This prespecified analysis of the Finerenone Trial to Investigate the Efficacy and Safety Superior to Placebo in Patients With Heart Failure (FINEARTS-HF) randomized clinical trial investigates the long-term effects of treatment with the nonsteroidal mineralocorticoid receptor antagonist, finerenone, in patients with heart failure with mildly reduced or preserved ejection fraction.
Key Points
Question
What are the long-term expected effects of treatment with the nonsteroidal mineralocorticoid receptor antagonist, finerenone, in patients with heart failure with mildly reduced or preserved ejection fraction?
Findings
In this prespecified analysis applying validated age-based methods to the Finerenone Trial to Investigate the Efficacy and Safety Superior to Placebo in Patients With Heart Failure (FINEARTS-HF) randomized clinical trial including 6001 participants, finerenone was estimated to extend survival free from cardiovascular death or a worsening heart failure event by up to 3 years.
Meaning
These data support the role of finerenone in modifying longitudinal risks of clinical events and provide estimates of its long-term expected therapeutic benefits that might be useful to aid clinical decision-making.
Abstract
Importance
People living with heart failure (HF) with mildly reduced or preserved ejection fraction have substantially curtailed life expectancy free from clinical events compared with their peers of comparable age. The nonsteroidal mineralocorticoid receptor antagonist, finerenone, was recently shown to reduce risks of cardiovascular events in this population over a median follow-up of 2.6 years; as patients with HF typically continue treatment beyond this time frame, estimating the potential long-term benefits of finerenone could inform shared clinical decision-making.
Objective
To estimate the projected long-term treatment effects of finerenone in patients with HF with mildly reduced or preserved ejection fraction if treated over a patient’s lifetime.
Design, Setting, and Participants
Prespecified analyses were conducted of the FINEARTS-HF trial, a phase 3 randomized clinical trial conducted across 653 sites in 37 countries. Adults 40 years and older with symptomatic HF and left ventricular ejection fraction of 40% or greater were randomized from September 2020 to January 2023. Median (IQR) follow-up was 2.6 (1.9-3.0) years.
Interventions
Finerenone (titrated to either 20 mg or 40 mg) or placebo.
Main Outcomes and Measures
The primary composite outcome was time to cardiovascular death or worsening HF event. The long-term gains in survival free from a primary end point with finerenone were iteratively estimated with age-based Kaplan-Meier curves using age at randomization rather than time from randomization. Differences in areas under the survival curves between the finerenone and placebo arms represented event-free survival gains.
Results
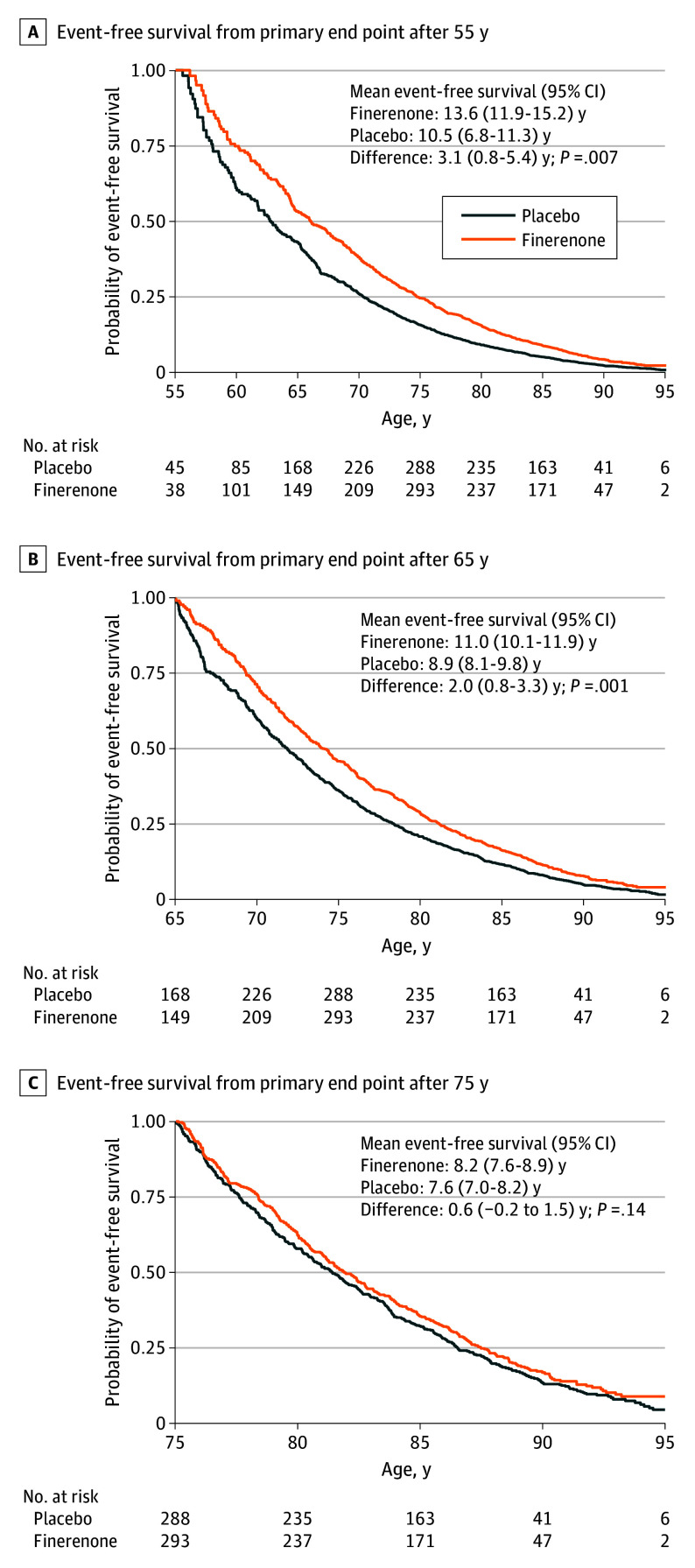
Among 6001 participants (median [IQR] age, 73 [66-79] years; 3269 male [54.5%]), mean survival free from the primary end point for a 55-year-old participant was 13.6 years (95% CI, 11.9-15.2 years) with finerenone and 10.5 years (95% CI, 6.8-11.3 years) with placebo, representing a gain in event-free survival of 3.1 years (95% CI, 0.8-5.4 years; P = .007). Mean event-free survival for a 65-year-old participant was 11.0 years (95% CI, 10.1-11.9 years) with finerenone and 8.9 years (95% CI, 8.1-9.8 years) with placebo, representing a gain of 2.0 years (95% CI, 0.8-3.3 years; P = .001). Projected mean event-free survival was numerically greater with finerenone than with placebo for every starting age between 50 to 80 years. Lifetime gains in event-free survival were observed even among individuals already treated with a sodium-glucose cotransporter 2 inhibitor (65-year-old participant: 3.1 years; 95% CI, 0.1-6.0 years; P = .04).
Conclusions and Relevance
In this prespecified secondary analysis of the FINEARTS-HF randomized clinical trial, long-term treatment with finerenone was estimated to extend event-free survival by up to 3 years among people with HF with mildly reduced or preserved ejection fraction.
Trial Registration
ClinicalTrials.gov Identifier: NCT04435626.
Introduction
Patients with heart failure with mildly reduced ejection fraction (HFmrEF) or HF with preserved ejection fraction (HFpEF) have median life expectancies that are up to 15 years shorter than persons of comparable age in community settings. Despite this survival gap, few truly disease-modifying therapies have been available in their care. Indeed, until recently, the management of HFpEF was largely empirical and centered around short-term control of symptoms, congestion, and blood pressure. Sodium-glucose cotransporter 2 (SGLT2) inhibitors were the first evidence-based therapies to be shown to definitively reduce clinically relevant cardiovascular events in this population and are now strongly guideline-recommended worldwide. More recently, the nonsteroidal mineralocorticoid receptor antagonist (MRA), finerenone, was demonstrated to reduce the risks of cardiovascular death and worsening HF events in this same population. Although these trials were conducted with mean follow-up durations of 2 to 3 years, patients with HF are often treated with medical therapies over longer-term horizons and even for their lifetimes. Because withdrawal of effective medical therapies in HF has led to early clinical deterioration, guidelines also recommend long-term treatment without interruption.
We previously developed and validated an age-based method to extrapolate within-trial observations to forecast long-term gains in event-free survival. These methods may allow for translation of traditional clinical trial reporting measures (eg, hazard ratios) to metrics that may be more interpretable by patients and clinicians (eg, years of life gained free from clinical events). In this prespecified analysis of the Finerenone Trial to Investigate the Efficacy and Safety Superior to Placebo in Patients with Heart Failure (FINEARTS-HF) trial, we estimated the long-term effects of finerenone on event-free survival in participants with HFmrEF or HFpEF.
Methods
FINEARTS-HF Trial Design
The design of the FINEARTS-HF trial has been previously described in detail (Supplement 1 and Supplement 2). In brief, the FINEARTS-HF trial was a global event-driven, placebo-controlled, randomized clinical trial examining finerenone in patients with HF and a left ventricular EF of 40% or greater. Key inclusion criteria included age of 40 years or older, New York Heart Association functional class II or greater symptoms, elevated natriuretic peptide levels, evidence of structural heart disease, and recent diuretic use. Key laboratory-based exclusion criteria included estimated glomerular filtration rate (eGFR) of less than 25 mL/min/1.73m2 or serum potassium level greater than 5.0 mmol/L (to convert to milliequivalents per liter, divide by 1). Additional relevant exclusion criteria included probable alternative causes to presenting symptoms other than HF, severe valvular heart disease requiring surgery, life-threatening or uncontrolled arrhythmia, or any condition limiting life expectancy to less than 12 months (such as active malignancy). Enrollment was permitted across major clinical care settings (whether hospitalized, recently hospitalized, or ambulatory). Patients self-identified with the following races and ethnicities: Asian, Black, White, and other, which included American Indian, Alaska Native, Native Hawaiian, Other Pacific Islander, or unreported. All patients provided written informed consent for participation, and the study protocol was approved by the institutional review boards or ethics committees at all sites. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization, Target Dosing, and End Points
Participants were randomized 1:1 to finerenone (which could be titrated to 20 mg if starting eGFR was ≤60 mL/min/1.73m2 or to 40 mg if starting eGFR was >60 mL/min/1.73m2) or matching placebo. The primary end point was cardiovascular death and total (first and recurrent) worsening HF events (inclusive of both hospitalizations and urgent ambulatory visits for HF). All-cause mortality was a secondary end point. In applying these actuarial methods, we considered survival time free from the first occurrence of the primary end point. All potential primary end points and deaths were adjudicated by a clinical end points committee.
Statistical Analysis
In this prespecified analysis of the FINEARTS-HF trial, we estimated long-term gains in survival free from a primary end point with finerenone by applying validated nonparametric actuarial methods. We iteratively calculated residual event-free survival at every age from 50 to 80 years in each arm using restricted mean survival time methods. Instead of time from randomization to a clinical event, we used age at randomization to a clinical event as the time horizon. We then created age-methods Kaplan-Meier lifetime event-free survival curves in the finerenone arm and the placebo arm. As randomized treatment was balanced across the age range, differences in areas under the survival curves (up to a maximum of 95 years) between arms represented event-free survival gains. Mean event-free survival in each arm by age at randomization was plotted. A locally weighted scatterplot smoothing procedure was applied to graphically display treatment differences in event-free survival across the age spectrum. As SGLT2 inhibitors are the only strongly recommended pharmacological therapy (other than diuretics) in HFpEF, in subgroup analysis, we further estimated event-free survival gains in participants who were treated (or not) with an SGLT2 inhibitor at baseline. In sensitivity analysis, we evaluated residual survival time free from the composite of all-cause death (rather than cardiovascular death) or a worsening HF event to address potential competing risks. We additionally estimated overall life expectancy in each treatment arm. All validly randomized participants were considered for analysis and no imputation was performed for data missingness. Application of these actuarial methods to estimate lifetime benefits were prespecified in the FINEARTS-HF academic statistical analysis plan before trial unblinding. Statistical significance was set at a 2-sided P value <.05. All statistical analyses were performed using Stata, version 18 (StataCorp).
Results
From September 2020 to January 2023, 6001 participants (median [IQR] age, 73 [66-79] years; 2732 female [45.5%]; 3269 male [54.5%]) were validly randomized to finerenone or placebo (eFigure 1 in Supplement 3). Participants self-identified with the following races and ethnicities: 996 Asian (16.6%), 88 Black (1.5%), 4735 White (78.9%), and 182 other (3.0%). The baseline clinical profiles of participants in the FINEARTS-HF trial have been previously described (eTable in Supplement 3). Participant age ranged from 40 to 97 years (eFigure 2 in Supplement 3). Participants were enrolled across 653 sites in 37 countries with all geographic regions represented. Baseline characteristics, including age distribution, were well balanced between treatment arms.
Event-Free Survival Gains With Finerenone
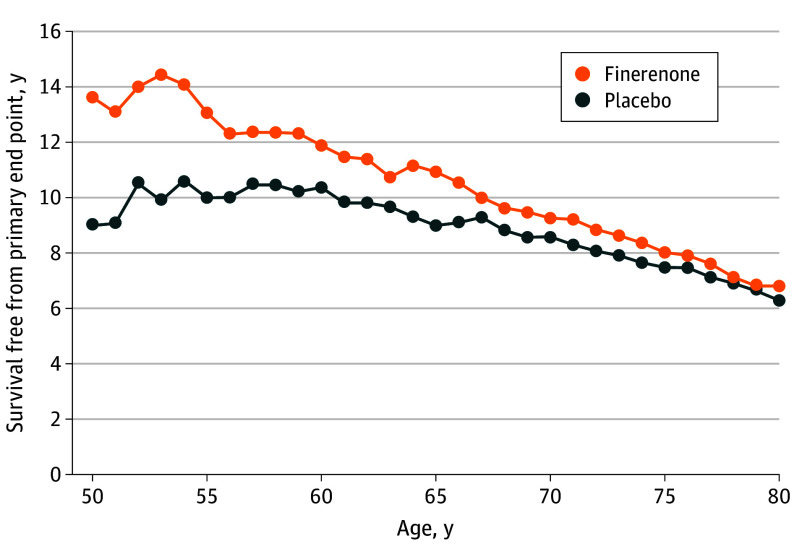
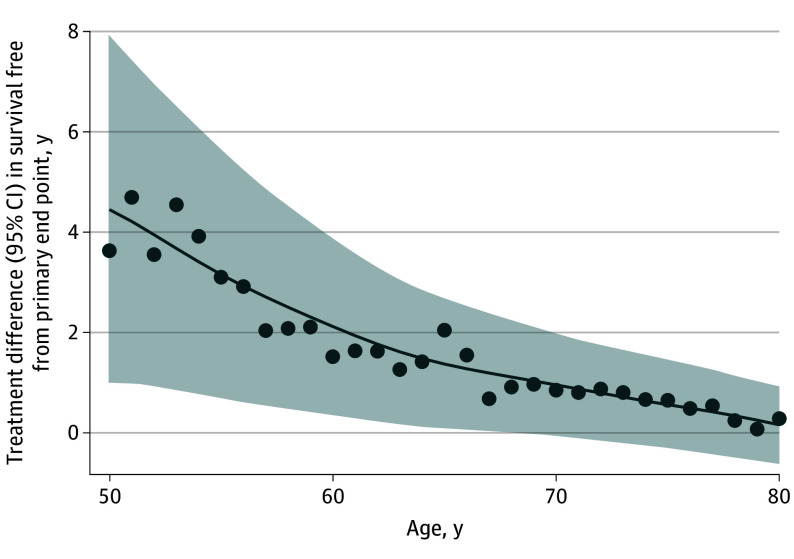
During median (IQR) follow-up of 2.6 (1.9-3.0) years, 1343 first primary end points of cardiovascular death or a worsening HF event were adjudicated. For a 55-year-old participant, mean residual survival free from the primary end point was 13.6 years (95% CI, 11.9-15.2 years) with finerenone and 10.5 years (95% CI, 6.8-11.3 years) with placebo, representing a gain in event-free survival of 3.1 years (95% CI, 0.8-5.4 years; P = .007) (Figure 1). Mean event-free survival for a 65-year-old participant was 11.0 years (95% CI, 10.1-11.9 years) with finerenone and 8.9 years (95% CI, 8.1-9.8 years) with placebo, representing a gain in event-free survival of 2.0 years (95% CI, 0.8-3.3 years; P = .001) (Figure 1). Mean event-free survival was numerically greater with finerenone than with placebo for every age from 50 to 80 years (Figure 2). As younger patients have longer expected life expectancies, the estimated gains in event-free survival were expectedly greatest among younger and middle-aged participants in the FINEARTS-HF trial (Figure 3). In sensitivity analyses, 1702 first composite events of all-cause death or worsening HF and 1013 all-cause deaths occurred in follow-up. For a 65-year-old participant, gains in survival free from all-cause death or worsening HF with finerenone were estimated to be 1.1 years (95% CI, 0.3-2.4 years; P = .01). For a 65-year-old participant, finerenone did not significantly extend overall long-term survival (between-arm difference of 0.2 years; 95% CI, −1.2 to 1.6 years; P = .75) (eFigure 3 in Supplement 3).
Figure 1. Projected Event-Free Survival Gains With Finerenone.
Survival free from the primary end point (cardiovascular death or worsening heart failure event) is displayed in the finerenone and placebo arms according to age at randomization. A, Age 55 years. B, Age 65 years. C, Age 75 years.
Figure 2. Mean Survival Free From Primary End Point.
Figure 3. Projected Long-Term Mean Event-Free Survival Gains With Finerenone According to Age at Initiation.
A locally weighted scatterplot smoothing procedure is applied to graphically display treatment differences in event-free survival.
Lifetime Benefits by Baseline SGLT2 Inhibitor Use
Overall, 817 participants (13.6%) were treated with an SGLT2 inhibitor at baseline. For a 65-year-old participant taking an SGLT2 inhibitor at baseline, lifetime gains in event-free survival from the primary end point were 3.1 years (95% CI, 0.1-6.0 years; P = .04). For a 65-year-old participant not taking an SGLT2 inhibitor at baseline, lifetime gains in event-free survival were 1.8 years (95% CI, 0.5-3.1 years; P = .009) (eFigure 4 in Supplement 3).
Discussion
In this prespecified analysis of the FINEARTS-HF trial, treatment with finerenone was estimated to yield clinically meaningful gains in event-free survival among individuals with HFmrEF or HFpEF. Extension in event-free survival was observed across a broad age range and in patients already treated with SGLT2 inhibitor therapy. Given longer anticipated lifetime therapeutic exposure, long-term gains in event-free survival were especially prominent when finerenone is initiated in younger and middle-aged patients. These data support the role of finerenone in modifying longitudinal risks of clinical events and provide estimates of its long-term expected therapeutic benefits that might be useful to aid clinical decision-making.
When considering initiation of a new medical therapy, patients and clinicians often are interested in the lifetime effects on morbidity and mortality. Yet, clinical trials are conducted over years rather than decades of follow-up due to resource limitations, the need to generate evidence more rapidly to inform practice, and the challenges in maintaining randomization (especially to a placebo comparator). Although real-world evidence may provide ancillary evidence of long-term safety with drug exposure, these data are subject to confounding due to selection bias of those treated in routine care. To address these limitations of existing data sources, we developed a novel age-based method to extrapolate within-trial observations to provide less biased and potentially informative estimates of long-term treatment benefits. In validating this approach in previous trials, estimated event-free survival based on these projections from within-trial follow-up closely aligned with actual event-free survival observed during extended post-trial follow-up. Since initial development and validation, these actuarial methods have been applied to a number of randomized clinical trials to aid in the interpretation of long-term effects of various therapies in HF.
The nonsteroidal MRA finerenone was estimated to extend event-free survival by up to 3 years, depending on the age of initiation, in patients with HFmrEF or HFpEF. These expected long-term benefits are comparable in magnitude to estimates derived for SGLT2 inhibitors in this target population and for medical therapies in HF with reduced ejection fraction. Extension in event-free survival with finerenone is driven by a meaningful delay in nonfatal worsening HF events as we did not observe a significant improvement in overall life expectancy. Although the evidence supporting the use of SGLT2 inhibitors in HFmrEF or HFpEF evolved during the course of the FINEARTS-HF trial, over 800 patients were treated with an SGLT2 inhibitor at baseline. We estimate meaningful extension in event-free survival with finerenone even among patients already treated with an SGLT2 inhibitor.
Study Limitations
Our projections rely on the important assumption that the treatment effects observed during the trial will persist for the lifetime of the patient. However, interval nonadherence, temporary interruption, or premature discontinuation may attenuate these optimistic projections of lifetime treatment-related benefits. Our estimates were derived from a clinical trial with stringent eligibility criteria that excluded individuals with life-threatening cardiovascular or noncardiovascular illness; as such, these trial-based projections might not generalize to all patients with various disease trajectories in usual care settings. Ultimately, these projections of lifetime benefits of finerenone should be contextualized alongside its relative safety, long-term cost, and incremental economic value.
Conclusions
In this prespecified secondary analysis of the FINEARTS-HF randomized clinical trial, long-term treatment with finerenone was estimated to extend event-free survival by up to 3 years among people with HFmrEF or HFpEF. The relative benefits reported during follow-up in the FINEARTS-HF trial can be interpreted together with these new projections of the expected absolute benefits with lifetime use of finerenone.
Trial Protocol.
Statistical Analysis Plan.
eTable. Baseline Characteristics
eFigure 1. FINEARTS-HF Consort Diagram
eFigure 2. Distribution of Age at Randomization in FINEARTS-HF
eFigure 3. Projected Overall Survival in the FINEARTS-HF Trial
eFigure 4. Projected Event-Free Survival Among Participants With and Without Baseline Use of an SGLT2 Inhibitor
Data Sharing Statement.
References
- 1.Shah KS, Xu H, Matsouaka RA, et al. Heart Failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486. doi: 10.1016/j.jacc.2017.08.074 [DOI] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of 5 randomized controlled trials. Lancet. 2022;400(10354):757-767. doi: 10.1016/S0140-6736(22)01429-5 [DOI] [PubMed] [Google Scholar]
- 3.McDonagh TA, Metra M, Adamo M, et al. ; ESC Scientific Document Group . 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44(37):3627-3639. doi: 10.1093/eurheartj/ehad195 [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, McMurray JJV, Vaduganathan M, et al. ; FINEARTS-HF Committees and Investigators . Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. in press. doi: 10.1056/NEJMoa2407107 [DOI] [PubMed] [Google Scholar]
- 6.Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomized trial. Lancet. 2019;393(10166):61-73. doi: 10.1016/S0140-6736(18)32484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claggett B, Packer M, McMurray JJ, et al. ; PARADIGM-HF Investigators . Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289-2290. doi: 10.1056/NEJMc1509753 [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Claggett BL, Lam CSP, et al. Finerenone in patients with heart failure with mildly reduced or preserved ejection fraction: rationale and design of the FINEARTS-HF trial. Eur J Heart Fail. 2024;26(6):1324-1333. doi: 10.1002/ejhf.3253 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Ostrominski JW, Vaduganathan M, et al. Baseline characteristics of patients with heart failure with mildly reduced or preserved ejection fraction: the FINEARTS-HF trial. Eur J Heart Fail. 2024;26(6):1334-1346. doi: 10.1002/ejhf.3266 [DOI] [PubMed] [Google Scholar]
- 10.Ferreira JP, Claggett BL, Docherty KF, et al. Within trial comparison of survival time projections from short-term follow-up with long-term follow-up findings. ESC Heart Fail. 2022;9(5):3655-3658. doi: 10.1002/ehf2.13731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty KF, Jhund PS, Claggett B, et al. ; DAPA-HF Investigators and Committees . Extrapolating long-term event-free and overall survival with dapagliflozin in patients with heart failure and reduced ejection fraction: an exploratory analysis of a phase 3 randomized clinical trial. JAMA Cardiol. 2021;6(11):1298-1305. doi: 10.1001/jamacardio.2021.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of 3 randomized controlled trials. Lancet. 2020;396(10244):121-128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 13.Ferreira JP, Docherty KF, Stienen S, et al. Estimating the lifetime benefits of treatments for heart failure. JACC Heart Fail. 2020;8(12):984-995. doi: 10.1016/j.jchf.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stienen S, Ferreira JP, Vincent J, et al. Estimated long-term survival with eplerenone. J Am Coll Cardiol. 2019;73(18):2357-2359. doi: 10.1016/j.jacc.2019.02.043 [DOI] [PubMed] [Google Scholar]
- 15.Vaduganathan M, Claggett BL, Jhund P, et al. Estimated long-term benefit of dapagliflozin in patients with heart failure. J Am Coll Cardiol. 2022;80(19):1775-1784. doi: 10.1016/j.jacc.2022.08.745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Statistical Analysis Plan.
eTable. Baseline Characteristics
eFigure 1. FINEARTS-HF Consort Diagram
eFigure 2. Distribution of Age at Randomization in FINEARTS-HF
eFigure 3. Projected Overall Survival in the FINEARTS-HF Trial
eFigure 4. Projected Event-Free Survival Among Participants With and Without Baseline Use of an SGLT2 Inhibitor
Data Sharing Statement.