Abstract
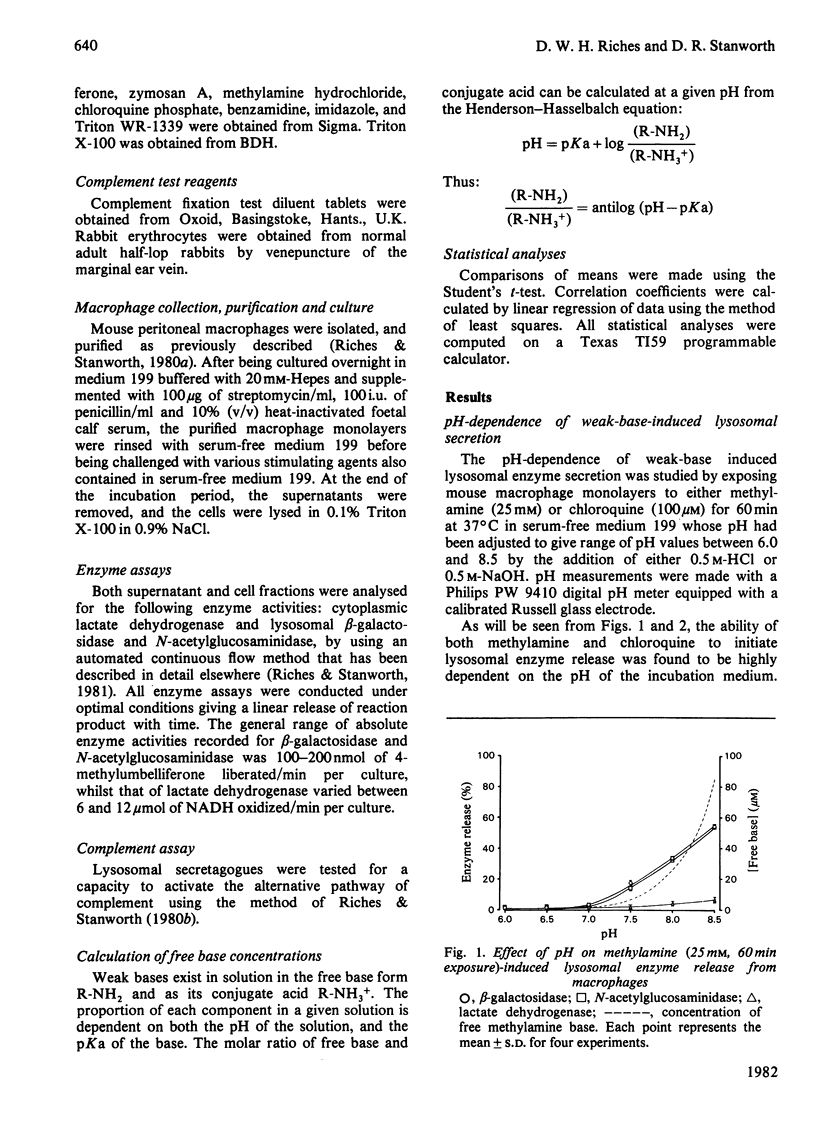
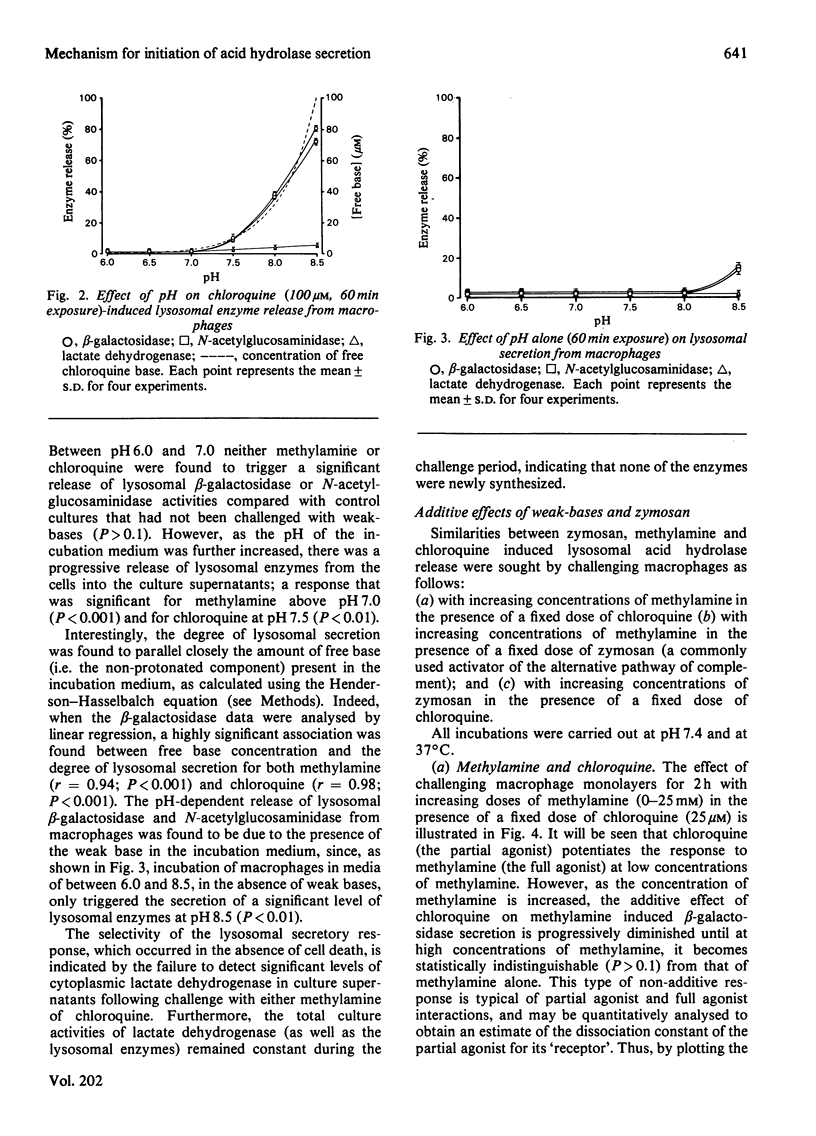
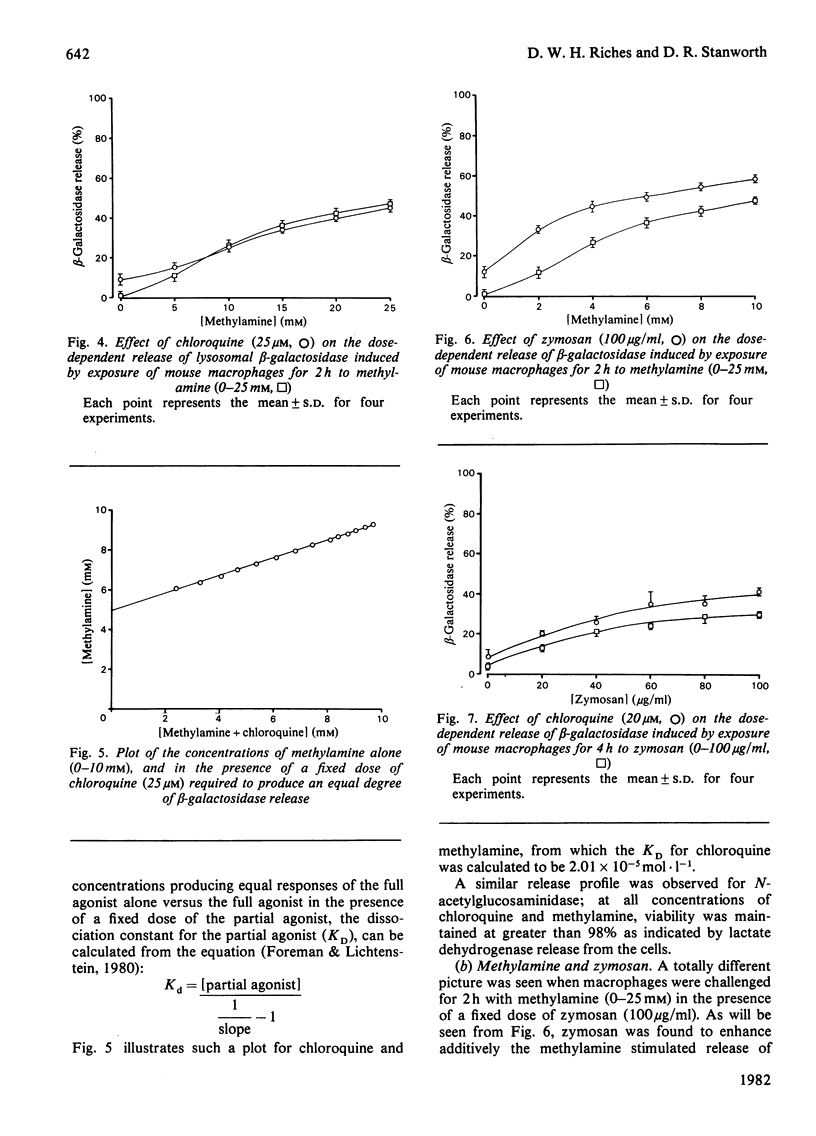
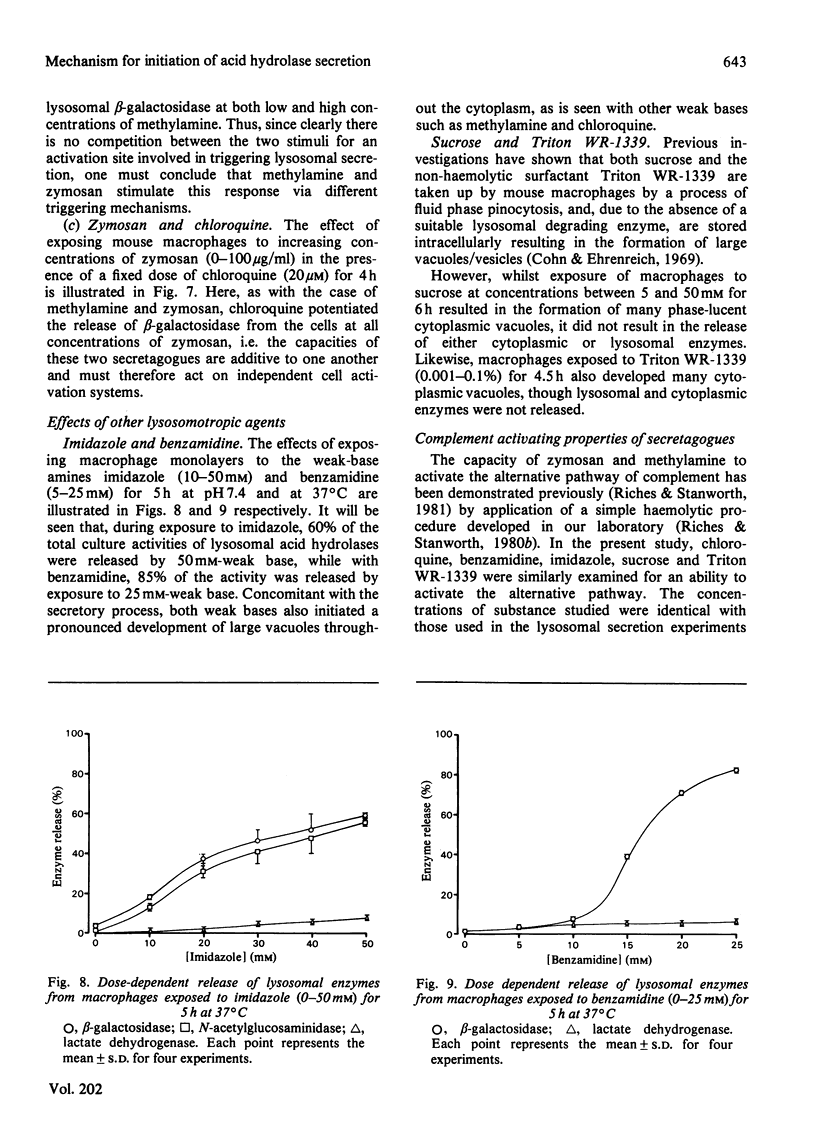
A mechanism for the initiation of selective acid hydrolase secretion with macrophages by weak bases that is functionally independent of complement activation is proposed on the basis of the following findings. (1) The release of beta-galactosidase from macrophages exposed to methylamine and chloroquine was found to be highly dependent on the pH of the incubation medium; the degree of lysosomal secretion correlated closely with the amount of free base in solution at each pH investigated. (2) The secretion of beta-galactosidase induced by methylamine was additively enhanced by a fixed dose of zymosan; likewise, chloroquine additively enhanced the secretion of beta-galactosidase during exposure to zymosan. By contrast, chloroquine did not additively enhance the release of lysosomal enzyme affected by exposure to methylamine. (3) Two new secretagogues, imidazole and benzamidine, like chloroquine, failed to initiate any activation of the alternative complement pathway. The possible relationship between secretagogue induced vacuolization and lysosomal secretion is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREWER D. B., HEATH D. DEVELOPMENT OF SUCROSE VACUOLES FROM LIVER-CELL LYSOSOMES. J Pathol Bacteriol. 1964 Apr;87:405–408. doi: 10.1002/path.1700870220. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A., Ehrenreich B. A. The uptake, storage, and intracellular hydrolysis of carbohydrates by macrophages. J Exp Med. 1969 Jan 1;129(1):201–225. doi: 10.1084/jem.129.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. H., Stanworth D. R. Characterization of calcium-ion-activated adenosine triphosphatase in the plasma membrane of rat mast cells. Biochem J. 1976 Jun 15;156(3):691–700. doi: 10.1042/bj1560691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Fell H. B., Glauert A. M. Endocytosis of sugars in embryonic skeletal tissues in organ culture. IV. Lysosomal and other biochemical effects. General discussion. J Cell Sci. 1969 Jan;4(1):139–153. doi: 10.1242/jcs.4.1.139. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. 5'-Nucleotidase activity of mouse peritoneal macrophages. I. Synthesis and degradation in resident and inflammatory populations. J Exp Med. 1976 Dec 1;144(6):1581–1595. doi: 10.1084/jem.144.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Lichtenstein L. M. Induction of histamine secretion by polycations. Biochim Biophys Acta. 1980 May 22;629(3):587–603. doi: 10.1016/0304-4165(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches D. W., Morris C. J., Stanworth D. R. Induction of selective acid hydrolase release from mouse macrophages during exposure to chloroquine and quinine. Biochem Pharmacol. 1981 Mar 15;30(6):629–634. doi: 10.1016/0006-2952(81)90136-2. [DOI] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Primary amines induce selective release of lysosomal enzymes from mouse macrophages. Biochem J. 1980 Jun 15;188(3):933–936. doi: 10.1042/bj1880933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Studies on the possible involvement of complement component C3 in the initiation of acid hydrolase secretion by macrophages. I. Correlation between enzyme-releasing and complement-activating capacities of several secretagogues. Immunology. 1981 Sep;44(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Bitter-Suermann D., Allison A. C. Complement activation by the alternative pathway and macrophage enzyme secretion in the pathogenesis of chronic inflammation. Immunology. 1977 Jun;32(6):929–940. [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Davies P., Allison A. C. Ability of activated complement components to induce lysosomal enzyme release from macrophages. Nature. 1976 May 6;261(5555):48–49. doi: 10.1038/261048a0. [DOI] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG W. C., STRASSER F. F., POMERAT C. M. MECHANISM OF DRUG-INDUCED VACUOLIZATION IN TISSUE CULTURE. Exp Cell Res. 1965 Jun;38:495–506. doi: 10.1016/0014-4827(65)90373-3. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


