Abstract
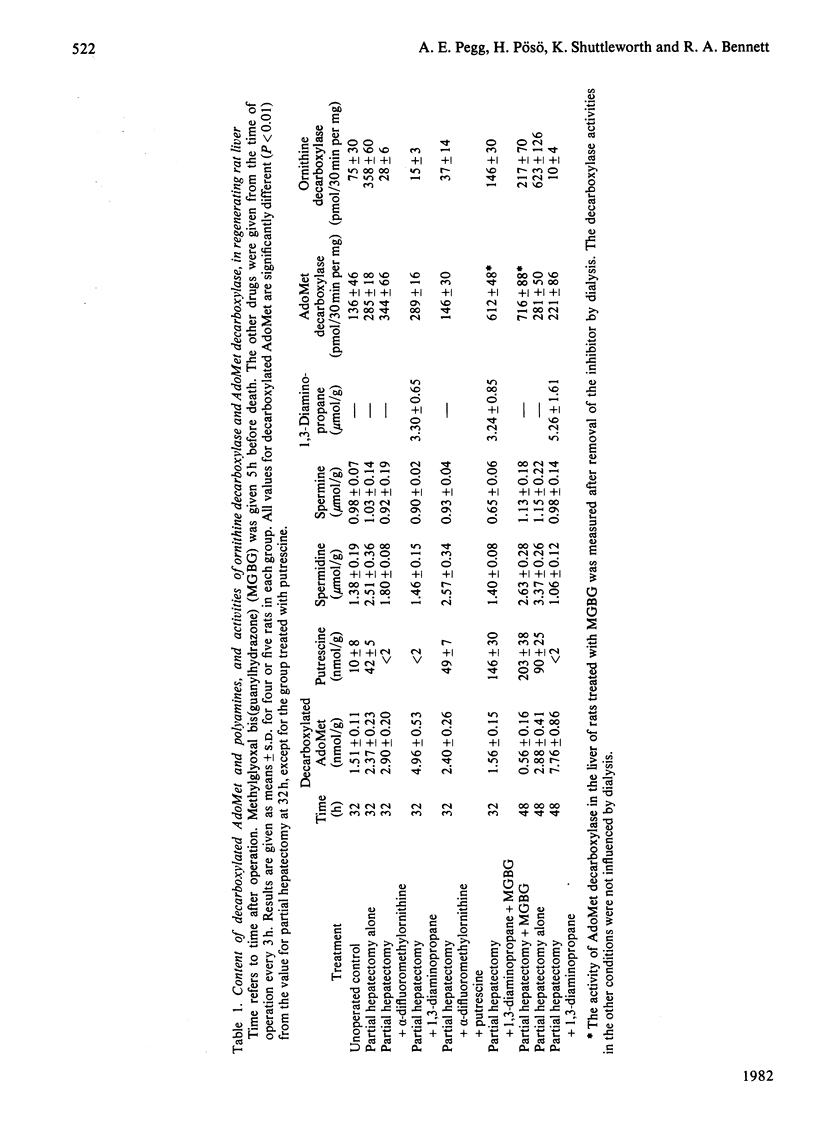
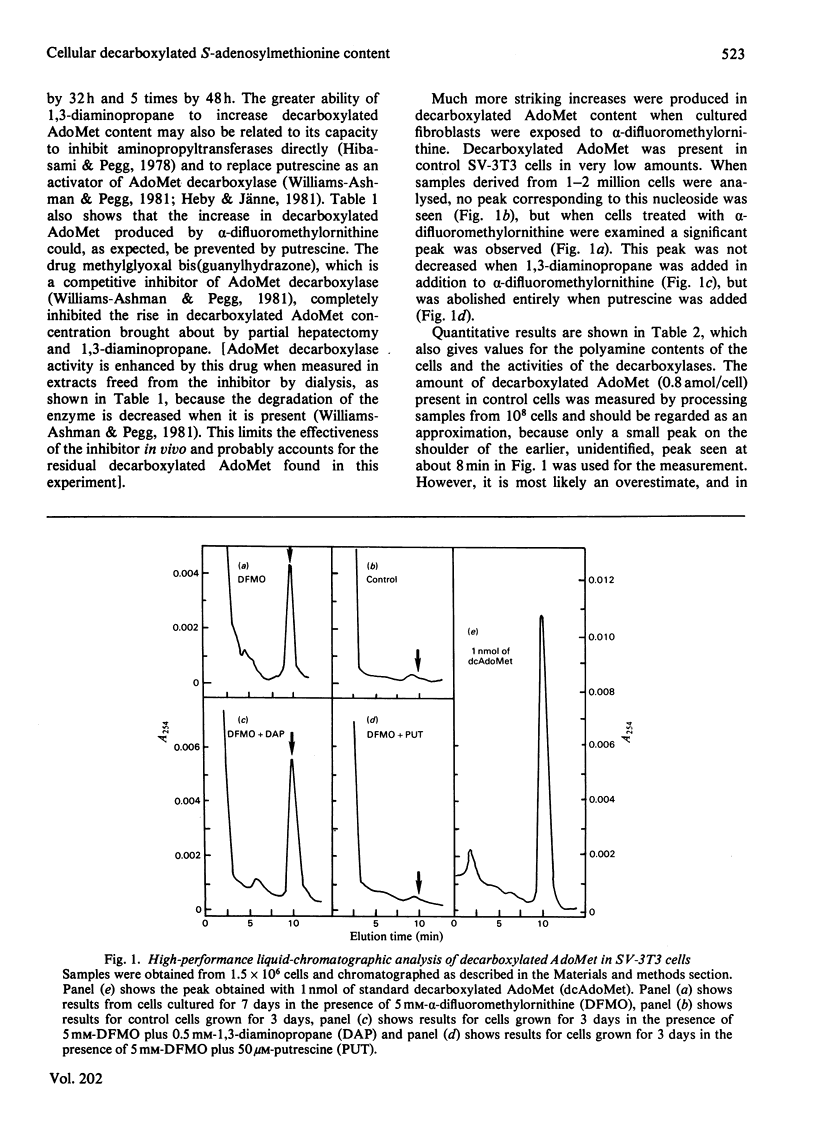
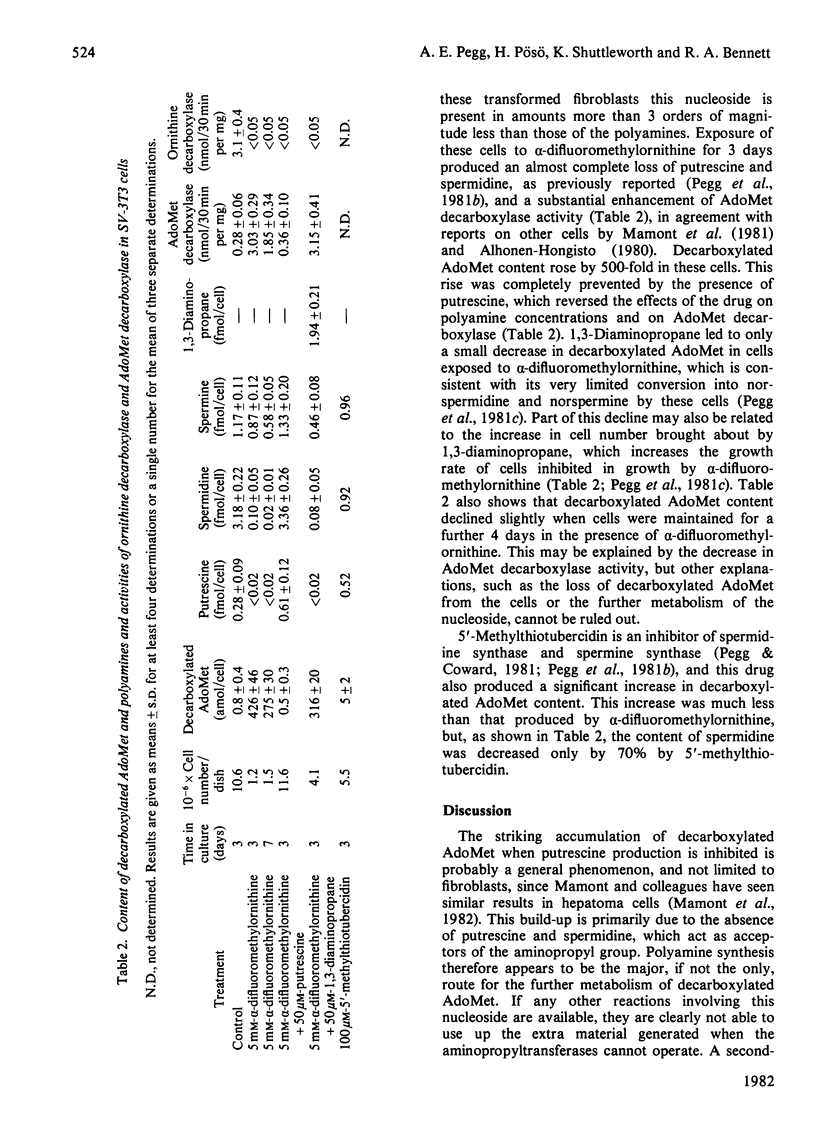
1. The content of decarboxylated S-adenosylmethionine (AdoMet) in transformed mouse fibroblasts (SV-3T3 cells) was increased 500-fold to about 0.4fmol/cell when ornithine decarboxylase was inhibited by α-difluoromethylornithine. This increase was due to the absence of putrescine and spermidine, which serve as substrates for aminopropyltransferases with decarboxylated AdoMet as an aminopropyl donor, and to the enhanced activity of AdoMet decarboxylase brought about by depletion of spermidine. The increase in decarboxylated AdoMet content was abolished by addition of putrescine, but not by 1,3-diaminopropane. 2. 5′-Methylthiotubercidin also increased decarboxylated AdoMet content, presumably by direct inhibition of aminopropyl-transferase activities, but the increase in its content and the decline in spermidine content were much less than those produced by α-difluoromethylornithine. 3. Decarboxylated AdoMet content of regenerating rat liver was measured in rats treated with inhibitors of ornithine decarboxylase. The content was increased by 60% 32h after partial hepatectomy in control rats, by 90% when α-difluoromethylornithine was given to the partially hepatectomized rats, and by 330% when 1,3-diaminopropane was used to inhibit putrescine and spermidine synthesis. After 48h of exposure to 1,3-diaminopropane, which completely prevented the increase in spermidine after partial hepatectomy, there was a 5-fold rise in hepatic decarboxylated AdoMet concentration. These increases were prevented by treatment with putrescine or with methylglyoxal bis(guanylhydrazone), an inhibitor of AdoMet decarboxylase. 4. These results show that changes in AdoMet metabolism result from the administration of specific inhibitors of polyamine synthesis. The possible consequences of the accumulation of decarboxylated AdoMet, which could, for example, interfere with normal cellular methylation or lead to depletion of cellular adenine nucleotides, should be considered in the interpretation of results obtained with such inhibitors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhonen-Hongisto L. Regulation of S-adenosylmethionine decarboxylase by polyamines in Ehrlich ascites-carcinoma cells grown in culture. Biochem J. 1980 Sep 15;190(3):747–754. doi: 10.1042/bj1900747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Bethell D. R., Pegg A. E. Effects of diamines on ornithine decarboxylase activity in control and virally transformed mouse fibroblasts. Biochem J. 1979 Apr 15;180(1):87–94. doi: 10.1042/bj1800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt R. T., Shiong Y., Huber J. A., Wycpalek A. F. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 6. Structural modifications of S-adenosylmethionine. J Med Chem. 1976 Sep;19(9):1104–1110. doi: 10.1021/jm00231a005. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Hibasami H., Hoffman J. L., Pegg A. E. Decarboxylated S-adenosylmethionine in mammalian cells. J Biol Chem. 1980 Jul 25;255(14):6675–6678. [PubMed] [Google Scholar]
- Hibasami H., Pegg A. E. Differential inhibition of mammalian aminopropyltransferase activities. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1398–1405. doi: 10.1016/0006-291x(78)91291-3. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Deen D. F., Seidenfeld J., Marton L. J. Sensitization of 9L rat brain gliosarcoma cells to 1,3-bis(2-chloroethyl)-1-nitrosourea by alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor. Cancer Res. 1981 Jul;41(7):2783–2785. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Joder-Ohlenbusch A. M., Nussli M., Grove J. Indirect evidence for a strict negative control of S-adenosyl-L-methionine decarboxylase by spermidine in rat hepatoma cells. Biochem J. 1981 May 15;196(2):411–422. doi: 10.1042/bj1960411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Borchardt R. T., Coward J. K. Effects of inhibitors of spermidine and spermine synthesis on polyamine concentrations and growth of transformed mouse fibroblasts. Biochem J. 1981 Jan 15;194(1):79–89. doi: 10.1042/bj1940079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Hibasami H. Polyamine metabolism during cardiac hypertrophy. Am J Physiol. 1980 Nov;239(5):E372–E378. doi: 10.1152/ajpendo.1980.239.5.E372. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Lockwood D. H., Williams-Ashman H. G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem J. 1970 Mar;117(1):17–31. doi: 10.1042/bj1170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Shuttleworth K., Hibasami H. Specificity of mammalian spermidine synthase and spermine synthase. Biochem J. 1981 Aug 1;197(2):315–320. doi: 10.1042/bj1970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pösö H., Pegg A. E. Differences between tissues in response of S-adenosylmethionine decarboxylase to administration of polyamines. Biochem J. 1981 Dec 15;200(3):629–637. doi: 10.1042/bj2000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Himberg J. J., Jänne J. Putrescine-insensitive S-adenosyl-L-methionine decarboxylase from Tetrahymena pyriformis. Acta Chem Scand B. 1975;29(9):932–936. doi: 10.3891/acta.chem.scand.29b-0932. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Zappia V., Galletti P., Porcelli M., Manna C., Ragione F. D. High-performance liquid chromatographic separation of natural adenosyl-sulphur compounds. J Chromatogr. 1980 Mar 7;189(3):399–405. doi: 10.1016/s0021-9673(00)80319-2. [DOI] [PubMed] [Google Scholar]
- Zappia V., Zydek-Cwick R., Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969 Aug 25;244(16):4499–4509. [PubMed] [Google Scholar]


