Zehnder M, Diogenes AR, Nair MK. Advances in endodontics: Potential applications in clinical practice. J Conserv Dent. 2016;19:199–206. doi: 10.4103/0972-0707.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alavi AM, Opasanon A, Ng YL, Gulabivala K. Root and canal morphology of Thai maxillary molars. Int Endod J. 2002;35:478–85. doi: 10.1046/j.1365-2591.2002.00511.x. [DOI] [PubMed] [Google Scholar]
- 3.Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J Endod. 2001;27:1–6. doi: 10.1097/00004770-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan LL, Vaishnavi C. Endodontic microbiology. J Conserv Dent. 2010;13:233–9. doi: 10.4103/0972-0707.73386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson KR, Goodell GG, McClanahan SB. Comparison of the antimicrobial activity of six irrigants on primary endodontic pathogens. J Endod. 2005;31:471–3. doi: 10.1097/01.don.0000148868.72833.62. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari TP, Bharti SK, Kaur HD, Dikshit RP, Hoondal GS. Synergistic antimicrobial activity of tea and antibiotics. Indian J Med Res. 2005;122:80–4. [PubMed] [Google Scholar]
- 7.Vinothkumar TS, Rubin MI, Balaji L, Kandaswamy D. In vitro evaluation of five different herbal extracts as an antimicrobial endodontic irrigant using real time quantitative polymerase chain reaction. J Conserv Dent. 2013;16:167–70. doi: 10.4103/0972-0707.108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayak A, Nayak RN, Soumya B, Bhat K, Kudalkar M. Evaluation of antibacterial and anticandidial efficacy of aqueous and alcoholic extract of Neem (Azadirachta indica) an in vitro study. Int J Res Ayurveda Pharm. 2011;2:230–5. [Google Scholar]
- 9.Ravikumar N, Mahalaxmi S, Jose P, Mohan A. Polymerase chain reaction (PCR) in endodontics. SRM Univ J Dent Sci. 2011;2:213–8. [Google Scholar]
- 10.Salem MR, Taha S, Abd El Gwad R. Evaluation of antimicrobial efficacy of green tea extract irrigant versus physiological saline following root canal treatment of infected primary molars in a group of Egyptian children: A randomized clinical trial. Egypt Dent J. 2017;63:101–10. [Google Scholar]
- 11.Martina LP, Ebenezar AV, Ghani MF, Narayanan A, Sundaram M, Mohan AG. An in vitro comparative antibacterial study of different concentrations of green tea extracts and 2% chlorhexidine on Enterococcus faecalis. Saudi Endod J. 2013;3:120–4. [Google Scholar]
- 12.Hosny NS, El Khodary SA, El Boghdadi RM, Shaker OG. Effect of Neem (Azadirachta indica) versus 2.5% sodium hypochlorite as root canal irrigants on the intensity of post-operative pain and the amount of endotoxins in mandibular molars with necrotic pulps: A randomized controlled trial. Int Endod J. 2021;54:1434–47. doi: 10.1111/iej.13532. [DOI] [PubMed] [Google Scholar]
- 13.Prasad SD, Goda PC, Reddy KS, Kumar CS, Hemadri M, Ranga Reddy DS. Evaluation of antimicrobial efficacy of neem and Aloe vera leaf extracts in comparison with 3% sodium hypochlorite and 2% chlorhexidine against E. faecalis and C. albicans. J NTR Univ Health Sci. 2016;5:104–10. [Google Scholar]
- 14.Gomes BP, Drucker DB, Lilley JD. Associations of specific bacteria with some endodontic signs and symptoms. Int Endod J. 1994;27:291–8. doi: 10.1111/j.1365-2591.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosaline H, Kandaswamy D, Gogulnath D, Rubin M. Influence of various herbal irrigants as a final rinse on the adherence of Enterococcus faecalis by fluorescence confocal laser scanning microscope. J Conserv Dent. 2013;16:352–5. doi: 10.4103/0972-0707.114365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel) 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Divya Kumari P, Shenoy SM, Khijmatgar S, Chowdhury A, Lynch E, Chowdhury CR. Antibacterial activity of new atraumatic restorative treatment materials incorporated with Azadirachta indica (Neem) against Streptococcus mutans. J Oral Biol Craniofac Res. 2019;9:321–5. doi: 10.1016/j.jobcr.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng YL, Spratt D, Sriskantharajah S, Gulabivala K. Evaluation of protocols for field decontamination before bacterial sampling of root canals for contemporary microbiology techniques. J Endod. 2003;29:317–20. doi: 10.1097/00004770-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kondreddi KC, Simhadri NS, Dola B, Moinuddin M. Polymerase chain reaction in exploring endodontic infections. Webmed Central Dentistryl. 2016;12:43–57. [Google Scholar]
- 20.Williams JM, Trope M, Caplan DJ, Shugars DC. Detection and quantitation of E. faecalis by real-time PCR (qPCR), reverse transcription-PCR (RT-PCR), and cultivation during endodontic treatment. J Endod. 2006;32:715–21. doi: 10.1016/j.joen.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Gomes BP, Pinheiro ET, Sousa EL, Jacinto RC, Zaia AA, Ferraz CC, et al. Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:247–53. doi: 10.1016/j.tripleo.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Pujar M, Patil C, Kadam A. Comparison of antimicrobial efficacy of Triphala, greentea polyphenols and 3% NaOCl on Enterococcus faecalis biofilms formed on tooth substrate in vitro. J Int Oral Health. 2011;39:235–40. [Google Scholar]
- 23.Ramezanali F, Samimi S, Kharazifard M, Afkhami F. The in vitro antibacterial efficacy of Persian green tea extract as an intracanal irrigant on Enterococcus faecalis biofilm. Iran Endod J. 2016;11:304–8. doi: 10.22037/iej.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta A, Kundabala M. Comparative anti-microbial efficacy of Azadirachta indica irrigant with standard endodontic irrigants: A preliminary study. J Conserv Dent. 2014;17:133–7. doi: 10.4103/0972-0707.128047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhargava K, Kumar T, Aggarwal S, Zinzarde S, Sanap A, Patil P. Comparative evaluation of the antimicrobial efficacy of neem, green tea, Triphala and NaOCl: An in vitro study. J Dent Res Rev. 2015;39:225–7. [Google Scholar]