Abstract
Background
Few antiviral therapies have been studied in patients with coronavirus disease 2019 (COVID-19) and kidney impairment. Herein, the efficacy, safety, and pharmacokinetics of remdesivir, its metabolites, and sulfobutylether-β-cyclodextrin excipient were evaluated in hospitalized patients with COVID-19 and severe kidney impairment.
Methods
In REDPINE, a phase 3, randomized, double-blind, placebo-controlled study, participants aged ≥12 years hospitalized for COVID-19 pneumonia with acute kidney injury, chronic kidney disease, or kidney failure were randomized 2:1 to receive intravenous remdesivir (200 mg on day 1; 100 mg daily up to day 5) or placebo (enrollment from March 2021 to March 2022). The primary efficacy end point was the composite of the all-cause mortality rate or invasive mechanical ventilation rate through day 29. Safety was evaluated through day 60.
Results
Although enrollment concluded early, 243 participants were enrolled and treated (remdesivir, n = 163; placebo, n = 80). At baseline, 90 participants (37.0%) had acute kidney injury (remdesivir, n = 60; placebo, n = 30), 64 (26.3%) had chronic kidney disease (remdesivir, n = 44; placebo, n = 20), and 89 (36.6%) had kidney failure (remdesivir, n = 59; placebo, n = 30); and 31 (12.8%) were vaccinated against COVID-19. Composite all-cause mortality or invasive mechanical ventilation rates through day 29 were 29.4% and 32.5% in the remdesivir and placebo group, respectively (P = .61). Treatment-emergent adverse events were reported in 80.4% for remdesivir versus 77.5% for placebo, and serious adverse events in 50.3% versus 50.0%, respectively. Pharmacokinetic plasma exposure to remdesivir was not affected by kidney function.
Conclusions
Although the study was underpowered, no significant difference in efficacy was observed between treatment groups. REDPINE demonstrated that remdesivir is safe in patients with COVID-19 and severe kidney impairment.
Clinical Trials Registration
EudraCT 2020-005416-22; Clinical Trials.gov NCT04745351.
Keywords: SARS-CoV-2, COVID-19, remdesivir, kidney impairment
Remdesivir was safe in hospitalized participants with COVID-19 and severe kidney impairment. No significant difference was found in composite all-cause mortality or invasive mechanical ventilation with remdesivir versus placebo, but the study was underpowered for these efficacy assessments.
The mortality risk of coronavirus disease 2019 (COVID-19) is high in patients with acute kidney injury (AKI) or chronic kidney disease (CKD), even those who are vaccinated [1, 2]. Individuals requiring treatment for COVID-19 often have preexisting CKD or experience AKI [3], yet uncertainty about the risks and benefits of antiviral therapy in patients with low estimated glomerular filtration rates (eGFRs) may limit their access to potentially life-saving therapies. Currently, few small-molecule antiviral treatment options are recommended for patients hospitalized with COVID-19 who have an eGFR <30 mL/min/1.73 m2.
Remdesivir is an antiviral that was approved before the availability of data collected through this trial for treating COVID-19 in patients with eGFR ≥30 mL/min/1.73 m2 who are either hospitalized or outpatients at risk for progression to severe disease [4]. When initially approved for the treatment of COVID-19, the pharmacokinetics and safety of remdesivir, its renally cleared metabolite (GS-441524), and its sulfobutylether-β-cyclodextrin (SBECD) excipient were not known in individuals with kidney impairment (Supplementary Figure 1) [5, 6]. The REDPINE trial was designed to investigate the efficacy, safety, and pharmacokinetics of remdesivir and its metabolites in hospitalized patients with severe COVID-19 and severe AKI, CKD, or kidney failure, including kidney transplant recipients.
METHODS
Trial Design and Oversight
REDPINE was a phase 3, randomized, double-blind, placebo-controlled, parallel-group study conducted internationally at 55 centers across 5 countries (Brazil, Portugal, Spain, the United Kingdom, and the United States; EudraCT registration no. 2020-005416-22; Clinical Trials.gov identifier NCT04745351). Trial protocols and amendments were approved by the ethics committee or institutional review board at each participating center, and the trial was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all participants or their legal representatives; in the case of emergency situations, participants (aged ≥18 years) could be enrolled under International Council of Harmonization E6(R2) 4.8.15 emergency use provisions by the investigator. An independent data monitoring committee evaluated safety outcomes. Data analysis was performed by the study sponsor.
Trial Participants and Procedures
Eligible participants were aged ≥12 years, weighed ≥40 kg, had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, were hospitalized with severe COVID-19, had oxygen saturation ≤94% with room air or required supplemental oxygen, and had either severely reduced kidney function (defined as eGFR <30 mL/min/1.73 m2 using the CKD Epidemiology Collaboration [CKD-EPI] equation [7], including individuals with kidney failure requiring chronic dialysis), or ongoing AKI (defined as a 50% increase in serum creatinine within 48 hours that was sustained for ≥6 hours, consistent with Kidney Disease: Improving Global Outcomes [KDIGO] criteria) [8]. Kidney or other solid-organ transplant recipients were permitted. Medications that had received emergency use authorization or regulatory approval, were broadly recommended for the treatment of hospitalized patients with COVID-19, and were medically appropriate in the investigator’s clinical opinion could be used in patients with severely reduced kidney function (eg, tocilizumab and systemic corticosteroids). Key exclusion criteria included requirements for invasive or noninvasive mechanical ventilation, extracorporeal membrane oxygenation, or dialysis for AKI (see Supplementary Table 1 for full inclusion and exclusion criteria).
Participants were randomly assigned (2:1) to receive intravenous remdesivir (200 mg on day 1, followed by 100 mg daily on days 2–5) or saline placebo to match [9], in addition to standard-of-care therapy. Participants were randomly assigned to treatment groups via the interactive voice or web response system in a 2:1 ratio using a stratified randomization schedule. Randomization was stratified by kidney failure requiring chronic dialysis, need for high-flow oxygen, and region (United States vs outside the United States). The first participant was screened on 31 March 2021; enrollment was halted on 31 March 2022 (last participant visit, 24 May 2022). Primary and secondary end points were evaluated through day 29. Investigators, participants, site personnel, and the sponsor were blinded to treatment.
Efficacy Assessments
The primary efficacy outcome was the composite of all-cause death or invasive mechanical ventilation through day 29. Secondary end points included all-cause death through day 29 and clinical status assessed by an 8-point ordinal scale [10] at days 15 and 29. Nasopharyngeal swab samples were collected until discharge at days 1, 3, 5, 7, 14, 21, and 29 to assess SARS-CoV-2 viral RNA copy number by means of quantitative reverse-transcription polymerase chain reaction.
Safety Assessments
Adverse events, serious adverse events, and adverse events leading to discontinuation of study intervention were collected daily for all participants until hospital discharge and by phone on days 29 and 60. Biochemical and hematologic evaluations were collected at screening and on days 1–29 until hospital discharge.
Pharmacokinetic Assessments
Sparse plasma samples were collected on day 2 (end of infusion and 4 hours after infusion), day 3 (before and 2 hours after infusion), and day 5 (middle of infusion and 6 hours after infusion).
Statistical Analysis
To achieve approximately 85% power to detect a hazard ratio (HR) of 0.70 at a 2-sided α level of .05 for the primary efficacy end point, enrollment of 1116 patients was planned. The study was halted early due to slow enrollment, including open-label use in the target population competing with trial enrollment; the study was thus underpowered for the primary efficacy end point. The primary analysis set for efficacy was the full analysis set (all participants who were randomly assigned and received ≥1 dose of study drug). The primary end point and key secondary end point (all-cause deaths through day 29) were analyzed using a stratified log-rank test according to the randomization strata. The HR and 95% confidence intervals (CIs) for all-cause mortality or invasive mechanical ventilation rates at day 29 were estimated using a Cox model with stratification factors as covariates. Participants with missing outcomes for any time-to-event analyses due to premature discontinuation before day 29 were censored at the date of the last known status for that end point.
Enrolled participants were also analyzed in the stratification subgroups of (1) ongoing AKI, with or without a history of CKD; (2) preexisting CKD without AKI; and (3) kidney failure requiring chronic dialysis. Clinical status by ordinal scale at days 15 and 29 was analyzed using a proportional odds model that included treatment as the independent variable. Adverse events, laboratory abnormalities, and pharmacokinetic exposures were summarized using descriptive statistics. The safety analysis set included randomized participants who received ≥1 dose of study drug. The virology analysis set included all participants who were randomized into the study, received ≥1 dose of remdesivir, and had detectable SARS-CoV-2 viral RNA copy number at baseline. Pharmacokinetic data were summarized using descriptive statistics.
RESULTS
Participants
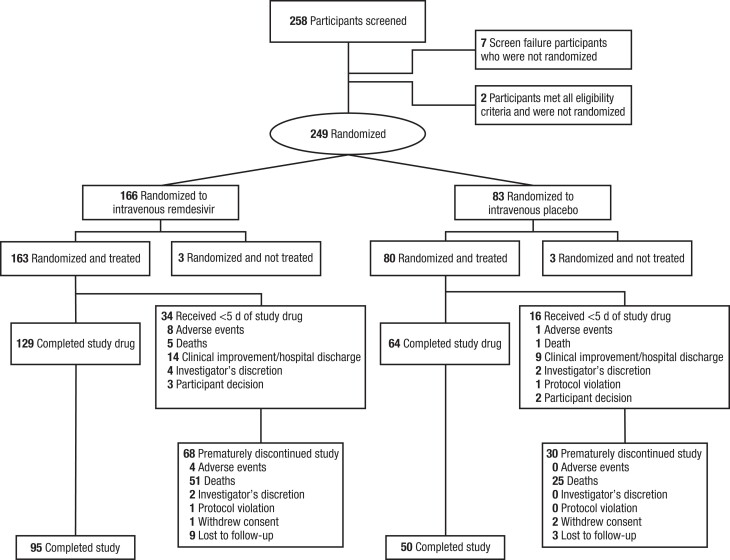
Of the 258 participants screened, 249 were randomly assigned, of whom 243 received ≥1 dose of study treatment (Figure 1). A total of 163 participants received remdesivir, and 80 received placebo. The mean interval (standard deviation) between COVID-19 symptom onset and administration of the first dose of study treatment was 5 (2.5) days (6 [2.5] days for the remdesivir and 5 [2.5] days for the placebo group). A total of 98 participants (40.3%) prematurely discontinued the study and were censored before day 60, mostly due to death (76 [31.3%]); discontinuation due to death before day 29 was captured in the primary end point. Few participants were lost to follow-up (12 [4.9%]), with 8 (3.3%) lost to follow-up before the day 29 primary end point (6 [3.7%] in the remdesivir and 2 [2.5%] in the placebo group). Fifty participants (20.6%) discontinued therapy early; common reasons for early discontinuation included clinical improvement/discharge (23 [9.5%]), adverse events (9 [3.7%]), and death (6 [2.5%]).
Figure 1.
Participant disposition. If participants did not meet all eligibility criteria, they were not randomized; the individual reasons for ineligibility were not captured. Two participants met all eligibility criteria and were not randomized at the investigator's discretion. The number of participants who completed the study corresponded to the number randomized and treated, excluding those who prematurely discontinued the study.
Demographics were balanced across both treatment groups, though the remdesivir group was younger (Table 1). At baseline, 90 participants (37.0%) had AKI (60 [36.8%] in the remdesivir and 30 [37.5%] in the placebo group), 64 (26.3%) had CKD (44 [27.0%] and 20 [25.0%], respectively), and 89 (36.6%) had kidney failure (59 [36.2%] and 30 [37.5%]). In total, 45 participants (18.5%) required high-flow oxygen, with no significant differences between treatment groups (P = .95). Overall, 31 participants (12.8%) were vaccinated against COVID-19 (21 [12.9%] in the remdesivir and 10 [12.5%] in the placebo group). There was imbalance introduced during randomization with more solid-organ transplant recipients assigned in the remdesivir than in the placebo group (35 [21.5%] vs 7 [8.8%], respectively). In all, 18 participants (7.4%) had received kidney transplants (13 [8.0%] in the remdesivir and 5 [6.3%] in the placebo group), 5 (2.1%) had received lung transplants (4 [2.5%] and 1 [1.3%], respectively), and 3 (1.2%) had received heart transplants (3 [1.8%] and 0).
Table 1.
Baseline Characteristics of Trial Participants in REDPINE in the Safety Analysis Set
| Characteristic | Participants, No. (%)a | P Value | ||
|---|---|---|---|---|
| Remdesivir (n = 163) |
Placebo (n = 80) |
Total (n = 243) |
||
| Age | ||||
| Mean (SD), y | 68 (14) | 71 (13) | 69 (14) | .054 |
| ≥18–64 y | 70 (42.9) | 22 (27.5) | 92 (37.9) | .02 |
| ≥65 y | 93 (57.1) | 58 (72.5) | 151 (62.1) | |
| Sex at birth | ||||
| Male | 92 (56.4) | 47 (58.8) | 139 (57.2) | .73 |
| Female | 71 (43.6) | 33 (41.3) | 104 (42.8) | |
| Raceb,c | ||||
| American Indian or Alaska Native | 1 (0.6) | 0 | 1 (0.4) | .89 |
| Asian | 4 (2.5) | 2 (2.6) | 6 (2.5) | |
| Black | 43 (26.7) | 18 (23.1) | 61 (25.5) | |
| White | 104 (64.6) | 55 (70.5) | 159 (66.5) | |
| Otherd | 11 (5.6) | 5 (3.8) | 16 (5.0) | |
| Ethnicityb | ||||
| Hispanic or Latino | 23 (14.6) | 8 (10.0) | 31 (13.0) | .32 |
| Not Hispanic or Latino | 135 (85.4) | 72 (90.0) | 207 (87.0) | |
| Not permittedd | 5 | 0 | 5 | |
| Body mass indexe | n = 158 | n = 77 | n = 235 | |
| Mean (SD) | 29.5 (7.1) | 28.9 (6.0) | 29.3 (6.8) | .63 |
| Kidney disease status | ||||
| AKI | 60 (36.8) | 30 (37.5) | 90 (37.0) | .95 |
| CKD | 44 (27.0) | 20 (25.0) | 64 (26.3) | |
| Kidney failure | 59 (36.2) | 30 (37.5) | 89 (36.6) | |
| Renal replacement therapy at baselinef | ||||
| Yes | 40 (24.5) | 21 (26.3) | 61 (25.1) | .77 |
| No | 123 (75.5) | 59 (73.8) | 182 (74.9) | |
| Serum creatinine by kidney disease statusg | ||||
| AKI | n = 60 | n = 30 | … | … |
| Mean (SD), mg/dL | 3.36 (1.8) | 3.45 (2.8) | … | .39 |
| CKD | n = 44 | n = 20 | … | … |
| Mean (SD), mg/dL | 3.28 (1.4) | 2.78 (0.8) | … | .17 |
| High-flow oxygen required | ||||
| Yes | 30 (18.4) | 15 (18.8) | 45 (18.5) | .95 |
| No | 133 (81.6) | 65 (81.3) | 198 (81.5) | |
| Clinical status (8-point ordinal scale)h | ||||
| Category 4 | 36 (22.1) | 18 (22.5) | 54 (22.2) | .99 |
| Category 5 | 97 (59.5) | 47 (58.8) | 144 (59.3) | |
| Category 6 | 30 (18.4) | 15 (18.8) | 45 (18.5) | |
| SARS-CoV-2 viral RNA copy number (NP swab sample) | n = 143 | n = 65 | n = 208 | |
| Mean (SD), log10 copies/mL | 5.80 (1.6) | 5.92 (1.7) | 5.84 (1.6) | .66 |
| Alanine aminotransferase | n = 161 | n = 80 | n = 241 | .98 |
| Mean (SD), U/L | 25 (20.9) | 28 (39.7) | 26 (28.5) | |
| History of solid organ transplant | ||||
| Yes | 35 (21.5) | 7 (8.8) | 42 (17.3) | .01 |
| No | 128 (78.5) | 73 (91.3) | 201 (82.7) | |
| Type of transplanti | ||||
| Kidney | 13 (8.0) | 5 (6.3) | 18 (7.4) | … |
| Lung | 4 (2.5) | 1 (1.3) | 5 (2.1) | |
| Heart | 3 (1.8) | 0 | 3 (1.2) | |
| Pancreas | 2 (1.2) | 0 | 2 (0.8) | |
| Kidney and pancreas | 1 (0.6) | 0 | 1 (0.4) | |
| Other immunocompromised statej | ||||
| Yes | 8 (4.9) | 1 (1.3) | 9 (3.7) | .16 |
| No | 155 (95.1) | 79 (98.8) | 234 (96.3) | |
| COVID-19 vaccine status at baseline | ||||
| Vaccinated | 21 (12.9) | 10 (12.5) | 31 (12.8) | .93 |
| Not vaccinated | 142 (87.1) | 70 (87.5) | 212 (87.2) | |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; NP, nasopharyngeal; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
aData represent no. (%) of participants unless otherwise specified.
bSelf-reported by participants.
cSome participants were not permitted to disclose their race or ethnicity because local regulators did not allow collection of such information. Values for those not permitted to disclose race or ethnicity were excluded from percentages.
dIncludes participants who were Native Hawaiian or Pacific Islander, other, or not permitted to disclose race.
eBody mass index calculated as weight in kilograms divided by height in meters squared.
fRenal replacement therapy occurring within the 3 days before the first dose of study treatment was considered renal replacement therapy at baseline; renal replacement therapy that occurred ≥3 days before or any time after the first dose was not.
gBaseline serum creatinine levels were the last available value recorded on or before dosing.
hCategory 1 was defined as not hospitalized with no limitation on activities; category 2, not hospitalized with limitations on activities and/or requirement for home oxygen; category 3, as hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (other than per-protocol remdesivir/saline as placebo administration); category 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care for COVID-19 (other than per-protocol remdesivir administration); category 5, hospitalized, with supplemental oxygen; category 6, hospitalized and on noninvasive ventilation or high-flow oxygen devices; category 7, hospitalized and receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and category 8, death. All participants had baseline scores of category 4, 5, or 6.
iThe numbers of participants with each type of transplant were not statistically compared between groups due to the small number of participants for each type.
jCoded based on high-level group term for “immunodeficiency syndromes,” using the Medical Dictionary for Regulatory Activities, version 25.0.
All participants received concomitant medications during the study. Full details regarding concomitant corticosteroid and monoclonal antibodies received by ≥1 participant during the study are provided in Supplementary Table 2. The most frequently administered concomitant medication was dexamethasone (108 [66.3%] in the remdesivir and 50 [62.5%] in the placebo group), and the most commonly used immunomodulatory monoclonal antibody therapy was tocilizumab (15 [9.2%] and 13 [16.3%], respectively).
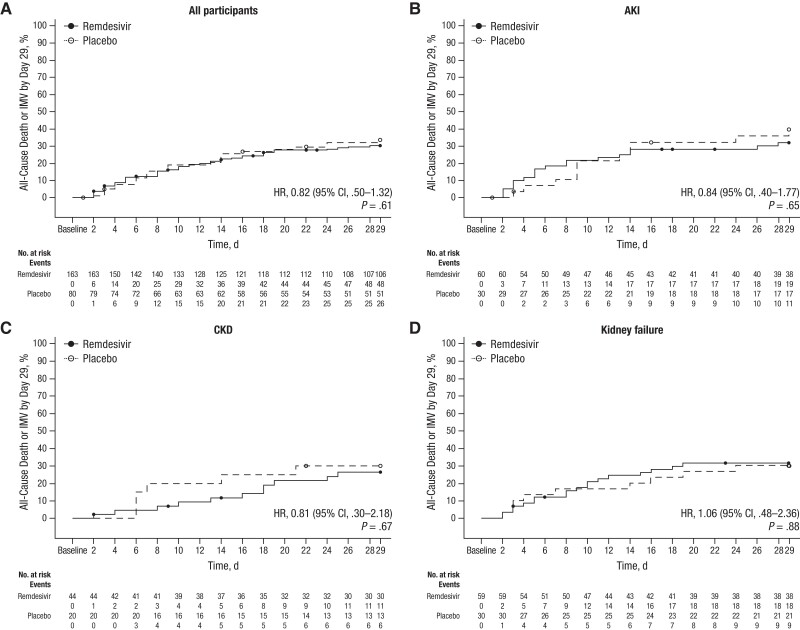
Efficacy
The primary analysis demonstrated no statistically significant differences in all-cause death or invasive mechanical ventilation rates by day 29 for participants in the remdesivir group compared with those in the placebo group (48 [29.4%] vs 26 [32.5%], respectively; HR, 0.82 [95% CI, .50–1.32]; P = .61) (Figure 2A). All-cause death by day 29 occurred in 41 (25.2%) and 23 (28.8%) participants in the remdesivir and placebo groups, respectively (HR, 0.83 [95% CI, .50–1.39]; P = .39). When outcomes were evaluated by kidney disease status, no significant differences were observed between the remdesivir and placebo groups for the primary efficacy end point (Figure 2B–2D and Supplementary Table 3). There was no significant difference between groups in the change of clinical status of participants on days 15 and 29, according to an 8-point ordinal scale (Supplementary Figure 2) or the SARS-CoV-2 viral RNA copy number over time (Supplementary Figure 3).
Figure 2.
Kaplan-Meier estimate of time to all-cause death or invasive mechanical ventilation (IMV). The number at risk represents the number of participants remaining at risk at the beginning of the interval. Participants who did not begin to receive IMV or die by day 29 were censored on their last study day or day 29, whichever was earlier. Abbreviations: AKI, acute kidney injury; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
Safety
The safety analysis set included 243 participants who received ≥1 dose of study treatment (163 in the remdesivir and 80 in the placebo group). Of these, 193 participants (79.4%) had ≥1 adverse event, including 131 (80.4%) in the remdesivir and 62 (77.5%) in the placebo group (Table 2). Overall, 151 participants (62.1%) had grade ≥3 adverse events, with similar frequencies for participants in the remdesivir and placebo groups (62.6% and 61.3%, respectively). Total adverse events considered related to the study drug were reported with a higher frequency for the remdesivir compared with the placebo group (13 [8.0%] vs 3 [3.8%], respectively), although serious adverse events were comparable (reported in 82 [50.3%] and 40 [50.0%], respectively). No serious adverse events were related to the study drug. Eight participants (4.9%) in the remdesivir group had adverse events leading to discontinuation, with 1 participant discontinuing due to a nonserious grade 4 adverse event (lipase increase) considered by the investigator to be related to the study drug; the remainder were not considered related to the study drug. One participant (1.3%) in the placebo group reported an adverse event leading to premature discontinuation of placebo.
Table 2.
Summary of Adverse Events
| Type of AEa | Participants, No. (%) | |
|---|---|---|
| Remdesivir (n = 163) |
Placebo (n = 80) |
|
| Any AE | 131 (80.4) | 62 (77.5) |
| Grade ≥3 AE | 102 (62.6) | 49 (61.3) |
| AE related to study drug | 13 (8.0) | 3 (3.8) |
| Grade ≥3 AE related to study drug | 2 (1.2) | 0 |
| SAE | 82 (50.3) | 40 (50.0) |
| SAE related to study drug | 0 | 0 |
| AE leading to premature discontinuation of study drug by preferred termb | 8 (4.9) | 1 (1.3) |
| AKI | 1 (0.6) | 0 |
| Acute pulmonary edema | 0 | 1 (1.3) |
| Delirium | 1 (0.6) | 0 |
| Lipase increased | 1 (0.6) | 0 |
| Multiple organ dysfunction syndrome | 1 (0.6) | 0 |
| Pneumonia | 1 (0.6) | 0 |
| Pyelonephritis acute | 1 (0.6) | 0 |
| Renal impairment | 1 (0.6) | 0 |
| Sepsis | 1 (0.6) | 0 |
| Subdural hematoma | 1 (0.6) | 0 |
| Treatment-emergent deathc | 47 (28.8) | 23 (28.8) |
| AEs occurring in ≥5% of participants by preferred term | ||
| Hypotension | 18 (11.0) | 4 (5.0) |
| Respiratory failure | 10 (6.1) | 10 (12.5) |
| Constipation | 12 (7.4) | 7 (8.8) |
| Acute respiratory failure | 13 (8.0) | 5 (6.3) |
| Hyperkalemia | 13 (8.0) | 3 (3.8) |
| Nausea | 12 (7.4) | 3 (3.8) |
| Atrial fibrillation | 10 (6.1) | 4 (5.0) |
| Metabolic acidosis | 8 (4.9) | 5 (6.3) |
| Anemia | 11 (6.7) | 1 (1.3) |
| Anxiety | 5 (3.1) | 7 (8.8) |
| Hyperglycemia | 7 (4.3) | 5 (6.3) |
| Hypoglycemia | 8 (4.9) | 4 (5.0) |
| Hypokalemia | 5 (3.1) | 7 (8.8) |
| Hypoxia | 8 (4.9) | 4 (5.0) |
| Hypertension | 4 (2.5) | 7 (8.8) |
| Agitation | 5 (3.1) | 4 (5.0) |
| COVID-19 pneumonia | 4 (2.5) | 4 (5.0) |
| Back pain | 3 (1.8) | 4 (5.0) |
| Headache | 3 (1.8) | 4 (5.0) |
| Hypomagnesemia | 3 (1.8) | 4 (5.0) |
Abbreviations: AE, adverse event; AKI, acute kidney injury; COVID-19, coronavirus disease 2019; SAE, serious AE.
aAEs were coded using the Medical Dictionary for Regulatory Activities, version 25.0. Severity grades were defined using the Division of AIDS Toxicity Grading Scale, version 2.1 (July 2017).
bParticipants could have >1 AE leading to premature discontinuation of study drug.
cDeaths that occurred between the first and last dose date plus 30 days (inclusive).
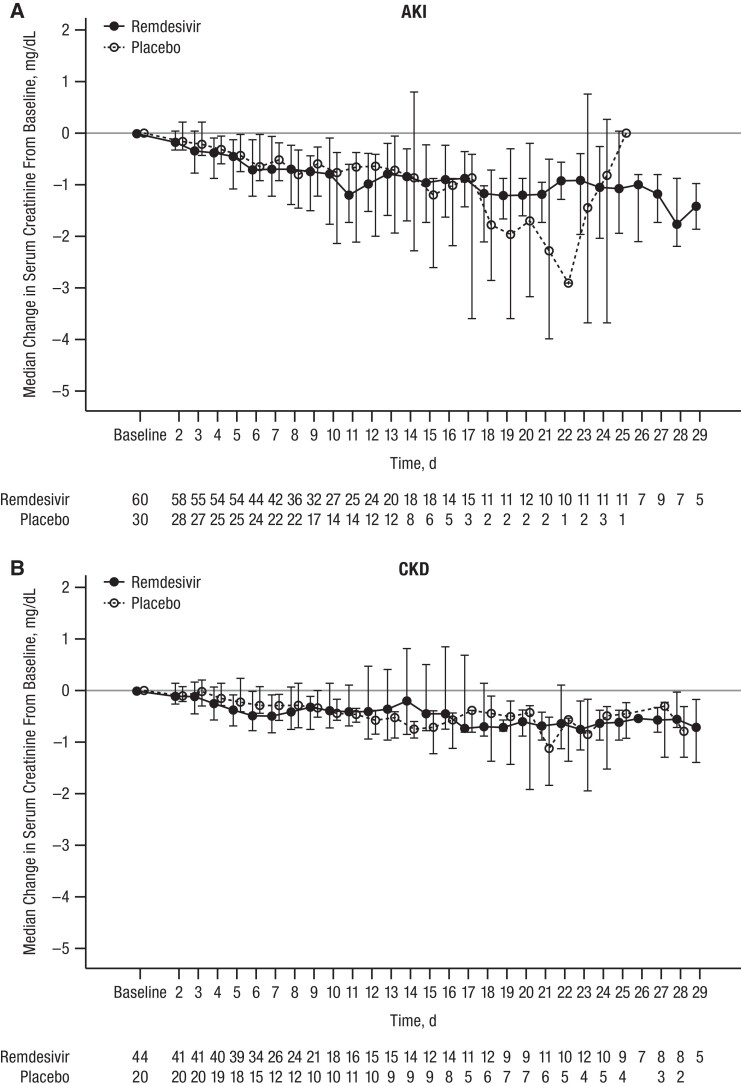
Treatment-emergent death occurred in 70 participants (28.8%), with equal frequency between groups, most commonly due to COVID-19, cardiac events, or respiratory events. Results for adverse events were similar when stratified by baseline kidney disease status, with similar percentages of grade ≥3 adverse events reported for participants in the remdesivir and placebo groups. Similar proportions of participants had baseline AKI (20 [33.3%] in the remdesivir and 12 [40.0%] in the placebo group; P = .32) or CKD (15 [34.1%] and 6 [30.0%], respectively; P = .81), or had new or progressive AKI, renal replacement therapy, or death by day 29 (Supplementary Table 4). Among those who were not receiving chronic dialysis at baseline, there were similar trends in median serum creatinine levels between baseline and day 29 for participants treated with remdesivir and placebo (Figure 3).
Figure 3.
Median (interquartile range) serum creatinine change in participants with (A) acute kidney injury (AKI) or (B) chronic kidney disease (CKD). Reference line represents no change from baseline (ie, y = 0). Kidney disease status categories are mutually exclusive; for participants without dialysis-treated chronic kidney failure at screening, the status was AKI in participants with ongoing AKI and CKD in those with a history of CKD.
Pharmacokinetics
Plasma concentrations of remdesivir and GS-704277 were generally consistent with those previously reported in adults receiving a clinical dose of remdesivir for 5 or 10 days [5]. GS-441524 and SBECD concentrations were increased, as expected, based on the predominant kidney elimination route of these analytes (Supplementary Table 5 and Supplementary Figure 4).
DISCUSSION
In the REDPINE study, participants with severely reduced kidney function who were hospitalized for COVID-19 exhibited no significant difference in rates of all-cause deaths or invasive mechanical ventilation by day 29 when treated with standard-dose remdesivir compared with placebo. With respect to safety, remdesivir was well tolerated, with no clinically relevant differences between the remdesivir and placebo groups observed. Furthermore, kidney-related adverse events and creatinine trends were similar between the remdesivir and placebo groups. Overall, no new safety signals were identified.
Enrollment was halted early when it became clear the study would not fully enroll, with many sites reporting increasingly frequent use of remdesivir in patients with kidney disease outside the study. Thus, the study was underpowered for the primary efficacy outcome. Nevertheless, the point estimate for difference in mortality rate for those treated with remdesivir versus placebo herein is similar to findings of other large retrospective analyses, including 2 studies demonstrating that remdesivir reduced inpatient mortality rates by 17% to 21% when comparing >24 000 and >67 000 remdesivir-exposed patients with matched controls [11, 12].
Due to a safety concern for potential accumulation of remdesivir metabolites or its excipient, SBECD, remdesivir was not recommended at the time of emergency use authorization in those with eGFRs <30 mL/min/1.73 m2 unless the potential benefit outweighed the potential risk [13]. The REDPINE study addresses a critical data gap by evaluating the efficacy, safety, and pharmacokinetics of remdesivir and its metabolites in participants with moderately and severely reduced kidney function, including those with kidney failure receiving dialysis. In earlier phases of the pandemic, monoclonal antibody therapy and convalescent plasma were used in patients with advanced kidney disease. However, loss of effectiveness of monoclonal antibodies against newer SARS-CoV-2 variants [14] and limited efficacy of convalescent plasma [15] now constrain their use.
In a trial in hospitalized adults with COVID-19, remdesivir reduced the median time to recovery compared with placebo, and the overall mortality rate among patients treated with remdesivir was 11.4%, compared with 15.2% among those treated with placebo [10]. In a trial of outpatients with risk factors for disease progression, remdesivir led to a large reduction in the risk of COVID-19–related hospitalization or death from any cause [16]. Finally, a large real-world analysis of >45 000 patients hospitalized with COVID-19, including >10 000 with kidney impairment, demonstrated that the initiation of remdesivir on hospital admission was associated with improved survival [17].
REDPINE included a broadly representative population of patients with severely reduced kidney function, including a high proportion of patients from racial minority groups (33.5%), solid-organ transplant recipients (11.9%), including kidney transplant recipients, and other immunocompromised participants (3.7%). REDPINE also provides key pharmacokinetic and safety data on SBECD. The total SBECD content in a 5-day course of remdesivir is 18 g, corresponding to an average of 51.4 mg/kg/d over 5 days for a 70-kg adult, well below the maximum safety dose of 250 mg/kg/d recommended by the European Medicines Agency safety review [4, 18]. We determined that SBECD pharmacokinetics after administration of remdesivir are consistent with previously published findings [6, 19–21]. These data are relevant to other medications that also use SBECD as an excipient, such as voriconazole [22].
The trial was limited by lower-than-planned enrollment. In addition, the concomitant use of immunomodulators, such as dexamethasone and tocilizumab, which were a part of the standard-of-care therapy that could have accompanied remdesivir, may have affected the true extent of remdesivir's viricidal effect.
In conclusion, though ultimately underpowered for efficacy, REDPINE demonstrated the safety of remdesivir in patients with COVID-19 and severe kidney impairment. No new safety signals were detected, and pharmacokinetic analyses suggest that dose adjustments are not required for remdesivir in individuals with COVID-19 and eGFR <30 mL/min/1.73 m2, whether due to AKI, CKD, or kidney failure, including dialysis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Meghan E Sise, Division of Nephrology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Jose Ramon Santos, Fight Infections Foundation, Service of Infectious Diseases, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Jason D Goldman, Swedish Center for Research and Innovation, Providence Swedish Medical Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA.
Katherine R Tuttle, Providence Medical Research Center, Providence Inland Northwest Health, Spokane, Washington, USA.
J Pedro Teixeira, Divisions of Nephrology and Pulmonary, Critical Care, and Sleep Medicine, University of New Mexico Hospital, Albuquerque, New Mexico, USA.
Allan F Seibert, Pulmonary Associates Research, Ascension Providence, Mobile, Alabama, USA.
Yiannis Koullias, Gilead Sciences, Foster City, California, USA.
Joe Llewellyn, Gilead Sciences, Foster City, California, USA.
Sean Regan, Gilead Sciences, Foster City, California, USA.
Yang Zhao, Gilead Sciences, Foster City, California, USA.
Hailin Huang, Gilead Sciences, Foster City, California, USA.
Robert H Hyland, Gilead Sciences, Foster City, California, USA.
Anu Osinusi, Gilead Sciences, Foster City, California, USA.
Helen Winter, Gilead Sciences, Foster City, California, USA.
Rita Humeniuk, Gilead Sciences, Foster City, California, USA.
Henry N Hulter, Department of Medicine, University of California, San Francisco, California, USA.
Robert L Gottlieb, Department of Internal Medicine, Baylor University Medical Center, Dallas, Texas, USA; Baylor Scott & White Research Institute, Dallas, Texas, USA.
Dahlene N Fusco, Department of Medicine, Tulane University School of Medicine, New Orleans, Louisiana, USA.
Rita Birne, Department of Nephrology, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal; NOVA Medical School, Lisbon, Portugal.
Fernando F Stancampiano, Department of Internal Medicine, Mayo Clinic College of Medicine and Science, Jacksonville, Florida, USA.
Claudia R Libertin, Department of Internal Medicine, Mayo Clinic College of Medicine and Science, Jacksonville, Florida, USA.
Catherine B Small, Department of Medicine, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA.
Markus Plate, Department of Medicine, Division of Infectious Diseases, Weill Cornell Medicine, New York, New York, USA.
Mark J McPhail, Institute of Liver Studies, King's College Hospital, London, United Kingdom.
for the REDPINE Investigators:
Rosa Ballesteros, Rita Birne, Luis Malheiro, Gil Silva, João Paulo Correia, Ana Vida, Andre Silva, Antonio Carujo, Moncef Belhassen Garcia, Jordi Carratala Fernandez, Gabriela Abelenda-Alonso, Josep M Cruzado, Alexander Rombauts, Diego A Sandoval, Miguel Garcia Deltoro, Fransesc Puchades Gimeno, Neus Gómez-Muñoz, Maria Martínez Roma, Juan Horcajada Gallego, Castañeda Pablo, Padilla Urrea Silvia, Rial Crestelo Sergio, Santos Fernandez David, Ramon Jose, Susanna Benet, Rosa Benítez, Carmen Bracke, Anna Chamorro, Sergio España, Fredzzia Graterol, Gemma LLadós, Cristina López, Lourdes Mateu, Roger Paredes, Boris Rebollo, Alba Romero, Laura Soldevila, Elena Abad, Anna Chamorro, Alba San José, Alex Soriano Viladomiu, Mark McPhail, Nicholas Medjeral-Thomas, Suzana Margareth Ajeje Lobo, Igor Abolnik, Anjali Acharya, Leland Allen, Keith A Bellovich, Mary Jane Burton, Miriam Cameron, Gerard J Criner, Lii-Yoong H Criner, Joseph Lambert, Marium Rashid, Heidi Shore-Brown, George A Diaz, David Dougherty, Nathaniel B Erdmann, Dahlene Fusco, Jason D Goldman, William Berrington, Christine Logar, Nidyanandh Vadivel, Allison Everett, Gonzalez Suarez Maria Lourdes, Robert L Gottlieb, Mezgebe Berhe, Gates Colbert, Christopher Hebert, Ankit Mehta, Cedric W Spak, Lorie Estrada, Richard Vargas, Jennifer Choe, Alex Pham, L Maria Mason, Catherine Tallmadge, Ariana Braddom, Maldonado Nicholas, Aayla Jamil, Ashley McAllister, Christina Guerra, Teena Sam, Edilia Solis, Deepa Gotur, Munish Goyal, Farrukh Koraishy, Brett Laurence, Vinay Malhotra, Luis A Manrique, James A McKinnell, Blaithin McMahon, Ruth Campbell, Caryn Morse, Jesus Navarro, Luis Ostrosky, Bela Patel, Carolyn Grimes, Maria Hernandez, Mehriban Mammadova, Laura Nielsen, Virginia Umana, Tobias Pusch, Philip Robinson, Arun J Sanyal, Harry Schrager, Jason Mallada, Allan F Seibert, Marc Siegel, Meghan Sise, Jihad Slim, Catherine Small, Peruvemba Sriram, Fernando Stancampiano, Joao Pedro Teixeira, Krystle D Apodaca, Michelle S Harkins, Amy G Cunningham, and Katherine R Tuttle
Notes
Acknowledgments. Medical writing and editorial support were provided by Laura Watts, PhD, of Lumanity Communications Inc. (Yardley, Pennsylvania, USA), and were funded by Gilead Sciences. Investigators thank the patients and their families for their participation
Author Contributions . M. E. S. and Y. K. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: M. E. S., K. R. T., J. L., A. O., R. H., H. N. H., and R. L. G. Acquisition, analysis, or interpretation of data: All authors. First draft of manuscript: M. E. S., Y. K., and R. H. Critical revision of the manuscript: All authors. Statistical analysis: Y. Z. and H. H. Supervision: M. E. S., Y. K., J. L., and R. H. H.
Role of the funder/sponsor. The funders/sponsors directed study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability . The study protocol and statistical analysis plan are available with the full text of this article. Study data will be made available to qualified external researchers on request.
Financial support. This work was supported by Gilead Sciences.
References
- 1. ERA-EDTA Council, ERACODA Working Group . Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell S, Campbell J, Lambourg E, et al. The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland. J Am Soc Nephrol 2022; 33:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marra F, Smolders EJ, El-Sherif O, et al. Recommendations for dosing of repurposed COVID-19 medications in patients with renal and hepatic impairment. Drugs R D 2021; 21:9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. VEKLURY® (remdesivir) [prescribing information] . Foster City, CA: Gilead Sciences, 2022. [Google Scholar]
- 5. Humeniuk R, Mathias A, Kirby BJ, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor. Clin Pharmacokinet 2021; 60:569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kiser TH, Fish DN, Aquilante CL, et al. Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care 2015; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kellum JA, Lameire N, Aspelin P, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2:1–138. [Google Scholar]
- 9. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med 2020; 383:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chokkalingam AP, Hayden J, Goldman JD, et al. Association of remdesivir treatment with mortality among hospitalized adults with COVID-19 in the United States. JAMA Netw Open 2022; 5:e2244505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffari E, Chandak A, Gottlieb RL, et al. Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants. Open Forum Infect Dis 2023; 10:ofad482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilead Sciences . Fact sheet for health care providers. Emergency use authorization (EUA) of VEKLURY® (remdesivir). Available at: https://www.samc.com/assets/documents/covid19/nursing/remdesivir_eua-hcp-fact-sheet-8-2020.pdf. Accessed 15 February 2023.
- 14. Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant. N Engl J Med 2022; 386:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Estcourt L, Callum J. Convalescent plasma for COVID-19—making sense of the inconsistencies. N Engl J Med 2022; 386:1753–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with coronavirus disease 2019 (COVID-19): a comparative analysis of in-hospital all-cause mortality in a large multicenter observational cohort. Clin Infect Dis 2022; 75:e450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Medicines Agency . Cyclodextrins used as excipients. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf. Accessed 10 October 2023.
- 19. Luke DR, Tomaszewski K, Damle B, Schlamm HT. Review of the basic and clinical pharmacology of sulfobutylether-β-cyclodextrin (SBECD). J Pharm Sci 2010; 99:3291–301. [DOI] [PubMed] [Google Scholar]
- 20. Luke DR, Wood ND, Tomaszewski KE, Damle B. Pharmacokinetics of sulfobutylether-β-cyclodextrin (SBECD) in subjects on hemodialysis. Nephrol Dial Transplant 2012; 27:1207–12. [DOI] [PubMed] [Google Scholar]
- 21. Hoover RK, Alcorn H Jr, Lawrence L, et al. Clinical pharmacokinetics of sulfobutylether-β-cyclodextrin in patients with varying degrees of renal impairment. J Clin Pharmacol, 2018; 58: 814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L, Zhang Z. Comment about the safety of intravenous voriconazole formulated with sulfobutylether beta-cyclodextrin. Exp Opin Drug Saf 2022; 21:133–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.