Key Points
Question
What are the effects of the Community Paramedicine at Clinic (CP@clinic) program on health care utilization compared with usual care?
Findings
In a cluster randomized clinical trial of 3695 residents aged 55 years or older in 30 social housing buildings, the rates of emergency department (ED) visits by ambulance (the primary outcome) were similar between control and intervention buildings; however the intervention group had higher odds of antihypertensive medication initiation, a higher rate of primary care visits, greater odds of receiving home care services, and lower odds of transferring to long-term care during the 12-month intervention, all statistically significant changes.
Meaning
While there was no significant difference in the primary outcome of ED visits by ambulance, CP@clinic did increase primary care visits and connections to home care services; this may have increased antihypertensive medication initiation and reduced long-term care transfers from social housing.
This randomized clinical trial of social housing building residents in Ontario, Canada, evaluates the effects of a community paramedicine program vs usual care on health service use.
Abstract
Importance
Community Paramedicine at Clinic (CP@clinic) is a chronic disease prevention program that decreases 911 calls for emergency medical services, but its wider system effects are unknown.
Objective
To evaluate the effects of CP@clinic vs usual care on individual-level health service utilization outcomes.
Design, Setting, and Participants
This open-label, pragmatic cluster randomized clinical trial evaluated all residents 55 years or older in 30 social housing buildings in Ontario, Canada, that had (1) a unique postal code, (2) at least 50 apartments, (3) 60% or more residents 55 years or older, and (4) a similar building for pairing (15 intervention and 15 control buildings, pair-matched randomization). The 12-month intervention had a staggered start date from January 1, 2015, to December 1, 2015, and ended between December 31, 2015, and November 30, 2016. Administrative health data analysis was conducted in May 2022.
Intervention
CP@clinic was a health promotion and disease prevention program led by specially trained community paramedics who held weekly drop-in sessions in social housing buildings. These paramedics conducted 1-on-1 risk assessments, provided health education and referrals to relevant community resources, and, with consent, sent assessments to family physicians. Control buildings received usual care (universal health care, including free primary and specialty medical care).
Main Outcome and Measures
Individual-level health service utilization was measured from administrative health data, with ED visits via ambulance as the primary outcome; secondary outcomes included ED visits for any reason, primary care visits, hospitalizations, length of hospital stay, laboratory tests, receipt of home care, transfer to long-term care, and medication initiation. Generalized estimating equations were used to estimate intervention effects on individual-level health service utilization, accounting for trial design and individual-level baselines.
Results
The 30 social housing buildings had 3695 residents (1846 control and 1849 intervention participants; mean [SD] age, 72.8 [9.1] years; 2400 [65.0%] female). Intention-to-treat analysis found no significant difference in ED visits by ambulance (445 of 1849 [24.1%] vs 463 of 1846 [25.1%]; adjusted odds ratio [AOR], 0.97; 95% CI, 0.89-1.05) but found higher antihypertensive medication initiation (74 of 500 [14.8%] vs 47 of 552 [8.5%]; AOR, 1.74; 95% CI, 1.19-2.53) and lower anticoagulant initiation (48 of 1481 [3.2%] vs 69 of 1442 [4.8%]; AOR, 0.68; 95% CI, 0.53-0.86) in the intervention arm vs the control arm. CP@clinic attendance was associated with higher incidence of primary care visits (adjusted incidence rate ratio, 1.10; 95% CI, 1.03-1.17), higher odds of receiving home care (AOR, 1.07; 95% CI, 1.01-1.13), and lower odds of long-term care transfers (AOR, 0.32; 95% CI, 0.13-0.81).
Conclusions and Relevance
In this cluster randomized clinical trial of CP@clinic, the intervention did not affect the rate of ED visits by ambulance; however, there were increased primary care visits and connections to home care services, which may have increased antihypertensive medication initiation and reduced long-term care transfers from social housing. Health policymakers should consider CP@clinic’s impact as an upstream approach to improve care for older adults with low income.
Trial Registration
ClinicalTrials.gov Identifier: NCT02152891
Introduction
Older adults are a rapidly increasing segment of the Canadian population.1 Low-income older adults experience the highest rates of chronic diseases and multimorbidity,2,3,4 lower health-related quality of life,5 and greater mortality rates6 compared with other older adults. Consequently, they account for one-third of emergency medical services (EMS) calls,4,7,8 some of which are preventable (eg, chronic disease and falls management).9 Community health promotion programs targeting low-income older adults have been proposed to address health inequities.10
The Community Paramedicine at Clinic (CP@clinic) program is an innovative community health program evaluated using a randomized clinical trial (RCT). CP@clinic led to 0.90 fewer calls per month per 100 apartment units in 5 communities in Ontario, Canada, and has demonstrated improved participant health-related quality of life and reduced chronic disease risk.11,12 A subsequent economic analysis found that CP@clinic was a cost-effective solution for the emergency health care system, resulting in a net resource gain,13 and many paramedic services have since adopted the program.14 However, CP@clinic’s effect on broader health service utilization has not been evaluated.
The objective of this multisite cluster RCT was to evaluate the effects of the CP@clinic program vs usual care on health service utilization outcomes. Universal health care is the default usual care in Ontario, with free primary and specialty medical health care provided to all ages. We hypothesized that health care utilization would be affected by the CP@clinic program. Because the CP@clinic intervention was a drop-in program open to all residents, building-level cluster randomization was the most appropriate design.15 These results provide an understanding of CP@clinic’s effect on the broader health system in Ontario and establish an evidence base to drive health system change as community-based, paramedicine-led health promotion programs become standard.16,17
Methods
Study Design
The study was an open-label cluster RCT randomized at the social housing building level in 30 buildings across 5 Ontario communities. These apartment buildings were for low-income seniors; and rent was capped at 30% of household income, with the remainder subsidized by the government. Details of the methods and intervention have been published elsewhere.11,12 This study was approved by the Hamilton Integrated Research Ethics Board, which granted a waiver of consent for cluster-level cohort creation at ICES (formerly known as the Institute for Clinical Evaluative Sciences). Those attending the CP@clinic program (attendees) signed written, informed consent forms to have their CP@clinic assessment data sent to ICES for linkage with their administrative health data at ICES. ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. CP@clinic participant data were probabilistically linked to their corresponding health care records at ICES according to name, sex, date of birth, and postal code; Ontario Health Insurance Plan (OHIP) numbers were not collected from participants. This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1.
Intervention
The intervention was a community paramedicine health promotion and disease prevention program: CP@clinic. Briefly, each week, 2 specially trained paramedics provided a half-day voluntary drop-in session for the social housing building residents in a common space (eg, wellness room) of each building. The community paramedics met with residents one on one to conduct validated health risk assessments (eg, physical measures, cardiometabolic risk screening questionnaires, and tools for assessing social determinants of health). On the basis of the individual’s risk profile, the paramedic worked with the resident to identify which risk factor(s) they wanted to focus on first (eg, low physical activity), provided tailored health education on lifestyle modifications, assisted in goal setting, and provided referrals to relevant community resources (eg, local walking programs, homecare services, and mobility device assessment). Residents were also encouraged to return to the CP@clinic program to discuss their progress and challenges, which allowed the community paramedic to assist in troubleshooting (eg, finding a different resource that may be a better fit), provide encouragement, and select another risk factor that the participant wanted to address next. For those who had a family physician and consented, assessment results were sent to the physician. The intervention had a staggered start date (between January 1 and December 1, 2015) and ran for 12 months (between December 31, 2015, and November 30, 2016, respectively).
Study Participants
Because the intervention was available to all building residents, the unit of randomization was the building. The building-level inclusion criteria were as follows: (1) a unique postal code not shared with neighboring residences, (2) at least 50 apartment units, (3) at least 60% of residents aged 55 years or older, and (4) presence of another building of similar size, demographic characteristics, and geographic location to permit paired randomization. There were no exclusion criteria.
The individual-level, closed cohort included all building residents 55 years or older who lived at the study postal codes in the quarter immediately preceding the intervention start date according to the OHIP Registered Persons Database (RPDB). All building residents were permitted to participate in the CP@clinic program regardless of age, but only those who met the age criterion were included in the analysis. Individuals were excluded from analysis if they were 120 years or older, ineligible for OHIP benefits, or had no encounters with the health care system in the preceding 7 years.
Randomization and Masking
Buildings were paired based on similar characteristics, such as location, number of units, percentage of seniors, previous year EMS call rate, and level of programming, in consultation with the municipal housing providers (see prior publication12 for a description of the matching process). Using a computer-generated randomization program (Research Randomizer; Social Psychology Network), the research team randomly allocated 1 building from each pair to receive the intervention (CP@clinic) and 1 building to usual care. Masking of intervention building residents was not feasible due to it being a drop-in program in a common space, although residents were not informed of the precise details of the trial outcome measures. In addition, control building residents were not informed they were study controls and would have only known about the intervention if made aware by intervention building residents or local media.
Procedures
The social housing building postal codes were sent to ICES for the building-level cohort creation for the intention-to-treat analysis. Demographic characteristics, health status profile, and health system utilization outcomes were obtained from multiple ICES-held datasets and ICES-derived cohorts, linked using unique encoded identifiers. For attendees, personal identifiers collected from the CP@clinic database were securely transmitted to ICES for linkage to facilitate the sensitivity analyses. All outcome data in this study are from administrative health datasets. The study team led the data analysis conducted by an ICES analyst to maintain privacy.
Demographic Characteristics and Health Status
Participant age and sex at the intervention start date were obtained from the OHIP RPDB; data on race and ethnicity were not available. Validated case definitions identified whether participants had 5 chronic diseases reliably captured in administrative health data: diabetes,18 congestive heart failure,19 chronic obstructive pulmonary disease,20 hypertension,21 and dementia.22 Cardiovascular disease and peripheral vascular disease status were determined using a 3-year lookback from the intervention start date based on physician service claims, emergency department (ED) records, and hospital discharge abstracts. Details are available in eTable 1 in Supplement 2. The Johns Hopkins ACG System, version 10 was used to quantify multimorbidity (number of aggregated diagnosis groups) and determine participants flagged as frail.23,24
Health Service Utilization Outcomes
For the 12-month preintervention and intervention periods, all unplanned ED visits for any cause and unplanned ED visits via ambulance for any cause were obtained from the Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System. Each outcome was dichotomized as having at least 1 ED visit vs none. Nonelective hospital admissions for any cause (ie, urgent or emergent) were obtained from the CIHI Discharge Abstract Database using an episode-of-care approach treating transfers among hospitals within 24 hours as a single event. Total length of stay for the episode of care was computed as the sum of days hospitalized.
Primary care visits during the 12-month preintervention and intervention periods were obtained from OHIP physician billings, capturing outpatient visits where the physician’s main specialty was family physician or general practitioner in the ICES Physician Database. Multiple billings from the same physician on the same date were considered a single visit. Diabetes, kidney function, and lipid laboratory tests were obtained from the Ontario Laboratories Information System (see eTable 1 in Supplement 2 for details).
Prescription drug claim data were available for those enrolled in the Ontario Drug Benefit (ODB) Plan, which covers individuals (1) receiving government financial benefits (eg, Ontario Works, Ontario Disability Support Program), (2) enrolled in the Trillium Drug Program (for those with very high prescription drug costs relative to income), or (3) aged 65 years or older. We grouped drug claims into 4 categories: diabetes, antihypertensives, anticoagulants, and statins or fibrates. New medication initiation was defined as having a medication initiated during the 12-month intervention period and having no prior ODB claim for medications within the same category during the 12 months before the intervention. Thus, individuals needed to have at least 1 year of medication data available before the intervention to be included. See eTable 2 in Supplement 1 for medications within each drug category.
Building residents with at least 1 visit from a home and community support service provider in the Ontario Homecare Database were classified as receiving homecare services during the 12-month preintervention or intervention period. Finally, transfers to long-term care (LTC) for short or long stays were defined by having at least 1 OHIP physician bill with an LTC-specific fee code or institution type indicating the service was provided within LTC during the intervention period.
Outcome Measures
The primary outcome was individual-level ED visits via ambulance as a main measure of health care utilization. Secondary outcomes included any ED visits, physician visits (primary care), hospitalizations, length of hospital stay, laboratory tests, receipt of home care, transfer to LTC, and prescription medication initiation among those with provincial drug benefits. Each of these individual-level outcomes was assessed through the ICES administrative datasets for 12 months during the intervention period (see the Procedures section for outcome definitions and details of data sources for each outcome).
Statistical Analysis
Data analysis was conducted in May 2022. Sample size was calculated based on the CP@clinic pilot3 in the same population, which reported a 25% decrease in EMS calls during the intervention year compared with the previous 2 years.3 Due to there being no other readily available data in this setting, we assumed a similar effect size in ED visits by ambulance adjusted for baseline and conservatively estimated a 15% effect size with an intracluster correlation coefficient (ICC) of 0.07. Using standard parameters (statistical power = 0.80, α = .05), the required sample size was a minimum of 1108 participants (11 buildings with 100 units) in each trial arm. All analyses were conducted using individual record–level data and 2-sided analyses and using a threshold of significance of P < .05. Demographic and health status characteristics were described using descriptive statistics.
For the study outcomes, descriptive analyses were performed for the 12-month preintervention period and the 12-month intervention period. The intervention start date was staggered, so the periods were relative and specific to each set of pair-randomized buildings. For the intention-to-treat analysis, we used generalized estimating equation models assuming an independent correlation matrix to estimate the individual-level effects of the CP@clinic intervention vs usual care, while accounting for clustering of residents within buildings and adjusting for the paired building trial design and individual-level baseline (ie, each resident’s preintervention year measure). For the transfers to LTC outcome, there was no adjustment for baseline because most residents who moved to LTC in the preintervention year were expected to no longer be residing at the building. A sensitivity analysis limited the modeling to the subset of intervention building residents who had attended at least 1 CP@clinic program session compared with control building residents. During the intervention, it was discovered that 2 buildings did not meet the eligibility criteria; this circumstance has been described in detail elsewhere.12 Therefore, further sensitivity analyses were conducted using the same methods but excluding these 2 pairs (Figure 1). The results are reported as adjusted odds ratio (AOR) for binary outcomes and adjusted incidence rate ratio (AIRR) for counts, with corresponding 95% CIs. All descriptive and generalized estimating equation analyses were performed using SAS software, version 9.4 (SAS Institute Inc).
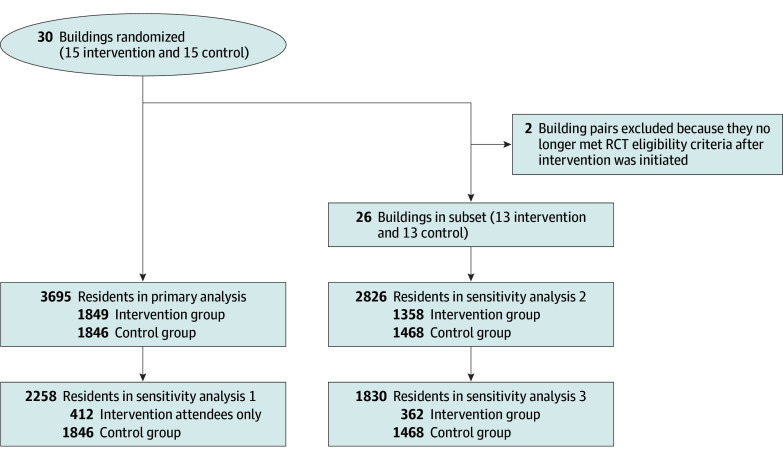
Figure 1. Community Paramedicine at Clinic (CP@clinic) Randomized Clinical Trial (RCT) Profile.
Results
Participant Characteristics
Per the RCT protocol,25 30 buildings were pair-randomized with 15 assigned to intervention and 15 to control (see Figure 1 for overview of study flow and participant numbers). Of the 3695 residents in the primary analysis (Figure 1), the success rate in linking individuals with their corresponding OHIP RPDB record was 92%. Cluster size ranged from 40 to 391 across the 30 sites. The primary outcome ICC was 0.004 and the secondary outcome ICCs ranged from −0.012 to 0.039.
The mean (SD) participant age was 72.8 (9.1) years; 2400 participants (65.0%) were female and 1295 (35.0%) were male; and 3482 (94.2%) were ODB eligible (eTable 3 in Supplement 2). The intervention and control groups had similar rates of most chronic diseases (Table 1).
Table 1. Participant Characteristics and Baseline Health Status of Intervention Building Residents, Control Building Residents, and Intervention Attendees.
| Characteristic | No. (%) of residentsa | |||||
|---|---|---|---|---|---|---|
| All buildings (N = 30) | Subset of buildings (n = 26) | |||||
| Control, all residents (n = 1846) | Intervention | Control, all residents (n = 1468) | Intervention | |||
| All residents (n = 1849) | Attendees only (n = 412) | All residents (n = 1358) | Attendees only (n = 362) | |||
| Demographics | ||||||
| Age, y | ||||||
| Mean (SD) | 72.5 (9.0) | 73.1 (9.1) | 73.5 (8.9) | 73.6 (8.9) | 73.7 (8.4) | 74.1 (8.6) |
| Median (IQR) | 72 (65-79) | 73 (66-79) | 74 (67-80) | 73 (67-80) | 74 (67-79) | 74 (67-80) |
| Sex | ||||||
| Female | 1148 (62.2) | 1252 (67.7) | 339 (82.3) | 963 (65.6) | 981 (72.2) | 302 (83.4) |
| Male | 698 (37.8) | 597 (32.3) | 73 (17.7) | 505 (34.4) | 377 (27.8) | 60 (16.6) |
| Health status | ||||||
| Diabetes | 637 (34.5) | 637 (34.5) | 150 (36.4) | 515 (35.1) | 469 (34.5) | 125 (34.5) |
| COPD | 722 (39.1) | 603 (32.6) | 168 (40.8) | 568 (38.7) | 437 (32.2) | 145 (40.1) |
| Congestive heart failure | 242 (13.1) | 223 (12.1) | 54 (13.1) | 214 (14.6) | 147 (10.8) | 45 (12.4) |
| Hypertension | 1269 (68.7) | 1326 (71.7) | 311 (75.5) | 1041 (70.9) | 1005 (74.0) | 277 (76.5) |
| Dementia | 118 (6.4) | 132 (7.1) | 29 (7.0) | 102 (6.9) | 89 (6.6) | 25 (6.9) |
| Cardiovascular disease | 104 (5.6) | 105 (5.7) | 19 (4.6) | 87 (5.9) | 77 (5.7) | 18 (5.0) |
| Frailtyb | 310 (16.8) | 301 (16.3) | 67 (16.3) | 266 (18.1) | 201 (14.8) | 56 (15.5) |
| Multimorbidity, No. of ADGs | ||||||
| 0-4 | 304 (16.5) | 263 (14.2) | 28 (6.8) | 226 (15.4) | 185 (13.6) | 24 (6.6) |
| 5-9 | 631 (34.2) | 614 (33.2) | 116 (28.2) | 494 (33.7) | 450 (33.1) | 103 (28.5) |
| ≥10 | 911 (49.3) | 972 (52.6) | 268 (65.0) | 748 (51.0) | 723 (53.2) | 235 (64.9) |
Abbreviations: ADGs, aggregated diagnosis groups (from the ACG System); COPD, chronic obstructive pulmonary disease.
Unless otherwise indicated.
Frailty flag from the Johns Hopkins ACG System.
Three sensitivity analyses were conducted: those who attended the program (n = 412) vs controls (n = 1846), all residents excluding 2 building pairs not meeting eligibility criteria (n = 1358 intervention building residents and n = 1468 controls), and combining the first 2 conditions with attendees (n = 362) compared with controls (n = 1468) (Figure 1). Attendees (n = 412) were predominantly female (339 [82.3%]), had greater multimorbidity (≥10 aggregated diagnosis groups, 268 [65.0%] vs 911 [49.3%]), and were more likely to have hypertension at baseline (311 [75.5%] vs 1269 [68.7%]) compared with controls (Table 1).
Health Service Utilization
Intention-to-Treat Analysis
The primary outcome comparing ED visits via ambulance in intervention building residents (attendees and nonattendees) vs control buildings demonstrated no significant difference (445 [24.1%] vs 463 [25.1%]; AOR, 0.97; 95% CI, 0.89-1.05) (Table 2). The secondary outcome analysis found that among residents who were ODB eligible, those in the intervention group had significantly higher odds of antihypertensive medication initiation (74 of 500 [14.8%] vs 47 of 552 [8.5%]; AOR, 1.74; 95% CI, 1.19-2.53) and lower odds of anticoagulant initiation (48 of 1481 [3.2%] vs 69 of 1442 [4.8%]; AOR, 0.68; 95% CI, 0.53-0.86) (eTable 4 in Supplement 2). For all other secondary outcomes, there was no significant difference observed (Table 2 and eTable 4 in Supplement 2).
Table 2. Intention-to-Treat Analysis of Health Care Utilization Outcomes, Except Medication Initiation, for All Intervention and Control Building Residents.
| Outcome | No. (%) of residentsa | AOR (95% CI)b | |||
|---|---|---|---|---|---|
| Baseline (N = 30 buildings) for all residents | Postintervention (N = 30 buildings) for all residents | ||||
| Control (n = 1846) | Intervention (n = 1849) | Control (n = 1846) | Intervention (n = 1849) | ||
| Binary outcomes | |||||
| ED visits | 689 (37.3) | 738 (39.9) | 764 (41.4) | 782 (42.3) | 0.99 (0.93-1.06) |
| ED visits by ambulance | 368 (19.9) | 368 (19.9) | 463 (25.1) | 445 (24.1) | 0.97 (0.89-1.05) |
| Hospital admissions | 264 (14.3) | 268 (14.5) | 338 (18.3) | 326 (17.6) | 0.98 (0.88-1.09) |
| Home care services | 430 (23.3) | 449 (24.3) | 499 (27.0) | 511 (27.6) | 0.99 (0.92-1.07) |
| Transfers to long-term care | NA | NA | 83 (4.5) | 75 (4.1) | 0.90 (0.68-1.20) |
| Kidney function laboratory test | 1229 (66.6) | 1302 (70.4) | 1277 (69.2) | 1324 (71.6) | 0.99 (0.95-1.04) |
| Electrolyte laboratory test | 1071 (58.0) | 1136 (61.4) | 1095 (59.3) | 1149 (62.1) | 1.01 (0.95-1.07) |
| Lipids laboratory test | 1011 (54.8) | 1047 (56.6) | 999 (54.1) | 1055 (57.1) | 1.02 (0.98-1.08) |
| Diabetes laboratory test | 946 (51.2) | 977 (52.8) | 1000 (54.2) | 1043 (56.4) | 1.01 (0.97-1.05) |
| Continuous outcomes | |||||
| No. of primary care visits, mean (SD) | 5.94 (6.59) | 6.04 (6.27) | 6.29 (6.77) | 6.22 (6.72) | AIRR, 1.00 (0.94-1.05) |
| Hospital length of stay, mean (SD), dc | 13.05 (21.89) | 12.16 (20.35) | 18.78 (37.20) | 17.93 (25.44) | AIRR, 0.94 (0.83-1.07) |
Abbreviations: AIRR, adjusted incidence rate ratio; AOR, adjusted odds ratio; ED, emergency department; NA, not applicable.
Unless otherwise indicated.
Except for the transfers to long-term care, all AORs and AIRRs were calculated using generalized estimated equation models adjusted for clustering of residents within buildings, building pairing (trial design), and the baseline value. For the transfers to long-term care outcome, the generalized estimated equation model was adjusted for clustering of residents within buildings and the building pairing only.
The hospital length of stay outcome was restricted to only those with a hospital admission.
Sensitivity Analysis 1
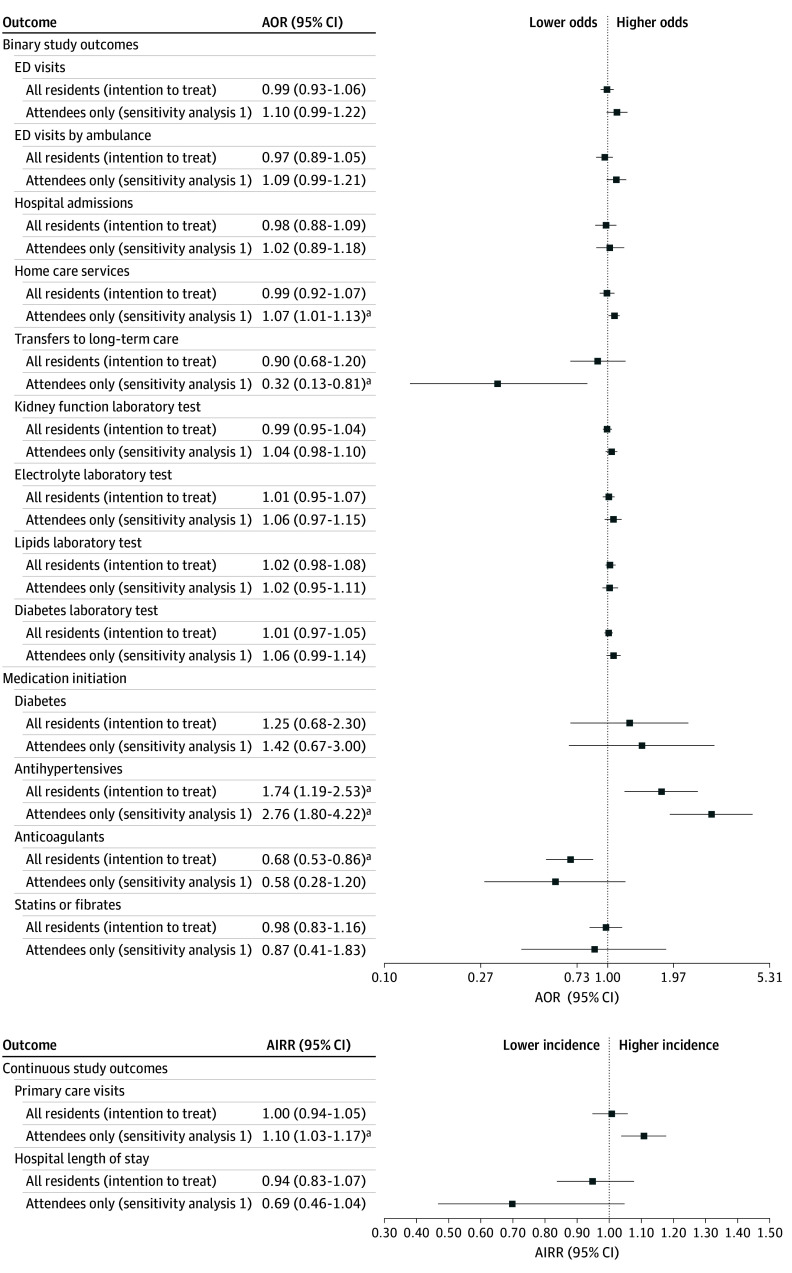
For those who attended the intervention compared with control building residents (Figure 2; eTable 5 in Supplement 2), no significant difference was observed in the primary outcome of ED visits via ambulance (AOR, 1.09; 95% CI, 0.99-1.21). However, for the secondary outcomes, attending the CP@clinic program was associated with a significantly higher incidence of primary care visits (AIRR, 1.10; 95% CI, 1.03-1.17), higher odds of receiving home care services (AOR, 1.07; 95% CI, 1.01-1.13), and lower odds of LTC transfers (AOR, 0.32; 95% CI, 0.13-0.81). Among those eligible for ODB, antihypertensive medications had significantly higher odds of being initiated (AOR, 2.76; 95% CI, 1.80-4.22). In addition, there were lower odds of anticoagulant initiation in the intervention attendees (AOR, 0.58; 95% CI, 0.28-1.20), but this finding was not statistically significant. For the remaining secondary outcomes, no significant differences were observed.
Figure 2. Effect of Community Paramedicine at Clinic (CP@clinic) on Health Care Utilization Outcomes Over 12 Months in the Full Sample of 30 Study Buildings.
AIRR indicates adjusted incident rate ratio; AOR, adjusted odds ratio; and ED, emergency department.
aSignificant at P < .05.
Sensitivity Analysis 2
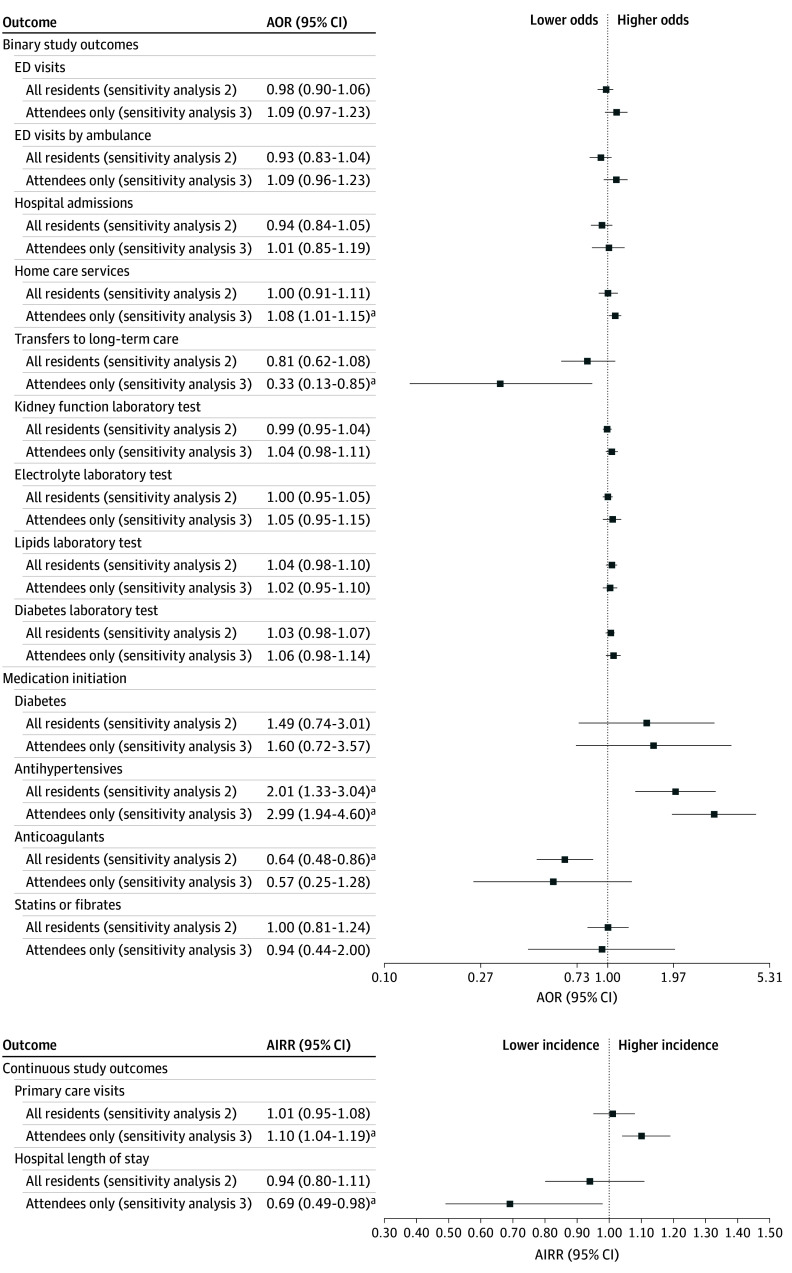
When 2 building pairs were excluded due to their eligibility changing during the RCT, the cohort descriptive analysis comparing building residents (both attendees and nonattendees) with the control buildings found no significant difference in the primary outcome (AOR, 0.93; 95% CI, 0.83-1.04) (Figure 3; eTable 6 in Supplement 2). For the secondary outcomes, there were significantly higher odds of antihypertensive medication initiation (AOR, 2.01; 95% CI, 1.33-3.04) and lower odds of anticoagulant medication initiation (AOR, 0.64; 95% CI, 0.48-0.86). No significant associations were observed in the remaining secondary outcomes.
Figure 3. Effect of Community Paramedicine at Clinic (CP@clinic) on Health Care Utilization Outcomes Over 12 Months in the Subset of 26 Study Buildings.
In the subset of study buildings (n = 26), 2 pairs were removed that no longer met eligibility criteria after intervention began. AIRR indicates adjusted incident rate ratio; AOR, adjusted odds ratio; and ED, emergency department.
aSignificant at P < .05.
Sensitivity Analysis 3
When comparing those who attended the intervention with the control building residents, with the 2 building pairs excluded, no significant difference was found in the primary outcome (AOR, 1.09; 95% CI, 0.96-1.23) (Figure 3; eTable 7 in Supplement 2). For the secondary outcomes, CP@clinic attendance was associated with a significantly higher incidence rate of primary care visits (AIRR, 1.10; 95% CI, 1.04-1.19), lower incidence rate for length of hospital stay (AIRR, 0.69; 95% CI, 0.49-0.98), lower odds of LTC transfers (AOR, 0.33; 95% CI, 0.13-0.85), and higher odds of home care services (AOR, 1.08; 95% CI, 1.01-1.15). Among those eligible for ODB, antihypertensive medications had significantly higher odds of being initiated (AOR, 2.99; 95% CI, 1.94-4.60). Odds of anticoagulant initiation were lower in CP@clinic attendees (AOR, 0.57; 95% CI, 0.25-1.28), but this finding was not significant. For all other secondary outcomes analyzed, no significant differences were observed.
Discussion
This multisite cluster RCT evaluated the effects of the CP@clinic program vs usual care on health service utilization outcomes. Our intention-to-treat analysis found that although residents of intervention buildings did not have significant differences in ED visits by ambulance, there was a significantly higher rate of antihypertensive medication initiation. This finding implies that uncontrolled or undiagnosed hypertension was being addressed by offering CP@clinic sessions in the social housing buildings, most likely through referrals to their primary care physicians. A qualitative study found that CP@clinic attendees who were reluctant to seek preventive medical care were more willing when it was recommended by the community paramedics, whom they highly respected.26 In addition, the relationship between medication initiation and primary care visits may account for the higher incidence rate of primary care visits for attendees vs nonattendees. This finding was consistent across sensitivity analyses. Hypertension is a risk factor for several cardiovascular diseases,27 and appropriate control in primary care is imperative for cardiac risk reduction.27
Unexpectedly, the rate of ED visits by ambulance was not significantly different between study groups. Previously, an RCT found that EMS calls significantly decreased in intervention buildings where CP@clinic was implemented12; therefore, a decrease in the rate of ED visits by ambulance was hypothesized. However, EMS calls and ED visits are vastly different, and the former may not always result in an ED visit. Indeed, the novel effect of the CP@clinic program on the health care system is that it reduces EMS demand for low-acuity conditions but not necessarily ED visits. Therefore, for paramedic services, the value in implementing the CP@clinic program remains because it reduces those low-acuity EMS calls for which transport is not necessary.
A common issue faced by community health programs is low participation rates, especially from marginalized populations,28,29,30 but, as previously reported, CP@clinic had a mean participation rate of 39%, and more than 80% of participants attended the program at least 3 times during the RCT.12 Our cohort descriptive analyses found that attendees had higher rates of multiple chronic diseases. Therefore, the population in greatest need of CP@clinic was receiving it appropriately. As a result of program attendance, attendees also had higher rates of primary care visits, hypertension medication initiation, and home care services. Conversely, attendees had fewer LTC transfers and fewer days in the hospital when admitted. In a qualitative study that interviewed CP@clinic attendees, attendees viewed the community paramedic role as being highly trusted professionals who advocated for their well-being and connected them with resources.26 This finding aligns with the present study’s findings that attendees had more homecare and fewer transfers to LTC in the administrative records. Therefore, all analyses imply that CP@clinic may increase attendees’ connections to important health care services, thereby averting health crises.
Community paramedicine interventions are rarely linked to outcomes from administrative data using RCT methods. A 2022 literature review identified 21 articles examining health system outcomes, of which 18 were peer reviewed, including 4 reporting RCTs (3 for the same trial) and 14 using administrative data.31 All administrative data studies in this review used observational methods, and community paramedicine was hypothesized as a potential reason for improved outcomes.32 Of the 2 RCTs, 1 was the trial currently being reported in this article,12 and the other, from the UK,33,34 examined paramedic practitioners as an alternate dispatch response. Therefore, our trial is, to our knowledge, the only cluster RCT to study a paramedic wellness program that did not require an emergency call to be initiated.
Strengths and Limitations
This study has several strengths, including the robust, pragmatic cluster randomization design and use of administrative data, which were not reliant on self-report or recall. Community programs are rarely evaluated using cluster RCT methods and administrative data because both are hard to execute in a pragmatic context.35,36 Another strength is that these results are generalizable to other Canadian locations and other countries (eg, UK, US, and Australia) with similar social housing buildings. Limitations include that our cohort may have slight errors because it was based on the RPDB, which can be inaccurate due to untimely address change reporting and update lags.
Conclusions
In this cluster randomized clinical trial, CP@clinic did not significantly affect the rate of ED visits by ambulance among participating social housing residents; however, the program did have significant effects on other aspects of resident health care utilization, decreasing system burden while directing people to appropriate primary care. Future research could determine the effectiveness of this preventive model in different populations and contexts. Health policymakers should consider CP@clinic’s impact as an upstream approach and promote widespread implementation in social housing.
Trial Protocol
eTable 1. Disease and Procedure Codes
eTable 2. Medication Categories
eTable 3. Participant Characteristics of Intervention Building Residents, Control Building Residents, and Intervention Attendees Who Had Prescription Drug Claim Records Available (≥66 Years or Eligible for the Ontario Drug Benefit)
eTable 4. Intention-to-Treat Analysis of Medication Initiation for All Intervention and Control Building Residents
eTable 5. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of Building Residents That Attended the Intervention Program
eTable 6. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of All Building Residents for a Subset of Buildings (N = 26)
eTable 7. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of Building Residents That Attended the Intervention Program for a Subset of Buildings (N = 26)
Data Sharing Statement
References
- 1.Martin D, Miller AP, Quesnel-Vallée A, Caron NR, Vissandjée B, Marchildon GP. Canada’s universal health-care system: achieving its potential. Lancet. 2018;391(10131):1718-1735. doi: 10.1016/S0140-6736(18)30181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seniors and the Health Care System: What Is the Impact of Multiple Chronic Conditions? Canadian Institute for Health Information; 2011. Accessed August 17, 2023. https://secure.cihi.ca/free_products/air-chronic_disease_aib_en.pdf
- 3.Agarwal G, Angeles R, Pirrie M, et al. Effectiveness of a community paramedic-led health assessment and education initiative in a seniors’ residence building: the Community Health Assessment Program through Emergency Medical Services (CHAP-EMS). BMC Emerg Med. 2017;17(1):8. doi: 10.1186/s12873-017-0119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Institute for Health Research. Pan-Canadian forum on high users of health care. 2014. Accessed August 17, 2023. https://secure.cihi.ca/free_products/highusers_summary_report_revised_EN_web.pdf
- 5.Huguet N, Kaplan MS, Feeny D. Socioeconomic status and health-related quality of life among elderly people: results from the Joint Canada/United States Survey of Health. Soc Sci Med. 2008;66(4):803-810. doi: 10.1016/j.socscimed.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 6.Bassuk SS, Berkman LF, Amick BC III. Socioeconomic status and mortality among the elderly: findings from four US communities. Am J Epidemiol. 2002;155(6):520-533. doi: 10.1093/aje/155.6.520 [DOI] [PubMed] [Google Scholar]
- 7.Platts-Mills TF, Leacock B, Cabañas JG, Shofer FS, McLean SA. Emergency medical services use by the elderly: analysis of a statewide database. Prehosp Emerg Care. 2010;14(3):329-333. doi: 10.3109/10903127.2010.481759 [DOI] [PubMed] [Google Scholar]
- 8.Svenson JE. Patterns of use of emergency medical transport: a population-based study. Am J Emerg Med. 2000;18(2):130-134. doi: 10.1016/S0735-6757(00)90002-0 [DOI] [PubMed] [Google Scholar]
- 9.Cone DC, Ahern J, Lee CH, Baker D, Murphy T, Bogucki S. A descriptive study of the “lift-assist” call. Prehosp Emerg Care. 2013;17(1):51-56. doi: 10.3109/10903127.2012.717168 [DOI] [PubMed] [Google Scholar]
- 10.Srivarathan A, Jensen AN, Kristiansen M. Community-based interventions to enhance healthy aging in disadvantaged areas: perceptions of older adults and health care professionals. BMC Health Serv Res. 2019;19(1):7. doi: 10.1186/s12913-018-3855-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal G, Angeles R, Pirrie M, et al. Evaluation of a community paramedicine health promotion and lifestyle risk assessment program for older adults who live in social housing: a cluster randomized trial. CMAJ. 2018;190(21):E638-E647. doi: 10.1503/cmaj.170740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal G, Angeles R, Pirrie M, et al. Reducing 9-1-1 emergency medical service calls by implementing a community paramedicine program for vulnerable older adults in public housing in Canada: a multi-site cluster randomized controlled trial. Prehosp Emerg Care. 2019;23(5):718-729. doi: 10.1080/10903127.2019.1566421 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal G, Pirrie M, Angeles R, Marzanek F, Thabane L, O’Reilly D. Cost-effectiveness analysis of a community paramedicine programme for low-income seniors living in subsidised housing: the community paramedicine at clinic programme (CP@clinic). BMJ Open. 2020;10(10):e037386. doi: 10.1136/bmjopen-2020-037386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMaster CP Research Team . CP@clinic 2023 Annual Report. Published 2024. Accessed August 28, 2024. https://cpatclinic.ca/media/cpclinic_annual_report_2023.pdf
- 15.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454-463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 16.Government of Ontario. Ontario Expands Community Paramedicine for Long-Term Care Program. 2021. Accessed September 10, 2024. https://news.ontario.ca/en/release/1001022/ontario-expands-community-paramedicine-for-long-term-care-program
- 17.Iezzoni LI, Dorner SC, Ajayi T. Community paramedicine–addressing questions as programs expand. N Engl J Med. 2016;374(12):1107-1109. doi: 10.1056/NEJMp1516100 [DOI] [PubMed] [Google Scholar]
- 18.Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res. 2018;18(1):316. doi: 10.1186/s12913-018-3148-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160-166. doi: 10.24095/hpcdp.33.3.06 [DOI] [PubMed] [Google Scholar]
- 20.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388-394. doi: 10.1080/15412550903140865 [DOI] [PubMed] [Google Scholar]
- 21.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18-e26. [PMC free article] [PubMed] [Google Scholar]
- 22.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54(1):337-349. doi: 10.3233/JAD-160105 [DOI] [PubMed] [Google Scholar]
- 23.Sternberg SA, Bentur N, Abrams C, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18(10):e392-e397. [PubMed] [Google Scholar]
- 24.Weiner JP, Abrams C. The Johns Hopkins ACG® System: Technical Reference Guide Version 10.0. Johns Hopkins Press; 2011.
- 25.Agarwal G, Pirrie M, McLeod B, et al. Rationale and methods of an evaluation of the effectiveness of the Community Paramedicine at Home (CP@home) program for frequent users of emergency medical services in multiple Ontario regions: a study protocol for a randomized controlled trial. Trials. 2019;20(1):75. doi: 10.1186/s13063-018-3107-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brydges M, Denton M, Agarwal G. The CHAP-EMS health promotion program: a qualitative study on participants’ views of the role of paramedics. BMC Health Serv Res. 2016;16(1):435. doi: 10.1186/s12913-016-1687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godwin M, Williamson T, Khan S, et al. Prevalence and management of hypertension in primary care practices with electronic medical records: a report from the Canadian Primary Care Sentinel Surveillance Network. CMAJ Open. 2015;3(1):E76-E82. doi: 10.9778/cmajo.20140038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagelhout GE, Abidi L, de Vries H. Reasons for (not) participating in a community-based health promotion program for low-income multi-problem households in the Netherlands: a qualitative study. Health Soc Care Community. 2021;29(1):241-249. doi: 10.1111/hsc.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karran EL, Grant AR, Lee H, et al. Do health education initiatives assist socioeconomically disadvantaged populations? A systematic review and meta-analyses. BMC Public Health. 2023;23(1):453. doi: 10.1186/s12889-023-15329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rong T, Ristevski E, Carroll M. Exploring community engagement in place-based approaches in areas of poor health and disadvantage: a scoping review. Health Place. 2023;81:103026. doi: 10.1016/j.healthplace.2023.103026 [DOI] [PubMed] [Google Scholar]
- 31.Shannon B, Eaton G, Lanos C, et al. The development of community paramedicine; a restricted review. Health Soc Care Community. 2022;30(6):e3547-e3561. doi: 10.1111/hsc.13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason S, O’Keeffe C, Coleman P, Edlin R, Nicholl J. Effectiveness of emergency care practitioners working within existing emergency service models of care. Emerg Med J. 2007;24(4):239-243. doi: 10.1136/emj.2006.035782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leduc S, Wells G, Thiruganasambandamoorthy V, et al. LO20: The characteristics, clinical course and disposition of long-term care patients treated by paramedics during an emergency call: exploring the potential impact of community paramedicine. CJEM. 2020;22(S1):S14. doi: 10.1017/cem.2020.76 [DOI] [PubMed] [Google Scholar]
- 34.Dixon S, Mason S, Knowles E, et al. Is it cost effective to introduce paramedic practitioners for older people to the ambulance service? results of a cluster randomised controlled trial. Emerg Med J. 2009;26(6):446-451. doi: 10.1136/emj.2008.061424 [DOI] [PubMed] [Google Scholar]
- 35.Randell E, McNamara R, Shaw C, Espinasse A, Simpson SA. Challenges of a community based pragmatic, randomised controlled trial of weight loss maintenance. BMC Res Notes. 2015;8(1):802. doi: 10.1186/s13104-015-1791-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Q, Chung H, Shambhu S, et al. Administrative claims data to support pragmatic clinical trial outcome ascertainment on cardiovascular health. Clin Trials. 2019;16(4):419-430. doi: 10.1177/1740774519846853 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Disease and Procedure Codes
eTable 2. Medication Categories
eTable 3. Participant Characteristics of Intervention Building Residents, Control Building Residents, and Intervention Attendees Who Had Prescription Drug Claim Records Available (≥66 Years or Eligible for the Ontario Drug Benefit)
eTable 4. Intention-to-Treat Analysis of Medication Initiation for All Intervention and Control Building Residents
eTable 5. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of Building Residents That Attended the Intervention Program
eTable 6. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of All Building Residents for a Subset of Buildings (N = 26)
eTable 7. Sensitivity Analysis of Healthcare Utilization and Medication Initiation Outcomes of Building Residents That Attended the Intervention Program for a Subset of Buildings (N = 26)
Data Sharing Statement