Key Points
Question
What proportion of pediatric intensive care unit (ICU) admissions are associated with respiratory syncytial virus (RSV) infections, and could nirsevimab and the maternal RSVpreF vaccine be associated with pediatric intensive care utilization?
Findings
In this cross-sectional study of 119 782 pediatric ICU encounters, 11.4% had RSV, of which 38.6% were eligible for RSV prevention. If typical vaccine uptake were achieved, pediatric ICU encounters would be reduced by an estimated 2.1% to 2.8%, and intensive care unit days would be reduced by an estimated 4.5% to 5.9%.
Meaning
These results suggest that nirsevimab and the maternal RSVpreF vaccine could be associated with a decrease in pediatric ICU utilization.
This cross-sectional study examines the potential association of novel respiratory syncytial virus (RSV) prevention strategies with pediatric intensive care unit utilization among children younger than 18 years of age.
Abstract
Importance
Bronchiolitis is the most common diagnosis necessitating respiratory support and pediatric intensive care, and respiratory syncytial virus (RSV) is the most common cause of bronchiolitis. In 2023, the monoclonal antibody nirsevimab and the maternal RSVpreF vaccine were implemented to prevent RSV in infants.
Objective
To determine the potential association of novel RSV prevention strategies with pediatric intensive care unit (ICU) utilization.
Design, Setting, and Participants
This retrospective cross-sectional study examined US pediatric ICU encounters with and without RSV from January 1, 2017, to June 1, 2023. Data were from Oracle Cerner RealWorld Data, a national electronic health record database. All children (aged >1 day and <18 years) admitted to an ICU during the study period were included in the analysis. Statistical analysis was performed from February to May 2024.
Main Outcomes and Measures
The primary outcomes were ICU encounters with RSV and ICU encounters with RSV eligible for RSV prevention.
Results
There were 119 782 ICU encounters from 53 hospitals; 65 757 encounters (54.9%) were male; median (IQR) age was 4.5 (1.1-12.5) years, median (IQR) ICU length of stay was 1.8 (1.0-3.9) days; 13 702 ICU encounters (11.4%) had RSV, of which 38.6% (5217) were eligible for prevention (4.4% of total ICU encounters). Encounters with RSV accounted for 21.1% (109 334) of ICU days, of which 43.8% (47 888) were eligible for prevention (9.2% of total ICU days). Encounters with RSV were associated with a greater use of positive pressure ventilation (4074 [29.7%] vs 18 821 [17.7%]; P < .001) and vasoactive medications (3057 [22.3%] vs 18 570 [17.5%]; P < .001) compared with those without RSV.
Conclusions and Relevance
The findings of this retrospective cross-sectional study of RSV and US pediatric intensive care utilization suggest that if 65% to 85% of eligible children received RSV prevention, an estimated 2.1% to 2.8% reduction in pediatric ICU encounters and an estimated 4.5% to 5.9% reduction in pediatric ICU days could be achieved.
Introduction
Vaccines have had an important impact in preventing morbidity and mortality from serious infections, especially in children.1,2 Following successful vaccination programs, infectious diseases that accounted for major mortality and morbidity in the early 20th century declined more than 90% from their peak incidences in the US.2 This reduction in morbidity and mortality also led to substantial health care savings.3 In the 21st century, bronchiolitis is the most common pediatric diagnosis necessitating respiratory support and intensive care.4 Respiratory syncytial virus (RSV), the most common viral cause of bronchiolitis, accounts for an estimated 3.6 million hospital admissions annually for children younger than 5 years and contributes substantially to pediatric intensive care unit (ICU) utilization, morbidity, and mortality.5,6 RSV infection during infancy is also independently associated with childhood asthma and contributes to ICU utilization after the initial infection.6
Two RSV prevention strategies were implemented in the US in 2023. First, in July 2023, nirsevimab (Beyfortus), an RSV monoclonal antibody with an extended half-life, was approved for infants under 8 months of age.7,8 Second, in August 2023, the recombinant, single-dose RSVpreF vaccine (Avrexy) was approved for pregnant people 32 to 36 weeks gestation to replace nirsevimab in full-term infants.9 Nirsevimab and the maternal RSVpreF vaccine were 83% and 67% effective at preventing hospitalization, respectively.10,11 This has also been observed by European countries that adopted nirsevimab early. Its efficacies at preventing ICU admission ranged from 70% to 86.9% in these settings.12,13,14,15,16 In the US, vaccine uptake is estimated to be between 65% and 85%.17,18,19 While prior studies established the effect of RSV infection on inpatient care needs in the US, the effect of RSV infection on pediatric ICU resource utilization is not known.20,21
This study is a national assessment of the monoclonal antibody nirsevimab and the maternal RSVpreF vaccine and their potential association with pediatric ICU resource utilization in the US.13,14,22 We conducted a retrospective analysis of a national multicenter database to determine the proportion of pediatric ICU encounters with RSV infection and the proportion of ICU encounters with RSV infection eligible for RSV prevention. We also explored the differences in clinical variables and markers of illness severity between these groups.
Methods
Data Source
This was a multicenter, retrospective cross-sectional study of Oracle’s Cerner RealWorld Data (CRWD), a deidentified national database of US hospitals.23 The database includes administrative and clinical data from the electronic health record (EHR), including diagnosis codes, medication administration, laboratory values, respiratory support, and hospital outcomes. Prior studies have used CRWD in pediatric and critical care settings.24,25,26 The Children’s National Hospital institutional review board approved the study with a waiver of informed consent because data were deidentified. This manuscript followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.27
Study Population, Variables, and Outcome Measures
Inclusion criteria were age greater than 1 day to younger than 18 years and admission to an ICU between January 1, 2017, and June 1, 2023. NICU encounters were excluded and planned surgical admissions were not excluded. Encounter variables collected or analyzed included admission date, age, weight, sex, insurance type, ICU and hospital length of stay, hospital type, and outcome (eTable 1 in Supplement 1). RSV infections were identified by RSV polymerase chain reaction or antigen test results or the presence of an RSV diagnosis code (International Statistical Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes: B974, J20.5, J12.1, or J21.0).28
Illness severity was categorized as respiratory failure, positive pressure ventilation (PPV), vasoactive medication, extracorporeal membrane oxygenation (ECMO), and death. Respiratory failure was identified by ICD-10-CM code (eTable 2 in Supplement 1). PPV included invasive and noninvasive mechanical ventilation and was identified by procedure codes and/or a charted ventilator setting (eTables 3 and 4 in Supplement 1). Receipt of vasoactive medication was determined from the medication administration record (eTable 5 in Supplement 1). ECMO was determined from procedure codes (eTable 6 in Supplement 1).
Encounters were classified as eligible for RSV prevention if they met the Centers for Disease Control and Prevention Health Alert Network’s 2023 to 2024 recommendations for nirsevimab administration: the child was younger than 1 year on admission or younger than 2 years on admission with a condition that places them at high risk for severe disease.9 ICD-10-CM diagnosis codes were used to identify high-risk conditions (eTable 7 in Supplement 1).9
Encounters admitted from 2017 to 2019 were used to estimate the RSV peak and RSV season to minimize the disruption of seasonality trends by the COVID-19 pandemic. Encounters were defined as occurring in RSV peak and RSV season. The North American RSV season was defined as October through April, and the North American RSV peak was defined as December and January.29,30
The estimated outcome of the RSV prevention strategies was determined by their expected efficacy and uptake. A 75% combined efficacy was used to represent nirsevimab (83.2% efficacy) and RSVpreF vaccine (63.7% efficacy) at preventing hospital admissions.10,11 Patient-level utilization was estimated to be between 65% and 85% based on national surveys of parental vaccine hesitancy and COVID-19 vaccine uptake.17,18
Statistical Analysis
Descriptive statistics (median [IQR] and number [percentage]) are provided for the variables of interest. Variables of interest were assessed in relation to encounter RSV infection status, and among encounters with RSV infection, variables were assessed in relation to RSV prevention eligibility status. Bivariate tests included Pearson χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. Post hoc multiple comparisons were performed if the primary comparison was significant. All tests were 2-tailed, and a significance level of P ≤ .05 was used. All statistical analyses were conducted using JMP version 16.1 (JMP Statistical Discovery LLC) from February to May 2024.
Results
Cohort Description
There were 119 782 ICU encounters from 53 hospitals during the study period (Table 1). The median (IQR) age was 4.5 (1.1-12.5) years, median (IQR) weight was 17.0 (9.4-43.5) kg, and 65 757 patients (54.9%) were male. ICU length of stay was 1.8 days (1.0-3.9), and hospital length of stay was 3.7 days (2.0-7.3). Approximately half of encounters (51.4%) took place in a children’s hospital, and 48.6% had public insurance. Hospital type, size, and location are further detailed in eTable 8 and the eFigure in Supplement 1. Respiratory failure was present in 36.9% (44 200 of 119 782) of all ICU encounters, 19.1% (22 895 of 119 782) required positive pressure ventilation, 18.1% (21 627 of 119 782) received vasoactive medications, and 4.9% (5854 of 119 782) died. High-risk conditions were identified in 19.1% (22 862 of 119 782), and the most frequent high-risk condition was congenital heart disease (11.8% [14 178 of 119 782]). Age-specific data are listed in eTables 9, 10, and 11 in Supplement 1.
Table 1. Demographics and Outcomes for All Pediatric ICU Encounters Between January 1, 2017, and June 1, 2023.
| Variables | Encounters, No. (% total) | P valuea | ||
|---|---|---|---|---|
| Total (N = 119 782) | RSV (n = 13 702) | No RSV (n = 106 080) | ||
| Sex | ||||
| Female | 54025 (45.1) | 5918 (43.2) | 48 107 (45.4) | .26 |
| Male | 65 757 (54.9) | 7784 (56.8) | 57 973 (54.6) | |
| Age, median (IQR), y | 4.5 (1.1-12.5) | 1.8 (0.6-6.0) | 5.1 (1.2-12.9) | <.001 |
| Weight, median (IQR), kg | 17.0 (9.4-43.5) | 11.4 (7.5-21.2) | 18.5 (9.9-45.9) | <.001 |
| Primary insurance | ||||
| Public | 58 197 (48.6) | 7011 (51.2) | 51 186 (48.3) | NA |
| Private | 28 291 (23.6) | 2761 (20.2) | 25 530 (24.1) | <.001b |
| Other or unknown | 33 294 (27.8) | 3930 (28.7) | 29 364 (27.7) | .28b |
| Hospital typec | ||||
| Children’s hospital | 61 512 (51.4) | 7118 (51.9) | 58 314 (55.0) | .14 |
| Nonchildren’s hospital | 58 270 (48.6) | 6584 (48.1) | 51 686 (45.0) | |
| ICU length of stay | ||||
| ICU days per encounter, median (IQR) | 1.8 (1.0-3.9) | 2.8 (1.5-6.2) | 1.7 (0.9-3.7) | <.001 |
| Sum of ICU days (% row) | 517 779 (100) | 109 334 (21.1) | 408 445 (78.9) | |
| Hospital length of stay | ||||
| Hospital days per encounter, median (IQR) | 3.7 (2.0-7.3) | 5.5 (3.0-11.9) | 3.5 (2.0-7.0) | <.001 |
| Sum of hospital days (% row) | 930 092 (100) | 189 408 (20.4) | 740 684 (79.6) | |
| Illness severity | ||||
| Respiratory failure | 44 200 (36.9) | 9723 (70.9) | 34 477 (32.5) | <.001 |
| Positive pressure ventilationd | 22 895 (19.1) | 4074 (29.7) | 18 821 (17.7) | <.001 |
| Vasoactive medication | 21 627 (18.1) | 3057 (22.3) | 18 570 (17.5) | <.001 |
| ECMO | 387 (0.32) | 124 (0.9) | 263 (0.3) | <.001 |
| Death | 5854 (4.9) | 735 (5.3) | 5119 (4.8) | .006 |
| High-risk condition | 22 862 (19.1) | 2561 (18.7) | 20 301 (19.1) | .21 |
| High-risk conditione | ||||
| Chronic respiratory failure | 3436 (2.9) | 388 (2.8) | 3048 (2.9) | .78 |
| Perinatal respiratory disease | 140 (0.1) | 10 (0.1) | 130 (0.1) | .11 |
| Congenital heart disease | 14 178 (11.8) | 1336 (9.7) | 12 842 (12.1) | <.001 |
| Prematurity | 3418 (2.8) | 493 (3.6) | 2925 (2.7) | <.001 |
| Immunodeficiency | 2748 (2.3) | 561 (4.1) | 2187 (2.1) | <.001 |
| Muscular dystrophy | 291 (0.2) | 28 (0.2) | 263 (2.5) | .33 |
| Cystic fibrosis | 256 (0.2) | 29 (0.2) | 227 (2.1) | .96 |
| Neuromuscular scoliosis | 1522 (1.3) | 113 (0.8) | 1409 (1.3) | <.001 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; NA, not applicable; RSV, respiratory syncytial virus.
RSV and non-RSV encounter characteristics were compared using 2-tailed Pearson χ2 tests for categorical variables and 2-tailed Wilcoxon rank-sum tests for continuous variables.
Primary, public, and unknown insurance types were compared with post hoc analysis. Public insurance was the reference group.
The percentage of total encounters in the children’s and nonchildren’s hospital groups is shown.
Positive pressure ventilation includes both invasive and noninvasive ventilation.
High-risk conditions were identified by International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes selected based on the Centers for Disease Control and Prevention recommendations for nirsevimab administration; see Supplement 1 for classification.9
Prevalence and Resource Utilization Associated With RSV Infection
RSV infection was identified in 13 702 ICU encounters (11.4%) (Table 1, Figure 1). Encounters with RSV infections were distributed similarly between children’s hospitals and nonchildren’s hospitals (7118 [51.9%] vs 58 314 [48.1%]; P = .14). Encounters with RSV infections had a lower median (IQR) age compared with encounters without RSV (1.8 [0.6-6.0] years vs 5.1 [1.2-12.9] years; P < .001) and were more likely to have public insurance (7011 [51.2%] vs 51 186 [48.3%]; P < .001). Encounters with RSV infections accounted for 21.1% of the total ICU days and 20.4% of the total hospital days (Table 1). During the North American RSV peak (December and January), 14.7% of all encounters had an RSV infection, accounting for 21.1% of all ICU days and 22.9% of all hospital days (Table 2). During this time 22.2% of all encounters with patients aged 0 to 2 years had an RSV infection, accounting for 30.1% of ICU days and 30.0% of hospital days of this age group (eTable 9 in Supplement 1).
Figure 1. Encounters and Intensive Care Unit (ICU) Days by Admission Month.
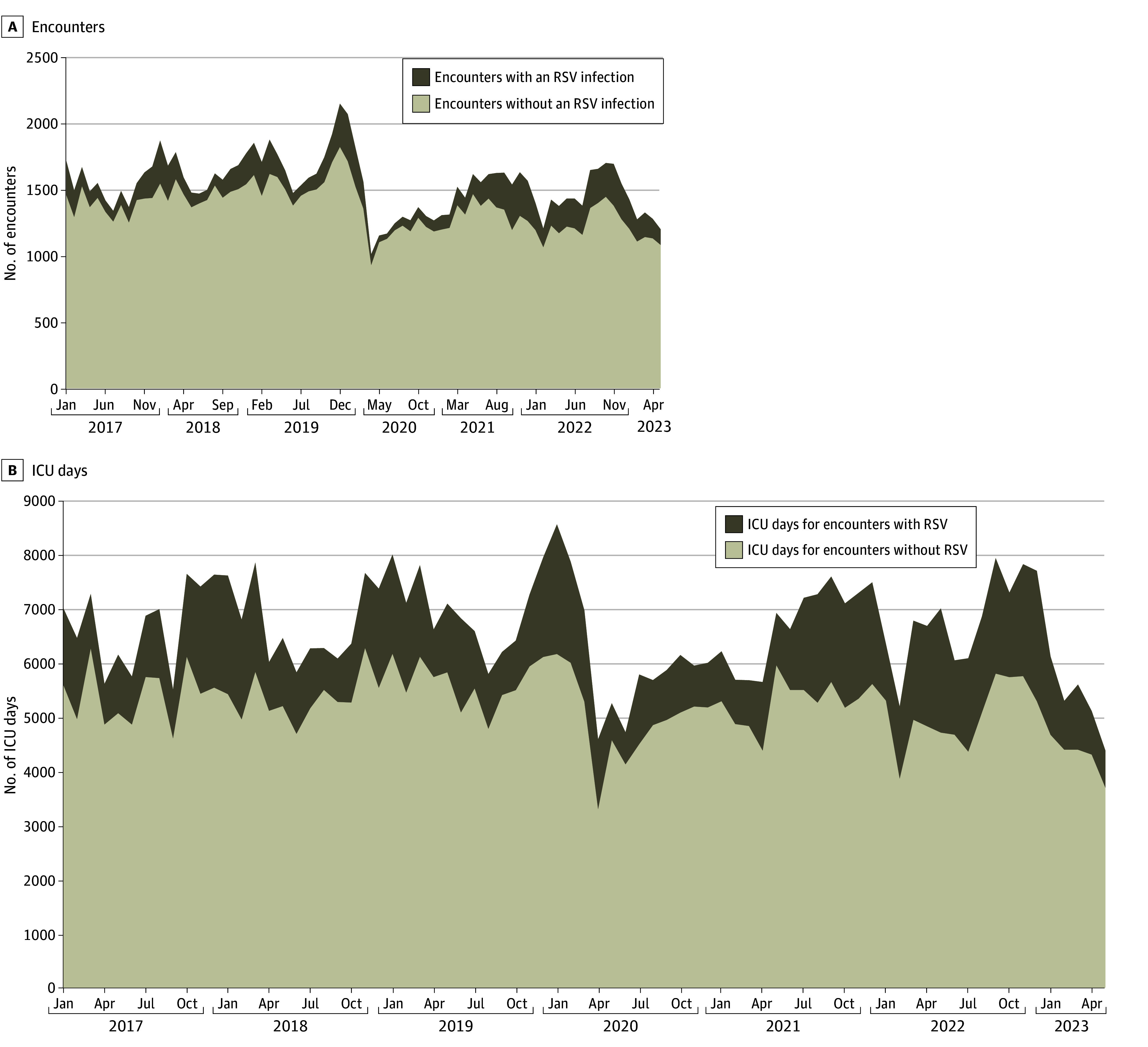
RSV indicates respiratory syncytial virus.
Table 2. Seasonality of All Pediatric ICU Encounters Between January 1, 2017, and December 31, 2019.
| Variables | Encounters | ||
|---|---|---|---|
| Total | RSV | No RSV | |
| All ICU encounters 2017-2019a | |||
| Encounters, No. (%) | 58 902 (100) | 5945 (10.1) | 52 957 (89.9) |
| ICU days, sum (%) | 244 386 (100) | 47 818 (19.6) | 196 567 (80.4) |
| Hospital days, sum (%) | 441 870 (100) | 81 245 (18.3) | 360 625 (81.6) |
| RSV seasonb | |||
| Encounters, No. (%) | 36 252 (100) | 4432 (12.2) | 31 820 (87.8) |
| ICU days, sum (%) | 149 767 (100) | 31 625 (21.1) | 118 142 (78.8) |
| Hospital days, sum (%) | 270 525 (100) | 54 680 (20.2) | 215 845 (79.8) |
| RSV peakb | |||
| Encounters, No. (%) | 11 029 (100) | 1618 (14.7) | 9411 (85.3) |
| ICU days, sum (%) | 45 550 (100) | 11 197 (24.6) | 34 353 (75.4) |
| Hospital days, sum (%) | 82 707 (100) | 18 900 (22.9) | 63 807 (77.1) |
Abbreviations: ICU, intensive care unit; RSV, respiratory syncytial virus.
Encounters admitted from 2017-2019 were used to represent the RSV season and the RSV peak to minimize alteration by the COVID-19 pandemic.45
North American RSV season is defined as October-April. The peak is defined as December to January.31
RSV infection was associated with greater illness severity including longer median (IQR) ICU length of stay (2.8 [1.5-6.2] vs 1.7 [0.9-3.7] days; P < .001), median (IQR) hospital length of stay (5.5 [3.0-11.9] vs 3.5 [2.0-7.0] days; P < .001); higher proportion of respiratory failure (9723 [70.9%] vs 34 477 [30.5%]; P < .001), positive pressure ventilation (4074 [29.7%] vs 18 821 [17.7%]; P < .001), vasoactive medication (3057 [22.3%] vs 18 570 [17.5%]; P < .001); ECMO (124 [0.9%] vs 263 [0.3%]; P < .001) and death (735 [5.3%] vs 5119 [4.8%]; P = .006) compared with encounters without RSV (Table 1).
Potential Association With Pediatric ICU Resource Utilization
Of the cohort, 13 702 ICU encounters (11.4%) had RSV infection, of which 5217 (38.6%) were eligible for RSV prevention. Encounters with RSV infections accounted for 21.1% of ICU days, of which 43.8% were eligible for RSV prevention. Therefore, 4.4% of ICU encounters and 9.2% of ICU days represent encounters that have RSV and are eligible for RSV prevention. Encounters with patients aged 0 to 2 years with RSV infection accounted for 10.9% of ICU days and 72.3% were eligible for RSV prevention (eTables 10 and 11 in Supplement 1). Encounters with RSV infections who were not eligible for RSV prevention were associated with more severe illness than those eligible for prevention, with more PPV (2573 [30.3%] vs 1501 [28.8%]; P < .001), vasoactive medication use (2060 [24.3%] vs 997 [19.1%]; P < .001), and deaths (545 [6.4%] vs 190 [4.8%]; P < .001) (Table 3). The results for PPV were consistent in age groups 0 to 2 years, 2 to 5 years, and greater than 5 years (eTable 10 in Supplement 1). Mortality was different between age groups. RSV was associated with decreased mortality in the age group of 0 to 2 years (251 [3.5%] vs 1461 [4.2%]; P = .005), not associated with mortality in the aged group of 2 to 5 years (149 [5.8%] vs 917 [5.2%]; P = .21), and associated with increased mortality in the age group of 5 years and older (335 [8.7%] vs 2741 [5.1%]; P < .001) (eTable 10 in Supplement 1).
Table 3. Eligibility for RSV Prevention Among Pediatric ICU Encounters With an RSV Infection Between January 1, 2017, and June 1, 2023.
| Variables | RSV infection present, No. (%) | P valueb | |
|---|---|---|---|
| Eligible for RSV preventiona | Not eligible for RSV preventiona | ||
| Total encountersc | 5217 (38.1) | 8485 (61.9) | NA |
| Hospital type | |||
| Children’s hospital | 2563 (35.7) | 4555 (63.4) | <.001 |
| Nonchildren’s hospital | 2654 (40.3) | 3930 (59.6) | |
| ICU days | |||
| Median (IQR) | 3.2 (1.8-6.8) | 2.6 (1.4-5.8) | <.001 |
| Sum (% total ICU days) | 47 888 (43.8) | 61 446 (56.2) | |
| Hospital days | |||
| Median (IQR) | 5.9 (3.6-11.9) | 5.1 (2.9-11.9) | <.001 |
| Sum (% total hospital days) | 75 030 (39.6) | 114 378 (60.4) | |
| Illness severity | |||
| Respiratory failure | 4023 (77.1) | 5700 (67.2) | <.001 |
| Positive pressure ventilationd | 1501 (28.8) | 2573 (30.3) | .05 |
| Vasoactive medication | 997 (19.1) | 2060 (24.3) | <.001 |
| ECMO | 54 (0.1) | 70 (0.1) | .21 |
| Death | 190 (3.6) | 545 (6.4) | <.001 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; NA, not applicable; RSV, respiratory syncytial virus.
Encounters with patients under 1 year of age on admission and encounters with patients under 2 years of age on admission with a high-risk condition were considered eligible for RSV prevention in accordance with the Centers for Disease Control and Prevention (CDC) guidelines.9 High-risk conditions were identified by International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes based on the CDC’s recommendations for nirsevimab administration; see eAppendix in Supplement 1 for classification.
Encounters with RSV eligible for prevention and encounters with RSV not eligible for prevention were compared using 2-tailed Pearson tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Percentage of total encounters with an RSV infection.
Positive pressure ventilation includes both invasive and noninvasive mechanical ventilation.
With a 75% efficacy at preventing hospital admission and 65% to 85% vaccine uptake, ICU encounters would be associated with an estimated 2.1% to 2.8% reduction and ICU days would be associated with an estimated 4.5% to 5.9% reduction (Figure 2). During the North American RSV peak, ICU encounters would be associated with an estimated 3.6% to 4.7% reduction and ICU days would be associated with an estimated 6.7% to 8.8% reduction. The estimated percentage decrease in ICU encounters by hospital type and season is shown in Figure 2.
Figure 2. Estimated Percentage Decrease in Intensive Care Unit (ICU) Encounters and ICU Days.
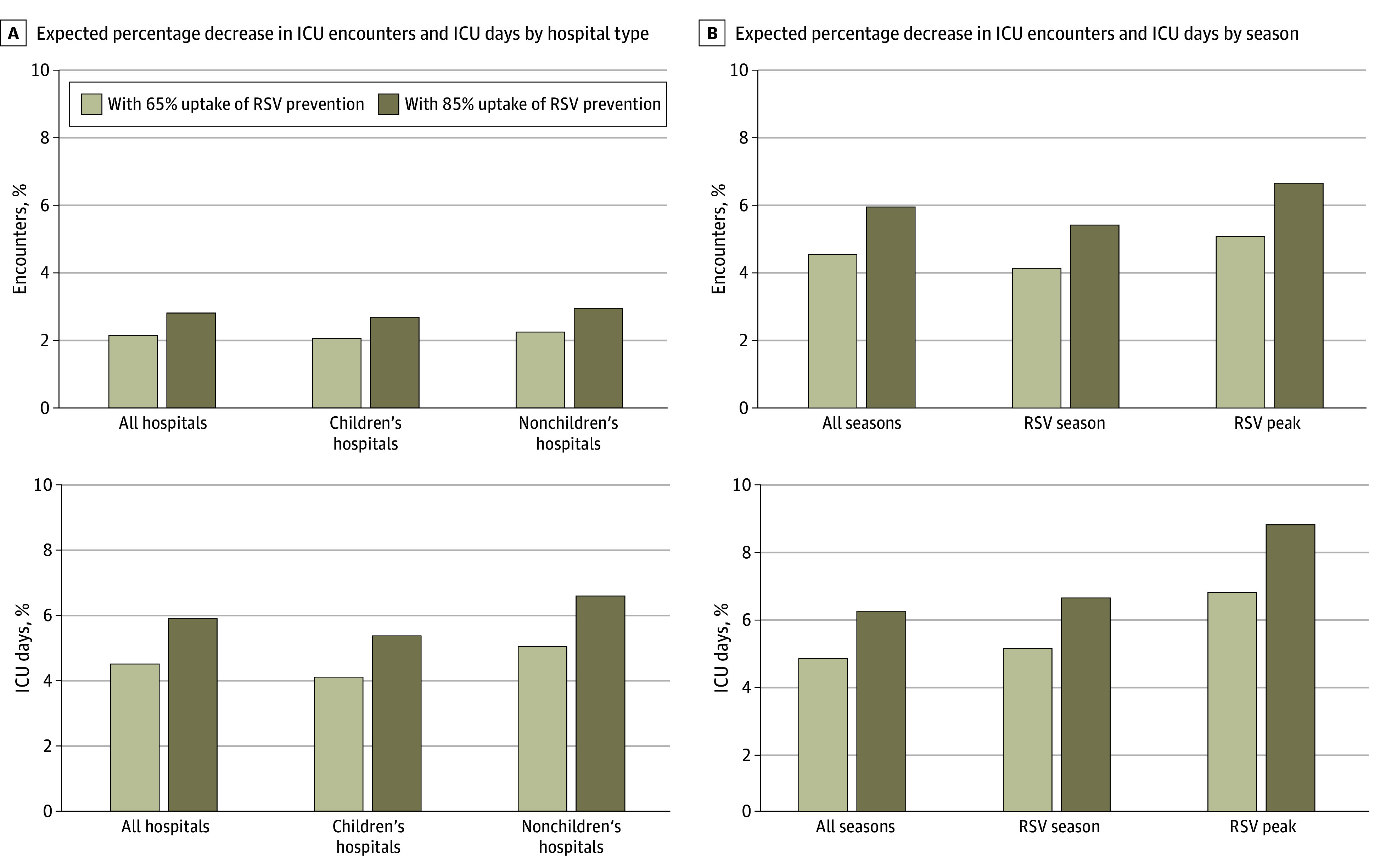
A, Estimated percent decrease in ICU encounters and ICU days with 65% and 85% uptake of the respiratory syncytial virus (RSV) prevention strategies. The estimated percentage decrease in total ICU encounters and total ICU days is illustrated by hospital type. B, The estimated percentage decrease in total ICU encounters and total ICU days by season. The North American RSV season is defined as October-April, and the peak is defined as December to January. Encounters admitted from 2017 to 2019 were used to estimate the RSV season and the RSV peak to minimize disruption by the COVID-19 pandemic.
Discussion
This study estimates the association of the new RSV preventive strategies with ICU utilization for children in the US. We examined 119 782 ICU encounters from the national multicenter CRWD database and identified that 11.4% had RSV infection, accounting for 21.1% of the total ICU days. RSV infection was associated with markers of greater severity of illness, including ICU length of stay, positive pressure ventilation, and death. Using 75% combined efficacy for reducing hospital admission and 65% to 85% uptake, we anticipate a 2.1% to 2.8% reduction in pediatric ICU encounters and a 4.5% to 5.9% reduction in ICU days.10,11,12,17,18,19,31
The novel preventive strategies for RSV follow a historical precedent of widespread vaccination. In the last century, the pertussis vaccine reduced the incidence of pertussis by over 90%,32 the Hemophilus influenzae type b (Hib) vaccine reduced the incidence of Hib meningitis in children under 5 years by over 90%,33,34 the pneumococcal vaccine reduced the incidence of invasive pneumococcal disease in US children by over 90%,34,35 the meningococcal vaccine reduced the incidence of meningococcal disease in United Kingdom adolescents by over 97%,35 and the oral rotavirus vaccine decreased hospitalization for diarrheal illnesses in eligible infants by over 50%.36 While it is too early to compare the RSV infection prevention strategies to these successes, they could emulate the effect of prior vaccination efforts.
The novel RSV prevention strategies could alter the recent trend of pediatric ICU expansion. Inpatient pediatric care has shifted from acute care beds to ICU beds over the past century, which has accelerated in recent years.37 Despite an overall decrease in inpatient pediatric care, pediatric ICU beds have increased by 46% in the past decade, increasing by 200 beds per year after 2016.31,37 Novel RSV prevention strategies could alter the demand for pediatric ICU beds.30 We conservatively estimated a 2.1% to 2.8% reduction in ICU encounters and a 4.5% to 5.9% reduction in ICU days, with a 6.7% to 8.8% reduction in ICU days at the RSV seasonal peak.10,11,17,18 Using a mean cost of $22 043 for each PICU encounter and 85% uptake, nirsevimab would have saved $14 772 340 per year on ICU care in our cohort.38
Vaccine uptake in the US is limited by access and acceptance. In the 2023 to 2024 season, pediatric clinics with higher proportions of privately insured patients and lower proportions of patients in lower-income zip codes were more likely to have access to nirsevimab.39 Acceptance of the therapy was also an issue. Only 47% of eligible patients at clinics with access to the drug received it, and patients from lower-income zip codes were less likely to do so.39 This experience indicates the potential for a substantial expansion of nirsevimab’s impact if these issues are addressed. If 100% of eligible infants receive nirsevimab, as they have in some parts of Spain, a 3.2% reduction in ICU encounters and a 6.9% reduction in ICU days may be possible.12,31
The novel RSV prevention strategies may also reduce pediatric ICU acuity. RSV infection was significantly associated with respiratory failure, positive pressure ventilation, and ECMO use in children aged 0 to 2 years, 2 to 5 years and greater than 5 years on admission (eTable 10 in Supplement 1). Additionally, 61.9% of encounters with RSV infection were not eligible for RSV prevention, and these encounters demonstrated greater illness severity than those eligible, with more positive pressure ventilation, vasoactive medication use, and death. Therefore, expanded eligibility for RSV prevention can potentially reduce ICU care utilization, morbidity, and mortality even further.
Limitations
This study had limitations. The efficacies of nirsevemab and the maternal RSV preF vaccine used in this analysis were based on early studies, which is a key limitation of this manuscript. When updated uptake and efficacy data are published, a more robust estimation will be possible.10,11 This manuscript had several other limitations. It was not possible to determine whether the RSV infection was the primary reason for admission vs an incidental finding. This is particularly important in patients with chronic critical illness, as they are often admitted to the ICU for a longer period and, therefore, have more opportunities to test positive for RSV.40,41 RSV infections in encounters with laboratory testing before hospitalization may have been missed, resulting in an underestimation of the impact of these preventive strategies. We did not account for RSV infections in neonatal ICUs, although there is a risk of nosocomial RSV infection within this population.42 Additionally, we did not account for decreased community spread, which could decrease the care needs of patients with RSV infection who are not eligible for prevention.43
Conclusions
In this retrospective cross-sectional study of RSV and US pediatric intensive care utilization, we estimated a 2.1% to 2.8% reduction in ICU encounters and a 4.5% to 5.9% reduction in ICU days if a 65% to 85% uptake of the novel RSV prevention strategies is achieved for eligible children, with a 3.6% to 4.7% reduction during the North American RSV Peak in December and January. Children with RSV infection who require ICU admission have a 29.7% chance of requiring positive pressure ventilation, a 22.3% chance of requiring vasoactive medications, and a 5.3% mortality. The novel RSV prevention strategies may reduce ICU morbidity and mortality for children. The results suggest that efforts to increase availability and acceptance of these therapies are warranted. Further expansion of the population eligible for RSV prevention could have a substantial impact.
eTable 1. Insurance Designations and Their Classification as Public, Private, or ‘Not Available’
eTable 2. ICD-10 Diagnosis Codes Used to Identify Acute or Unspecified Respiratory Failure
eTable 3. ICD-10 Procedure Codes Used to Identify Positive Pressure or Mechanical Ventilation
eTable 4. ICD-10 Clinical Events Used to Identify Noninvasive and Invasive Positive Pressure Ventilation
eTable 5. Medication Administration Drug IDs Used to Identify the Administration of a Vasoactive Medication
eTable 6. ICD-10 Procedure Codes Used to Identify ECMO
eTable 7. International Classification of Diseases, Tenth Revision (ICD-10) Codes Used to Identify High-Risk Conditions, Defined as Medical Conditions That Make Patients Eligible For Respiratory Syncytial Virus (RSV) Prevention in Their 2nd RSV Season per the CDC Recommendations
eTable 8. Hospital Type, Size, and Location of All Pediatric ICU Encounters Between January 1st, 2017, and December 31st, 2019, Stratified by RSV Infection
eTable 9. Seasonality of All Pediatric ICU Encounters Between January 1st, 2017, and December 31st, 2019, Stratified by Age
eTable 10. Demographics and Outcomes for All Pediatric ICU Encounters Between January 1st, 2017, and June 1st, 2023, Stratified by Age
eTable 11. Demographics and Outcomes for All Pediatric ICU Encounters With RSV Between January 1st, 2017, and June 1st, 2023, Stratified by Positive Pressure Ventilation and Age
eFigure. Number of Encounters in Each United States Zip Code Region
eReferences.
Data Sharing Statement
References
- 1.Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526. doi: 10.3389/fmicb.2020.01526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roush SW, Murphy TV; Vaccine-Preventable Disease Table Working Group . Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298(18):2155-2163. doi: 10.1001/jama.298.18.2155 [DOI] [PubMed] [Google Scholar]
- 3.Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12(1):878. doi: 10.1186/1471-2458-12-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000-2016. Pediatrics. 2019;144(6):e20192614. doi: 10.1542/peds.2019-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Wang X, Blau DM, et al. ; Respiratory Virus Global Epidemiology Network; RESCEU investigators . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047-2064. doi: 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosas-Salazar C, Chirkova T, Gebretsadik T, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet. 2023;401(10389):1669-1680. doi: 10.1016/S0140-6736(23)00811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammitt LL, Dagan R, Yuan Y, et al. ; MELODY Study Group . Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837-846. doi: 10.1056/NEJMoa2110275 [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, Kang TH, Godon O, et al. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat Commun. 2019;10(1):5031. doi: 10.1038/s41467-019-13108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Health Alert Network . Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. Accessed September 27, 2024. http://emergency.cdc.gov
- 10.Drysdale SB, Cathie K, Flamein F, et al. ; HARMONIE Study Group . Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med. 2023;389(26):2425-2435. doi: 10.1056/NEJMoa2309189 [DOI] [PubMed] [Google Scholar]
- 11.Kampmann B, Madhi SA, Munjal I, et al. ; MATISSE Study Group . Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451-1464. doi: 10.1056/NEJMoa2216480 [DOI] [PubMed] [Google Scholar]
- 12.Ares-Gómez S, Mallah N, Santiago-Pérez MI, et al. ; NIRSE-GAL study group . Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study. Lancet Infect Dis. 2024;24(8):817-828. doi: 10.1016/S1473-3099(24)00215-9 [DOI] [PubMed] [Google Scholar]
- 13.Estrella-Porter P, Blanco-Calvo C, Lameiras-Azevedo AS, et al. Effectiveness of nirsevimab introduction against respiratory syncytial virus in the Valencian Community: A preliminary assessment. Vaccine. 2024;42(22):126030. doi: 10.1016/j.vaccine.2024.05.078 [DOI] [PubMed] [Google Scholar]
- 14.Paireau J, Durand C, Raimbault S, et al. Nirsevimab effectiveness against cases of respiratory syncytial virus bronchiolitis hospitalised in paediatric intensive care units in France, September 2023-January 2024. Influenza Other Respir Viruses. 2024;18(6):e13311. doi: 10.1111/irv.13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consolati A, Farinelli M, Serravalle P, et al. Safety and Efficacy of Nirsevimab in a Universal Prevention Program of Respiratory Syncytial Virus Bronchiolitis in Newborns and Infants in the First Year of Life in the Valle d’Aosta Region, Italy, in the 2023–2024 Epidemic Season. Vaccines. 2024;12(5):549. doi: 10.3390/vaccines12050549 [DOI] [PMC free article] [PubMed]
- 16.Agüera M, Soler-Garcia A, Alejandre C, et al. Nirsevimab immunization’s real-world effectiveness in preventing severe bronchiolitis: a test-negative case-control study. Pediatr Allergy Immunol. 2024;35(6):e14175. doi: 10.1111/pai.14175 [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez VJ, Kozlova S, LaBarrie DL, Liu Q. Parental anxiety and pediatric vaccine refusal in a US national sample of parents. Vaccine. 2023;41(48):7072-7075. doi: 10.1016/j.vaccine.2023.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanath K, Bekalu M, Dhawan D, Pinnamaneni R, Lang J, McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health. 2021;21(1):818. doi: 10.1186/s12889-021-10862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill HA, Yankey D, Elam-Evans LD, Chen M, Singleton JA. Vaccination coverage by age 24 months among children born in 2019 and 2020 - National Immunization Survey-Child, United States, 2020-2022. MMWR Morb Mortal Wkly Rep. 2023;72(44):1190-1196. doi: 10.15585/mmwr.mm7244a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson EK, Sylte D, Chaves SS, et al. Hospital utilization rates for influenza and RSV: a novel approach and critical assessment. Popul Health Metr. 2021;19(1):31. doi: 10.1186/s12963-021-00252-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krilov LR, Forbes ML, Goldstein M, Wadhawan R, Stewart DL. Severity and cost of RSV hospitalization among US preterm infants following the 2014 American Academy of Pediatrics policy change. Infect Dis Ther. 2021;10(S1)(suppl 1):27-34. doi: 10.1007/s40121-020-00389-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis S, Ehwerhemuepha L, Feaster W, et al. Standardized Health data and Research Exchange (SHaRE): promoting a learning health system. JAMIA Open. 2022;5(1):ooab120. doi: 10.1093/jamiaopen/ooab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehwerhemuepha L, Carlson K, Moog R, et al. Cerner real-world data (CRWD) - a de-identified multicenter electronic health records database. Data Brief. 2022;42:108120. doi: 10.1016/j.dib.2022.108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qeadan F, Tingey B, Egbert J, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner Real-World Data. PLoS One. 2022;17(4):e0266809. doi: 10.1371/journal.pone.0266809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepper DJ, Demirkale CY, Sun J, et al. Does obesity protect against death in sepsis? a retrospective cohort study of 55,038 adult patients. Crit Care Med. 2019;47(5):643-650. doi: 10.1097/CCM.0000000000003692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gouel-Cheron A, Swihart BJ, Warner S, et al. Epidemiology of ICU-onset bloodstream infection: prevalence, pathogens, and risk factors among 150,948 ICU patients at 85 U.S. hospitals. Crit Care Med. 2022;50(12):1725-1736. doi: 10.1097/CCM.0000000000005662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)Statement: guidelines for reporting observational studies. UK EQUATOR Centre. [DOI] [PMC free article] [PubMed]
- 28.Cai W, Tolksdorf K, Hirve S, et al. Evaluation of using ICD-10 code data for respiratory syncytial virus surveillance. Influenza Other Respir Viruses. 2020;14(6):630-637. doi: 10.1111/irv.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356-1364. doi: 10.1093/infdis/jiy056 [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Berger N, Davis PB, Kaelber DC, Volkow N, Xu R. Time trend and seasonality in medically attended respiratory syncytial virus (RSV) infections in US children aged 0-5 years, January 2010-January 2023. Fam Med Community Health. 2023;11(4):e002453. doi: 10.1136/fmch-2023-002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos-Fernández JM, Moreno-Pérez D, Gutiérrez-Bedmar M, et al. Prediction of severe course in infants with RSV bronchiolitis under 6 months. Spain. Article in Spanish. Rev Esp Salud Publica. 2017;91:e201701006. [PubMed] [Google Scholar]
- 32.Burr JS, Jenkins TL, Harrison R, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN) . The Collaborative Pediatric Critical Care Research Network Critical Pertussis Study: collaborative research in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12(4):387-392. doi: 10.1097/PCC.0b013e3181fe4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georges S, Lepoutre A, Dabernat H, Levy-Bruhl D. Impact of Haemophilus influenzae type B vaccination on the incidence of invasive Haemophilus influenzae disease in France, 15 years after its introduction. Epidemiol Infect. 2013;141(9):1787-1796. doi: 10.1017/S0950268813000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grijalva CG, Griffin MR. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Rev Vaccines. 2008;7(1):83-95. doi: 10.1586/14760584.7.1.83 [DOI] [PubMed] [Google Scholar]
- 35.Borrow R, Abad R, Trotter C, van der Klis FRM, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31(41):4477-4486. doi: 10.1016/j.vaccine.2013.07.083 [DOI] [PubMed] [Google Scholar]
- 36.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1)(suppl):S56-S60. doi: 10.1097/INF.0b013e3181fefdc0 [DOI] [PubMed] [Google Scholar]
- 37.Cushing AM, Bucholz EM, Chien AT, Rauch DA, Michelson KA. Availability of pediatric inpatient services in the United States. Pediatrics. 2021;148(1):e2020041723. doi: 10.1542/peds.2020-041723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heneghan JA, Rogerson C, Goodman DM, Hall M, Kohne JG, Kane JM. Epidemiology of pediatric critical care admissions in 43 United States children’s hospitals, 2014-2019. Pediatr Crit Care Med. 2022;23(7):484-492. doi: 10.1097/PCC.0000000000002956 [DOI] [PubMed] [Google Scholar]
- 39.Abraham C, Hatoun J, Correa ET, Rabbani N, Vernacchio L. Disparities in the availability and acceptance of nirsevimab in Massachusetts. Pediatrics. 2024;154(1):e2023065425. doi: 10.1542/peds.2023-065425 [DOI] [PubMed] [Google Scholar]
- 40.Marcus KL, Henderson CM, Boss RD. Chronic critical illness in infants and children: a speculative synthesis on adapting ICU care to meet the needs of long-stay patients. Pediatr Crit Care Med. 2016;17(8):743-752. doi: 10.1097/PCC.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 41.Marcin JP, Slonim AD, Pollack MM, Ruttimann UE. Long-stay patients in the pediatric intensive care unit. Crit Care Med. 2001;29(3):652-657. doi: 10.1097/00003246-200103000-00035 [DOI] [PubMed] [Google Scholar]
- 42.Johnson J, Quach C, Fshea MF. Outbreaks in the neonatal intensive care unit: a review of the literature HHS public access. Curr Opin Infect Dis. 2017;30(4):395-403. doi: 10.1097/QCO.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang JC. Use of mathematical modelling to assess respiratory syncytial virus epidemiology and interventions: a literature review. J Math Biol. 2022;84(4):26. doi: 10.1007/s00285-021-01706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Insurance Designations and Their Classification as Public, Private, or ‘Not Available’
eTable 2. ICD-10 Diagnosis Codes Used to Identify Acute or Unspecified Respiratory Failure
eTable 3. ICD-10 Procedure Codes Used to Identify Positive Pressure or Mechanical Ventilation
eTable 4. ICD-10 Clinical Events Used to Identify Noninvasive and Invasive Positive Pressure Ventilation
eTable 5. Medication Administration Drug IDs Used to Identify the Administration of a Vasoactive Medication
eTable 6. ICD-10 Procedure Codes Used to Identify ECMO
eTable 7. International Classification of Diseases, Tenth Revision (ICD-10) Codes Used to Identify High-Risk Conditions, Defined as Medical Conditions That Make Patients Eligible For Respiratory Syncytial Virus (RSV) Prevention in Their 2nd RSV Season per the CDC Recommendations
eTable 8. Hospital Type, Size, and Location of All Pediatric ICU Encounters Between January 1st, 2017, and December 31st, 2019, Stratified by RSV Infection
eTable 9. Seasonality of All Pediatric ICU Encounters Between January 1st, 2017, and December 31st, 2019, Stratified by Age
eTable 10. Demographics and Outcomes for All Pediatric ICU Encounters Between January 1st, 2017, and June 1st, 2023, Stratified by Age
eTable 11. Demographics and Outcomes for All Pediatric ICU Encounters With RSV Between January 1st, 2017, and June 1st, 2023, Stratified by Positive Pressure Ventilation and Age
eFigure. Number of Encounters in Each United States Zip Code Region
eReferences.
Data Sharing Statement


