Abstract
Context:
Concerns about adverse reactions and the development of antibiotic resistance have prompted an alternative treatment strategy that utilizes traditional medicinal herbs.
Aim:
This randomized control trial assessed the antimicrobial efficacy of 2% chlorhexidine (CHX) gel, Triphala, and Azadirachta indica as intracanal medicaments against Enterococcus faecalis.
Materials and Methods:
Forty patients with nonvital teeth and single root canals were selected (n = 10). Following the initial access opening, the first microbial samples (S1) were collected using paper points. Second microbial samples (S2) were collected following the chemo-mechanical preparation and 1 week after introducing the intracanal medicaments. Group I: 2% CHX, Group II: A. indica, Group III: Triphala, and Group IV: Calcium hydroxide (Ca(OH)2). S1 and S2 samples were collected, and bacterial growth was observed using the colony-forming unit (CFU) count.
Statistical Analysis:
Comparison of the difference in CFU count among four groups was performed using one-way ANOVA test (P < 0.05) followed by post hoc Tukey test.
Results:
Reduction in CFU count postmedication S2 from S1 in each group was statistically significant. Percentage reduction in CFU count was highest in Triphala group followed by A. indica and 2% CHX group. Percentage reduction in CFU count was least in Ca(OH)2 group. The post hoc pairwise comparison of % reduction in CFU count among four groups showed that the percent reduction was highest in Triphala group.
Conclusion:
Triphala has considerable antimicrobial efficacy against E. faecalis.
Keywords: 2%, chlorhexidine, Azadirachta indica, calcium hydroxide, intracanal medicaments, triphala
INTRODUCTION
The primary objectives of root canal therapy include the eradication of microorganisms from the root canal system and the establishment of a secure seal to prevent reinfection and promote complete periradicular healing.[1] Achieving thorough disinfection poses challenges due to the intricate anatomy of root canals and the complex architecture of dentinal tubules. To mitigate microbial flora, various strategies such as canal shaping procedures, specific irrigation protocols, and the administration of intracanal medications have been recommended.[2,3,4,5,6] Enterococcus faecalis commonly infects root canals associated with apical periodontitis, representing a significant challenge in endodontic treatment.[7,8] This facultative anaerobic Gram-positive bacterium is particularly concerning due to its resistance to intracanal medicaments, contributing to treatment failures.[7,9] E. faecalis is a biological indication that has been thoroughly studied. Numerous laboratory experiments examining E. faecalis’s susceptibility to endodontic therapy revealed the bacteria’s strong tolerance to antimicrobial treatments. Moreover, E. faecalis can endure under extremely unforgiving conditions with a low food supply and a high alkaline pH of up to 11.5. The ability of E. faecalis to develop as a mono-infection in treated canals and as a biofilm on root canal walls without the synergistic support of other bacteria renders the pathogen highly resistant to antimicrobial agents and root canal therapy.[10]
Calcium hydroxide (Ca(OH)2) was introduced by Hermann in 1920 as an intracanal medication, primarily functioning by increasing pH levels through hydroxyl ion release. This action aids in deactivating bacterial lipopolysaccharides, particularly effective against Gram-negative bacterial outer membranes.[8] Despite its popularity, Ca(OH)2 exhibits low solubility in water, which further decreases with temperature increases. It possesses both bacteriostatic and bactericidal properties owing to its high pH, with additional benefits including tissue healing and inflammation reduction.[11] However, studies have demonstrated that E. faecalis can develop resistance to Ca(OH)2 after 10 days of intracanal application.[9]
Chlorhexidine (CHX) has gained prominence as both an irrigant and intracanal medication due to its minimal toxicity and broad-spectrum antimicrobial activity. Its cationic nature interacts with the microbial cell wall’s negative charge, disrupting cellular osmotic balance and leading to microbial inhibition.[12,13] Effective against both Gram-positive and Gram-negative microorganisms, CHX is valued for its enduring antimicrobial effects, though it is associated with tooth discoloration and taste disturbances and has limited tissue-dissolving ability.[12,14] Given concerns over synthetic medication’s adverse effects and the rising challenge of antibiotic resistance, there is a growing interest in herbal alternatives recognized for their safety and potent antibacterial properties.[15] Triphala composed of Emblica officinalis, Terminalia chebula, and Terminalia bellirica has established antimicrobial efficacy rooted in Ayurvedic tradition. Beyond antimicrobial benefits, Triphala exhibits diverse pharmacological activities including anti-inflammatory, antioxidant, and digestive properties.[15] Similarly, neem (Azadirachta indica) possesses broad-spectrum antimicrobial properties attributed to nimbidin, a potent active compound found in its seeds.[16]
In response to the need for safer and more effective alternatives, Triphala has been formulated to address microbial challenges in endodontics.[15] This study aimed to evaluate and compare the antibacterial effectiveness of Ca(OH)2, 2% CHX, A. indica, and Triphala as intracanal medications against E. faecalis through an in vivo investigation.
MATERIALS AND METHODS
Methods
A parallel randomized single-blind clinical trial was conducted, with 1-week follow-up. The patients were selected from the outpatient department of conservative dentistry and endodontics who reported for root canal treatment. The intervention group received Group I: 2% CHX, Group II: A. indica, Group III: Triphala, as intracanal medicament, whereas Group IV (control) received Ca (OH)2 as intracanal medicament between endodontic treatment sessions.
Inclusion criteria
Maxillary or mandibular teeth with necrotic pulp and single root canal as diagnosed by clinical examination and radiography
Completely formed apices
Straight roots without severe curvature
The presence of PA lesions with a minimum diameter of 2 mm.
Exclusion criteria
Teeth with cracks or fractures, root caries, or shape and size anomalies
Severe mobility
External/internal resorption
Calcified canals
Periapical radiolucency >5 mm single operator conducted the procedure on patients.
Sample size calculation
The sample size was estimated using the data obtained from a previous study conducted by Dutta et al.[17] (Int J Clin Pediatr Dent 2017;10[3]:267-271). The sample size was calculated using the following formula:
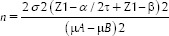
(μA and μB: Mean scores of the two out of four groups to be 3.53 and 2.65; σ: Pooled standard deviation of the two groups to be 0.64; τ: The number of pairwise comparisons to be made = 6; α error to be 5% and power to be 95%).
A sample size of 10 was required in each group.
Considering the process of randomization total sample size was taken to be 48 considering that there might be dropouts in each group.
Randomization and blinding
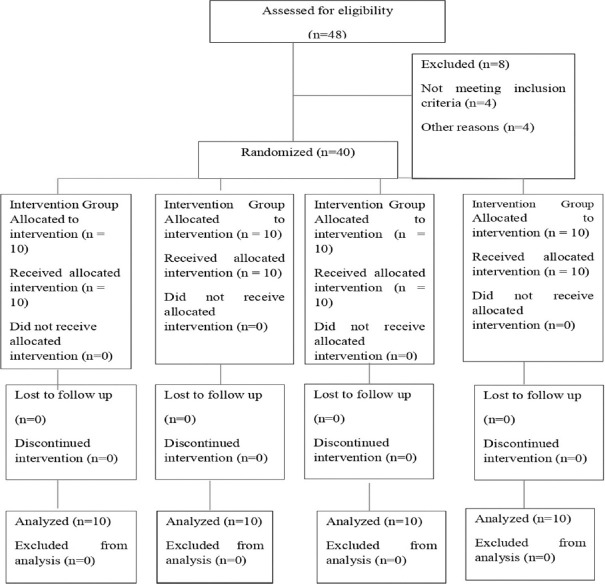
This parallel randomized clinical trial was conducted on 40 patients. The study was approved by the Ethical Committee of Inderprastha Dental College and Hospital, Ghaziabad, India IPDC/SS/2023/10190(4) and was conducted at the institute. Informed consent was obtained from the patients after explaining them the procedure. After ethical approval, the study was registered in Clinical Trials Registry-India (CTRI), REF/2024/01/078480 Registration no-CTRI/2024/03/063499. After initial screening, patients with nonvital teeth and single-root canals were selected for the study and divided into four groups. A total of 40 patients were selected by convenience sampling and were randomly divided into four groups using the using simple random allocation [Figure 1]. The allocation was concealed by placing the sequence in a sealed envelope, which was then given to the dental assistant. The study had a single-blind design, and the patients were unaware of the type of medicament applied to their root canals.
Figure 1.
CONSORT diagram showing the flow of participants through each stage of a randomized trail
Treatment procedure
Blinding
The study had a single-blind design, and the patients were unaware of the type of medicament applied to their root canals.
Standard endodontic procedures were performed under rubber dam isolation after oral prophylaxis. After the access opening, orifices were enlarged using Sx hand file (Dentsply) to facilitate easy entry of paper points. Initially, microbiological samples were obtained by placing #10 (2%) sterile paper points into the canal for 30 s, then transferring them into a test tube containing brain heart infusion broth. Within 2 h, the preoperative samples labeled as (S1) were delivered to the microbiological laboratory. K-files (mani) of suitable size were introduced into the root canal, and working lengths were verified with apex locator (J Morita, mini). Root canals were instrumented using K-files along with irrigation using 2.5% sodium hypochlorite and normal saline. After chemomechanical preparation, the following intracanal medicaments were introduced in the canal using lentulospiral. Group 1: The 20% solution of CHX digluconate (Sigma Aldrich, Bangalore, C9394) was initially diluted to 2%. Then, the 2% CHX gel was made keeping the vehicle as polyethylene glycol and the thickening agent as methylcellulose. Group 2: A. indica (Dabur Neem Churna) 150 mg of powder of neem was mixed with 0.15 ml of distilled water. Group 3: Triphala (Zandu Triphala Churna) 150 mg of Triphala powder was mixed with 0.15 mL of distilled water), Group 4: Ca(OH)2 (Control group) (Ca(OH)2 [Deepashree Products, India, B. No.051]) 150 mg of Ca(OH)2 powder was blended with 0.15 mL of distilled water and in Groups 2, 3, and 4 methylcellulose was used as thickening agent. Intermediate restoration was used to seal the coronal access. Following a week, the patients were recalled. Copious irrigation was done to remove the medication. In the same way as the preoperative sampling protocol, postoperative microbiological samples (S2) were collected. A skilled worker who was unaware of the disinfection protocols processed the bacterial cultures. Before being inoculated onto a blood agar plate and allowed to incubate for up to 48 h at 37°C, the paper tip in the brain heart infusion broth was vortexed for 2 min. A colony counter was used to detect bacterial growths and to count the colony-forming units (CFUs). The data recording sheet was utilized to document the colony counts of each sample both before and after surgery. After tabulating and statistical analysis, the data were analyzed.
Statistical analysis
Data were analyzed using SPSS v26 software (IBM corporation, Aromonk, New York, USA). The level of significance was kept at 5%. Results were presented using descriptive statistics. A comparison of CFU count between two different phases was performed using a paired t-test. Comparison of difference in CFU count among four groups was performed using one-way ANOVA test followed by post hoc Tukey test.
Outcomes
The main objective of this study was to compare the antimicrobial efficacy of 2% CHX gel, Triphala, and A. indica as intracanal medicaments against E. faecalis. The samples (S1 and S2) were collected before and after placement of the intracanal medicament and sent to the laboratory. Bacterial growth was observed using the colony counter and the CFU count.
RESULTS
The sample consisted of 40 patients with nonvital teeth and single root canals; Figure 1 presents the flow diagram of the study. Forty-eight patients were initially selected for the study from which 8 patients were excluded from the study 4 patients did not meet the inclusion criteria, 1 patient had other reasons, and 4 patients refused to participate in the study. A total of 40 patients were randomly divided into four groups.
Patients who had teeth with external/internal resorption, calcified canals, or periapical radiolucency >5 mm were excluded from the study. Following the initial access opening, the first microbial samples (S1) were collected using paper points. Second microbial samples (S2) were collected following the chemo-mechanical preparation and 1 week after introducing the intracanal medicaments. The total duration of the study was 1 week. No patients were harmed during the study. A statistically significant difference was exhibited by all test groups. Reduction in CFU count postmedication S2 from S1 in each group was statistically significant.
Table 1 compares the % reduction in CFU count among four groups. Percentage reduction in CFU count was highest in the Triphala group followed by A. indica and 2% CHX group. Percentage reduction in CFU count was least in Ca(OH)2 group.
Table 1.
Compares the percentage reduction in colony forming unit count among four groups
| Group | Mean | SD | P |
|---|---|---|---|
| 2% CHX | 77.06 | 2.12 | <0.001* |
| A. indica | 77.58 | 1.45 | |
| Triphala | 79.80 | 1.81 | |
| Calcium hydroxide | 53.70 | 1.70 |
*A significant difference at P<0.05. Percentage reduction in CFU count was highest in the triphala group followed by A. indica and 2% CHX group. Percentage reduction in CFU count was least in the calcium hydroxide group. One-way ANOVA test. SD: Standard deviation, CFU: Colony forming unit, A. indica: Azadirachta indica, CHX: Chlorhexidine
Table 2 presents the post hoc pairwise comparison of % reduction in CFU count among four groups. Percent reduction in the Triphala group was significantly greater than the other three groups. A. indica showed a significantly greater reduction in CFU as compared to Ca(OH)2 and a similar reduction to that of 2% CHX.
Table 2.
Presents the post hoc pairwise comparison of percentage reduction in colony forming unit count among four groups
| Group | P |
|---|---|
| 2% CHX versus A. indica | 1.000 |
| 2% CHX versus triphala | 0.009* |
| 2% CHX versus calcium hydroxide | <0.001* |
| A. indica versus triphala | 0.050* |
| A. indica versus calcium hydroxide | <0.001* |
| Triphala versus calcium hydroxide | <0.001* |
*A significant difference at P<0.05. Percent reduction in triphala group was significantly greater than the other three groups. A. indica showed a significantly greater reduction in CFU as compared to calcium hydroxide and a similar reduction to that of 2% CHX. Post hoc Bonferroni test. CFU: Colony forming unit, A. indica: Azadirachta indica, CHX: Chlorhexidine
None of the groups showed any harmful effects.
DISCUSSION
Persistent root canal infections are primarily associated with the presence of E. faecalis, and therefore, it was taken as the test organism.[18] It is resistant to chemo-mechanical instrumentation and can persist inside root canals, thereby affecting the outcome of endodontic treatment.[19]
Colony-forming units, or CFUs, are employed in a variety of microbiological investigations to quantify the findings. Compared to stereomicroscopic examination, which counts every single cell, living or dead, this method is more accurate since it counts the amount of cells that are still viable enough to multiply and form small colonies.[19,20]
Triphala was found to be the most effective medication against E. faecalis in this investigation. Triphala is rich in tannins, quinoline, and phenol, which are potent antibacterial chemical components. Similar to the results of this investigation, a previously conducted study showed that the application of Triphala as an irrigation solution against a 3-week biofilm resulted in a complete reduction in bacterial growth and demonstrated statistically significant antibacterial activity.[15]
Tetranortriterpenes found in the neem leaf extract have antibacterial properties that work by preventing the production of cell membranes. In this study, A. indica (neem) showed a statistically significant reduction in CFU counts. These results are in accordance with the previously conducted studies where the zone of inhibition in the agar diffusion test showed the antimicrobial efficiency of the neem extract was comparable to that of 2% CHX and 3% NaOCl.[19]
CHX is an effective intracanal medicament in endodontic, with substantivity. It exhibits a broad range of antimicrobial efficacy and is also effective against E. faecalis.[21] Prior research compared the antibacterial effectiveness of propolis and 2% CHX with other medications and found that 2% CHX demonstrated the highest level of microbial inhibition against E. faecalis, up to 200–400 micrometers deep.[21]
Ca(OH)2 requires a minimum period of seven days as an intracanal medicament; therefore, the intracanal medicaments were kept for 7 days.[22,23]
In the current investigation, Ca(OH)2 proved ineffective against E. faecalis. The outcomes matched with the previously conducted studies.[19,21] Due to the proton pump’s role as a major resistance mechanism, E. faecalis is resistant to strongly alkaline environments.[18]
Till now, many in vitro studies have been conducted to compare the antimicrobial effects of herbal medicaments against E. faecalis, so this in vivo was conducted so that the results could be directly extrapolated to the clinical scenarios.
CONCLUSION
According to the study, it was concluded that Triphala showed substantial antimicrobial effectiveness against E. faecalis followed by A. indica and 2% CHX. Herbal alternatives are more effective against E. faecalis and show promising results in reducing the antimicrobial activity compared to Ca(OH)2. For future research and clinical scenarios, herbal intracanal medicaments may be preferred compared to synthetic counterparts because of their fewer/nil side effects and potent antimicrobial actions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Shreshtha Sharma (B. D. S., Meerut) and Dr. Mahi Singh (B. D. S., Meerut) for their valuable support.
REFERENCES
- 1.Donyavi Z, Ghahari P, Esmaeilzadeh M, Kharazifard M, Yousefi-Mashouf R. Antibacterial efficacy of calcium hydroxide and chlorhexidine mixture for treatment of teeth with primary endodontic lesions: A randomized clinical trial. Iran Endod J. 2016;11:255–60. doi: 10.22037/iej.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–12. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 3.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–5. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 4.Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 5.Sjögren U, Sundqvist G. Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surg Oral Med Oral Pathol. 1987;63:366–70. doi: 10.1016/0030-4220(87)90208-8. [DOI] [PubMed] [Google Scholar]
- 6.Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751–5. doi: 10.1097/00004770-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Babaji P, Jagtap K, Lau H, Bansal N, Thajuraj S, Sondhi P. Comparative evaluation of antimicrobial effect of herbal root canal irrigants (Morinda citrifolia, Azadirachta indica, Aloe vera) with sodium hypochlorite: An in vitro study. J Int Soc Prev Community Dent. 2016;6:196–9. doi: 10.4103/2231-0762.183104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinothkumar TS, Rubin MI, Balaji L, Kandaswamy D. In vitro evaluation of five different herbal extracts as an antimicrobial endodontic irrigant using real time quantitative polymerase chain reaction. J Conserv Dent. 2013;16:167–70. doi: 10.4103/0972-0707.108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta-Wadhwa A, Wadhwa J, Duhan J. Comparative evaluation of antimicrobial efficacy of three herbal irrigants in reducing intracanal E. faecalis populations: An in vitro study. J Clin Exp Dent. 2016;8:e230–5. doi: 10.4317/jced.52339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142–9. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 11.Goel S, Sinha DJ, Singh UP, Ahuja U, Haider N, Sharma N. Comparative evaluation of effect of chlorhexidine, Azadirachta indica (neem) and Aloe barbadensis miller (aloe vera) on resin - sesdentin bond stabilization using shear bond testing: An in vitro study. J Conserv Dent. 2019;22:300–4. doi: 10.4103/JCD.JCD_11_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi H, Dave DS, Shah N, Pathak A, Patel KR, Martin P. Comparative evaluation of antimicrobial efficacy of herbal versus synthetic irrigating solutions: An in vitro study. Int J Oral Care Res. 2021;9:8–10. [Google Scholar]
- 13.Nirmala S, Surender LR, Reddy N, Reddy SD, Chukka RR, Kumar KN. Antimicrobial efficacy of Morinda citrifolia, Nisin, and 2% Chlorhexidine against Enterococcus faecalis: An in-vitro study. Cureus. 2022;14:e23206. doi: 10.7759/cureus.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil V, Akal N, Biradar S, Ratnakar P, Rairam S, Batta O. Comparative evalution of antimicrobial efficacy of mushroom, aloevera, and curcuma longa with calcium hydroxide as intracanal medicament against Enterococcus faecails: An in vitro study. J Conserv Dent. 2022;25:415–9. doi: 10.4103/jcd.jcd_208_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and Green Tea Polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J Endod. 2010;36:43–9. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Tyagi SP, Sinha DJ, Garg P, Singh UP, Mishra CC, Nagpal R. Comparison of antimicrobial efficacy of propolis, Morinda citrifolia, Azadirachta indica (Neem) and 5% sodium hypochlorite on candida albicans biofilm formed on tooth substrate: An in-vitro study. J Conserv Dent. 2013;16:532–5. doi: 10.4103/0972-0707.120973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta B, Dhull KS, Das D, Samir PV, Verma RK, Singh N. Evaluation of antimicrobial efficacy of various intracanal medicaments in primary teeth: An in vivo study. Int J Clin Pediatr Dent. 2017;10:267. doi: 10.5005/jp-journals-10005-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Vaghela DJ, Kandaswamy D, Venkateshbabu N, Jamini N, Ganesh A. Disinfection of dentinal tubules with two different formulations of calcium hydroxide as compared to 2% chlorhexidine: As intracanal medicaments against Enterococcus faecalis and Candida albicans: An in vitro study. J Conserv Dent. 2011;14:182–6. doi: 10.4103/0972-0707.82625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wassel M, Radwan M, Elghazawy R. Direct and residual antimicrobial efficacy of 2% chlorhexidine gel, double antibiotic paste and chitosan- chlorhexidine nanoparticles as intracanal medicaments against Enterococcus faecalis and Candida albicans in primary molars: an in-vitro study. BMC Oral Health. 2023;23:296. doi: 10.1186/s12903-023-02862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasudeva A, Sinha DJ, Tyagi SP, Singh NN, Garg P, Upadhyay D. Disinfection of dentinal tubules with 2% Chlorhexidine gel, calcium hydroxide and herbal intracanal medicaments against Enterococcus faecalis: An in-vitro study. Singapore Dent J. 2017;38:39–44. doi: 10.1016/j.sdj.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Chandwani ND, Maurya N, Nikhade P, Chandwani J. Comparative evaluation of antimicrobial efficacy of calcium hydroxide, triple antibiotic paste and bromelain against enterococcus faecalis: an in vitro study. J Conserv Dent. 2022;25:63–7. doi: 10.4103/jcd.jcd_461_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love RM. Enterococcus faecalis - A mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]