Abstract
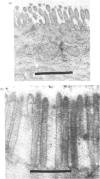
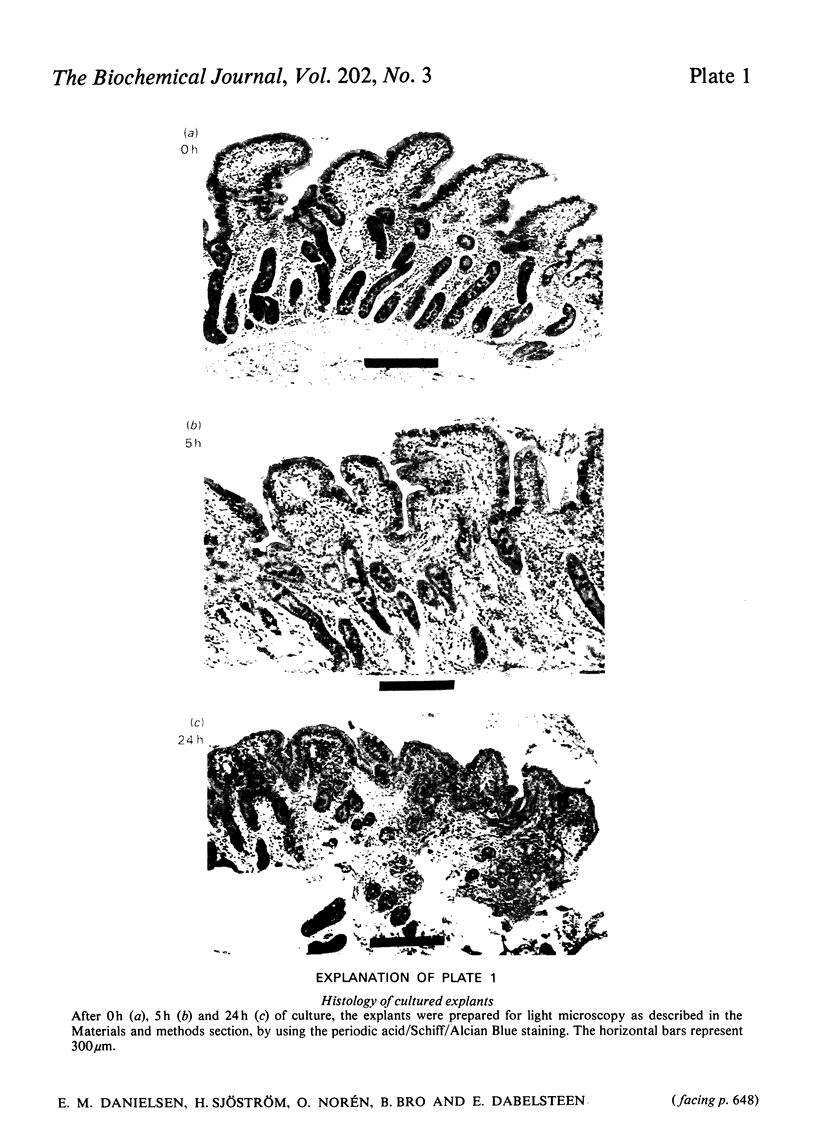
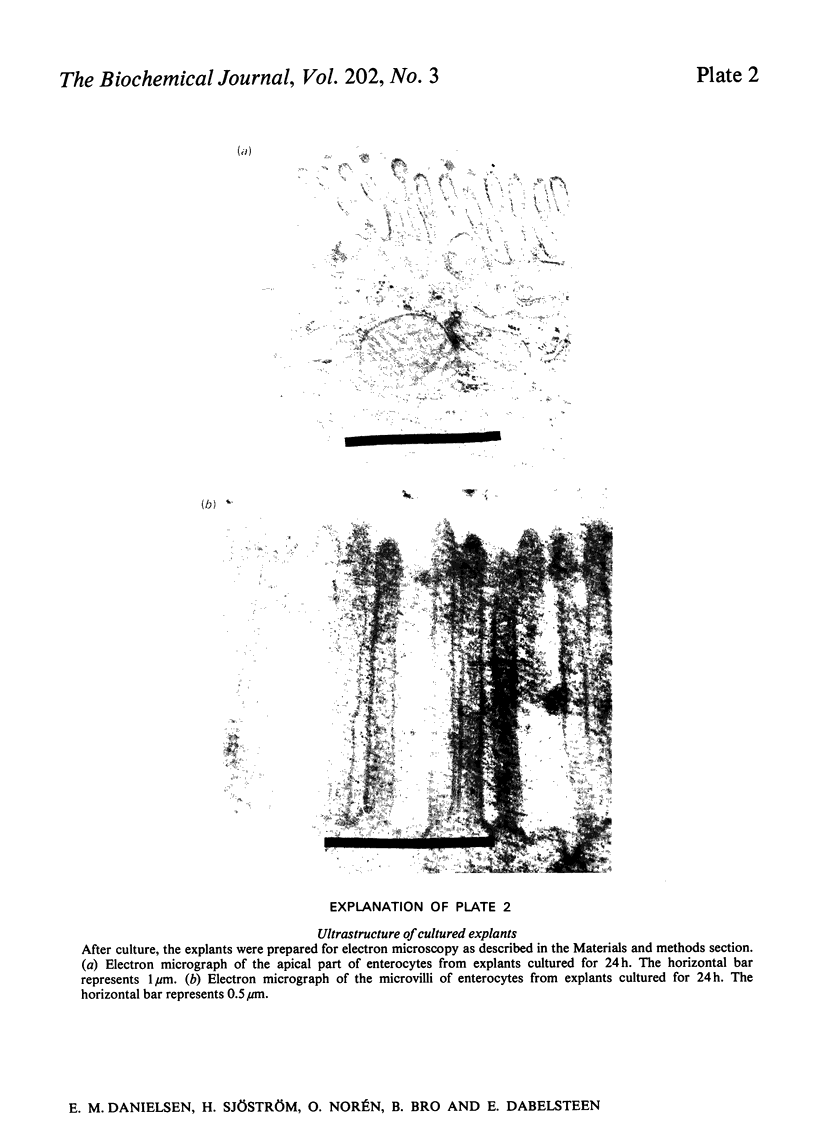
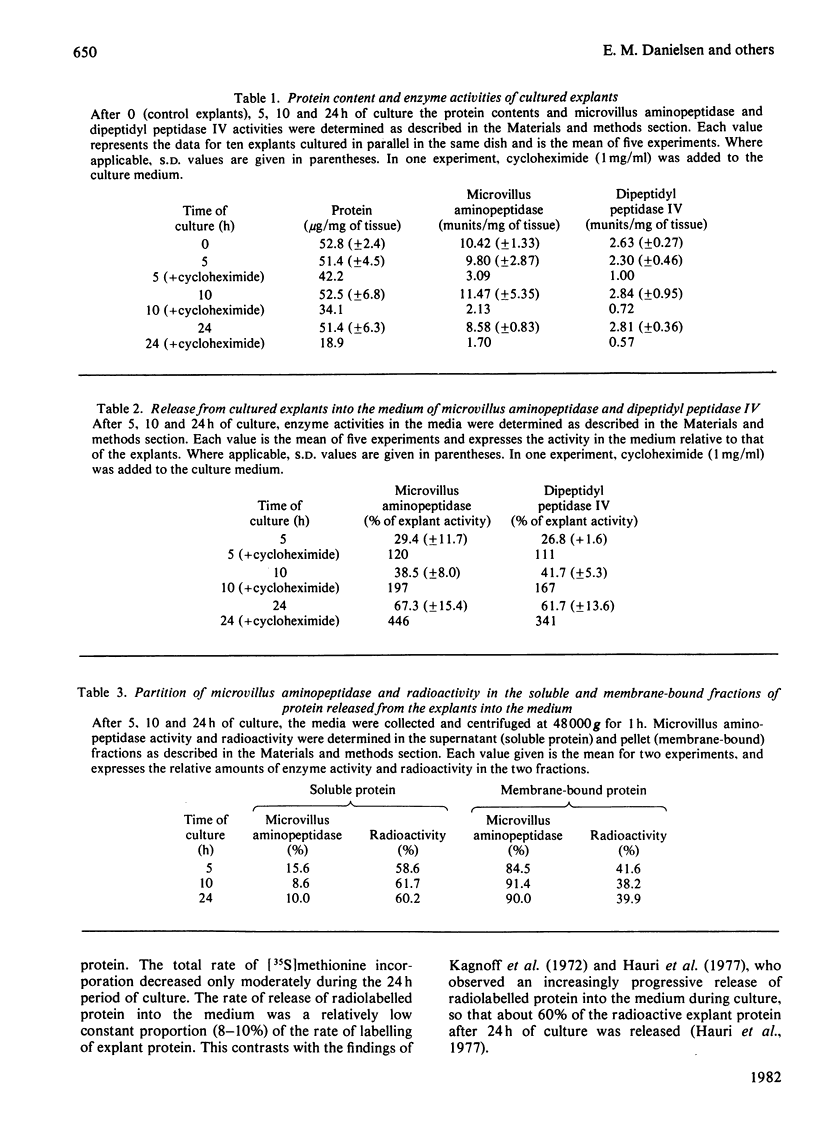
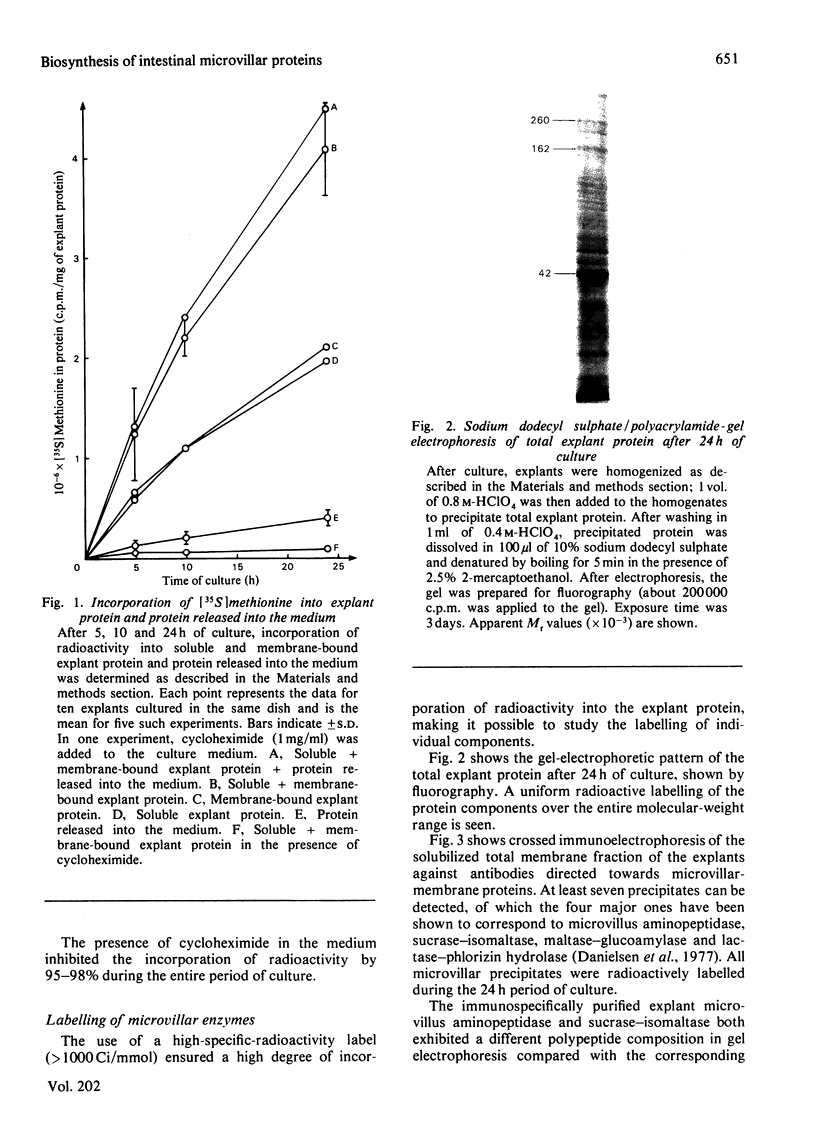
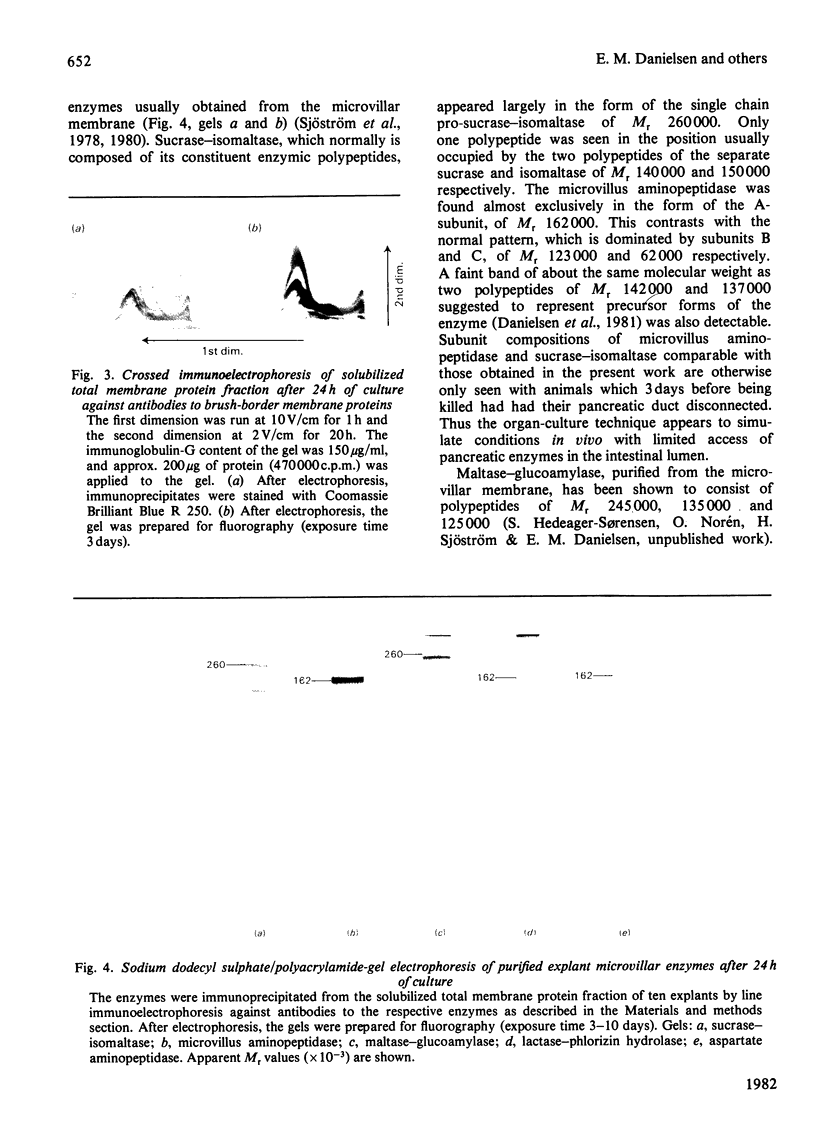
Explants of pig small intestine were maintained at 37 degrees C in organ culture for periods up to 24 h in a system using Trowell T-8 medium supplemented with 10% foetal-calf serum. The mucosal morphology was well preserved during culture, as judged by light and electron microscopy. The explant contents of protein and two brush-border enzymes, microvillus aminopeptidase (EC 3.4.11.2) and dipeptidyl peptidase IV (EC 3.4.14.5), were not significantly modified during culture compared with controls, but a moderate, continuous release of both protein and enzyme activities into the medium was observed. Continuous labelling with [35S]methionine resulted in an even incorporation of radioactivity in the protein components, and the rate of labelling only moderately decreased over the 24 h period. The polypeptide compositions of sucrase (EC 3.2.1.48)--isomaltase (EC 3.2.1.10), maltase--glucoamylase (EC 3.2.1.20) lactase (EC 3.2.1.23)--phlorizin hydrolase (EC 3.2.1.62), microvillus aminopeptidase and aspartate aminopeptidase (EC 3.4.11.7) synthesized during culture were studied, and some were found to be similar to those of the pro-forms of the enzymes isolated from animals that had had their pancreatic duct disconnected 3 days before being killed. These results confirmed earlier findings of the existence of pro-forms of some of the microvillar enzymes and thus indicate a low activity of pancreatic proteinases in the culture system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benajiba A., Maroux S. Purification and characterization of an aminopeptidase A from hog intestinal brush-border membrane. Eur J Biochem. 1980 Jun;107(2):381–388. doi: 10.1111/j.1432-1033.1980.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Berteloot A., Chabot J. G., Menard D., Hugon J. S. Organ culture of adult mouse intestine. III. Behavior of the proteins, DNA content and brush border membrane enzymatic activities. In Vitro. 1979 Apr;15(4):294–299. doi: 10.1007/BF02618954. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O. Biosynthesis of intestinal microvillar proteins. Putative precursor forms of microvillus aminopeptidase and sucrase--isomaltase isolated from Ca2+-precipitated enterocyte membranes. FEBS Lett. 1981 May 5;127(1):129–132. doi: 10.1016/0014-5793(81)80358-4. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Dabelsteen E. Immunoelectrophoretic studies on pig intestinal brush border proteins. Biochim Biophys Acta. 1977 Oct 26;494(2):332–342. doi: 10.1016/0005-2795(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Ferland S., Hugon J. S. Organ culture of adult mouse intestine. I. Morphological results after 24 and 48 hours of culture. In Vitro. 1979 Apr;15(4):278–287. doi: 10.1007/BF02618952. [DOI] [PubMed] [Google Scholar]
- Ferland S., Hugon J. S. Organ culture of adult mouse intestine. II. Mitotic activity, DNA synthesis and cellular migration after 24 and 48 hours of culture. In Vitro. 1979 Apr;15(4):288–293. doi: 10.1007/BF02618953. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Freiburghaus A., Grenier J. F., Hadorn B. Biosynthesis of brush border glycoproteins by human small intestinal mucosa in organ culture. Biochim Biophys Acta. 1977 Jun 16;467(3):327–339. doi: 10.1016/0005-2736(77)90310-8. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Grenier J. F., Hadorn B. Organ culture of human duodenum and jejunum. Biol Gastroenterol (Paris) 1975 Oct-Nov;8(4):307–319. [PubMed] [Google Scholar]
- Kagnoff M. F., Donaldson R. M., Jr, Trier J. S. Organ culture of rabbit small intestine: prolonged in vitro steady state protein synthesis and secretion and secretory IgA secretion. Gastroenterology. 1972 Oct;63(4):541–551. [PubMed] [Google Scholar]
- Kedinger M., Berteloot A., Haffen K., Hugon J. S. Organ culture of adult guinea-pig intestine. II. Biochemical and ultracytochemical findings. Histochemistry. 1974;40(4):311–321. doi: 10.1007/BF00495038. [DOI] [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Hugon J. S. Organ culture of adult guinea-pig intestine. I. Ultrastructural aspect after 24 and 48 hours of culture. Z Zellforsch Mikrosk Anat. 1974 Feb 27;147(2):169–181. doi: 10.1007/BF00582792. [DOI] [PubMed] [Google Scholar]
- Kedinger M., Hauri H. P., Haffen K., Green J. R., Grenier J. F., Hadorn B. Turnover studies of human intestinal brush border membrane glycoproteins in organ culture. Enzyme. 1979;24(2):96–106. doi: 10.1159/000458637. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Misch D. W., Giebel P. E., Faust R. G. Intestinal microvilli: responses to feeding and fasting. Eur J Cell Biol. 1980 Aug;21(3):269–279. [PubMed] [Google Scholar]
- Norén O., Sjöström H. Fluorography of tritium-labelled proteins in immunoelectrophoresis. J Biochem Biophys Methods. 1979;1(1):59–64. doi: 10.1016/0165-022x(79)90046-0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields H. M., Yedlin S. T., Bair F. A., Goodwin C. L., Alpers D. H. Successful maintenance of suckling rat ileum in organ culture. Am J Anat. 1979 Jul;155(3):375–389. doi: 10.1002/aja.1001550307. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
- Sjöström H., Norén O., Jeppesen L., Staun M., Svensson B., Christiansen L. Purification of different amphiphilic forms of a microvillus aminopeptidase from pig small intestine using immunoadsorbent chromatography. Eur J Biochem. 1978 Aug 1;88(2):503–511. doi: 10.1111/j.1432-1033.1978.tb12476.x. [DOI] [PubMed] [Google Scholar]
- Skovbjerg H., Sjöström H., Norén O. Purification and characterisation of amphiphilic lactase/phlorizin hydrolase from human small intestine. Eur J Biochem. 1981 Mar;114(3):653–661. doi: 10.1111/j.1432-1033.1981.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]