Abstract
Solid organ transplantation (SOT) is considered the optimal treatment for children with end-stage organ failure; however, increased efforts are needed to understand the gap surrounding equitable access to and health outcomes of SOT for Indigenous children. This scoping review summarizes the literature on the characteristics of access to and health outcomes of pediatric SOT among Indigenous children in the settler-colonial states of Canada, Aotearoa New Zealand, Australia, and the United States. A search was performed on MEDLINE, EMBASE, PsycINFO, and CINAHL for studies matching preestablished eligibility criteria from inception to November 2021. A preliminary gray literature search was also conducted. Twenty-four studies published between 1996 and 2021 were included. Studies addressed Indigenous pediatric populations within the United States (n = 7), Canada (n = 6), Aotearoa New Zealand (n = 5), Australia (n = 5), and Aotearoa New Zealand and Australia combined (n = 1). Findings showed that Indigenous children experienced longer time on dialysis, lower rates of preemptive and living donor kidney transplantation, and disparities in patient and graft outcomes after kidney transplantation. There were mixed findings about access to liver transplantation for Indigenous children and comparable findings for graft and patient outcomes after liver transplantation. Social determinants of health, such as geographic remoteness, lack of living donors, and traditional spiritual beliefs, may affect SOT access and outcomes for Indigenous children. Evidence gaps emphasize the need for action-based initiatives within SOT that prioritize research with and for Indigenous pediatric populations. Future research should include community-engaged methodologies, situated within local community contexts, to inform culturally safe care for Indigenous children.
INTRODUCTION
Indigenous peoples are the original inhabitants of present-day Canada, Aotearoa New Zealand, Australia, and the United States,1 and are diverse groups with cultures, traditions, and knowledge systems that are foundational to the health of Indigenous children.2 Yet, settler-colonial relations persist in these former European colonies, including land dispossession, state paternalism, loss of culture, racism, and violence, which are documented to impact the health and well-being of Indigenous children, families, and communities.3-5 Indigenous children have reported disproportionately higher rates of kidney, liver, cardiovascular, and respiratory diseases compared with non-Indigenous children2,6-8 that may progress to organ failure. Solid organ transplantation (SOT) is the optimal treatment for children with end-stage organ failure9 and is associated with markedly improved clinical and patient-reported outcomes.10,11 However, SOT is a complicated process, from an initial referral to a transplant center for evaluation to a strict regimen of immunosuppressant drug therapy and life-long medical follow-up, amid risks of severe complications posttransplant.9,12 The barriers for patients and families proceeding through these stages are multifaceted and ethno-racial disparities exist in access to and health outcomes of pediatric SOT.13
Recent studies have suggested that Indigenous pediatric patients have lower rates of access to kidney transplantation (KTx)14 and worse long-term graft outcomes than non-Indigenous patients15; however, research in this field is limited. Increased efforts are needed to understand and address the gap surrounding equitable access to and health outcomes of SOT between Indigenous and non-Indigenous children. Indigenous-focused and Indigenous-led data collection, through meaningful community-engaged partnerships, is the first step to understanding how to support Indigenous child health in pediatric SOT.
In recognizing this need, this scoping review addresses the question: What are the characteristics of access to care and health outcomes of pediatric SOT among Indigenous children in the settler-colonial states of Canada, Aotearoa New Zealand, Australia, and the United States? This exploratory review aims to summarize the current body of knowledge within pediatric SOT to identify evidence gaps and inform future research priorities to optimize the health and well-being of Indigenous children.
MATERIALS AND METHODS
This review was conducted following Arksey and O’Malley’s framework.16,17 In addition, a Patient Advisory Committee composed of First Nations and Métis SOT patient and family partners was established.18 Patient Advisory Committee members engaged in research processes, including codeveloping the search strategy and providing feedback on results and interpretation. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews was referred to for study reporting.19
Identifying the Research Question
The Participants, Concept, and Context framework was used for research question development.17 These elements are identified as follows: (a) Participants: Indigenous children; (b) Concept: characteristics of access to care and health outcomes of pediatric SOT; and (c) Context: Canada, Aotearoa New Zealand, Australia, and the United States. Access to care was defined as the ability to use timely and available healthcare services to achieve the best health outcomes,20 including being placed on the SOT waitlist, time to SOT, and access to living or deceased transplantation.
Setting Inclusion Criteria
Articles were included if the study (1) was English language and peer-reviewed; (2) was a primary study; (3) participants were <19 y old; (4) described access to or health outcomes of SOT (ie, heart, kidney, liver, lung, pancreas, and multiorgan); and (5) participants included Indigenous peoples in Canada, Aotearoa New Zealand, Australia, or the United States. Articles that included a participant population different from the population described above were included if the results were disaggregated within the article. Publication year was not a parameter for inclusion because this is the first known review on this topic.
Identifying Relevant Studies
Search Strategy
A comprehensive search of articles was performed on MEDLINE, EMBASE, PsycINFO, and CINAHL from inception to November 17, 2021. The search strategy was developed collaboratively among the research team, Patient Advisory Committee, and a medical librarian to identify a combination of most relevant search terms (Appendix 1, SDC, http://links.lww.com/TP/D65).21
Article Selection
Two independent reviewers (J.L. and I.S.) screened the title and abstracts and examined the full text of potentially relevant articles to determine eligibility. Discrepancies were reviewed and resolved, and if necessary, a third reviewer (S.J.A.) collaborated. The Kappa value for full-text review was scored at 0.77. Title, abstract, and full-text article screening were conducted on Covidence software.22
Extracting Data
A data extraction sheet was created and piloted by extracting data from 3 articles independently and comparing for alignment. Data extraction was then continued independently by the reviewers (J.L., I.S.) for the remaining articles, followed by a comparison for reliability and consistency. Data extracted from each article included as follows: (1) study characteristics, (2) participant characteristics, and (3) summary of results (eg, access to SOT, health outcomes, social determinants of health [SDOH]).
Summarizing and Reporting Results
Results were summarized and co-interpreted with the Patient Advisory Committee, who shared their lived experiences and feedback that provided patient and family perspectives on the included articles, aggregated results, research gaps, and clinical interpretations of the larger research team.
Gray Literature Search
A preliminary gray literature search was performed using Google Advanced Search to scope for published gray literature addressing SDOH affecting pediatric SOT among Indigenous children in Canada, Aotearoa New Zealand, Australia, and the United States. Searches were conducted with various word combinations of the same search terms as the peer-reviewed literature, using advanced search features for domain types (eg, .org and .gov) and search boxes (eg, 'all these words' and 'any of these words'). Four searches were completed for website landing pages, and the author (J.L.) reviewed the first 60 results of each search for relevance. Because of the lack of relevant gray literature found, this search was not continued past these preliminary steps.
RESULTS
Study Characteristics
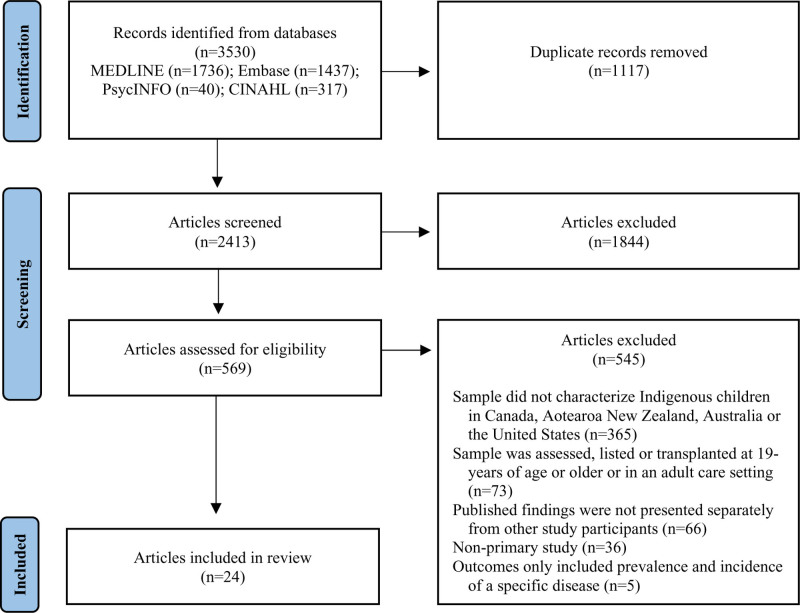
The original search yielded 3530 articles, of which 1117 articles were removed as duplicates. A total of 2413 articles were screened for eligibility at the title and abstract level, and 569 articles were reviewed at the full-text screening level. See Figure 1 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews diagram.19
FIGURE 1.
PRISMA-ScR: Flow of information through different phases of the scoping review. PRISMA-ScR, Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews.
Twenty-four articles were retained for analysis and included Indigenous populations within the United States23-29 (n = 7), Canada15,30-34 (n = 6), Aotearoa New Zealand35-39 (n = 5), Australia6,14,40-42 (n = 5), and Aotearoa New Zealand and Australia combined43 (n = 1). Article publication years ranged from 1996 to 2021, with 6 articles (25%) published within the last 5 y. Most articles used a retrospective cohort design (n = 21); 2 used a multiple case study design and 1 used a case study design. Several articles gathered participant data from the same databases: Australia and New Zealand Dialysis and Transplant Registry (n = 5), US Renal Data System (n = 3), and United Network for Organ Sharing (n = 2). A summary of the included articles is outlined in Table 1.
TABLE 1.
Overview of included articles
| Author, year; country | Organ | Objectives | Design and data source | Sample and characteristics |
|---|---|---|---|---|
| Chaturvedi et al,6 2021; Australia | Kidney | Described trends in incidence, prevalence, cause, and modality selection of KRT for First Nation Australian children and young adults in Australia | Retrospective cohort study using ANZDATA, between 1963 and 2017 | First Nation Australian (n = 178) |
| Other (n = 3451) | ||||
| Ferris et al,24 2006; United States | Kidney | Characterized the survival and outcomes of patients starting ESRD therapy and assessed specific outcomes in different ethnic groups | Retrospective cohort study using USRDS, between 1978 and 2002 | Native American (n = 208) |
| White (n = 11 329) | ||||
| Black (n = 4639) | ||||
| Asian (n = 587) | ||||
| Other (n = 272) | ||||
| Grace et al,40 2014; Australia | Kidney | Investigated racial disparities in uptake of KTx for pediatric patients in Australia and investigated clinical factors likely to influence KTx | Retrospective cohort study using ANZDATA, between 1990 and 2011 | First Nation Australian (n = 35) |
| Caucasian (n = 655) | ||||
| Other (n = 133) | ||||
| Grace et al,39 2014; Aotearoa New Zealand | Kidney | Investigated racial disparities in access and outcomes to KTx for pediatric patients treated for ESRD in New Zealand | Retrospective cohort study using ANZDATA, between 1990 and 2012 | Māori (n = 48) |
| European (n = 125) | ||||
| Asian (n = 12) | ||||
| Pacific (n = 30) | ||||
| Hiraki et al,23 2011; United States | Kidney | Identified predictors of waitlisting for KTx, KTx, and mortality among children with ESRD | Retrospective cohort study using USRDS, between 1995 and 2006 | American Indian (n = 14) |
| White (n = 219) | ||||
| African American (n = 287) | ||||
| Asian (n = 46) | ||||
| Hispanic (n = 142) | ||||
| Non-Hispanic (n = 441) | ||||
| Le Page et al,41 2021; Australia | Kidney | Described the incidence of new-onset vascular disease and vascular death in children receiving KRT and identified risk factors | Retrospective cohort study using ANZDATA, between 1991 and 2017 | First Nation Australian (n = 47) |
| Caucasian (n = 915) | ||||
| Other (n = 306) | ||||
| Matsuda-Abedini et al,15 2009; Canada | Kidney | Determined the short- and long-term outcomes of KTx among Aboriginal and non-Aboriginal children | Retrospective cohort study using British Columbia Children’s Hospital Transplant Program database, between 1985 and 2005 | Aboriginal (n = 24) |
| Non-Aboriginal (n = 135) | ||||
| McDonald and Russ,43 2003; Australia and Aotearoa New Zealand | Kidney | Reported the incidence and outcomes of Indigenous peoples who began KRT in Australia or New Zealand | Retrospective cohort study using ANZDATA, between 1991 and 2000 | First Nation Australian (n = 11) |
| Māori (n = 10) | ||||
| Non-Indigenous (n = 253) | ||||
| Pacific Islander (n = 10) | ||||
| Narva et al,25 1996; United States | Kidney | Investigated reported Arizona and New Mexico resident cases to understand more about Native American and White ESRD patients | Retrospective cohort study using ESRD Network No. 15; follow-up period not reported | Native American in Arizona (n = 1039) |
| Native American in New Mexico (n = 533) | ||||
| White in Arizona (n = 5448) | ||||
| White in New Mexico (n = 1811) | ||||
| Samuel et al,33 2011; Canada | Kidney | Evaluated differences in dialysis modality, time on dialysis, rates of KTx, and patient and graft survival between Aboriginal and non-Aboriginal children | Retrospective cohort study using the Canadian Pediatric End-stage Renal Disease database, between1992 and 2007 | Aboriginal (n = 104) |
| White (n = 521) | ||||
| Other (n = 218) | ||||
| Samuel et al,34 2011; Canada | Kidney | Estimated age-specific graft failure rates among KTx recipients and determined the influence of the adaptation period after adult care transfer on graft failure risk | Retrospective cohort study using the Canadian Organ Replacement Register, between 1992 and 2007 | Aboriginal (n = 35) |
| White (n = 256) | ||||
| Black (n = 9) | ||||
| Other (n = 113) | ||||
| Verghese et al,28 2020; United States | Kidney | Assessed rehospitalization rates within 1 y of KTx for recipients, risk factors associated with early and late rehospitalization, and the impact of rehospitalizations on patient and graft survival | Retrospective cohort study using USRDS, between 2006 and 2016 | American Indian (n = 14) |
| Caucasian (n = 153) | ||||
| African American (n = 13) | ||||
| Asian (n = 15) | ||||
| Unknown (n = 2) | ||||
| Weststrate et al,38 2021; Aotearoa New Zealand | Kidney | Determined the rates of preemptive KTx in the New Zealand pediatric chronic kidney disease population and sought potential barriers to preemptive KTx | Retrospective cohort study using the New Zealand National Paediatric Nephrology KF clinical database, between 2005 and 2017 | Māori (n = 21) |
| European (n = 34) | ||||
| Asian (n = 7) | ||||
| Pacific Islander (n = 13) | ||||
| Arnon et al,26 2013; United States | Liver | Investigated government insurance and outcomes after first LTx for children with biliary atresia | Retrospective cohort study using UNOS Standard Transplant Analysis and Research files, between 2003 and 2011 | American Indian or Alaskan Native, Native Hawaiian, Pacific Islander (n = 73) |
| White (n = 758) | ||||
| Black (n = 302) | ||||
| Hispanic (n = 335) | ||||
| Asian (n = 111) | ||||
| Chinnaratha et al,14 2014; Australia | Liver | Compared overall and graft survival after LTx between First Nation Australian and non-Indigenous patients, assessed the factors influencing survival, and examined the proportion of First Nation Australian patients undergoing LTx | Retrospective cohort study using Australian and New Zealand LTx registry, between 1985 and 2012 | First Nation Australian (n = 14) |
| Non-Indigenous (n = 622) | ||||
| Drouin et al,30 2000; Canada | Liver | Described the features of Aboriginal patients with North American Indian cirrhosis | Retrospective cohort study using clinical records from 1981 | Northern Ojibway or Cree descent (n = 30) |
| Evans et al,36 2018; Aotearoa New Zealand | Liver | Determined the incidence and outcome of biliary atresia with specific reference to whether these differed between ethnic groups | Retrospective cohort study using surgical records, databases and coding data, between 2002 and 2014 | Māori (n = 45) |
| Pacific Islander (n = 11) | ||||
| Asian (n = 9) | ||||
| European (n = 25) | ||||
| Hanna et al,42 2000; Australia | Liver | Described 3 cases of fulminant hepatitis A in Indigenous children in north Queensland | Multiple case study; data source not reported | First Nation Australian (n = 3) |
| Hsu et al,27 2015; United States | Liver | Described trends in the use of exceptions among the pediatric liver waitlist population | Retrospective cohort study using UNOS Organ Procurement and Transplant Network database, between 2002 and 2013 | American Indian/Alaskan Native (n = 28) |
| Native Hawaiian/Pacific Islander (n = 16) | ||||
| White (n = 1911) | ||||
| Black (n = 663) | ||||
| Hispanic (n = 814) | ||||
| Asian (n = 206) | ||||
| Multiracial (n = 90) | ||||
| Phillips et al,31 1996; Canada | Liver | Reported on 6 Native Canadian children with severe chronic cholestatic liver disease | Multiple case study using clinical records; follow-up period not reported | Native Canadian of Cree, Ojibwa-Cree, or unspecified descent (n = 6) |
| Shapiro,32 2005; Canada | Liver | Discussed whether parents should consent to having their child undergo LTx if considered medically necessary | Case study; data source not reported | First Nations (n = 1) |
| Smith et al,35 2002; Aotearoa New Zealand | Liver | Determined numbers and indications for LTx at present, current outcomes and estimated the likely demand for the service in the future | Retrospective cohort study using medical records, the Queensland LTx Unit database or the New Zealand LTx Trust, between 1990 and 2000 | Māori (n = 6) |
| Pacific Islander (n = 4) | ||||
| Other (n = 7) | ||||
| Wilde et al,37 2007; Aotearoa New Zealand | Liver | Reported the first 5 y of pediatric LTx undertaken by the New Zealand LTx Unit | Retrospective cohort study; data source not reported | Māori (n = 10) |
| European (n = 14) | ||||
| Pacific (n = 3) | ||||
| Asian (n = 1) | ||||
| Kleinmahon et al,29 2019; United States | Heart | Identified risk factors for rejection with severe hemodynamic compromise and predictors of graft failure | Retrospective cohort study using data collected from the Pediatric Heart Transplant Study, between 2005 and 2015 | American Indian/Alaskan Native/Pacific Islander (n = 55) |
| African American (n = 622) | ||||
| Asian (n = 120) | ||||
| White (n = 2211) | ||||
| Other (n = 251) |
ANZDATA, Australia and New Zealand Dialysis and Transplant Registry; ESRD, end-stage renal disease; KRT, kidney replacement therapy; KTx, kidney transplantation; LTx, liver transplantation; UNOS, United Network for Organ Sharing; USRDS, US Renal Data System.
Participant Characteristics
Articles included kidney (n = 13), liver (n = 10), and heart (n = 1) organ groups. No articles included participants across multiple organ groups or addressed lung or pancreas organ groups. Studies composed of 43 767 pediatric patients overall, consisting of Native Americans, including American Indians and Alaska Natives (n = 1980), Aboriginal and Torres Strait Islander peoples, hereafter respectfully referred to as First Nation Australians (n = 288), First Nations, Métis, and Inuit peoples (n = 200), and Māori peoples (n = 140).
Access to Pediatric SOT
Kidney
Ten articles reported on access to pediatric KTx among Indigenous children in Canada15,33 (n = 2), Aotearoa New Zealand38,39 (n = 2), Australia6,40 (n = 2), Aotearoa New Zealand and Australia combined43 (n = 1), and the United States23-25 (n = 3). Overall, articles showed that Indigenous children with kidney failure spent longer time on dialysis, were less likely to receive a preemptive transplant—the preferred kidney replacement therapy modality, and were less likely to receive a KTx from any source (living or deceased), especially a living donor KTx. Table 2 summarizes findings related to access to KTx.
TABLE 2.
Access to pediatric kidney transplantation
| Variable | Source, year | Country | Relevant results |
|---|---|---|---|
| Kidney replacement therapy | |||
| Disparities identified | McDonald and Russ,43 2023 | Aotearoa New Zealand and Australia | Proportion of First Nation Australian patients <15 y old at start of KRT was lower compared with non-Indigenous patients (P < 0.05) |
| Ferris et al,24 2006 | United States | Native American patients were more likely to be hemodialysis-dependant than White patients but fared better than Black patients | |
| Disparities not identified | Samuel et al,33 2011 | Canada | No difference in median age at start of KRT among Aboriginal, White, and patients of other ethnicities; hemodialysis was the initial modality for 48% of Aboriginal patients, 43% of White patients, and 63% of patients of other ethnicities (P < 0.001) |
| Grace et al,39 2014 | Aotearoa New Zealand | No difference in patient age at start of KRT among Māori, European, Asian, and Pacific Islander patients | |
| Weststrate et al,38 2021 | Aotearoa New Zealand | No difference in early vs late referral to KRT among Māori, European, Asian, and Pacific Islander patients | |
| Time to KTx | |||
| Disparities identified | Grace et al,40 2014 | Australia | Median time from KRT to deceased donor KTx was 4.3 y for First Nation Australian patients, 3.3 y for Caucasian patients, and 3.4 y for patients of other ethnicities; First Nation Australian patients were more likely to be referred late for a KTx than Caucasian patients and patients of other ethnicities (P < 0.001) |
| Chaturvedi et al,6 2021 | Australia | First Nation Australian patients spent over double the time on dialysis before receiving a KTx compared with patients of other ethnicities (P < 0.001) | |
| Samuel et al,33 2011 | Canada | Time from start of dialysis to first KTx was longer for Aboriginal than non-Aboriginal patients (P < 0.001) | |
| Hiraki et al,23 2011 | United States | American Indian patients had a trend of lower age- and sex-adjusted odds ratio of waitlisting for KTx compared with White patients | |
| Disparities not identified | Grace et al,39 2014 | Aotearoa New Zealand | No differences in time to first KTx listing among Māori, European, Asian, and Pacific Islander patients on dialysis |
| Grace et al,40 2014 | Australia | Median time from KRT to deceased donor KTx was 4.3 y for Indigenous patients, 3.3 y for Caucasian patients, and 3.4 y for patients of other ethnicities | |
| Matsuda-Abedini et al,15 2009 | Canada | No differences in waitlisting to KTx among Aboriginal and non-Aboriginal patients | |
| Preemptive KTx | |||
| Disparities identified | Matsuda-Abedini et al,15 2009 | Canada | No Aboriginal patients received a preemptive Tx compared with 22% of non-Aboriginal patients |
| Samuel et al,33 2011 | Canada | Aboriginal patients were less likely to receive a preemptive KTx than White patients (P < 0.001) | |
| Weststrate et al,38 2021 | Aotearoa New Zealand | No Māori patients received a (living or deceased) preemptive KTx; Māori patients were more likely to be “missed opportunities” for preemptive KTx (P = 0.0009) | |
| Grace et al,39 2014 | Aotearoa New Zealand | Māori patients did not receive any (living or deceased) preemptive KTx compared with 30% of European patients (P < 0.009) | |
| Grace et al,40 2014 | Australia | No First Nation Australian patients received a (living or deceased) preemptive KTx compared with 19% of Caucasian patients (P < 0.001) | |
| Chaturvedi et al,6 2021 | Australia | Preemptive KTx was performed at a lower rate among First Nation Australian patients than patients of other ethnicities; a total of 6 First Nation Australian patients received a preemptive KTx | |
| Living vs deceased KTx | |||
| Disparities identified | Grace et al,39 2014 | Aotearoa New Zealand | Māori patients had the highest mortality on KRT (P = 0.008) and were more likely to be dead without a KTx (P < 0.001); Māori patients were less likely to receive a living donor KTx but more likely to receive a deceased donor KTx than European patients (P < 0.001); there were proportionally fewer KTx of any type among Māori patients |
| Weststrate et al,38 2021 | Aotearoa New Zealand | There was a lower proportion of living donor KTx for Māori patients (71%) than Asian (100%) and European patients (88%); among 7 Māori patients who did not receive a KTx, 5 did not have a suitable living donor present for donor evaluation | |
| Chaturvedi et al,6 2021 | Australia | A higher percentage of First Nation Australian patients did not receive a KTx (P < 0.001) and transitioned to adult care on dialysis (P < 0.001); living donor KTx was performed at a lower rate among First Nation Australian patients than patients of other ethnicities (11% vs 36%; P < 0.001) | |
| Grace et al,40 2014 | Australia | First Nation Australian patients were less likely to receive a living donor KTx but had higher rates of deceased donor KTx than Caucasian patients (P < 0.001); First Nation Australian patients had proportionally fewer KTxs of any type | |
| Samuel et al,33 2011 | Canada | Aboriginal patients were less likely to receive a KTx from any source (HR = 0.54), a living donor (HR = 0.36), and a deceased donor (HR = 0.62) compared with White patients | |
| Narva et al,25 1996 | United States | Age-specific KTx rate was lower for Native American than White American patients in Arizona and New Mexico, although the significance was not tested | |
| Ferris et al,24 2006 | United States | Native American patients were less likely to receive a KTx compared with White patients (72% vs 82%; P = 0.0001) but more likely than Black patients | |
| Hiraki et al,23 2011 | United States | American Indian patients had a trend of lower age- and sex-adjusted odds ratio of receiving a KTx compared with White patients | |
| Disparities not identified | Matsuda-Abedini et al,15 2009 | Canada | No difference in living donor KTx among Aboriginal and non-Aboriginal patients |
HR, hazard ratio; KRT, kidney replacement therapy; KTx, kidney transplantation; Tx, transplantation.
Liver
Nine articles reported on access to pediatric liver transplantation (LTx) among Indigenous children with liver disease in Canada30-32 (n = 3), Aotearoa New Zealand35-37 (n = 3), Australia14,42 (n = 2), and the United States27 (n = 1). Overall, articles showed mixed findings with respect to disparities in referral for LTx, time to LTx, and access to LTx among Indigenous and non-Indigenous children. Table 3 summarizes findings related to access to LTx.
TABLE 3.
Access to pediatric liver transplantation
| Variable | Source, year | Country | Relevant results |
|---|---|---|---|
| Referral for LTx | |||
| Case studies | Hanna et al,42 2000 | Australia | Reported cases of fulminant hepatitis A in First Nation Australian patients; a total of 3 patients were transferred to a specialist and 2 patients were referred and listed for urgent LTx; however, all cases died before a donor organ was found; availability of a suitable donor organ may be a critical issue |
| Shapiro,32 2005 | Canada | Reported on a First Nations family’s reasons for foregoing LTx for their child; the parents were informed that LTx was the only available life-saving therapy but their traditional spiritual beliefs precluded introducing another person’s organ into their child, and they had concerns about the long-term effects of the immunosuppressive drugs; LTx was declined to allow the child to live their natural life | |
| Time to LTx | |||
| Disparities identified | Hsu et al,27 2015 | United States | Exception requests to MELD/PELD scores can be made among waitlisted candidates to expedite LTx; American Indian and Alaska Native patients represented 1% of all pediatric LTx exception requests and only 18% of patients received exception requested compared with 29%–37% of non-Indigenous patients |
| Disparities not identified | Chinnaratha et al,14 2014 | Australia | No difference in waitlisting for LTx and waitlist mortality among First Nation Australian and non-Indigenous patients; a greater proportion of First Nation Australian patients were too sick to receive an LTx, although the significance was not tested |
| Access to LTx | |||
| Disparities identified | Smith et al,35 2002 | Aotearoa New Zealand | Māori children were overrepresented in the pediatric LTx population as a higher proportion of Māori patients received LTx than would be expected from their proportion in the pediatric population (59% vs 29%; P < 0.01) |
| Wilde et al,37 2007 | Aotearoa New Zealand | Māori patients were overrepresented among pediatric LTx recipients, primarily because of the high incidence of extrahepatic biliary atresia in the pediatric Māori population | |
| Evans et al,36 2018 | Aotearoa New Zealand | Of 90 children born with biliary atresia, 56% of Māori patients were transplanted at a median age of 1.92 y compared with 72% of European patients at 1.01 y (P < 0.02); Māori patients had better “transplant-free survival” than European patients (P < 0.04) despite later age at Kasai portoenterostomy, possibly because of a unique form of biliary atresia with later presentation and more favorable outcomes | |
| Chinnaratha et al,14 2014 | Australia | First Nation Australian patients were older at age of LTx than non-Indigenous patients (P = 0.048), despite similar cause; approximately 2.2% of First Nation Australian patients received a LTx compared with 4.7% of non-Indigenous children in the general population, suggesting that First Nation Australian children are underrepresented in the Australian pediatric LTx population | |
| Disparities not identified | Hsu et al,27 2015 | United States | No difference in rates of LTx across racial groups that included American Indian and Alaska Native patients |
| Case studies | Phillips et al,31 1996 | Canada | Reported cases of severe chronic cholestatic liver disease among First Nations patients; all 6 patients were referred for LTx; a total of 5 patients underwent LTx, whereas 1 was assessed for LTx during the study period |
| Drouin et al,30 2000 | Canada | Reported cases of North American Indian cirrhosis among First Nations children; a total of 9 patients underwent LTx and 4 could not for various reasons; of the 21 patients who did not undergo LTx, 9 were alive at the time of the study and 12 passed away because of liver failure, accidents, or sepsis | |
LTx, liver transplantation; MELD, Model for End-stage Liver Disease; PELD, Pediatric End-stage Liver Disease.
Health Outcomes Following Pediatric SOT
Kidney
Eight articles reported on outcomes of pediatric KTx among Indigenous children in Canada15,33,34 (n = 3), Aotearoa New Zealand39 (n = 1), Australia41 (n = 1), and the United States23,24,28 (n = 3). Overall, articles showed disparities in long-term KTx graft outcomes and patient survival for Indigenous children compared with non-Indigenous children. Table 4 summarizes KTx graft and patient outcomes.
TABLE 4.
Health outcomes after pediatric kidney transplantation
| Variable | Source, year | Country | Relevant results |
|---|---|---|---|
| Graft outcomes | |||
| Disparities identified | Grace et al,39 2014 | Aotearoa New Zealand | Māori patients were more likely to experience delayed graft function among all primary KTx (P < 0.05), all primary deceased KTx (P < 0.05), but not living donor KTx compared with European patients; the 5-y death-censored graft survival was 61% for Māori patients and 88% for European patients; Māori patients reported lower rates of retransplantation compared with European patients (14% vs 36%), potentially because of noncompliance |
| Matsuda-Abedini et al,15 2009 | Canada | The estimated glomerular filtration rate at 2- and 5-y and long-term graft survival were worse for Aboriginal compared with non-Aboriginal patients (P < 0.005); Aboriginal patients also had increased rate of late rejections (P = 0.03) | |
| Samuel et al,34 2011 | Canada | Aboriginal patients had a higher risk of graft failure at the 3-y adaptation interval during the transition period to adult care compared with White patients (HR = 3.26) | |
| Verghese et al,28 2020 | United States | American Indian patients had a trend of higher rates of early rehospitalization compared with Caucasian patients (57% vs 39%) | |
| Disparities not identified | Matsuda-Abedini et al,15 2009 | Canada | No difference in early graft outcomes, number of acute rejection episodes, and estimated glomerular filtration rate at 1-y among Aboriginal and non-Aboriginal patients |
| Samuel et al,33 2011 | Canada | No difference in median time from first KTx to graft failure and overall unadjusted kidney graft failure at 5 and 10 y among Aboriginal, Black, White, and patients of other ethnicities | |
| Verghese et al,28 2020 | United States | No difference in rates of late rehospitalization after KTx among American Indian, Caucasian, African American, and Asian patients | |
| Patient outcomes | |||
| Disparities identified | Grace et al,39 2014 | Aotearoa New Zealand | Among children who started KRT, including those with and without a KTx, Māori patients had a higher adjusted mortality rate than European patients (HR = 4.26) |
| Le Page et al,41 2021 | Australia | For patients followed into adulthood receiving KRT, including those with and without a KTx, Indigenous ethnicity was associated with vascular disease (HR = 3.60), vascular mortality (HR = 4.51), and composite vascular outcome (HR = 3.62) | |
| Matsuda-Abedini et al,15 2009 | Canada | There were 5.7% deaths in the study population, comprising 12.5% of Aboriginal patients and 4.4% of non-Aboriginal patients | |
| Ferris et al,24 2006 | United States | Native American patients had lower 10-y KTx survival rates compared with White and Asian patients (P = 0.0001) but higher than Black patients | |
| Hiraki et al,23 2011 | United States | American Indian patients had a trend of higher overall mortality compared with White patients | |
| Disparities not identified | Le Page et al,41 2021 | Australia | No difference in childhood vascular disease, vascular mortality, and composite vascular outcome among First Nation Australian and non-Indigenous patients receiving KRT, including KTx |
| Samuel et al,33 2011 | Canada | No differences in 5- and 10-y patient survival rates across Indigenous, Black, White, and patients of other ethnicities | |
HR, hazard ratio; KRT, kidney replacement therapy; KTx, kidney transplantation.
Liver
Six articles reported on outcomes of pediatric LTx among Indigenous children in Canada30 (n = 1), Aotearoa New Zealand35-37 (n = 3), Australia14 (n = 1), and the United States26 (n = 1). Overall, articles showed that LTx graft outcomes and patient survival were comparable between Indigenous and non-Indigenous children. Table 5 summarizes LTx graft and patient outcomes.
TABLE 5.
Health outcomes after pediatric liver transplantation
| Variable | Source, year | Countries | Relevant results |
|---|---|---|---|
| Graft outcomes | |||
| Disparities not identified | Wilde et al,37 2007 | Aotearoa New Zealand | Graft survival was 97% in the sample, of whom 36% were Māori, during the 5-y study period |
| Chinnaratha et al,14 2014 | Australia | No difference in cumulative 5- and 10-y graft survival between First Nation Australian and non-First Nation Australian patients (72.4% and 63.4%) | |
| Arnon et al,26 2013 | United States | No difference in 1- and 5-y liver graft survival among American Indian or Alaskan Native, White, Black, Hispanic, and Asian patients | |
| Patient outcomes | |||
| Disparities identified | Chinnaratha et al,14 2014 | Australia | The remoteness of the family residence was the only predictor of death and re-Tx (P = 0.03), with a trend toward increased death and re-Tx rates in First Nation Australian patients from remote areas (P = 0.08) |
| Disparities not identified | Smith et al,35 2002 | Aotearoa New Zealand | All 17 patients in the sample, including 6 Māori children, were participating in a full-time school program |
| Wilde et al,37 2007 | Aotearoa New Zealand | All 28 patients in the sample, including 10 Māori children, were living at home and those who were of school age attended school | |
| Evans et al,36 2018 | Aotearoa New Zealand | Comparatively, 11% of Māori patients died, 8% of European patients died, 44% of Asian patients died, and 9% of Pacific Islander patients died | |
| Chinnaratha et al,14 2014 | Australia | No differences in overall survival rate between First Nation Australian and non-Indigenous patients | |
| Case studies | Drouin et al,30 2000 | Canada | Of the 9 First Nations LTx recipients, 7 were alive at the time of the study with no reoccurrence of disease, whereas 2 patients did not survive LTx |
LTx, liver transplantation; Tx, transplantation.
Heart
One article reported on outcomes of pediatric heart transplantation from an international registry including Indigenous children from the United States.29 Analysis did not find an association between American Indian and Alaskan Native ethnicity with rejection with severe hemodynamic compromise after pediatric heart transplantation.29
SDOH Affecting Pediatric SOT
Seven articles reported on SDOH affecting pediatric SOT for Indigenous patients. According to Samuel et al,33 a greater proportion of Indigenous children with kidney failure in Canada lived >300 km away from the nearest pediatric kidney center (P < 0.001) and were in the lowest income quintile (P < 0.001). Similarly, Chaturvedi et al6 reported that First Nation Australian patients with kidney failure in Australia were more likely to reside in remote locations (P < 0.001). Another Australian study found that the remoteness of family residence was the only predictor of death and retransplantation among pediatric LTx recipients (P = 0.03), with a trend of increased death and retransplantation among First Nation Australian patients from remote areas.14
A lack of suitable donors and traditional beliefs were also highlighted to affect access to pediatric SOT. Weststrate et al38 and Hanna et al42 noted that the availability of a suitable donor organ may be a critical issue for Māori and First Nation Australian patients who were not transplanted in Aotearoa New Zealand and Australia. Another study suggested that disparities in graft outcomes for Māori KTx patients may be because of a lack of living donors and well-matched deceased kidney donors.39 In 1 article, traditional spiritual beliefs was reported to impact the acceptance of SOT as a life-saving therapy.32
DISCUSSION
This is the first scoping review to synthesize the literature on the characteristics of access to and health outcomes of pediatric SOT for Indigenous children in Canada, Aotearoa New Zealand, Australia, and the United States. Twenty-four articles met inclusion criteria, and there was a paucity of relevant gray literature. This review identified that Indigenous children with kidney failure experience longer time on dialysis, lower rates of preemptive and living donor KTx, and disparities in long-term graft and patient outcomes. There were mixed findings about access to pediatric LTx and comparable findings for LTx graft and patient outcomes among Indigenous children. In 1 article addressing pediatric heart transplantation, there was no information about access and limited evidence about outcomes among Indigenous patients.
Although this review focused on Indigenous pediatric patients, it is notable that findings align with current research examining health disparities in access to and outcomes of SOT for Indigenous adult populations. In their review addressing Indigenous adults, Yeates et al44 reported that Indigenous patients in Australia, Canada, Aotearoa New Zealand, and the United States faced significantly longer wait times for KTx and lower rates of KTx compared with Caucasian patients. The authors cited potential barriers as delayed referral for KTx evaluation and from referral to KTx waitlist,44 which is also reflected in the current findings showing low rates of preemptive KTx among Indigenous children.33,38-40 Zhang et al45 reported that the incidence of rejection, graft, and patient survival between adult First Nations and non-First Nations populations undergoing LTx were similar; however, they cited the reasons as unclear. The absence of disparities in access to and outcomes of pediatric LTx among Indigenous children in the current review is also unexplained, but notable.
It is apparent that additional research is needed to investigate the scope in topic that illuminates access to and outcomes of pediatric KTx and LTx among Indigenous patients. Namely, considerations of historical, contextual, and sociocultural determinants are essential if we are to provide an informed discussion on the biomedical outcomes of SOT for Indigenous pediatric populations. Findings from this review suggest determinants of Indigenous health,46,47 similar to findings within the adult population,48 that may affect pediatric SOT access and outcomes, including the lack of living organ donors, traditional spiritual beliefs surrounding SOT, and the geographic remoteness of patients and families. Specifically, the lack of specialized medical support in remote communities is a known barrier to accessing healthcare for Indigenous peoples.49 Indigenous patients living in remote communities must often relocate to an urban center to await and undergo SOT, leaving behind their cultural support systems while incurring additional financial stressors,48 both potentially affecting their SOT experience and subsequent outcomes. Furthermore, in the adult literature, Indigenous patients have reported that pursuing preemptive or living donor KTx is complicated by a reluctance to accept an organ from a family or community member,50 concerns that the donor may experience negative health outcomes,51 and a need to integrate cultural practices within the SOT experience to honor the donor (eg, smudges, healing circles).52 These are areas to further explore within the Indigenous pediatric population.
There is value in acknowledging the marked absence of meaningful discussion within the included articles of the impacts of settler colonialism, generational trauma, institutional racism, and cultural bias as critical barriers to accessing and receiving appropriate healthcare for Indigenous populations, including access to and health outcomes of pediatric SOT for Indigenous children.3-5 Of interest, Gerrald and McDonald53 assessed the multiple steps to receiving a KTx and identified several systemic biases for adult First Nation Australian patients, including culturally biased healthcare practices, that contributed to the lower rates of KTx within this population.54 Their findings draw attention to the lack of access to traditional ceremony within Western-based clinical care and the need for patient education and healthcare provider training that incorporates considerations of Indigenous worldviews and value systems.55 We discern that a similar need in pediatric care is present, and future research is warranted to prioritize exploration and understanding of the impacts of systemic biases within pediatric SOT care for Indigenous patients and families.
Although this scoping review identified useful, albeit limited, evidence about the characteristics of access to and health outcomes of pediatric SOT among Indigenous children, published findings and gray literature illustrate substantial gaps in evidence with regards to organ groups (eg, heart, lung, pancreas, multiorgan), holistic patient-reported outcomes (eg, quality of life, strengths-based outcomes), and research approaches (eg, community-based and participatory research methodologies). Challenges in identifying and (mis)reporting Indigenous patients within healthcare and health research,56 including because of stigma or historic lack of safety, may contribute to this population’s erasure in research,1 especially within studies that only use population health database registries. Of the included articles, only one explicitly reported working with Indigenous partners (eg, the Australia and New Zealand Dialysis and Transplant Registry Aboriginal and Torres Strait Islander Health Working Group41). Community-based and participatory approaches with Indigenous stakeholders, including community leaders, are necessary for research concerning Indigenous peoples and may support local Indigenous self-determination, nurture strengths-based interpretations inclusive of Indigenous worldviews, and research co-ownership.57-59
Furthermore, qualitative methodologies, including ethnography and narrative inquiry, may be valuable within future work that aims to elucidate social and historical determinants of care, such as the impact of colonialism, trauma, racism, and cultural bias on SOT experiences for Indigenous children. Their value lies in an understanding that Indigenous knowledge is often shared through unique representations of life experiences, such as storytelling, ceremonies, and artistic expression.60
This research demonstrates that exploring the experiences of Indigenous people globally can offer a shared narrative. Alternatively, it emphasizes a need to recognize the significant diversity within Indigenous communities and groups, including the distinct cultural, spiritual, historical, and political contexts that shape Indigenous health and well-being beyond geography.1 Although this scoping review included Indigenous children across 4 settler-colonial countries, Indigenous peoples and communities are not homogenous, and researchers and healthcare providers must be attuned to how an individual or community identifies, offering respect for the diversity of history and culture within communities.56 Research, care, and interventions need to reflect the distinct cultural values and specific needs of the local patient and community population and be community-engaged to offer impact on health outcomes for Indigenous children. As an exploratory approach, this scoping review used a global perspective as a necessary starting point to increase our understanding of the breadth of barriers and outcomes for Indigenous children accessing pediatric SOT within culturally-based lived experiences. Future research could build on this work by situating research questions within local community-led contexts to capture the depth of characteristics, experiences, and outcomes necessary to improve Indigenous health equity for children accessing SOT.
Limitations
It is possible that relevant articles were missed for review because of limited terminology that captures the broad range of community names within Indigenous populations.1,21 Furthermore, we did not exclude articles based on publication date, yet we acknowledge that data published 20 y past or more may have limited relevance given changes in contemporary contexts among Indigenous groups (eg, population demographics, disease incidence, clinical practices, and policies). Other research design approaches, such as an environmental scan, may have allowed us to be more culturally responsive to the contextual realities and perspectives of pediatric SOT underlying the lived experience of Indigenous communities through interviews with key stakeholders.61,62
CONCLUSIONS
This scoping review identified disparities and mixed findings concerning access to and health outcomes of pediatric SOT among Indigenous children in the settler-colonial states of Canada, Aotearoa New Zealand, Australia, and the United States. There was an absence of meaningful discussion within the included articles of historical and sociocultural determinants, including the impacts of settler colonialism, generational trauma, cultural bias, and institutional racism, that may enact as barriers to access and health outcomes of pediatric SOT for Indigenous children. Identifying substantial evidence gaps in the current review emphasizes the timeliness of action-based conversations and funding opportunities that prioritize research with and for Indigenous populations. Research initiatives that place value on community-engaged and participatory methodologies, situated within local community-led contexts, have the potential to inform culturally safe and strengths-based care for Indigenous children in need of SOT.
Supplementary Material
Footnotes
This research was supported by the Canadian Institutes of Health Research and the Canadian Blood Services.
The authors declare no conflicts of interest.
All authors were involved in study concept and design. J.L., E.K.S., I.S., and S.J.A. were involved in acquisition of data, analysis, or interpretation of data. J.L., E.K.S., I.S., and S.J.A. drafted the article, and all authors participated in critical revision for important intellectual content. All authors read and approved the final article and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Bartlett JG, Madariaga-Vignudo L, O’Neil JD, et al. Identifying indigenous peoples for health research in a global context: a review of perspectives and challenges. Int J Circumpolar Health. 2007;66:287–307. [DOI] [PubMed] [Google Scholar]
- 2.Brewster DR, Morris PS. Indigenous child health: are we making progress? J Paediatr Child Health. 2015;51:40–47. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe P. Settler colonialism and the elimination of the native. J Genocide Res. 2006;8:387–409. [Google Scholar]
- 4.Allan B, Smylie J. First peoples, second class treatment: the role of racism in the health and well-being of indigenous peoples in Canada [The Wellesley Institute]. Available at https://www.wellesleyinstitute.com/wp-content/uploads/2015/02/Summary-First-Peoples-Second-Class-Treatment-Final.pdf. Accessed January 9, 2023.
- 5.Cooke M, Mitrou F, Lawrence D, et al. Indigenous well-being in four countries: an application of the UNDP’S human development index to indigenous peoples in Australia, Canada, New Zealand, and the United States. BMC Int Health Hum Rights. 2007;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi S, Ullah S, LePage AK, et al. Rising incidence of end-stage kidney disease and poorer access to kidney transplant among Australian Aboriginal and Torres Strait Islander children and young adults. Kidney Int Rep. 2021;6:1704–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang AB, Brown N, Toombs M, et al. Lung disease in indigenous children. Paediatr Respir Rev. 2014;15:325–332. [DOI] [PubMed] [Google Scholar]
- 8.Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75. [DOI] [PubMed] [Google Scholar]
- 9.Black CK, Termanini KM, Aguirre O, et al. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6:409–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rosa C, Jorge Baluarte H, Meyers KE. Outcomes in pediatric solid-organ transplantation. Pediatr Transplant. 2011;15:128–141. [DOI] [PubMed] [Google Scholar]
- 11.Kim JJ, Marks SD. Long-term outcomes of children after solid organ transplantation. Clinics (Sao Paulo). 2014;69:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shellmer D, Brosig C, Wray J. The start of the transplant journey: referral for pediatric solid organ transplantation. Pediatr Transplant. 2014;18:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggers PW. Racial disparities in access to transplantation: a tough nut to crack. Kidney Int. 2009;76:589–590. [DOI] [PubMed] [Google Scholar]
- 14.Chinnaratha MA, Chelvaratnam U, Stuart KA, et al. ; Australia and New Zealand Liver Transplant Clinical Study Group. Liver transplantation outcomes for Australian Aboriginal and Torres Strait Islanders. Liver Transpl. 2014;20:798–806. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda-Abedini M, Al-AlSheikh K, Hurley RM, et al. Outcome of kidney transplantation in Canadian Aboriginal children in the province of British Columbia. Pediatr Transplant. 2009;13:856–860. [DOI] [PubMed] [Google Scholar]
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 17.Peters MD, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18:2119–2126. [DOI] [PubMed] [Google Scholar]
- 18.Smylie J. The ethics of research involving Canada’s Aboriginal populations. CMAJ. 2005;172:977; author reply 977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplantation Network. Organ transplantation: report on equity in access. Available at https://optn.transplant.hrsa.gov/media/2260/equity_in_access_report_201708.pdf. Accessed October 12, 2021.
- 21.University of Alberta. Health sciences search filters, indigenous peoples. Available at https://guides.library.ualberta.ca/search-filters. Accessed October 12, 2021. [Google Scholar]
- 22.Babineau J. Product review: Covidence (systematic review software). J Can Health Libr Assoc. 2014;35:68–71. [Google Scholar]
- 23.Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum. 2011;63:1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris ME, Gipson DS, Kimmel PL, et al. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21:1020–1026. [DOI] [PubMed] [Google Scholar]
- 25.Narva A, Stiles S, Karp S, et al. Access of Native Americans to renal transplantation in Arizona and New Mexico. Blood Purif. 1996;14:293–304. [DOI] [PubMed] [Google Scholar]
- 26.Arnon R, Annunziato RA, Willis A, et al. Liver transplantation for children with biliary atresia in the pediatric end-stage liver disease era: the role of insurance status. Liver Transpl. 2013;19:543–550. [DOI] [PubMed] [Google Scholar]
- 27.Hsu E, Shaffer M, Bradford M, et al. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015;15:436–444. [DOI] [PubMed] [Google Scholar]
- 28.Verghese PS, Chinnakotla S, Berglund D, et al. Re-hospitalization after pediatric kidney transplant: a single-center study. Pediatr Transplant. 2020;24:e13717. [DOI] [PubMed] [Google Scholar]
- 29.Kleinmahon JA, Gralla J, Kirk R, et al. Cardiac allograft vasculopathy and graft failure in pediatric heart transplant recipients after rejection with severe hemodynamic compromise. J Heart Lung Transplant. 2019;38:277–284. [DOI] [PubMed] [Google Scholar]
- 30.Drouin E, Russo P, Tuchweber B, et al. North American Indian cirrhosis in children: a review of 30 cases. J Pediatr Gastroenterol Nutr. 2000;31:395–404. [DOI] [PubMed] [Google Scholar]
- 31.Phillips MJ, Ackerley CA, Superina RA, et al. Excess zinc associated with severe progressive cholestasis in Cree and Ojibwa-Cree children. Lancet. 1996;347:866–868. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro C. Organ transplantation in infants and children—necessity or choice: the case of K’aila Paulette. Pediatr Nurs. 2005;31:121–122. [PubMed] [Google Scholar]
- 33.Samuel SM, Foster BJ, Tonelli MA, et al. ; Pediatric Renal Outcomes Canada Group. Dialysis and transplantation among Aboriginal children with kidney failure. CMAJ. 2011;183:E665–E672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, et al. ; Pediatric Renal Outcomes Canada Group. Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation. 2011;91:1380–1385. [DOI] [PubMed] [Google Scholar]
- 35.Smith J, Wesley A, Chin S, et al. Auckland paediatric liver transplant experience 1990-2000. N Z Med J. 2002;115:244–245. [PubMed] [Google Scholar]
- 36.Evans HM, Asher MI, Cameron-Christie S, et al. Ethnic disparity in the incidence and outcome of biliary atresia in New Zealand. J Pediatr Gastroenterol Nutr. 2018;66:218–221. [DOI] [PubMed] [Google Scholar]
- 37.Wilde J, Chin S, Johnston P, et al. Paediatric liver transplantation in New Zealand: the first 5 years. N Z Med J. 2007;120:U2679. [PubMed] [Google Scholar]
- 38.Weststrate H, Ronaldson J, Yonge G, et al. Barriers to pre-emptive kidney transplantation in New Zealand children. J Paediatr Child Health. 2021;57:1490–1497. [DOI] [PubMed] [Google Scholar]
- 39.Grace BS, Kara T, Kennedy SE, et al. Racial disparities in pediatric kidney transplantation in New Zealand. Pediatr Transplant. 2014;18:689–697. [DOI] [PubMed] [Google Scholar]
- 40.Grace BS, Kennedy SE, Clayton PA, et al. Racial disparities in paediatric kidney transplantation. Pediatr Nephrol. 2014;29:125–132. [DOI] [PubMed] [Google Scholar]
- 41.Le Page AK, Kennedy SE, Durkan A, et al. Incidence and predictors of vascular events following end-stage kidney disease in childhood. Nephrology (Carlton). 2021;26:715–724. [DOI] [PubMed] [Google Scholar]
- 42.Hanna JN, Warnock TH, Shepherd RW, et al. Fulminant hepatitis A in indigenous children in north Queensland. Med J Aust. 2000;172:19–21. [DOI] [PubMed] [Google Scholar]
- 43.McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton). 2003;8:42–48. [DOI] [PubMed] [Google Scholar]
- 44.Yeates KE, Cass A, Sequist TD, et al. Indigenous people in Australia, Canada, New Zealand and the United States are less likely to receive renal transplantation. Kidney Int. 2009;76:659–664. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Uhanova J, Minuk G. Liver transplant outcomes in a Canadian First Nations population. Can J Gastroenterol Hepatol. 2011;25:307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenwood ML, de Leeuw SN. Social determinants of health and the future well-being of Aboriginal children in Canada. Paediatr Child Health. 2012;17:381–384. [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll SR, Suina M, Jäger MB, et al. Reclaiming indigenous health in the US: moving beyond the social determinants of health. Int J Environ Res Public Health. 2022;19:7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Dassouki N, Wong D, Toews DM, et al. Barriers to accessing kidney transplantation among populations marginalized by race and ethnicity in Canada: a scoping review part 1-Indigenous communities in Canada. Can J Kidney Health Dis. 2021;8:2054358121996835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374:76–85. [DOI] [PubMed] [Google Scholar]
- 50.Keddis M, Finnie D, Kim WS. Native American patients’ perception and attitude about kidney transplant: a qualitative assessment of patients presenting for kidney transplant evaluation. BMJ Open. 2019;9:e024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mollins CJ. The impact of urban relocation on native kidney transplant patients and their families: a retrospective study [University of Manitoba]. Available at https://mspace.lib.umanitoba.ca/server/api/core/bitstreams/cadb43c3-e036-40fe-9d13-d406a339b944/content. Accessed March 1, 2024.
- 52.Smith M. Nagweyaab Geebawug: a retrospective autoethnography of the lived experience of kidney donation. CANNT J. 2015;25:13–18. [PubMed] [Google Scholar]
- 53.Garrard E, McDonald S. Improving access to and outcomes of kidney transplantation for Aboriginal and Torres Strait Islander people in Australia: performance report [The Transplantation Society of Australia and New Zealand]. Available at https://www.anzdata.org.au/wp-content/uploads/2019/07/TSANZ-Performance-Report-Improving-Indigenous-Transplant-Outcomes-Final-edited-1.pdf. Accessed March 1, 2024. [Google Scholar]
- 54.Kelly J, Dent P, Owen K, et al. Cultural bias Indigenous kidney care and kidney transplantation report [National Indigenous Kidney Transplantation Taskforce]. Available at https://tsanz.com.au/storage/NIKTT/CulturalBias_FinalReport.pdf. Accessed March 1, 2024. [Google Scholar]
- 55.Hughes JT, Owen KJ, Kelly J, et al. Cultural bias in kidney care and transplantation: review and recommendations to improve kidney care for Aboriginal and Torres Strait Islander people. Med J Aust. 2023;219:S11–S14. [DOI] [PubMed] [Google Scholar]
- 56.Canuto K, Finlay SM. I am not here for your convenience. Aust N Z J Public Health. 2021;45:305–306. [DOI] [PubMed] [Google Scholar]
- 57.Wilson S. Research Is Ceremony: Indigenous Research Methods. Fernwood Publishing; 2020. [Google Scholar]
- 58.Robinson-Settee H, Settee C, King M, et al. Wabishki Bizhiko Skaanj: a learning pathway to foster better Indigenous cultural competence in Canadian health research. Can J Public Health. 2021;112:912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huria T, Palmer SC, Pitama S, et al. Consolidated criteria for strengthening reporting of health research involving Indigenous peoples: the CONSIDER statement. BMC Med Res Methodol. 2019;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iseke J. Indigenous storytelling as research. Int Rev Qual Res. 2013;6:559–577. [Google Scholar]
- 61.Graham P, Evitts T, Thomas-MacLean R. Environmental scans: how useful are they for primary care research? Can Fam Physician. 2008;54:1022–1023. [PMC free article] [PubMed] [Google Scholar]
- 62.National Collaborating Centre for Aboriginal Health. Landscapes of First Nations, Inuit, and Métis health: an environmental scan of organizations, literature and research, 3rd edition. Available at https://www.nccih.ca/docs/context/RPT-LandscapesofHealth2014-EN.pdf. Accessed March 1, 2024. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.