Abstract
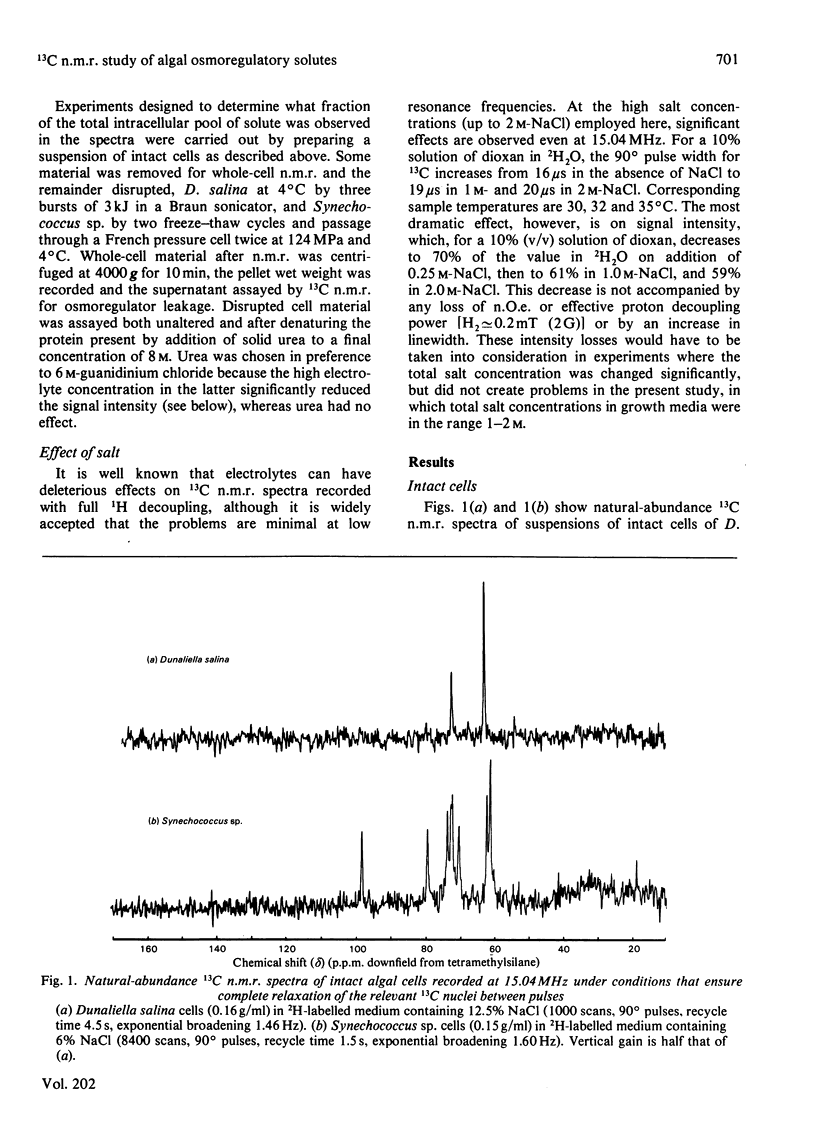
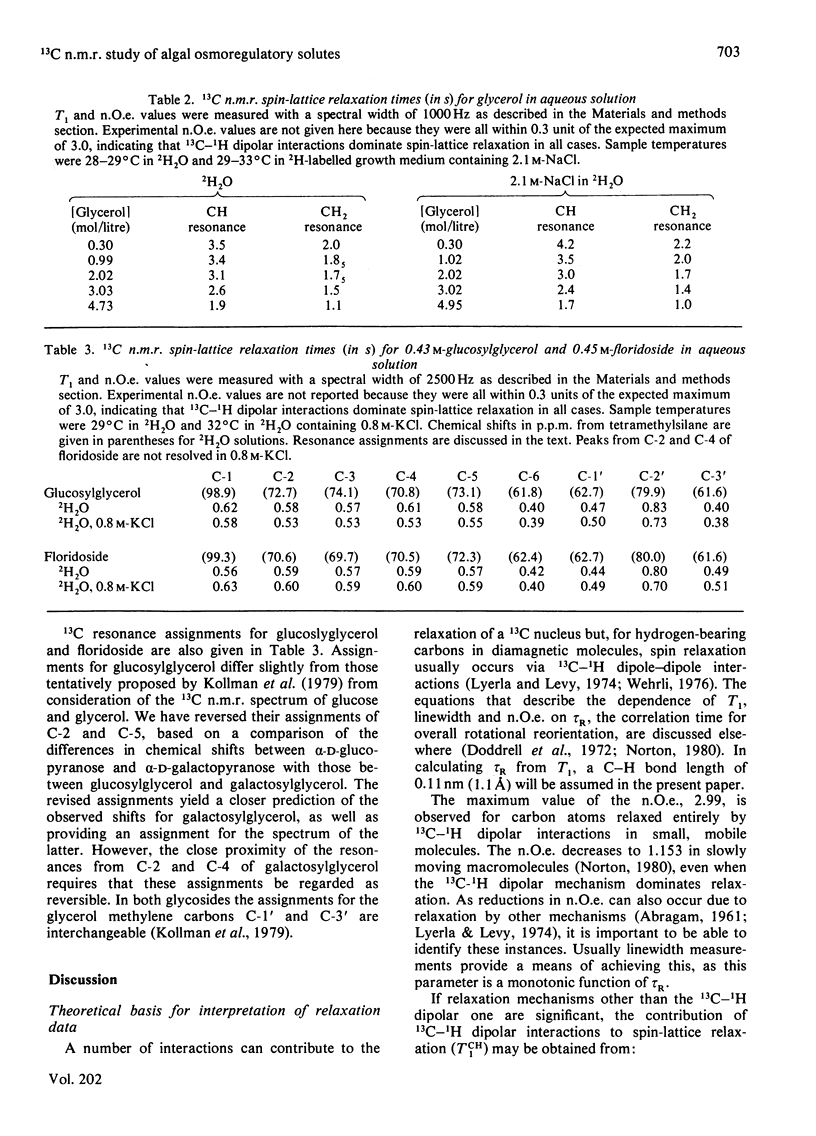
Natural-abundance 13C n.m.r. spin-lattice relaxation-time measurements have been carried out on intact cells of the unicellular blue--green alga Synechococcus sp. and the unicellular green alga Dunaliella salina, with the aim of characterizing the environments of the organic osmoregulatory solutes in these salt-tolerant organisms. In Synechococcus sp., all of the major organic osmoregulatory solute, 2-O-alpha-D-glucopyranosylglycerol, is visible in spectra of intact cells. Its rotational motion in the cell is slower by a factor of approx. 2.4 than in aqueous solution, but the molecule is still freely mobile and therefore able to contribute to the osmotic balance. In D. salina, only about 60% of the osmoregulatory solute glycerol is visible in spectra of intact cells. The rotational mobility of this observable fraction is approximately half that found in aqueous solution, but the data also indicate that there is a significant concentration of some paramagnetic species in D. salina which contributes to the overall spin-lattice relaxation of the glycerol carbon atoms. The non-observable fraction, which must correspond to glycerol molecules that have very broad 13C resonances and that are in slow exchange with bulk glycerol, has not been properly characterized as yet, but may represent glycerol in the chloroplast. The implications of these findings in relation to the physical state of the cytoplasm and the mechanism of osmoregulation in these cells are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowitzka L. J., Demmerle S., Mackay M. A., Norton R. S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980 Nov 7;210(4470):650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Viscosity of the internal aqueous phase of unilamellar phospholipid vesicles. Arch Biochem Biophys. 1980 Jul;202(2):650–652. doi: 10.1016/0003-9861(80)90473-7. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Johnson E. J., MacElroy R. D., Speer H. L., Bruff B. S. Effects of salts on the halophilic alga Dunaliella viridis. J Bacteriol. 1968 Apr;95(4):1461–1468. doi: 10.1128/jb.95.4.1461-1468.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Lusczakoski D. M., Simpson D. A. Internal microviscosity of red blood cells and hemoglobin-free resealed ghosts: a spin-label study. Biochemistry. 1979 Oct 30;18(22):5021–5029. doi: 10.1021/bi00589a033. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Wyssbrod H. R. Spin-lattice relaxation times for 13C in isotope-enriched glycine accumulated in frog muscle. Biophys J. 1977 Mar;17(3):255–267. doi: 10.1016/S0006-3495(77)85654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTNER I. J., PROVASOLI L. Artificial cultivation of a red-pigmented marine blue-green alga, Phormidium persicinum. J Gen Microbiol. 1958 Feb;18(1):190–197. doi: 10.1099/00221287-18-1-190. [DOI] [PubMed] [Google Scholar]
- Wilbur D. J., Norton R. S., Clouse A. O., Addleman R., Allerhand A. Determination of rotational correlation times of proteins in solution from carbon-13 spin-lattice relaxation measurements. Effect of magnetic field strength and anisotropic rotation. J Am Chem Soc. 1976 Dec 8;98(25):8250–8254. doi: 10.1021/ja00441a059. [DOI] [PubMed] [Google Scholar]
- Wydrzynski T. J., Marks S. B., Schmidt P. G., Govindjee, Gutowsky H. S. Nuclear magnetic relaxation by the manganese in aqueous suspensions of chloroplasts. Biochemistry. 1978 May 30;17(11):2155–2162. doi: 10.1021/bi00604a020. [DOI] [PubMed] [Google Scholar]


