Key Points
Question
Have reproductive patterns changed within generations of women diagnosed with breast cancer in Caribbean countries?
Findings
In this cross-sectional study including 995 women diagnosed with breast cancer in 7 countries in the Caribbean, reproductive patterns shifted. Within four 10-year generations, significant decreases were observed in the mean number of siblings, age at menarche, number of pregnancies, and number of full-term pregnancies.
Meaning
This study observed changes in reproductive patterns in women with breast cancer in the Caribbean region, suggesting that interventions targeting other breast cancer risk factors need to be implemented.
Abstract
Importance
Breast cancer (BC) is commonly diagnosed among Caribbean women. Shifts in reproductive patterns modify the incidence of BC diagnosis and age at BC diagnosis in population-based studies; however, reproductive patterns in Caribbean women remain understudied.
Objective
To describe the temporal trends in reproductive patterns and age at BC diagnosis in Caribbean-born women.
Design, Setting, and Participants
A cross-sectional observational study—the Caribbean Women’s Cancer Study—was conducted, with data on reproductive patterns known to affect BC risk collected in The Bahamas, Barbados, Cayman Islands, Dominica, Haiti, Jamaica, and Trinidad and Tobago. Participants were recruited prospectively. The sample included women born in Caribbean countries and diagnosed with invasive BC and/or ovarian cancer from June 1, 2010, to June 30, 2018, and was divided into 4 birth cohorts (born before 1950, 1950-1959, 1960-1969, and in or after 1970). Data were analyzed between August 1, 2023, and July 31, 2024.
Exposures
Receipt of a BC diagnosis and birth in a Caribbean country.
Main Outcomes and Measures
Change in reproductive patterns between birth cohorts, including age at BC diagnosis, family history of cancer, age at first pregnancy, number of pregnancies, number of full-term pregnancies, number of siblings, age at menarche and menopause, estrogen receptor status, and germline pathogenic/likely pathogenic variants.
Results
Of 1015 participants diagnosed with BC and ovarian cancer, 995 women (mean [SD] age, 46.6 [10.8] years; 605 [81.8%] Afro-Caribbean, 98 [13.2%] East Indian, 22 [3.0%] White, and 12 [1.6%] >1 race) received a diagnosis of invasive BC. Comparison from older to younger birth cohorts (presented in the order of born before 1950, 1950-1959, 1960-1969 and in or after 1970) showed an increased proportion of women experiencing menarche at age 12 years or younger (33.0% vs 47.3% vs 45.5% vs 57.9%; P < .001), women with no pregnancies (6.8% vs 6.8% vs 10.5% vs 22.8%; P < .001), and nulliparous women (8.6% vs 9.2% vs 13.9% vs 27.6%; P < .001). Younger age at BC diagnosis was observed in women experiencing menarche at age 12 years or younger (mean [SD], 45.0 [10.5] years) vs 15 years or older (mean [SD], 49.1 [11.2] years) and in nulliparous women (mean [SD], 42.1 [11.2] years) vs 3 or more full-term pregnancies (mean [SD], 49.9 [10.6] years; P < .001). For every year of first pregnancy delay, women had a 4% increased chance of being diagnosed with estrogen receptor–positive tumors (odds ratio, 1.04; 95% CI, 1.01-1.08; P = .02).
Conclusions and Relevance
In this cross-sectional study, between each 10-year birth cohort, women diagnosed with BC had a lower age at menarche, number of pregnancies, and number of full-term pregnancies. These findings suggest that interventions targeting other BC risk factors need to be implemented.
This cross-sectional study examines changes over 4 decades in factors related to reproduction in women in Caribbean countries with a diagnosis of invasive breast cancer.
Introduction
Breast cancer (BC) accounts for the largest proportion of all cancer cases and is the fourth leading cause of cancer mortality worldwide.1 Although the incidence of BC is 88% higher in high-income countries, women living in low- to middle-income countries have 17% higher mortality rates.1 The Caribbean region has one of the highest BC mortality rates, with The Bahamas, Barbados, and Jamaica experiencing the world’s highest mortality.2,3 In the Caribbean, BC is prevalent among premenopausal women and is commonly diagnosed at a younger age (mean age: The Bahamas, 56.6; Barbados, 57.9; Haiti, 49; Jamaica, 49; and Trinidad and Tobago, 43 years)4,5,6,7 compared with the US (median age, 63 years).8
Several factors can explain the disproportionate burden of BC diagnoses in the Caribbean, such as obesity,9,10 genetics, parity, age at menarche, age at first pregnancy, and family size.11,12,13 These patterns differ between countries, oftentimes reflecting varying levels of economic development. The intersection of socioeconomic growth and changing lifestyle patterns plays a major role in shifting health outcomes. As low- to middle-income countries undergo economic transitions toward a higher development index, their populations tend to adopt a more sedentary lifestyle, gain weight, and display increased smoking and alcohol intake.14,15 Reproductive patterns also lean toward modern lifestyle norms, characterized by delayed first childbirth and fewer overall pregnancies.16,17
In a previous study examining the prevalence of hereditary breast and ovarian cancer syndrome (HBOC) in the Caribbean, individuals from The Bahamas had the highest prevalence of HBOC.18 Furthermore, 80% of Caribbean women diagnosed with BC and carrying a pathogenic/likely pathogenic germline variant had a relative diagnosed with BC.19 This initial Caribbean-based cross-sectional study observed early onset and highly aggressive disease in women with HBOC.19
The rich cultural and ethnic diversity of the Caribbean region also provides a multifaceted lens to examine these health outcomes. The Caribbean population is predominantly of African ancestry but also includes Asian, East Indian, European, Indigenous, and Middle Eastern ancestries.18 This admixture of genomic ancestries potentially affects the disease patterns we observe.
The interplay of genetic variants, genomic ancestries, and reproductive patterns within this uniquely diverse population is a largely understudied area, representing a major gap in the literature that our study aims to address. Considering the high rates of BC in the Caribbean, we sought to examine reproductive patterns across 7 countries in the region.
Methods
Cohort and Eligibility Criteria
This cross-sectional study is a subset of the Caribbean Women’s Cancer Study (CWCS), which prospectively enrolled patients with invasive BC and/or ovarian cancer born in Caribbean countries from June 1, 2010, to June 30, 2018. Details of the CWCS protocol approval were published previously.18 From the CWCS we selected participants who self-identified as female and received a diagnosis of invasive primary BC at any age. The study was approved by the institutional review boards at the Ministries of Health in The Bahamas, Barbados, Cayman Islands, Dominica, and Haiti; and at the University of West Indies, Mona, Jamaica; the Northwest Regional Health Authority of Trinidad and Tobago; and the University of Miami. Participants provided written informed consent; no financial compensation was given. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Reporting
Eligible women were divided into 4 birth cohorts: before 1950, between 1950 and 1959, between 1960 and 1969, and in or after 1970. The following variables were collected from the participants through a survey: family history of BC and ovarian cancer, age at first pregnancy, number of pregnancies, number of full-term pregnancies (FTPs), number of biologic siblings, age at menarche, menopausal status, and age at menopause. For race and ethnicity identification, participants chose among 12 categories; selecting multiple options was allowed. If the option Other was selected, they could write how they self-identified. For analysis purposes, categories were combined as Asian (includes Chinese or Other part of Asia), Black or Afro-Caribbean (includes African American, Afro-Caribbean, and Black) East Indian (includes India), 2 or more races (includes when participants selected ≥2 or more categories [eg, Chinese or other part of Asia, Afro-Caribbean, and White/Caucasian]), White or Caucasian (includes White/Caucasian and European).
Body mass index (BMI) at the time of diagnosis, age at BC diagnosis, and total abdominal hysterectomy with salpingo-oophorectomy (TAH-BSO) status were abstracted from the medical records. Estrogen receptor (ER) was measured by immunohistochemistry when available and BC was classified as ER-positive (≥1%) or ER negative (<1%) as determined by a local pathologist. Age at first pregnancy was reported in a subset of women with at least 1 FTP (parous). Age at natural menopause was the age at menopause reported in a subset of women who were postmenopausal and did not undergo TAH-BSO. Women underwent genetic testing as described previously18; for germline variant status, benign variants and variants of unknown significance were grouped as negative germline pathogenic/likely pathogenic variant, whereas likely pathogenic and pathogenic variants were characterized as positive germline pathogenic/likely pathogenic variant.18
The term fertility is used herein when referring to total reproductive experience, without accounting for its physiologic or social determinants, which is consistent with use of the term in other studies associating BC risk with fertility.20 Age at menarche was categorized as age 12 or less, 13, 14, and 15 years or older; number of pregnancies as 0, 1, 2, and 3 or more; number of FTP as 0 (nulliparous), 1, 2, and 3 or more; age at natural menopause as age 45 or younger, 46 to 50, and older than 50 years; age at first pregnancy as less than 20, 20 to 24, 25 to 29, and 30 years or older; and number of siblings as none, 1 to 5, and more than 5. These variables were also reported as means. Age at BC diagnosis was reported as a continuous variable. Body mass index was classified as less than 25.0, 25.0 to 29.9, and 30.0 or greater (calculated as weight in kilograms divided by height in meters squared). Family history of BC or ovarian cancer and germline pathogenic/likely pathogenic variant status were dichotomous (yes/positive or no/negative). We analyzed associations of reproductive factors with age at BC diagnosis using the defined categories. We assessed associations between age at BC diagnosis, germline variant status, ER-positive BC, and reproductive patterns.
Statistical Analysis
Descriptive statistics were used to describe the 4 birth cohorts. Continuous variables were compared using a 2-tailed independent t test or 1-way analysis of variance when appropriate. Categorical variables were compared using the χ2 test or Fisher exact test. Missing data accounted for less than 10% of the data; therefore, missing data were excluded in analyses and the total number of observations used in the analysis is reported in each table. Correlation between age at BC diagnosis and other continuous variables was explored using the Spearman rank correlation coefficient (ρ). Logistic regression tested reproductive factors as estimators for the likelihood of positive germline variant or ER-positive BC. All tests were 2-tailed, and P < .05 was considered statistically significant. Data analysis was conducted using SAS software, version 9.4 (SAS Institute Inc) and R, version 4.3.2 (R Foundation for Statistical Computing). Data were analyzed between August 1, 2023, and July 31, 2024.
Results
From the 1015 women who were enrolled in the CWCS, the analytical sample included 995 self-identified women who had a primary diagnosis of BC. Mean (SD) age at BC diagnosis was 46.6 (10.8) years; 3 (0.4%) women self-identified as Asian, 605 (81.8%) as Black or Afro-Caribbean, 98 (13.2%) as East Indian, 22 (3.0%) as White, and 12 (1.6%) as 2 or more races. Most women were premenopausal (558 [57.5%]), had a BMI greater than or equal to 30 (399 [41.5%]), experienced menarche before age 12 years (453 [46.7%]), reported 3 or more FTPs (404 [41.1%]), and experienced pregnancy between ages 20 and 24 years (289 [35.1%]); further demographic information is reported in Table 1. The 4 birth cohorts included 177 women born before 1950, 254 born between 1950 and 1959, 330 between 1960 and 1969, and 234 born in or after 1970. Excluding the cohort born in or after 1970, the mean (SD) age at BC diagnosis decreased steadily with each successive decade of birth from before 1950, 1950 to 1959, and 1960 to 1969 (60.7 [9.4] vs 50.4 [7.6] vs 44.3 [4.9]; P < .001). Women born between 1960 and 1969 received a BC diagnosis at a younger mean age (44.3 years) compared with women born between 1950 and 1959 (50.4 years) and women born before 1950 (60.7 years). For each of the 4 birth groups, most participants had overweight (BMI 25.0-29.9) or obesity (BMI ≥30). The proportion of women with obesity was lowest among those born in or after 1970 (34.9%) compared with those born before 1950 (45.4%), 1950 to 1959 (45.3%) and 1960 to 1969 (41.1 %) (P = .02); however, stratification of BMI by menopausal status revealed no association between BMI and birth groups (eTable 1 in Supplement 1). There was no association across successive decade of birth in the order of born before 1950, 1950-1959, 1960-1969 and in or after 1970 for positive family history of BC (52.5% vs 54.6% vs 49.7% vs 45.3%; P = .02) or ovarian cancer (8.6% vs 10.5% vs 12.0% vs 11.2%; P = .71). Across the 4 birth cohorts (born before 1950, 1950-1959, 1960-1969 and in or after 1970), there were significant differences in the means of the number of biological siblings (6.6 vs 5.8 vs 6.1 vs 4.5; P < .001), suggesting a transition from larger to smaller families. Other notable differences included an increased proportion of women experiencing menarche at or before age 12 years (33.0% vs 47.3% vs 45.5% vs 57.9%; P < .001), an increased proportion of no pregnancies (6.8% vs 6.8% vs 10.5% vs 22.8%; P < .001), an increased proportion of nulliparous women (8.6% vs 9.2% vs 13.9% vs 27.6%; P < .001), and an increased proportion of women undergoing natural menopause at or before age 45 years (22.7% vs 30.6% vs 59.6%; P < .001 [group born in or after 1970 excluded]) in women born in later decades. Fewer women underwent TAH-BSO in younger birth groups (32% vs 20.8% vs 13.2% vs 5.1%; P<.001). There was an increasing proportion of BCs associated with positive germline variants over decades (9.6% vs 11.8% vs 15.8% vs 17.1%) and ER-negative BC (32.1% vs 37.6% vs 42.6% vs 43.9%), although the finding was not statistically significant. These results are summarized in Table 2.
Table 1. Demographic Characteristics of the Entire Study Population.
| Variable | Participants, No. (%) |
|---|---|
| Age at BC diagnosis, mean (SD), y | 46.6 (10.8) |
| Self-identified racea | |
| Asian | 3 (0.4) |
| Black or Afro-Caribbean | 605 (81.8) |
| East Indian | 98 (13.2) |
| ≥2 Races | 12 (1.6) |
| White or Caucasian | 22 (3.0) |
| Unknown/missing | 250 |
| BMI | |
| <25.0 | 243 (25.3) |
| 25.0-29.9 | 320 (33.3) |
| ≥30.0 | 399 (41.5) |
| Unknown/missing | 66 |
| Family history of breast cancer | |
| Yes | 497 (50.4) |
| No | 489 (49.6) |
| Unknown/missing | 18 |
| Family history of ovarian cancer | |
| Yes | 106 (10.8) |
| No | 874 (89.2) |
| Unknown/missing | 30 |
| No. of siblings | |
| Mean (SD) | 5.8 (4.1) |
| 0 | 24 (2.9) |
| 1-5 | 411 (50.3) |
| >5 | 382 (46.8) |
| Unknown/missing | 178 |
| Age at menarche, y | |
| Mean (SD) | 12.8 (1.9) |
| ≤12 | 453 (46.7) |
| 13 | 202 (20.8) |
| 14 | 144 (14.8) |
| ≥15 | 172 (17.7) |
| Unknown/missing | 24 |
| No. of pregnancies (gravida) | |
| Mean (SD) | 3.1 (2.2) |
| 0 | 116 (11.8) |
| 1 | 117 (11.9) |
| 2 | 197 (20.1) |
| ≥3 | 551 (56.2) |
| Unknown/missing | 14 |
| No. of full-term pregnancies (para) | |
| Mean (SD) | 2.4 (1.9) |
| 0 | 147 (15.0) |
| 1 | 158 (16.1) |
| 2 | 273 (27.8) |
| ≥3 | 404 (41.1) |
| Unknown/missing | 13 |
| Age at first pregnancy, parous women, y (n = 824) | |
| Mean (SD) | 22.8 (5.3) |
| <20 | 260 (31.5) |
| 20-24 | 289 (35.1) |
| 25-29 | 185 (22.5) |
| ≥30 | 90 (10.9) |
| TAH-BSO (n = 715) | |
| Yes | 139 (16.3) |
| No | 716 (83.7) |
| Unknown/missing | 280 |
| Menopausal status (n = 947) | |
| Premenopausal | 558 (57.5) |
| Postmenopausal | 413 (42.5) |
| Unknown/missing | 48 |
| Age at natural menopause (n = 236), y | |
| Mean (SD) | 47.2 (5.6) |
| ≤45 | 81 (34.3) |
| 46-50 | 93 (39.4) |
| >50 | 62 (26.3) |
| Germline likely pathogenic/pathogenic variant status | |
| Positive | 886 (86) |
| Negative | 139 (14) |
| Estrogen receptor | |
| Positive | 342 (59.5) |
| Negative | 233 (40.5) |
| Unknown/missing | 420 |
Abbreviations: BC, breast cancer; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TAH-BSO, total abdominal hysterectomy with bilateral oophorectomy.
Participants chose among 12 categories; selecting multiple options was allowed. If the option Other was selected, they could write how they self-identified. For analysis purposes, categories were combined as Asian (includes Chinese or Other part of Asia), Black or Afro-Caribbean (includes African American, Afro-Caribbean, and Black), East Indian (includes India), 2 or more races (includes when participants selected ≥2 categories [eg, Chinese or other part of Asia, Afro-Caribbean, and White/Caucasian]), White or Caucasian (includes White/Caucasian and European).
Table 2. Demographic Characteristics of the Study Population Divided by Birth Group.
| Variable | Year of birth, No. (%) | P value | |||
|---|---|---|---|---|---|
| <1950 (n = 177) | 1950-1959 (n = 254) | 1960-1969 (n = 330) | ≥1970 (n = 234) | ||
| Age at BC diagnosis, mean (SD), y | 60.7 (9.4) | 50.4 (7.6) | 44.3 (4.9) | 35.0 (5.0)a | <.001 |
| Raceb | |||||
| Asianc | 0 | 0 | 0 | 3 | <.001 |
| Black or Afro-Caribbean | 96 (83.5) | 144 (88.3) | 199 (77.1) | 166 (82.6) | |
| East Indian | 8 (7.0) | 10 (6.1) | 48 (18.6) | 32 (15.9) | |
| ≥ Races | 1 (0.8) | 3 (1.9) | 6 (2.3) | 2 (1) | |
| White or Caucasian | 10 (8.7) | 6 (3.7) | 5 (2.0) | 1 (0.5) | |
| Unknown/missingc | 62 | 91 | 72 | 30 | |
| BMI (n = 962) | |||||
| <25.0 | 42 (24.1) | 52 (21.9) | 71 (22.1) | 78 (34.1) | .02 |
| 25.0-29.9 | 53 (30.5) | 78 (32.8) | 118 (36.8) | 71 (31) | |
| ≥30.0 | 79 (45.4) | 108 (45.3) | 132 (41.1) | 80 (34.9) | |
| Family history of breast cancer (n = 986) | |||||
| Yes | 93 (52.5) | 136 (54.6) | 163 (49.7) | 105 (45.3) | .20 |
| No | 84 (47.5) | 113 (45.4) | 165 (50.3) | 127 (54.7) | |
| Family history of ovarian cancer (n = 980) | |||||
| Yes | 15 (8.6) | 26 (10.5) | 39 (12.0) | 26 (11.2) | .71 |
| No | 159 (91.4) | 222 (89.5) | 287 (88) | 206 (88.8) | |
| No. of siblings (n = 817) | |||||
| 0 | 7 (4.2) | 8 (3.5) | 5 (2) | 4 (2.4) | <.001 |
| 1-5 | 71 (42.5) | 112 (48.5) | 110 (44) | 118 (69.8) | |
| >5 | 89 (53.3) | 111 (48) | 135 (54) | 47 (27.8) | |
| Mean (SD) | 6.6 (5.4) | 5.8 (4.0) | 6.1 (3.6) | 4.5 (3.1) | <.001 |
| Age at menarche, y (n = 971) | |||||
| ≤12 | 57 (33.0) | 116 (47.3) | 148 (45.5) | 132 (57.9) | <.001 |
| 13 | 36 (20.8) | 51 (20.8) | 68 (20.9) | 47 (20.6) | |
| 14 | 41 (23.7) | 35 (14.3) | 49 (15.1) | 19 (8.3) | |
| ≥15 | 39 (22.5) | 43 (17.6) | 60 (18.5) | 30 (13.2) | |
| Mean (SD) | 13.4 (1.9) | 12.8 (1.9) | 12.9 (1.8) | 12.3 (1.7) | |
| No. of pregnancies (gravida) (n = 981) | |||||
| 0 | 12 (6.8) | 17 (6.8) | 34 (10.5) | 53 (22.8) | <.001 |
| 1 | 15 (8.6) | 20 (8.0) | 37 (11.4) | 45 (19.4) | |
| 2 | 28 (16) | 56 (22.5) | 70 (21.5) | 43 (18.5) | |
| ≥3 | 120 (68.6) | 156 (62.7) | 184 (56.6) | 91 (39.2) | |
| Mean (SD) | 4.0 (2.7) | 3.5 (2.2) | 2.9 (2.0) | 2.1 (1.8) | |
| No. of full-term pregnancies (para) (n = 982) | |||||
| 0 | 15 (8.6) | 23 (9.2) | 45 (13.9) | 64 (27.6) | <.001 |
| 1 | 18 (10.3) | 30 (12.0) | 57 (17.5) | 53 (22.8) | |
| 2 | 41 (23.4) | 73 (29.2) | 94 (28.9) | 65 (28.0) | |
| ≥3 | 101 (57.7) | 124 (49.6) | 129 (39.7) | 50 (21.6) | |
| Mean (SD) | 3.5 (2.5) | 2.8 (1.8) | 2.2 (1.5) | 1.6 (1.4) | |
| Age at 1st pregnancy, parous women, y (n = 824) | |||||
| <20 | 53 (33.3) | 70 (31.5) | 94 (33.7) | 43 (26.2) | .12 |
| 20-24 | 58 (36.5) | 92 (41.4) | 83 (29.7) | 56 (34.2) | |
| 25-29 | 35 (22.0) | 41 (18.5) | 66 (23.7) | 43 (26.2) | |
| ≥30 | 13 (8.2) | 19 (8.6) | 36 (12.9) | 22 (13.4) | |
| Mean (SD) | 22.2 (5.1) | 22.6 (5.2) | 23.1 (5.8) | 23.7 (5) | .06 |
| TAH-BSO (n = 855) | |||||
| Yes | 48 (32) | 43 (20.8) | 37 (13.2) | 11 (5.1) | <.001 |
| No | 102 (68) | 164 (79.2) | 244 (86.8) | 206 (94.9) | |
| Menopausal status (n = 947) | |||||
| Premenopausal | 14 (8.3) | 74 (30.3) | 247 (75.5) | 223 (96.5) | <.001 |
| Postmenopausal | 155 (91.7) | 170 (69.7) | 80 (24.5) | 8 (3.5) | |
| Age at natural menopause, postmenopausal women, y (n = 236) | |||||
| ≤45 | 20 (22.7) | 30 (30.6) | 28 (59.6) | 3 (100)a | <.001 |
| 46-50 | 33 (37.5) | 42 (42.9) | 18 (38.3) | 0a | |
| >50 | 35 (39.8) | 26 (26.5) | 1 (2.13) | 0a | |
| Mean (SD) | 49.1 (5.6) | 47.6 (5) | 43.3 (5) | 41 (3.6)a | <.001 |
| Germline likely pathogenic/pathogenic variant status | |||||
| Positive | 17 (9.6) | 30 (11.8) | 52 (15.8) | 40 (17.1) | .09 |
| Negative | 160 (90.4) | 224 (88.2) | 278 (84.2) | 194 (82.9) | |
| Estrogen receptor | |||||
| Positive | 55 (67.9) | 73 (62.4) | 117 (57.4) | 97 (56.1) | .26 |
| Negative | 26 (32.1) | 44 (37.6) | 87 (42.6) | 76 (43.9) | |
| Unknown/missingc | 96 | 137 | 126 | 61 | |
Abbreviations: BC, breast cancer; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TAH-BSO, total abdominal hysterectomy with bilateral oophorectomy.
The birth group that was born in or after 1970 was excluded from this analysis.
Participants chose among 12 categories; selecting multiple options was allowed. If the option Other was selected, they could write how they self-identified. For analysis purposes, categories were combined as Asian (includes Chinese or Other part of Asia) Black or Afro-Caribbean (includes African American, Afro-Caribbean, and Black) East Indian (includes India), Mixed (includes when participants selected ≥2 or more categories [eg, Chinese or other part of Asia], Afro-Caribbean, and White/Caucasian), White or Caucasian (includes White/Caucasian and European).
The category was excluded from the analysis.
Correlation analysis between age at BC diagnosis and other continuous variables showed that younger age at BC diagnosis correlated with younger age at menarche, fewer pregnancies, fewer FTPs, younger age at menopause, and older age at first pregnancy (correlation coefficient ρ or P value) (eTable 2 in Supplement 1). Mean (SD) age at BC diagnosis differed significantly between women who experienced menarche at or before age 12 (45 [10.5]) years compared with women who experienced menarche at 14 (49.9 [10.4]) years and at or after 15 (49.1 [11.2]) years (P < .001) (Figure, A). Women who were never pregnant had a lower mean (SD) age of BC (41.5 [11.4] years) compared with women with 2 pregnancies (46.1 [9.36] years) or those with 3 or more pregnancies (49.9 [11.2] years; P < .001) (Figure, B). Nulliparous women had BC diagnosed at a mean (SD) age of 42.1 (11.2) years compared with women with more than 2 FTPs who were diagnosed a mean of 7 years later (49.9 [10.6] years; P < .001) (Figure, C). There were no associations in age at BC diagnosis with age at first pregnancy (eFigure 1 in Supplement 1). Women who experienced natural menopause at or before age 45 years presented with a mean (SD) age at BC diagnosis of 52.6 (9.5) years, which differed from the age at BC diagnosis in women undergoing natural menopause between ages 46 and 50 years (57.1 [9.5] years; P < .001) and after 50 years (58.6 [8.3] years; P < .001) (Figure, D).
Figure. Comparison of Age at Breast Cancer (BC) Diagnosis Across Reproductive Factors.
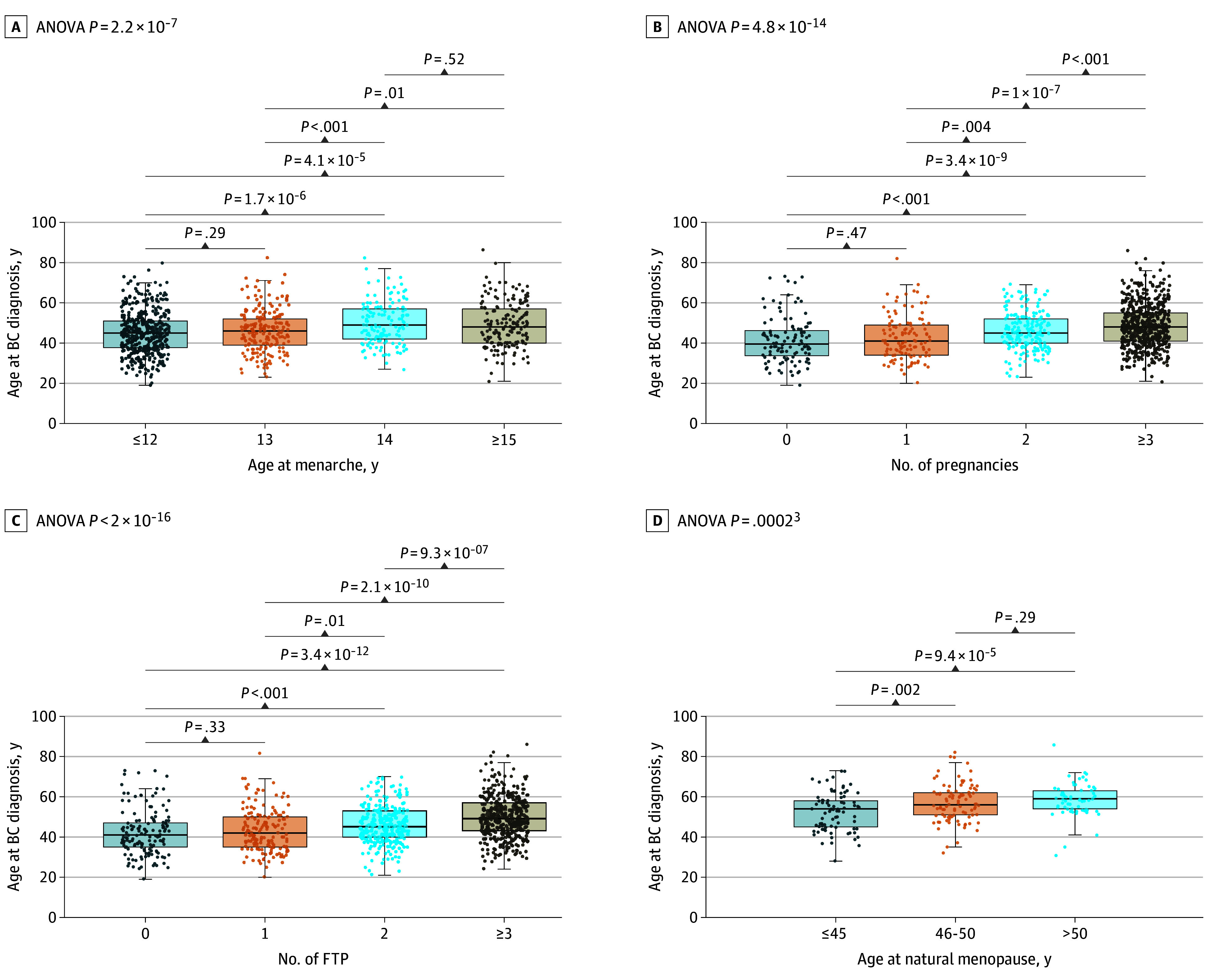
A, Differences in women reporting menarche at or before age 12 (mean, 45) years, 13 (mean, 45.9) years, 14 (mean, 49.9) years, and at or after 15 (mean, 49.1) years. B, Differences among number of pregnancies, women with no pregnancies (mean age, 41.5 years), 1 pregnancy (mean age, 42.5 years), 2 pregnancies (mean age, 46.1 years), and 3 or more pregnancies (mean age, 48.7 years). C, Differences among number of full-term pregnancies (FTPs): nulliparous women (mean age, 42.1 years), 1 FTP (mean age, 43.4 years), 2 FTPs (mean age, 46 years) and 3 or more FTPs (mean age, 49.9 years). D, Differences among age of natural menopause: women experiencing menopause at or before 45 years (mean age, 52.6 years), between 46 and 50 years (mean age, 57.1 years), and after 50 years (mean age, 58.6 years). ANOVA indicates analysis of variance.
We explored age and BC diagnosis across each birth cohort, stratified by country. The distribution was uniform and similar (eFigure 2 in Supplement 1). The overall trend across all countries showed a decrease in the age at BC diagnosis as the birth cohort increased. Compared with women in the other countries, Bahamian women consistently presented at a younger mean age of BC diagnosis in each birth cohort.
Based on HBOC germline variant status, as expected, women with germline-positive variants had a lower mean age at BC diagnosis (40.6 years) compared with women with germline-negative variants (47.6 years), and family history differed by germline variant for BC (80.2% vs 45.7%; P < .001) or ovarian cancer (18.9% vs 9.6%; P = .001). There was a higher proportion of women who underwent TAH-BSO in the group with a positive germline variant (23.3%) compared with the group with a negative germline variant (15.3%; P = .04), as well as a higher proportion of ER-negative BC in women with a positive germline variant (53%) compared with those with none (38.9%; P = .03). There were no significant differences in the proportions or means for other variables, such as birth year, BMI, number of siblings, age at menarche, number of pregnancies, number of FTPs, age at first pregnancy, and age at natural menopause (Table 3).
Table 3. Characteristics of Study Population by Genetic Germline Variant Status.
| Variable | Germline likely pathogenic/pathogenic variant status, No. (%) | P value | |
|---|---|---|---|
| Positive | Negative | ||
| Age at BC diagnosis, mean (SD), y | 40.6 (9.0) | 47.6 (10.7) | <.001 |
| BMI (n = 962) | |||
| <25.0 | 26 (19.9) | 217 (26.1) | .09 |
| 25.0-29.9 | 50 (38.2) | 270 (32.5) | |
| ≥30.0 | 55 (42.0) | 344 (41.4) | |
| Family history of breast cancer (n = 986) | |||
| Yes | 109 (80.2) | 388 (45.7) | <.001 |
| No | 27 (19.9) | 462 (54.4) | |
| Family history of ovarian cancer (n = 980) | |||
| Yes | 25 (18.9) | 81 (9.6) | .001 |
| No | 107 (81.1) | 767 (90.5) | |
| No. of siblings (n = 817) | |||
| 0 | 6 (4.4) | 18 (2.7) | .40 |
| 1-5 | 72 (52.6) | 339 (49.8) | |
| >5 | 59 (43.0) | 323 (47.5) | |
| Mean (SD) | 5.3 (3.5) | 5.9 (4.2) | .07 |
| Age at menarche, y (n = 971) | |||
| ≤12 | 64 (46.7) | 389 (46.6) | .73 |
| 13 | 28 (20.4) | 174 (20.9) | |
| 14 | 24 (17.5) | 120 (14.4) | |
| ≥15 | 21 (15.3) | 151 (18.1) | |
| Mean (SD) | 12.8 (1.9) | 12.8 (1.9) | .94 |
| No. of pregnancies (gravida) (n = 981) | |||
| 0 | 15 (10.9) | 101 (12) | .49 |
| 1 | 20 (14.5) | 97 (11.5) | |
| 2 | 32 (23.2) | 165 (19.6) | |
| ≥3 | 71 (51.4) | 480 (56.9) | |
| Mean (SD) | 2.9 (2.1) | 3.1 (2.3) | .20 |
| No. of full-term pregnancies (para) (n = 982) | |||
| 0 | 19 (13.8) | 128 (15.2) | .35 |
| 1 | 29 (21) | 129 (15.3) | |
| 2 | 39 (28.2) | 234 (27.7) | |
| ≥3 | 51 (37) | 353 (41.8) | |
| Mean (SD) | 2.3 (1.7) | 2.5 (1.9) | .22 |
| Age at 1st pregnancy, parous women, y (n = 824) | |||
| <20 | 37 (32.2) | 223 (31.5) | .72 |
| 20-24 | 37 (32.2) | 252 (35.5) | |
| 25-29 | 30 (26.1) | 155 (21.9) | |
| ≥30 | 11 (9.5) | 79 (11.1) | |
| Mean (SD) | 23.3 (5.6) | 22.9 (5.3) | .43 |
| TAH-BSO (n = 855) | |||
| Yes | 24 (23.3) | 115 (15.3) | .04 |
| No | 79 (76.7) | 637 (84.7) | |
| Age at natural menopause, postmenopausal women, y (n = 236) | |||
| ≤45 | 10 (55.6) | 71 (32.6) | .11 |
| 46-50 | 6 (33.3) | 87 (39.9) | |
| >50 | 2 (11.1) | 60 (27.5) | |
| Mean (SD) | 44.2 (7.4) | 47.5 (5.4) | .07 |
| Estrogen receptor | |||
| Positive | 31 (47.0) | 311 (61.1) | .03 |
| Negative | 35 (53.0) | 198 (38.9) | |
| Unknown/missing | 73 | 347 | |
Abbreviations: BC, breast cancer; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); TAH-BSO, total abdominal hysterectomy with bilateral oophorectomy.
Univariable and multivariable logistic regression models the likelihood of testing for a positive genetic variant in women across all birth groups overall and in The Bahamas are presented in eTable 3 in Supplement 1. As expected, women with a family history of BC or ovarian cancer were more likely to have a positive genetic germline variant (odds ratio [OR], 4.98; 95% CI, 3.13-7.92; P < .001). Furthermore, for every 1-year increase in age at first pregnancy, women had a 4% increased odds of receiving a diagnosis of ER-positive tumors (OR, 1.04; 95% CI, 1.01-1.08; P = .02) (eTable 4 in Supplement 1); other reproductive factors were not significant in the model testing the likelihood of testing for a positive germline variant or ER-positive tumors.
Discussion
Several factors increase the risk of developing BC. Hormonal drivers and lifestyle changes noted with economic development and reproductive patterns all resulted in a decrease in the age at BC diagnosis.21,22 Our findings suggest a decreasing mean age in age at BC diagnosis in younger generations of Caribbean women. The youngest cohort was excluded from this analysis as women in this cohort were not old enough to develop postmenopausal BC. However, the fact that age at BC diagnosis decreased in the 3 oldest birth groups aligns with a broader global trend of increasing BC incidence rates across various age groups and regions from 1990 to 2017, as reported by Lima et al.23 Decreasing age at BC diagnosis is linked to shifts in reproductive factor patterns, such as age at menarche, age at first pregnancy, number of pregnancies, and parity, as seen in the oldest 3 birth cohorts of our study and other studies.24 In the Caribbean, this decrease in the age at BC diagnosis can likely be attributed to changing habits of women as their countries become increasingly westernized.25,26
In this study, age at menarche decreased with each generation and the proportion of women who experienced menarche at age 12 years or younger increased with each generation. These changes have also been observed in high-income countries, such as the UK, where age at menarche decreased from 13.4 years for women born between 1925 and 1929 to 12.8 years for women born between 1950 and 1955.27 The decrease in mean age at menarche seen in the cohort in our study is further evidence of the increase in the human development index and corresponding behaviors of individuals in Caribbean countries. Age at menarche and menopause are major determinants of endogenous hormone exposure, associated with cancer risk, but also with osteoporosis28 and heart disease.29,30 A Collaborative Group on Hormonal Factors in Breast Cancer published a meta-analysis of more than 100 000 women showing the risk of BC increasing by lengthening a woman’s reproductive years, where early menarche played a greater role than later menopause.31 In addition, younger age at menarche has been reported to increase the odds of triple-negative BC compared with luminal A (ER-positive/progesterone receptor–negative), particularly in African American women.32 Diet and lifestyle changes play an important role in the decrease in age at menarche and BC onset.33 With modern lifestyle changes, such as diet and sedentarism, the rate of obesity is increasing, which contributes to the risk of developing BC.34
Further evidence of the shift in dietary habits and exercise patterns is seen in the proportion of women with obesity (BMI≥30) decreasing over the decades in the cohort in this study. High BMI is a well-known risk factor for breast, ovarian, and many other cancers.35 Two reviews suggested that high BMI may either be a risk factor or a protective one, depending on menopausal status of women at the time of BC diagnosis. In premenopausal women, a high BMI is a protective factor,36 whereas in postmenopausal women, it is a risk factor.37 In one study examining postmenopausal BC in 28 409 women in the UK, researchers found a 5% increase in the incidence of cancer per 5-unit increase in BMI.35 Our study assessed BMI and menopausal status at the time of BC diagnosis, with most premenopausal and postmenopausal women having obesity; however, no further association was found between BMI and year of birth when stratified by menopausal status in our study. Although high BMI showed a positive correlation with the incidence of BC diagnosis, in the cohort in our study, it is not clear how BMI affects BC age at onset.
In our study, for each year of delayed pregnancy, there was an increased chance of ER-positive BC diagnosis, by 4%. The improvement of the human development index in the Caribbean experienced by more-recent birth cohorts in the 1970s, for example, may explain the shifts in other reproductive factors, such as age at first pregnancy. Age at first pregnancy has been reported as a major fertility risk factor for BC since the publication of findings in an international multicenter case-control study in 1970.38 Early menarche and later age at first pregnancy have been shown to synergistically contribute to an earlier and longer exposure to estrogen during the initial development of the breast gland, a crucial time that modulates the risk for BC.26,39 These factors likely contribute to the decrease seen in the age at BC diagnosis in younger generations.
The decreasing number of siblings seen in our study is indicative of transitioning of larger to smaller families. Family history is associated with an increased risk of BC; however, in the cohort in our study, there was no significant change in positive family history proportions when stratified by year of birth, suggesting that family history has been an unchanged risk factor across generations.
In our study, the proportion of nulliparous women significantly increased from older to younger generations. However, a study comparing parity and BC incidence showed that a change of increased incidence of BC occurred more than 3 decades before the secular decrease in parity, concluding that the increase in BC incidence cannot be attributed to decreasing changes in parity.40 Furthermore, women with a germline pathogenic variant had a lower mean age at BC diagnosis compared with women without a germline variant, a finding supported by previously published cohorts.41,42 When the cohorts were stratified by germline variant status, we found no significant difference in the number of siblings, age at menarche, number of pregnancies, number of FTPs, or age at first pregnancy. This finding is similar to that of a study by Pal et al,43 reporting that the fertility patterns experienced by BRCA variant carriers is similar to that of noncarriers. However, the percent of women who had a TAH-BSO was significantly different between both groups, with germline variant carriers having a higher percent of TAH-BSO, which can be attributed to the fact that most women with a positive germline variant had undergone risk-reducing surgery. Additionally, there is a higher percentage of ER-negative BC in germline variant carriers (women with positive germline variants); this finding is consistent with the literature reporting high rates of triple-negative BC in BRCA1 variant carriers.44
Reproductive factors affecting BC have been studied in other populations. For example, in a separate cohort in Barbados,45 researchers found that older age at first pregnancy and nulliparity were significant risk factors for BC diagnosis, with an apparent dose effect of higher parity reducing risk, with similar results in Puerto Rico.46 However, the literature on social determinants for BC in these populations is very limited by study variability.45 In The Bahamas, BC is diagnosed in women at a younger age (mean, 42 years).18,47 Bahamian women were more likely to report a genetic germline variant, which explains the younger age seen in our Bahamian cohort. Reproductive factors did not appear to influence age at BC presentation in The Bahamas, as the genetic germline variant was strong enough to change BC presentation. As reported by Anders et al,48 younger age adjusted for all well-known prognostic factors is a powerful estimator for higher rates of recurrence risk and lower survival. The Bahamas showed the highest prevalence of BRCA1 variants of any population studied to date by our group.19 In sub-Saharan Africa, changes in lifestyle and reproductive patterns have been influenced by improved nutrition and education.49 Furthermore, these changes have been associated with a shift in the incidence and age of onset of BC.50,51 Better nutrition leads to earlier menarche, while increased educational levels among women delay the age at first pregnancy.52 The Caribbean represents a transitioning region in which reproductive patterns and behaviors linked to BC are rapidly changing.
Limitations
Our study is not void of limitations. Although our results show a decrease in the mean age at diagnosis of the first BC concurrent with changes in reproductive patterns, these trends may be associated with several other factors. First, we did not obtain a representative sample of all women unaffected by BC and our analyses focused on women with BC without a control group; therefore, certain well-known factors that affect BC incidence, such as age at first pregnancy, were not significant within cases of BC only. Second, since this was a cross-sectional and not a longitudinal study, we only surveyed women who survived long enough to participate in our study; therefore, the youngest cohort (born in or after 1970) was represented mainly by premenopausal women with BC. Furthermore, women with premenopausal BC may have experienced pregnancy after data were captured. Third, the main sources of exogenous estrogen exposure, such as hormone replacement therapy and oral contraceptive use, can increase the risk of BC,53 and these variables were not captured in this study. Fourth, a limited number of participants had immunohistochemistry test results available, so BC molecular subtypes and reproductive patterns could not be robustly assessed. Fifth, participants in the study included women from 7 countries of the Caribbean; the observed changes in reproductive patterns may differ in other populations.
Conclusions
The data from this cross-sectional study suggest that The Bahamas, Barbados, Cayman Islands, Dominica, Haiti, Jamaica, and Trinidad and Tobago have undergone changes in reproductive patterns, specifically a decrease in the number of siblings, age at menarche, number of pregnancies, number of FTPs, and age at menopause, within the 4 age cohorts studied. These changes provide insight into risk factor patterns for BC incidence associated with younger age at BC diagnosis. As Caribbean countries experience this transition, interventions targeting other modifiable risk factors for BC, such as dietary intake of fruits and vegetables, increased physical activity, weight loss, and decreased alcohol consumption, are needed to compensate for the shift in reproductive patterns.
eTable 1. Stratification of BMI by Menopausal Status
eTable 2. Spearman Correlation Between Age at BC Diagnosis and Other Variables
eFigure 1. No Differences Seen Comparing Age at BC Diagnosis in Women Reporting Age at First Pregnancy Before 20y (Mean 48.4y), Between 20-24y (Mean 48.6y), Between 25-29y (mean 47.9y), and at or After 30y (Mean 48.7y)
eFigure 2. Mean Age at Breast Cancer Diagnosis by Decade of Birth and 95% Confidence Interval Bars Are Shown
eTable 3. Logistic Regression Testing the Likelihood of a Positive Germline Pathogenic/Likely Pathogenic Variant
eTable 4. Logistic Regression Testing the Likelihood of ER-Positive BC Diagnosis
Data Sharing Statement
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263. doi: 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- 2.Razzaghi H, Quesnel-Crooks S, Sherman R, et al. Leading causes of cancer mortality—Caribbean region, 2003-2013. MMWR Morb Mortal Wkly Rep. 2016;65(49):1395-1400. doi: 10.15585/mmwr.mm6549a3 [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15-23. doi: 10.1016/j.breast.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samaroo K, Hosein A, Olivier LK, Ali J. Breast cancer in the Caribbean. Cureus. 2021;13(8):e17042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez A, DeGennaro V, George SHL, et al. Presentation, treatment, and outcomes of Haitian women with breast cancer in Miami and Haiti: disparities in breast cancer—a retrospective cohort study. J Glob Oncol. 2016;3(4):389-399. doi: 10.1200/JGO.2016.005975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragin C, Banydeen R, Zhang C, et al. Breast cancer research in the Caribbean: analysis of reports from 1975 to 2017. J Glob Oncol. 2018;4:1-21. doi: 10.1200/JGO.18.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hercules SM, Hercules JC, Ansari A, et al. High triple-negative breast cancer prevalence and aggressive prognostic factors in Barbadian women with breast cancer. Cancer. 2020;126(10):2217-2224. doi: 10.1002/cncr.32771 [DOI] [PubMed] [Google Scholar]
- 8.Surveillance Research Program National Cancer Institute . SEER*Explorer: an interactive website for SEER cancer statistics. November 16, 2023. Accessed April 14, 2024. https://seer.cancer.gov/statistics-network/explorer/
- 9.Dehesh T, Fadaghi S, Seyedi M, et al. The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: a systematic review and meta-analysis. BMC Womens Health. 2023;23(1):392. doi: 10.1186/s12905-023-02543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Wang YX, Sandoval-Insausti H, et al. Menstrual cycle characteristics and incident cancer: a prospective cohort study. Hum Reprod. 2022;37(2):341-351. doi: 10.1093/humrep/deab251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444-457. doi: 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 12.Zahed H, Feng X, Sheikh M, et al. Age at diagnosis for lung, colon, breast and prostate cancers: an international comparative study. Int J Cancer. 2024;154(1):28-40. doi: 10.1002/ijc.34671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore J, Pal T, Beeghly-Fadiel A, et al. A pooled case-only analysis of obesity and breast cancer subtype among Black women in the southeastern United States. Cancer Causes Control. 2022;33(4):515-524. doi: 10.1007/s10552-021-01545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumith SC, Hallal PC, Reis RS, Kohl HW III. Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med. 2011;53(1-2):24-28. doi: 10.1016/j.ypmed.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 15.Riahi M, Mohammadi AA, Rohani H, Bidkhori M. Dataset on the prevalence of tobacco smoking in men and women of selected countries whit difference human development. Data Brief. 2018;18:506-511. doi: 10.1016/j.dib.2018.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Šprocha B, Tišliar P, Šídlo L. A cohort perspective on the fertility postponement transition and low fertility in Central Europe. Moravian Geographical Reports. 2018;26(2):109-120. doi: 10.2478/mgr-2018-0009 [DOI] [Google Scholar]
- 17.Safdari-Dehcheshmeh F, Noroozi M, Taleghani F, Memar S. Factors influencing the delay in childbearing: a narrative review. Iran J Nurs Midwifery Res. 2023;28(1):10-19. doi: 10.4103/ijnmr.ijnmr_65_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George SHL, Donenberg T, Alexis C, et al. Gene sequencing for pathogenic variants among adults with breast and ovarian cancer in the Caribbean. JAMA Netw Open. 2021;4(3):e210307. doi: 10.1001/jamanetworkopen.2021.0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from The Bahamas. Breast Cancer Res Treat. 2011;125(2):591-596. doi: 10.1007/s10549-010-1156-9 [DOI] [PubMed] [Google Scholar]
- 20.Marklund A, Lundberg FE, Eloranta S, Hedayati E, Pettersson K, Rodriguez-Wallberg KA. Reproductive outcomes after breast cancer in women with vs without fertility preservation. JAMA Oncol. 2021;7(1):86-91. doi: 10.1001/jamaoncol.2020.5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM, Phipps AI, Hines LM, et al. Menstrual and reproductive characteristics and breast cancer risk by hormone receptor status and ethnicity: the Breast Cancer Etiology in Minorities study. Int J Cancer. 2020;147(7):1808-1822. doi: 10.1002/ijc.32923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John EM, Koo J, Phipps AI, et al. Reproductive characteristics, menopausal status, race and ethnicity, and risk of breast cancer subtypes defined by ER, PR and HER2 status: the Breast Cancer Etiology in Minorities study. Breast Cancer Res. 2024;26(1):88. doi: 10.1186/s13058-024-01834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine. 2021;38:100985. doi: 10.1016/j.eclinm.2021.100985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C, Elsworth GR, Osborne RH. Co-designing eHealth and equity solutions: application of the Ophelia (Optimizing Health Literacy and Access) process. Front Public Health. 2020;8:604401. doi: 10.3389/fpubh.2020.604401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray F, Soerjomataram I. The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: Disease Control Priorities, Third Edition (Volume 3). The International Bank for Reconstruction and Development; 2015. [PubMed] [Google Scholar]
- 26.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400-2409. doi: [DOI] [PubMed] [Google Scholar]
- 27.Gentry-Maharaj A, Glazer C, Burnell M, et al. Changing trends in reproductive/lifestyle factors in UK women: descriptive study within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). BMJ Open. 2017;7(3):e011822. doi: 10.1136/bmjopen-2016-011822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sioka C, Fotopoulos A, Georgiou A, Xourgia X, Papadopoulos A, Kalef-Ezra JA. Age at menarche, age at menopause and duration of fertility as risk factors for osteoporosis. Climacteric. 2010;13(1):63-71. doi: 10.3109/13697130903075337 [DOI] [PubMed] [Google Scholar]
- 29.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3-15. doi: 10.1023/A:1015714305420 [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Cook-Wiens G, Johnson BD, et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart Lung and Blood Institute–sponsored women’s ischemia syndrome evaluation. J Am Heart Assoc. 2019;8(12):e012406. doi: 10.1161/JAHA.119.012406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collaborative Group on Hormonal Factors in Breast Cancer . Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141-1151. doi: 10.1016/S1470-2045(12)70425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123-139. doi: 10.1007/s10549-007-9632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhary S, Khichar S, Dabi D, et al. Urban rural comparison of anthropometry and menarcheal status of adolescent school going girls of Jodhpur, Rajasthan, India. J Clin Diagn Res. 2016;10(10):SC08-SC12. doi: 10.7860/JCDR/2016/21882.8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akarolo-Anthony SN, Ogundiran TO, Adebamowo CA. Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Res. 2010;12(Suppl 4)(suppl 4):S8. doi: 10.1186/bcr2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755-765. doi: 10.1016/S0140-6736(14)60892-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fortner RT, Katzke V, Kühn T, Kaaks R. Obesity and breast cancer. Recent Results Cancer Res. 2016;208:43-65. doi: 10.1007/978-3-319-42542-9_3 [DOI] [PubMed] [Google Scholar]
- 37.Gravena AAF, Romeiro Lopes TC, Demitto MO, et al. The obesity and the risk of breast cancer among pre and postmenopausal women. Asian Pac J Cancer Prev. 2018;19(9):2429-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacMahon B, Cole P, Lin TM, et al. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209-221. [PMC free article] [PubMed] [Google Scholar]
- 39.Britt K. Menarche, menopause, and breast cancer risk. Lancet Oncol. 2012;13(11):1071-1072. doi: 10.1016/S1470-2045(12)70456-4 [DOI] [PubMed] [Google Scholar]
- 40.Lima SM, Kehm RD, Swett K, Gonsalves L, Terry MB. Trends in parity and breast cancer incidence in US women younger than 40 years from 1935 to 2015. JAMA Netw Open. 2020;3(3):e200929. doi: 10.1001/jamanetworkopen.2020.0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169-180. doi: 10.1016/S1470-2045(17)30891-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakkach J, Mansouri M, Derkaoui T, et al. Contribution of BRCA1 and BRCA2 germline mutations to early onset breast cancer: a series from north of Morocco. BMC Cancer. 2020;20(1):859. doi: 10.1186/s12885-020-07352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal T, Keefe D, Sun P, Narod SA; Hereditary Breast Cancer Clinical Study Group . Fertility in women with BRCA mutations: a case-control study. Fertil Steril. 2010;93(6):1805-1808. doi: 10.1016/j.fertnstert.2008.12.052 [DOI] [PubMed] [Google Scholar]
- 44.Krammer J, Pinker-Domenig K, Robson ME, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(3):565-571. doi: 10.1007/s10549-017-4198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemesure B, Wu SY, Hambleton IR, Leske MC, Hennis AJ; Barbados National Cancer Study Group . Risk factors for breast cancer in a Black population—the Barbados National Cancer Study. Int J Cancer. 2009;124(1):174-179. doi: 10.1002/ijc.23827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales L, Alvarez-Garriga C, Matta J, et al. Factors associated with breast cancer in Puerto Rican women. J Epidemiol Glob Health. 2013;3(4):205-215. doi: 10.1016/j.jegh.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbari MR, Donenberg T, Lunn J, et al. The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in The Bahamas. Clin Genet. 2014;85(1):64-67. doi: 10.1111/cge.12132 [DOI] [PubMed] [Google Scholar]
- 48.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324-3330. doi: 10.1200/JCO.2007.14.2471 [DOI] [PubMed] [Google Scholar]
- 49.GBD 2016 Healthcare Access and Quality Collaborators . Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391(10136):2236-2271. doi: 10.1016/S0140-6736(18)30994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapil U, Bhadoria AS, Sareen N, Singh P, Dwivedi SN. Reproductive factors and risk of breast cancer: a review. Indian J Cancer. 2014;51(4):571-576. doi: 10.4103/0019-509X.175345 [DOI] [PubMed] [Google Scholar]
- 51.Colditz GA, Bohlke K. Preventing breast cancer now by acting on what we already know. NPJ Breast Cancer. 2015;1:15009. doi: 10.1038/npjbcancer.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Y, Walsh T, Gulsuner S, et al. Inherited breast cancer in Nigerian women. J Clin Oncol. 2018;36(28):2820-2825. doi: 10.1200/JCO.2018.78.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearlman MD. Exogenous hormones and breast cancer risk: contraception, menopausal hormone therapy, and breast cancer survivors. Clin Obstet Gynecol. 2022;65(3):510-523. doi: 10.1097/GRF.0000000000000718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Stratification of BMI by Menopausal Status
eTable 2. Spearman Correlation Between Age at BC Diagnosis and Other Variables
eFigure 1. No Differences Seen Comparing Age at BC Diagnosis in Women Reporting Age at First Pregnancy Before 20y (Mean 48.4y), Between 20-24y (Mean 48.6y), Between 25-29y (mean 47.9y), and at or After 30y (Mean 48.7y)
eFigure 2. Mean Age at Breast Cancer Diagnosis by Decade of Birth and 95% Confidence Interval Bars Are Shown
eTable 3. Logistic Regression Testing the Likelihood of a Positive Germline Pathogenic/Likely Pathogenic Variant
eTable 4. Logistic Regression Testing the Likelihood of ER-Positive BC Diagnosis
Data Sharing Statement


